Abstract
Pressure generated by patient’s inspiratory muscles (Pmus) during assisted mechanical ventilation is of significant relevance. However, Pmus is not commonly measured since an esophageal balloon catheter is required. We have previously shown that Pmus can be estimated by measuring the electrical activity of the diaphragm (EAdi) through the Pmus/EAdi index (PEI). We investigated whether PEI could be reliably measured by a brief end-expiratory occlusion maneuver to propose an automated PEI measurement performed by the ventilator. Pmus, EAdi, airway pressure (Paw), and flow waveforms of 12 critically ill patients undergoing assisted mechanical ventilation were recorded. Repeated end-expiratory occlusion maneuvers were performed. PEI was measured at 100 ms (PEI0.1) and 200 ms (PEI0.2) from the start of the occlusion and compared to the PEI measured at the maximum Paw deflection (PEIoccl, reference). PEI0.1 and PEI0.2 tightly correlated with PEIoccl, (p < 0.001, R2 = 0.843 and 0.847). At a patient-level analysis, the highest percentage error was -64% and 50% for PEI0.1 and PEI0.2, respectively, suggesting that PEI0.2 might be a more reliable measurement. After correcting the error bias, the PEI0.2 percentage error was lower than ± 30% in all but one subjects (range − 39 to + 29%). It is possible to calculate PEI over a brief airway occlusion of 200 ms at inspiratory onset without the need for a full patient's inspiratory effort. Automated and repeated brief airway occlusions performed by the ventilator can provide a real time measurement of PEI; combining the automatically measured PEI with the EAdi trace could be used to continuously display the Pmus waveform at the bedside without the need of an esophageal balloon catheter.
Electronic supplementary material
The online version of this article (10.1007/s10877-020-00459-1) contains supplementary material, which is available to authorized users.
Keywords: Pressure generated by inspiratory muscles over electrical activity of the diaphragm index, Patient spontaneous breathing effort, Diaphragm neuromuscular efficiency, Patient inspiratory effort
Introduction
Assisted mechanical ventilation is commonly used for the treatment of critically ill patients, particularly when the acute phase is resolving and the patient needs to be weaned from the ventilator [1]. During assisted ventilation, patient’s inspiratory activity is partly reduced by the pressure generated by the ventilator [2]. However, patient’s inspiratory efforts occur and are difficult to control. They can lead to alveolar overdistension, reduction of airway pressure within the alveoli below the set peep level, and development of the pendelluft phenomenon [3, 4]. The esophageal balloon catheter is currently considered the gold standard to measure the pressure generated by the inspiratory muscles (Pmus), although other methods such as the analysis of the respiratory equation of motion are feasible [5, 6]. The measurement of the electrical activity of the diaphragm (EAdi) is available at the bedside through a specifically designed nasogastric catheter, allowing the physician to continuously monitor the patient’s main inspiratory muscle; however, no normal EAdi values are available due to the extreme variability among patients [7, 8].
We have previously demonstrated that it is possible to estimate the patient’s inspiratory effort (Pmus) during assisted ventilation without the need of an esophageal balloon catheter by measuring the Pmus/EAdi index (PEI), also known as neuromuscular efficiency index, and using the PEI to convert the EAdi waveform into an estimate of Pmus (Pmus ≈ PEI*EAdi) [9]. To measure PEI, a full inspiratory effort should occur during an end-expiratory occlusion maneuver lasting few seconds. In this condition, airway pressure deflections are due to Pmus and match esophageal pressure variations; therefore, the deflection measured on the airway pressure signal can be used to calculate PEI [10]. The PEI measured during occlusion is highly correlated with the PEI during assisted ventilation and could be used to convert the EAdi signal into a continuous Pmus signal [9]. We also showed that there is a high variability among patients, but in the same patient, the PEI is quite constant over intensive care unit stay [11].
In the present study, we hypothesize that it is possible to calculate PEI over a brief airway occlusion (100–200 ms) without the need for a full patient’s inspiratory effort. Performing a brief airway occlusion is widely accepted by clinicians; it is repeatable with no risk of harm to the patient and has been used for many years to measure P0.1, a marker of inspiratory effort [12, 13].
Methods
Patients
A cohort of 12 acute respiratory failure adult patients admitted to our intensive care unit who were intubated and undergoing assisted mechanical ventilation with PEEP > 5 cmH2O were included. Exclusion criteria were FiO2 > 60%, use of vasopressors, known chronic obstructive pulmonary disease, and Richmond agitation and sedation score > − 1. The patients were recruited for a previously published study, designed to investigate the PEI in patients undergoing assisted mechanical ventilation; detailed description of the population is available in the Sect. 2 of the previously published article [9].
Data acquisition
Briefly, the EAdi signal was measured by an esophageal NAVA catheter (Edi catheter, Getinge, Sweden). An esophageal balloon catheter was used to record the esophageal pressure trace depicted in Fig. 1; esophageal pressure was not considered in this study elsewhere. Airway pressure, flow, and EAdi, waveforms were recorded on a PC-based acquisition system by a dedicated software with an acquisition rate of 20 Hz (LabChart 7.0, ADInstruments, UK). The signals were directly imported from the ventilator; therefore, no additional synchronization was required after acquisition. Several end-expiratory occlusion maneuvers were performed during patients’ inspiratory efforts as follows: during expiration, the “expiratory hold” button was pressed and kept pressed until the patients completed an entire inspiratory effort, seen on the airway waveform as a negative deflection and a return towards the set peep value.
Fig. 1.

During an airway occlusion, patient’s inspiratory effort is equally reflected by airway pressure (Paw) deflections and esophageal pressure (Pes) variations; see Sect. 1 for further details. The thin continuous line is set at the beginning of the exemplary inspiratory effort (t0, see Sect. 2 section in the text for details) based on the airway pressure waveform. PEI0.1 and PEI0.2 were calculated as the ratio of the airway pressure deflection and electrical activity of the diaphragm (EAdi) increment at 100 and 200 ms from t0, respectively (dotted lines). The reference PEI value (PEIoccl in the text) was calculated at the maximum airway deflection point (dashed line)
Data analysis
We analyzed the recorded traces offline by the aforementioned waveform analysis software. We identified the end-expiratory occlusions by the zero-flow waveform and placed the t0 marker just before the beginning of the Paw deflection. More precisely, we identified the physiological variability of the Paw waveform during expiration in the 1-s period before the occlusion (e.g., 10.75–10.96 cmH2O, minimum value 10.75 cmH2O); the t0 marker was placed in proximity of the zero-flow point when the Paw waveform reached a value inferior of 0.1 cmH2O compared to the recorded minimum (e.g. t0 at 10.65 cmH2O). We then identified the t0.1 and t0.2 timepoints at 100 and 200 ms after t0 (Fig. 1). The last identified timepoint was at the maximum airway negative deflection during the end-expiratory airway occlusion maneuver and served for the calculation of the reference PEI (PEIoccl). On the same end-expiratory occlusion waveforms, PEI0.1 and PEI0.2 were calculated as the absolute value of the ratio between the deflection of the Paw (ΔPaw) and the increase of EAdi (ΔEAdi) waveforms at t0.1 and t0.2 as compared to t0.
Due to the aforementioned method to calculate the PEI ratio, a deflection of the airway pressure signal was always present at t0.1 and t0.2. We did not control for variations of the EAdi signal. Since the diaphragm is the major inspiratory muscle, during an airway occlusion ΔPaw and ΔEAdi usually occur simultaneously. However, in some cases such as the of activation of accessory inspiratory muscles or filtering of the electrocardiogram signal occurring during the first 100 or 200 ms, the increase of the EAdi signal might be delayed, resulting in abnormally high PEI values. Therefore, the definition of a ΔEAdi minimum cutoff value was mandatory to properly calculate PEI. We analyzed the variance of PEI values of the entire study population obtained at 100 and 200 ms by 0.05-µV intervals of ΔEAdi. Variance markedly increased for ΔEAdi lower than 0.3 µV, suggesting that the very small denominator (ΔEAdi) led to spurious increase of PEI (Figure S1, Electronic supplementary material 1). Thus, a minimum ΔEAdi of 0.3 µV was required to include the end-expiratory occlusion data in the analysis.
Statistical analysis
Due to the intrinsic variability of spontaneous breathing, the presence of intrasubject variability of measured PEI values is expected. Therefore, we opted for the analysis of the median PEI values at the three timepoints (PEIoccl, PEI0.1 and PEI0.2). Statistical analysis was performed using SPSS 17.0 (SPSS Inc). Data are reported as means ± standard deviation (SD) unless otherwise specified. Correlations were assessed by means of linear regression. Agreement between PEI0.1 or PEI0.2 and PEIoccl was tested by calculation of the systemic error (bias) and the 95% limits of agreement as bias ± 2 SD, as reported by Bland and Altman; percentage error was defined as PEI0.x − PEIoccl/[(PEI0.x + PEIoccl)/2] [14]. A p-value of < 0.05 was considered statistically significant. Artworks were created with SigmaPlot 11.0 (Systat Software, San Jose, CA, USA).
Results
We analyzed a total of 443 end-expiratory occlusions from the 12 patients enrolled, ranging from 31 to 53 occlusions per patient. Three-hundred and fourteen (314) maneuvers (71%) were considered suitable for PEI calculation, while the others were not included due to insufficient ΔEAdi (< 0.3 µV). Patients’ data and the number of included end-expiratory occlusions are reported in Table 1. In one patient among the 12 enrolled, adequate ΔEAdi was present only in one occlusion, while the other 37 occlusions could not be included in the analysis (ΔEAdi < 0.3 µV). However, PEI0.1 and PEI0.2 measured for this patient were similar as compared to PEIoccl (respectively 5.6 and 4.3 vs. 4.9 µV/cmH2O; corresponding to a percentage error of + 12% and − 14%).
Table 1.
Individual data for the included study patients
| Patient ID | Age, years | Diagnosis | PaO2/FiO2 | Cpl, mL/cmH2O | Analyzed occlusions | Included occlusions (%) |
|---|---|---|---|---|---|---|
| 1 | 78 | Septic shock | 363 | 54 | 44 | 40 (91) |
| 2 | 74 | ARDS | 255 | 36 | 33 | 28 (85) |
| 3 | 74 | Post-surgical | 233 | 45 | 31 | 29 (94) |
| 4 | 43 | ARDS | 198 | 30 | 32 | 12 (38) |
| 5 | 43 | ARDS | 241 | 52 | 32 | 23 (72) |
| 6 | 78 | Pneumonia | 126 | 55 | 38 | 1 (3) |
| 7 | 76 | Septic shock | 344 | 34 | 34 | 33 (97) |
| 8 | 54 | Septic shock | 185 | 30 | 35 | 33 (94) |
| 9 | 83 | Septic shock | 273 | 43 | 53 | 29 (55) |
| 10 | 58 | Septic shock | 240 | 40 | 36 | 24 (67) |
| 11 | 70 | Cardiac arrest | 189 | 40 | 38 | 30 (79) |
| 12 | 77 | Pneumonia | 163 | 65 | 32 | 32 (100) |
| Average | 67 | 232 | 44 | 37 | 26 | |
| SD | 14 | 69 | 11 | 6 | 10 |
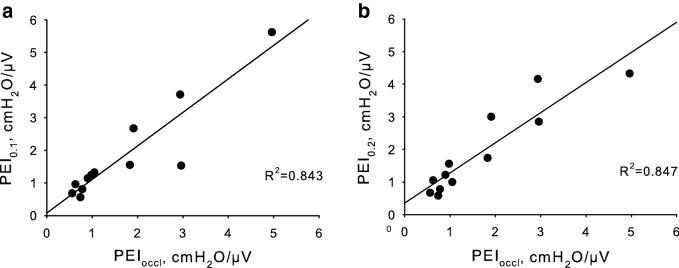
Considering the entire population, both the median PEI0.1 and PEI0.2 tightly correlated with the median PEIoccl (p < 0.001, R2 = 0.843 and 0.847, respectively, Fig. 2). However, at a patient-level analysis, the highest percentage error was − 64% and 50% for PEI0.1 and PEI0.2, respectively, suggesting that PEI0.2 might be a more reliable measurement. PEI0.2 was fairly related to the measured PEIoccl, as shown by the Bland–Altman plot (bias = 0.22, upper limit = 1.27, lower limit = − 0.82 cmH2O/µV, Fig. 3). The agreement between the two PEI measurements tended to be reduced at high PEI levels; the percentage error was instead more homogeneously spread between patients (Fig. 4).
Fig. 2.
The median Pmus/EAdi index measured in the first 100 ms of an end-expiratory airway occlusion maneuver (PEI0.1), is correlated with the same index measured at the maximum airway deflection (PEIoccl; p < 0.001, R2 = 0.843, panel a). Panel b shows the correlation between PEI measured at 200 ms (PEI0.2) and PEIoccl (p < 0.001, R2 = 0.847)
Fig. 3.

Bland–Altman plot for the PEI measured at 200 ms (PEI0.2) and the PEI measured at the maximum airway deflection (PEIoccl). Estimated error tended to increase for high PEI values
Fig. 4.
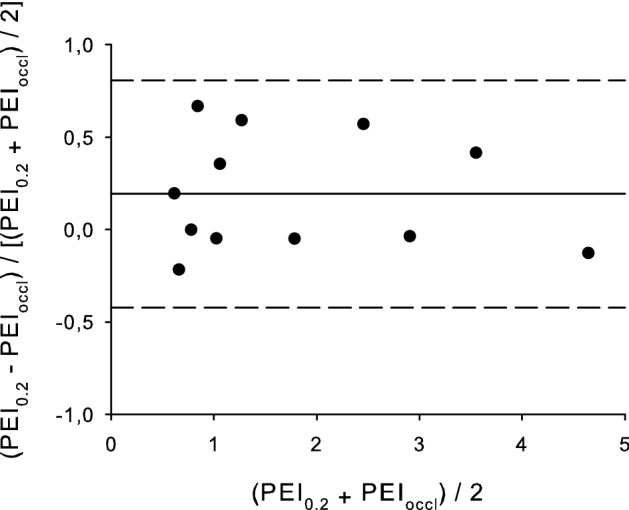
Percentage error Bland–Altman plot for the PEI measured at 200 ms (PEI0.2) and the PEI measured at the maximum airway deflection (PEIoccl)
Discussion
In the present study, we show that PEI median values measured at the beginning of an end-expiratory airway occlusion are similar to PEI values obtained after a prolonged end-expiratory occlusion. Therefore, a short end-expiratory occlusion maneuver could be used to estimate pressure generated by patient’s inspiratory muscles during ventilation by EAdi monitoring.
The PEI median value was calculated on approximately 70% of the analyzed occlusions, while the other 30% were discarded due to insufficient ΔEAdi (< 0.3 µV). As expected by definition of the timepoints, a minimum ΔPaw was always present, but the corresponding ΔEAdi was not always relevant. The phenomenon of Paw deflection without relevant increase of EAdi might be due both to EAdi signal processing and physiological reasons. The EAdi signal is automatically processed for ECG artifacts elimination, which may lead to a brief flattening of the EAdi waveform possibly masking an increase of EAdi in the first milliseconds of inspiratory efforts. Furthermore, the EAdi signal records the activity of the diaphragm, which is not the only inspiratory muscle; in theory, if the patients would inspire using only the accessory muscles, the EAdi signal would be flat. Another physiological reason could be the recruitment of different muscle groups, as we noticed in the patient who showed an intense use of the abdominal expiratory muscles at the clinical examination, resulting in the passive inflation of the respiratory system during the initial phase of the inspiration. Thus, in that specific condition, ΔPaw during the first 100–200 ms depended on the relaxation of the expiratory muscles rather than the activation of the diaphragm, resulting in insufficient ΔEAdi for adequate PEI calculation [15]. However, PEI measurement was accurate when ΔEAdi was higher than the proposed cutoff.
A previous report showed a high within-patient variability of the neuromuscular efficiency index, an index similar to the PEI, over repeated measures [16]. Thus, we focused on the median value, rather than the mean value, of the measurements collected in every patient to estimate the PEI during brief occlusions. Obtaining brief occlusions is feasible and safe for the patients: if the patient is ventilated with a pressure-based trigger, a brief occlusion with sufficient drop of the airway pressure is present at the beginning of every breath and is necessary to trigger the ventilator. Moreover, repeated brief occlusion is a widely used maneuver to measure P0.1, an index used for many years with no reports of any associated harms.
In our study, the PEI measured at 100 ms (PEI0.1) was less reliable, but the measurement of PEI at 200 ms (PEI0.2) showed a fair agreement with PEIoccl, the index we previously showed can be reliably used to transform the EAdi signal into a Pmus waveform. PEI0.2 tended to overestimate PEIoccl, therefore we suggest to implement a correcting factor to improve agreement: since a 20% overestimation bias was present, we propose that PEI0.2 should be corrected for a fixed factor (PEIoccl ≈ PEI0.2/1.2) for better PEIoccl estimation. Such correction led to a PEI0.2 percentage error lower than ± 30% in all but one subject (range − 39 to + 29%). The phenomenon of PEI overestimation for measurement performed early during an occlusion occurs also during non-occluded breathing, as previously described [9]. A PEIoccl estimation error of approximately 35% could be considered acceptable: translated into practice, the Pmus estimation error for a patient with PEI = 1 and EAdi peak = 5 µV would be lower than 2 cmH2O.
PEIoccl requires a manual prolonged end-expiratory occlusion to be measured, a maneuver considered safe but that generates patient’s discomfort and thus cannot be repeated often. Instead, PEI0.2 measurement could be embedded into the ventilator software, resulting in automated repeated measures providing a reliable value with no discomfort for the patient. For example, if the maneuver was automatically performed every two minutes, PEI could be robustly calculated over the last hour. Then, an estimation of the Pmus trace could be continuously displayed without the need of an esophageal balloon, possibly helping the clinician to modulate patient’s effort, maintaining a low level of effort in the acute phase to prevent alveolar overdistension and a higher level during the weaning phase to prevent diaphragm atrophy. The measurement of PEI by an occlusion longer than 200 ms might increase the agreement with PEIoccl; however, the measurement might not be repeatable many times without causing patient’s discomfort.
Pmus estimation could not be accurate in some patients, such as severe COPD patients who were excluded from our study to limit confounding factors. Patients affected by COPD usually present considerable levels of auto-peep; the presence of auto-peep is associated with a relevant ΔEadi, which is not immediately reflected in a detectable ΔPaw [17]. It is unknown whether PEI could be reliably measured by a short occlusion maneuver in patients affected by auto-peep.
Conclusions
The present study demonstrates that it is possible to calculate PEI over a brief airway occlusion of 200 ms at inspiratory onset without the need for a full patient's inspiratory effort. Automated and repeated brief airway occlusions performed by the ventilator could provide a real time measurement of PEI with little to no discomfort for the patient. PEI measured at 200 ms could be used in place of PEI measured during a full inspiratory effort to obtain a continuous display of pressure generated by patient’s inspiratory muscles, estimated by EAdi monitoring without the need of an esophageal balloon catheter.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Funding
Departmental funds, University of Milan-Bicocca.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Informed consent was obtained from all individual participants included in the study, according to the Institutional Ethical Committee recommendations.
Research involving human participants and/or animals
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Andrea Coppadoro, Email: andrea.coppadoro@gmail.com.
Roberto Rona, Email: roberto.rona@libero.it.
Giacomo Bellani, Email: giacomo.bellani1@unimib.it.
Giuseppe Foti, Email: giuseppe.foti@unimib.it.
References
- 1.Esteban A, Anzueto A, Frutos F, Alia I, Brochard L, Stewart TE, Benito S, Epstein SK, Apezteguia C, Nightingale P, Arroliga AC, Tobin MJ. Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28-day international study. JAMA. 2002;287(3):345–355. doi: 10.1001/jama.287.3.345. [DOI] [PubMed] [Google Scholar]
- 2.Brochard L, Harf A, Lorino H, Lemaire F. Inspiratory pressure support prevents diaphragmatic fatigue during weaning from mechanical ventilation. Am Rev Respir Dis. 1989;139(2):513–521. doi: 10.1164/ajrccm/139.2.513. [DOI] [PubMed] [Google Scholar]
- 3.Bellani G, Grasselli G, Teggia-Droghi M, Mauri T, Coppadoro A, Brochard L, Pesenti A. Do spontaneous and mechanical breathing have similar effects on average transpulmonary and alveolar pressure? A clinical crossover study. Crit Care. 2016;20(1):142. doi: 10.1186/s13054-016-1290-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoshida T, Torsani V, Gomes S, De Santis RR, Beraldo MA, Costa EL, Tucci MR, Zin WA, Kavanagh BP, Amato MB. Spontaneous effort causes occult pendelluft during mechanical ventilation. Am J Respir Crit Care Med. 2013;188(12):1420–1427. doi: 10.1164/rccm.201303-0539OC. [DOI] [PubMed] [Google Scholar]
- 5.ATS/ERS Statement on respiratory muscle testing (2002). Am J Respir Crit Care Med 166 (4):518–624. 10.1164/rccm.166.4.518 [DOI] [PubMed]
- 6.Akoumianaki E, Maggiore SM, Valenza F, Bellani G, Jubran A, Loring SH, Pelosi P, Talmor D, Grasso S, Chiumello D, Guerin C, Patroniti N, Ranieri VM, Gattinoni L, Nava S, Terragni PP, Pesenti A, Tobin M, Mancebo J, Brochard L. The application of esophageal pressure measurement in patients with respiratory failure. Am J Respir Crit Care Med. 2014;189(5):520–531. doi: 10.1164/rccm.201312-2193CI. [DOI] [PubMed] [Google Scholar]
- 7.Sinderby C, Navalesi P, Beck J, Skrobik Y, Comtois N, Friberg S, Gottfried SB, Lindstrom L. Neural control of mechanical ventilation in respiratory failure. Nat Med. 1999;5(12):1433–1436. doi: 10.1038/71012. [DOI] [PubMed] [Google Scholar]
- 8.Doorduin J, van Hees HW, van der Hoeven JG, Heunks LM. Monitoring of the respiratory muscles in the critically ill. Am J Respir Crit Care Med. 2013;187(1):20–27. doi: 10.1164/rccm.201206-1117CP. [DOI] [PubMed] [Google Scholar]
- 9.Bellani G, Mauri T, Coppadoro A, Grasselli G, Patroniti N, Spadaro S, Sala V, Foti G, Pesenti A. Estimation of patient's inspiratory effort from the electrical activity of the diaphragm. Crit Care Med. 2013;41(6):1483–1491. doi: 10.1097/CCM.0b013e31827caba0. [DOI] [PubMed] [Google Scholar]
- 10.Baydur A, Behrakis PK, Zin WA, Jaeger M, Milic-Emili J. A simple method for assessing the validity of the esophageal balloon technique. Am Rev Respir Dis. 1982;126(5):788–791. doi: 10.1164/arrd.1982.126.5.788. [DOI] [PubMed] [Google Scholar]
- 11.Bellani G, Coppadoro A, Pozzi M, Bronco A, Albiero D, Eronia N, Meroni V, Grasselli G, Pesenti A. The Ratio of Inspiratory Pressure Over Electrical Activity of the Diaphragm Remains Stable During ICU Stay and is not Related to Clinical Outcome. Respir Care. 2016;61(4):495–501. doi: 10.4187/respcare.04400. [DOI] [PubMed] [Google Scholar]
- 12.Kuhlen R, Hausmann S, Pappert D, Slama K, Rossaint R, Falke K. A new method for P0.1 measurement using standard respiratory equipment. Intensive Care Med. 1995;21(7):554–560. doi: 10.1007/BF01700159. [DOI] [PubMed] [Google Scholar]
- 13.Whitelaw WA, Derenne JP, Milic-Emili J. Occlusion pressure as a measure of respiratory center output in conscious man. Respir Physiol. 1975;23(2):181–199. doi: 10.1016/0034-5687(75)90059-6. [DOI] [PubMed] [Google Scholar]
- 14.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–310. doi: 10.1016/S0140-6736(86)90837-8. [DOI] [PubMed] [Google Scholar]
- 15.Doorduin J, Roesthuis LH, Jansen D, van der Hoeven JG, van Hees HWH, Heunks LMA. Respiratory Muscle Effort during Expiration in Successful and Failed Weaning from Mechanical Ventilation. Anesthesiology. 2018;129(3):490–501. doi: 10.1097/aln.0000000000002256. [DOI] [PubMed] [Google Scholar]
- 16.Jansen D, Jonkman AH, Roesthuis L, Gadgil S, van der Hoeven JG, Scheffer GJ, Girbes A, Doorduin J, Sinderby CS, Heunks LMA. Estimation of the diaphragm neuromuscular efficiency index in mechanically ventilated critically ill patients. Crit Care. 2018;22(1):238. doi: 10.1186/s13054-018-2172-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellani G, Coppadoro A, Patroniti N, Turella M, Arrigoni Marocco S, Grasselli G, Mauri T, Pesenti A. Clinical assessment of auto-positive end-expiratory pressure by diaphragmatic electrical activity during pressure support and neurally adjusted ventilatory assist. Anesthesiology. 2014;121(3):563–571. doi: 10.1097/aln.0000000000000371. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.