Abstract
Epstein–Barr virus (EBV) was the first human tumor virus being discovered and remains to date the only human pathogen that can transform cells in vitro. 55 years of EBV research have now brought us to the brink of an EBV vaccine. For this purpose, recombinant viral vectors and their heterologous prime-boost vaccinations, EBV-derived virus-like particles and viral envelope glycoprotein formulations are explored and are discussed in this review. Even so, cell-mediated immune control by cytotoxic lymphocytes protects healthy virus carriers from EBV-associated malignancies, antibodies might be able to prevent symptomatic primary infection, the most likely EBV-associated pathology against which EBV vaccines will be initially tested. Thus, the variety of EBV vaccines reflects the sophisticated life cycle of this human tumor virus and only vaccination in humans will finally be able to reveal the efficacy of these candidates. Nevertheless, the recently renewed efforts to develop an EBV vaccine and the long history of safe adoptive T cell transfer to treat EBV-associated malignancies suggest that this oncogenic γ-herpesvirus can be targeted by immunotherapies. Such vaccination should ideally implement the very same immune control that protects healthy EBV carriers.
Keywords: Virus-like particles, Recombinant viral vectors, Glycoprotein multimers, Infectious mononucleosis, Cytotoxic lymphocytes, Neutralizing antibodies, Prophylactic, Therapeutic, Lymphoma, Carcinoma
Importance of EBV as a vaccination target
The Epstein–Barr virus (EBV) is a common human γ-herpesvirus with the most potent host cell transforming capacity of all infectious disease agents in vitro [1]. It was discovered 55 years ago in Burkitt’s lymphoma [2, 3] and is associated with epithelial-, lymphocyte- and smooth muscle-derived tumors in humans [4]. The most prominent EBV-associated tumors are in addition to the still most common Sub-Saharan childhood tumor Burkitt’s lymphoma, post-transplant lymphoproliferative disease (PTLD), diffuse large B cell lymphomas (DLBCL), Hodgkin’s lymphoma, nasopharyngeal carcinoma and the 10% of gastric carcinoma that are positive for this virus [5]. Around 2% of all malignancies in humans are associated with EBV with an annual incidence rate of 200,000 [6]. In addition to these EBV-associated malignancies, this virus causes immune pathologies that result from a hyperactivation of EBV-induced T cell responses [7]. These include syndromes that result from CD8+ T cell lymphocytosis during symptomatic primary EBV infection called infectious mononucleosis (IM) [8], from virus-induced cytokine production for the hyperactivation of myeloid cells resulting in hemophagocytic lymphohistiocytosis (HLH) [9] and possibly also the autoimmune disease multiple sclerosis (MS) [10]. Along the lines of EBV possibly setting up a pro-inflammatory environment in the brain of some MS patients, it was recently reported that encephalitis in at least one patient under immune checkpoint treatment blocking the inhibitory receptor PD-1 on T cells was associated with elevated EBV loads in blood and cerebrospinal fluid, as well as clonal expansion of T cells with EBV-specific T cell receptors in the brain [11]. Accordingly, loss of EBV-specific T cell-mediated immune control was observed upon PD-1 blockade in a preclinical model of EBV infection in mice with reconstituted human immune system components (HIS mice) [12]. Thus, both EBV-associated malignancies and immune pathologies justify the development of a vaccine against EBV, but which individual or combination of viral antigens should be targeted.
For the choice of vaccine antigen, the life cycle of EBV and its gene expression in the various virus-associated diseases needs to be considered. EBV is primarily transmitted via saliva exchange and most likely crosses the mucosal epithelial cell barrier by transcytosis to infect B cells in submucosal secondary lymphoid tissues like tonsils [13, 14]. In B cells EBV expresses latent viral gene products from its circularized and increasingly chromatinized multi-copy extrachromosomal DNA [15]. Initial expression of six nuclear antigens (EBNAs) and two membrane proteins (LMPs) in the so called latency III program is curtailed with further B cell differentiation to just EBNA1, LMP1 and 2 (latency II) in germinal center B cells and to finally no viral protein expression in memory B cells (latency 0), the site of EBV persistence [16]. In homeostatically proliferating memory B cells, EBNA1 is transiently expressed as the only viral protein (latency I) [17]. The latent EBV proteins drive B cell proliferation allowing dissemination of the virus in the human body. From the reservoir in memory B cells EBV can reactivate upon plasma cell differentiation [18], and then presumably amplifies virion production by lytic replication in epithelial cells for more efficient shedding into the saliva and further transmission [19]. EBV-associated pathologies originate from these different stages of the EBV life cycle. For example, PTLD and some DLBCL express latency III, Hodgkin’s and Burkitt’s lymphoma emerge from germinal centers with latency II or latency I, respectively, and early lytic EBV antigen-specific CD8+ T cells expand mainly during IM. Furthermore, early lytic EBV antigen expression has recently been recognized to enhance virus-associated tumor formation [1]. These considerations identify latent and early lytic EBV antigens as promising candidates for vaccines, but also envelope proteins are explored as targets of neutralizing antibody responses that could curb transmission.
Protective immune responses against EBV infection
With the classes of EBV antigens that could be targeted for vaccination against EBV- associated diseases in mind, the question arises which type of immune responses should be elicited. Information about protective immune responses against EBV can be gleaned from preclinical in vitro and in vivo models and clinical observations. Among the most informative clinical observations are primary immunodeficiencies that identify genetic lesions that predispose for EBV-associated diseases [20, 21]. These point towards cytotoxic lymphocytes as the main immune compartment that exerts immune control over EBV infection. The respective lymphocytes need to be positive for the cytotoxic granule machinery, including perforin, Munc13-4 and Munc18-2 [22–24]. They need to carry the co-stimulatory molecules CD27, SLAM protein family members like 2B4, 4-1BB and NKG2D, as well as the co-inhibitory CTLA-4 receptor in combination with the main activating receptors CD16 or the T cell receptor [25–35]. Furthermore, they need to expand well after activation and depend on GATA2 and MCM4 for their differentiation [36–39]. In contrast, EBV-specific immune control does not seem to depend on type I and II interferons, antibody production and MHC class II restricted T cell responses [20]. Particularly the absence of EBV-associated pathologies in patients with B+ hypogammaglobunemia and Ig class-switch recombination deficiencies is surprising [40, 41]. Furthermore, since MHC class II deficiencies do not predispose for complications with EBV infection [42, 43], MHC class I-restricted helper T cell functions might compensate to maintain cytotoxic lymphocytes. These considerations point to CD8+ T cells, natural killer (NK), NKT and γδ T cells as pillars of EBV-specific immune control. Indeed, all of these cytotoxic lymphocyte populations have been shown to restrict EBV infection in the preclinical model of HIS mice [44–49]. In addition the EBV- specific CD8+ T cells might have a particular PD-1+Tim-3+KLRG1+CXCR5+TCF-1+ and BATF3+ phenotype that allows them to control EBV-infected B cells in germinal centers [12, 50, 51]. These CD8+ T cells recognize predominantly latent and early lytic EBV antigens [7]. T cell lines have also been adoptively transferred to treat EBV-associated malignancies, initially primarily PTLD [52]. With respect to individual antigens EBNA1, LMP1- and LMP2-specific T cell lines have proven clinically efficacious in EBV- associated lymphomas and nasopharyngeal carcinoma [53–55]. Interestingly, T cells with these specificities have also been infused into MS patients with some clinical success [56, 57]. Lytic EBV replication is in addition targeted by early differentiated CD56dimNKG2A+KIR− partially CD16+ NK cells [45, 58, 59]. Both CD8+ T cells and NK cells significantly expand during IM [8, 59–62]. In addition to early differentiated NK cells, Vγ8Vδ2 T cells are elevated in a subset of children [63]. They preferentially respond to Burkitt lymphoma cells with a latency I EBV gene expression. Finally, NKT cells preferentially respond to Hodgkin’s lymphoma and nasopharyngeal carcinoma cell lines [64]. Thus, while CD8+ T cells target all EBV latencies and early lytic EBV replication, NK, NKT and γδ T cells seem to restrict lytic, latency II and latency I EBV infection, respectively. These might be the cytotoxic lymphocyte compartments on which immune control of EBV infection depends and that should be stimulated by EBV-specific vaccination.
Recombinant viral vector vaccines
Recombinant viral vector vaccines are live viruses that are engineered to express additional proteins, against which immune responses are desired [65, 66]. These vaccine platforms are relatively new and have several advantages over traditional vaccines. First, viral vector vaccines can induce a broad range of immune responses, particularly in CD8+ cytotoxic T lymphocyte (CTL) responses that are important in clearing virally infected and tumor cells. This contrasts with most of the existing vaccine formulations that are designed to elicit primarily a humoral antibody response. The viral vector infects target cells and leads to antigen expression in the cytosol, where it can gain easy access to the classical MHC class I-processing pathway, and subsequent presentation of the resulting peptide epitopes on MHC class I molecules to stimulate an antigen-specific CD8+ CTL response. Second, viruses are naturally immunogenic and therefore adjuvants themselves as they express a range of pathogen-associated molecular patterns (PAMPs) to initiate an inflammatory response. This adjuvant effect is crucial for enhancing the protective immune response elicited by vaccines. Third, viral vector vaccines have a high gene transduction efficiency [67] and can deliver the antigens to different cell types depending on the tropism of the used viral vectors.
Many different viral vectors have been developed to use as vaccine candidates, including poxviruses, adenoviruses and yellow fever virus [65]. The choice of viral vectors for vaccine development mostly depends on the vector’s properties with respect to immunogenicity, safety and infectivity. Furthermore, the pre-existing immunity against the viral vectors in humans is often considered. Vaccinia virus and adenovirus are among the most widely used viral vectors, mainly due to their ability to induce antigen-specific T cell responses. Currently many clinical trials are ongoing to test diverse viral vector vaccines in different disease settings, mainly infectious diseases [68, 69].
The first EBV vaccine tested in humans used live recombinant vaccinia virus expressing the EBV membrane antigen BLLF1 (gp350) [70]. While there were no significant EBV titer variations between vaccinated and unvaccinated adults, only three of nine vaccinated infants were infected with EBV within 16 months after vaccination compared to ten out of ten in the unvaccinated control. However, this vaccine platform is no longer accepted due to the risk of adverse effects [71]. A safer alternative is the multiplication-incompetent-attenuated pox viral vector of modified vaccinia virus Ankara (MVA) [72, 73]. Indeed, a MVA vaccine encoding the EBV antigens EBNA1 and LMP2 (MVA-EL) has been developed as a therapeutic vaccine against EBV-positive cancer [74, 75]. This vaccine has been evaluated for safety and immunogenicity in phase I clinical trials in EBV-positive nasopharyngeal carcinoma (NPC) patients. MVA-EL was well tolerated and there was an increase in T cell responses against at least one antigen after vaccination in 8 of 14 patients in the UK and 15 of 18 patients in Hong Kong. However, the therapeutic efficacy of the MVA-EL has yet to be shown. A recombinant adenovirus vector has also been developed to induce EBV-specific T cell responses. However, instead of using it as a direct vaccination, facing pre-existing adenovirus immunity, the respective vectors encoding LMP polyepitopes with or without EBNA1 were used to infect DCs or EBV-transformed B lymphoblastoid cell lines in vitro, to either expand EBV-specific T cells and infuse these back into patients or to adoptively transfer the infected DCs as cellular vaccination [55, 76–78]. Considering the complexity of cellular vaccine approaches, adenovirus vectors that are shielded against pre-existing immunity and encoded EBV antigens should be explored for direct vaccination against EBV (Fig. 1).
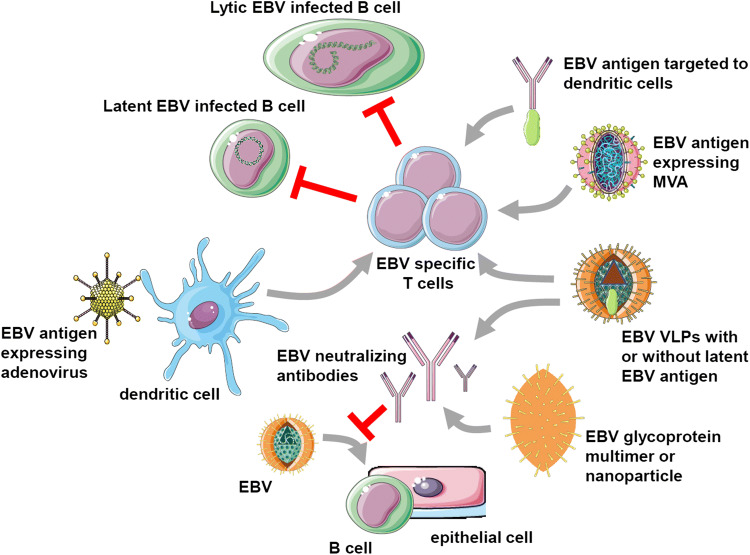
Fig. 1.
EBV vaccine candidates. EBV-specific vaccination aims to either stimulate protective T cell responses (top half) or neutralizing antibodies (bottom half), that target latent and lytic EBV-infected B cells or prevent B and epithelial cell infection, respectively. For EBV-specific T cell stimulation, recombinant adenoviruses encoding latent EBV antigens are explored for dendritic cell infection, followed by T cell expansion in vitro for adoptive transfer or injection into patients with EBV-associated malignancies. Furthermore, latent EBV antigen targeting to dendritic cells with antibodies is investigated. Moreover, recombinant modified vaccinia virus Ankara (MVA) vectors expressing latent EBV antigens have been developed and tested in patients. Finally, EBV-derived virus-like particles (VLPs) have shown promising results in preclinical models, lowering EBV titers when a latent EBV antigen was transgenically expressed in the viral tegument. Neutralizing antibodies were also elicited with VLPs or EBV envelope proteins. These antibody responses were more potent after multimerization of the respective glycoproteins or their incorporation into nanoparticles. This figure was created in part with modified Servier Medical Art templates, which are licensed under a Creative Commons Attribution 3.0 Unported License: https://smart.servier.com
Heterologous prime-boost vaccination
Early work on adenovirus vaccines used serotypes such as human adenovirus 5 (hAd5), but pre-existing immunity that can neutralize the viral vector is widespread in the human population, thus limiting its potency and hampering its clinical use. Chimpanzee adenovirus vectors were then developed to avoid this pre-existing neutralizing immunity [79, 80]. Unfortunately, the immunogenicity of these vectors can establish neutralizing responses that limit its capacity for secondary injections, requiring the use of different viral vectors during boost vaccination. Indeed, heterologous prime-boost strategies using two antigen formulations have been regarded as an improved way of immunization [81, 82].
Different combinations of heterologous prime-boost vaccines have been tested in animal models and some are undergoing efficacy testing in clinical trials, mainly against infectious diseases [82–84]. Among these, the combination of chimpanzee adenovirus and MVA has been shown to induce a strong CD8+ T cell response that correlates with efficacy in humans against a liver-stage malaria antigen [85]. The same strategy has been applied to vaccine development against additional diseases, including hepatitis C virus (HCV), Ebola virus and prostate cancer [86–88]. Our group has also demonstrated that adenovirus prime and MVA boost vaccination against EBNA1 are efficient in eliciting comprehensive CD4+ and CD8+ T cell responses which can translate into protection against EBV antigen expressing lymphomas [89].
Though viral vectors generally elicit a higher magnitude of T cell responses, they are expensive to produce and usually take a long time to manufacture. In contrast to these, protein-based vaccines are generally safer and cheaper to produce. Our lab has developed a vaccine platform to deliver the EBV antigen EBNA1 to antigen presenting cells by fusing the antigen to a monoclonal antibody against the DC endocytic receptor DEC-205 [90–92]. This recombinant protein vaccine, adjuvanted with the double-stranded RNA polyI:C, has been shown to induce robust T cell responses, but mostly CD4+ T cell responses and lacking CD8+ T cell responses when tested in vivo [89, 91]. As viral vector vaccines are known for their superiority in inducing CD8+ T cell responses, we combined this approach with viral vector vaccines to stimulate strong CD4+ and CD8+ T cell responses [89]. We have shown that this heterologous prime boost vaccination strategy is more efficient in inducing a protective T cell response than the homologous prime boost. The combination of the protein vaccine targeting DEC-205 and the adenovirus is only slightly less efficient than the adenovirus prime and MVA boost in protecting mice from T cell lymphoma challenge, with the later, however, being superior against B cell lymphomas. These findings are consistent with previous studies, indicating that human immunodeficiency virus (HIV) antigen targeting to DEC-205 had to be boosted with a recombinant poxviral vaccine to elicit protective responses in nonhuman primates [93]. Thus, heterologous prime-boost approaches should be considered in the future for the development of a vaccine against EBV that aims to elicit T cell mediated immune control.
Virus-like particles
Virus-like particles (VLPs) are defined as virus particles which do not contain any viral nucleic acids. The research efforts of the last decades led to the development of VLP vaccines, including human papillomavirus VLPs against cervical carcinoma and Plasmodium falciparum antigen displaying alfalfa mosaic virus VLPs against malaria [94, 95]. Because of their safety attributes and their ability to elicit virus-specific innate and adaptive immune responses without harming the host, VLPs were also investigated as versatile tools for EBV vaccine development.
In 2015, a novel Newcastle disease virus (NDV) VLP platform displaying the EBVgp350/220 ectodomain was shown to elicit strong, long-lasting neutralizing antibody responses in BALB/c mice, which were, however, not significantly higher than responses induced by soluble gp350/220 [96]. The NDV VLP platform was subsequently used to incorporate additional EBV envelope and latent antigens. The combination of gH/gL-EBNA1 and gB/LMP2 into VLPs both led to the generation of high neutralizing titers and EBV-specific T cell responses in vaccinated BALB/c mice [97]. A different, but possibly even more promising approach, is to use VLPs based on the EBV particle. To reduce oncogenicity of EBV for vaccination, genetic elements and/or proteins involved in DNA packaging were deleted [98]. Already 20 years ago, the first generation of cell lines that produce EBV VLPs was created by removing the terminal repeats (TRs), which previously had been identified as packaging signals of EBVs DNA [99–101]. Those first EBV VLPs were able to bind human B and epithelial cells and did contain large amounts of viral particles, but no viral DNA. In 2011, Ruiss et al. developed EBV-derived VLPs in which the deletion of TRs was complemented with the deletion of potential EBV oncogenes namely EBNA2, 3A, 3B and 3C, LMP1 and BZLF1 for additional safety [102]. Those EBV VLPs were shown to be assembled and released via the endosomal sorting complex for transport (ESCRT). Infected B cells were capable of presenting multiple EBV antigens to CD8+ and CD4+ T cells, which led to significant T cell expansions in vitro. In immunized BALB/c mice, the EBV VLPs elicited EBV-specific humoral and cellular immune responses [102].
Despite strong evidence of immune activation and a good safety profile in mice, the risk of remaining infectious oncogenic genomes in the early EBV VLPs remained high. Therefore, the development of EBV VLPs was further improved through the deletion of the viral packaging and nuclear egress proteins BFLF1/BFRF1A or the portal protein BBRF1 for viral DNA insertion into the capsid. In 2012, Pavlova et al. managed to create fully DNA-free EBV VLPs. The BFLF1/BFRF1A mutant EBV strain elicited comparable CD4+ T cell responses as the EBV wildtype in vitro [103]. Through these deletions, the pathogenic potential of the EBV VLPs was reduced, however the responses against structural and lytic components of EBV may not be sufficient for the creation of an effective EBV vaccine.
Therefore, more immunogenic EBV VLPs were created by fusing latent antigens such as EBNA1 and EBNA3C to the abundant major tegument protein BNRF1. Through this approach, the EBV VLPs were able to stimulate potent CD4+ T cell responses against structural as well as latent EBV epitopes. In ex vivo cultures with human peripheral blood mononuclear cells, the EBV VLPs, which contained EBNA1 latent EBV antigen, could inhibit the outgrowth of EBV-infected B cells more proficiently than their counterparts without latent antigen. This partial inhibition of EBV infection in B cells could also be shown in vivo in HIS mice, while 100% of the PBS-treated mice got infected after EBV challenge, only 14% of the VLP-EBNA1-immunized mice had detectable viral loads in their peripheral blood [104]. Therefore, EBV-derived VLPs might need to contain latent antigens in addition to the structural proteins to elicit protective immune responses. Despite the improved safety profile of EBV-derived VLPs themselves, the low titers of these that can be produced by most cell lines and contaminants in the respective preparations that derive from the human producer cells remain concerns for this vaccination approach.
Envelope protein formulations to elicit neutralizing antibodies
Gp350/220 is an EBV glycoprotein, which initiates the attachment of EBV to susceptible host, primarily B cells expressing the complement receptor type 2 (CD21) and/or type 1 (CD35) [105]. Binding is further strengthened by the gp42 envelope protein interacting with MHC class II [106]. While these glycoproteins are specific for EBV, fusion of the viral envelope with cellular membranes is finally mediated by the gH/gL and gB proteins that are conserved among the herpesviruses [107]. Being crucial in the first step of EBV latent infection, gp350/220 is one of the antigenic candidates often in the focus of exploration for the development of a prophylactic EBV vaccine. In the past, multiple potent antibodies against the EBV gp350 protein were found in human blood [108]. The neutralizing antibody that has been mainly characterized is the monoclonal 72A1 antibody. The broad interest in the 72A1 antibody led to the development of a humanized anti-gp350 antibody which blocked EBV infection of B cells in vitro to equivalent levels as the mouse-human chimeric 72A1 antibody construct [109]. However, immunizing with the gp350 protein alone did not lead to a prevention of infection with EBV in a phase II clinical trial, but only to a partial reduction of acute IM [110, 111]. Therefore, improvements of the gp350 protein vaccination were conceived [112] and dimers, trimers and tetramers of gp350 elicited significantly higher neutralizing antibody titers in mice [113, 114]. Multimerized gp350 therefore seems to elicit more potent B cell responses.
Improvement of gp350 protein vaccines was not only achieved by multimerization, but also by the addition of immune-stimulating adjuvants. A study of Heeke et al. included the use of GLA/SE as an adjuvant in addition to the vaccination with gp350 in mice and rabbits. GLA/SE is composed of the synthetic TLR4 agonist glucopyranosyl lipid A (GLA) integrated into a stable emulsion (SE). Mice and rabbits that were vaccinated with GLA/SE-adjuvanted gp350 vaccines showed elevated EBV-neutralizing antibody titers. Also, high IgG titers and robust anti-gp350 CD4+ T cell responses could be detected in vaccinated mice [115]. Furthermore, by epitope mapping, it was found that the immune response against EBV’s gp350 protein is mainly directed against one dominant neutralizing epitope of gp350. In an approach to focus the antibody response on this potent epitope, gp350 mimetic peptides with strong ionic, electrostatic or hydrogen bonds to the neutralizing region of the monoclonal antibody 72A1 [116] were generated by computer modeling [117]. In mice, those gp350 mimetic peptides elicited antibody responses that were able to block the interaction of 72A1 antibody and gp350. This technique may lead to more potent peptide vaccines which could contain the neutralizing epitopes of multiple EBV envelope glycoproteins.
In addition to improving EBV gp350-specific vaccination, antigen formulations have been extended to the other envelope proteins. Cui et al. compared the vaccination of rabbits with recombinant monomeric as well as multimeric EBV gH/gL and gB proteins to gp350 protein vaccines. The group found that vaccination with EBV gH/gL or gB protein vaccines elicited higher neutralization titers than gp350 protein vaccines [118]. These antibody titers were even increased when gH/gL and gB proteins were multimerized. Recently, Snijder et al. also used the proteins from the EBV fusion machinery as targets and the group isolated neutralizing human antibodies from memory B cells [119]. An anti-gH/gL antibody, AMMO1, showed potent inhibition of infection of B and epithelial cells in vitro. Therefore, vaccination for gp350 plus the herpesviral fusion complex might elicit the most comprehensive humoral immune responses to EBV,
Another promising approach for EBV vaccination, which also mainly focuses on the generation of neutralizing antibodies against viral glycoproteins, is the use of nanoparticles for the delivery of mutimerized and optimally spaced EBV antigens. In 2015, nanoparticles containing a portion of the ectodomain of gp350 including the complement receptor 2 binding site were used to vaccinate mice and monkeys [120]. Vaccinated mice developed anti-gp350 titers that were about 1000-fold higher than in mice that received a soluble monomeric gp350 vaccine and were protected against a challenge with vaccinia virus expressing gp350. Cynomolgus macaques immunized with the gp350 nanoparticles also generated anti-gp350 titers that were three- to ten-fold higher than with soluble monomeric gp350 protein [120]. More recently, the same group investigated the immunization of nonhuman primates with gH/gL- and gH/gL/gp42-based nanoparticles. Those highly immunogenic vaccines elicited virus-neutralizing antibody responses that were maintained for at least 3 months after vaccination. It could be shown that the vaccination-induced antibodies were able to inhibit the viral fusion with B and epithelial cells [121]. Because the vaccinated animals cannot be infected with EBV, it remains unclear whether these neutralizing antibody titers would inhibit EBV infection in vivo.
Conclusions and outlook
From the many approaches summarized above, it is clear that the time is ripe for vaccination against EBV-associated pathologies. From the frequent reinfections of healthy virus carriers with EBV [122, 123], it seems also clear that sterilizing immunity against EBV infection is probably utopic. Such immune protection would also have to be watertight, because if it would be transient and just delay primary EBV infection, the ensuing initial encounters with the virus would carry a higher risk for IM [8]. Therefore, establishing or maintaining immune control of asymptomatic persistent EBV infection should probably be the goal for EBV vaccination. In patients with already established EBV-associated malignancies, therapeutic vaccination might be an uphill battle due to established immunosuppressive mechanisms. Furthermore, prophylactic vaccination against these pathologies might be difficult to assess in initial clinical trials due to their low incidence rate, usually ranging below 50 per 100′000 individuals [4]. Therefore, the most likely scenario to test EBV-specific vaccine candidates is adolescents or young adults that are still EBV seronegative (around one third of this population) and who have a high risk to acquiring EBV with IM (30–50%) [8], followed by an increased risk for Hodgkin’s lymphoma and MS [124, 125]. Even so natural immune control of EBV primarily relies on cytotoxic lymphocytes [20, 21], vaccine-induced EBV-neutralizing antibodies could convert IM into asymptomatic infection, because the elevated viral shedding into the saliva and CD8+ T cell lymphocytosis driven by early lytic EBV antigens suggest that uncontrolled lytic replication contributes to IM [7]. Therefore, all the above discussed EBV vaccine candidates could prevent IM and provide the proof of concept that immunization against EBV is possible. In the end, however, vaccination or the endogenous immune response to asymptomatic EBV infection probably needs to establish long-lived immune control by cytotoxic CD8+ T cells, which form the required cornerstone of natural immunity to this tumor virus. Thus, the development of an EBV-specific vaccine offers the possibility to design formulations that selectively elicit such cell-mediated immune control, which then also could be adapted to tumors that are not associated with viruses.
Acknowledgements
Research in our laboratories is supported by Cancer Research Switzerland (KFS-4091-02-2017), KFSP-PrecisionMS , HMZ ImmunoTargET and Cancer Research Center Zurich of the University of Zurich, the Vontobel Foundation, the Baugarten Foundation, the Sobek Foundation, the Swiss Vaccine Research Institute, Roche, Novartis and the Swiss National Science Foundation (310030B_182827 and CRSII5_180323) to C.M., and Swiss National Science Foundation (P300P3_155374) to C.S.L.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Münz C. Latency and lytic replication in the oncogenesis of the Epstein Barr virus. Nat Rev Micobiol. 2019;17:691–700. doi: 10.1038/s41579-019-0249-7. [DOI] [PubMed] [Google Scholar]
- 2.Epstein MA, Achong BG, Barr YM. Virus particles in cultured lymphoblasts from Burkitt's lymphoma. Lancet. 1964;1:702–703. doi: 10.1016/s0140-6736(64)91524-7. [DOI] [PubMed] [Google Scholar]
- 3.Epstein MA, Henle G, Achong BG, Barr YM. Morphological and biological studies on a virus in cultured lymphoblasts from Burkitt's lymphoma. J Exp Med. 1964;121:761–770. doi: 10.1084/jem.121.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shannon-Lowe C, Rickinson A. The global landscape of EBV-associated tumors. Front Oncol. 2019;9:713. doi: 10.3389/fonc.2019.00713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kutok JL, Wang F. Spectrum of Epstein–Barr virus-associated diseases. Annu Rev Pathol. 2006;1:375–404. doi: 10.1146/annurev.pathol.1.110304.100209. [DOI] [PubMed] [Google Scholar]
- 6.Cohen JI, Fauci AS, Varmus H, Nabel GJ. Epstein–Barr virus: an important vaccine target for cancer prevention. Sci Transl Med. 2011;3(107):107fs7. doi: 10.1126/scitranslmed.3002878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor GS, Long HM, Brooks JM, Rickinson AB, Hislop AD. The immunology of Epstein–Barr virus-induced disease. Annu Rev Immunol. 2015;33:787–821. doi: 10.1146/annurev-immunol-032414-112326. [DOI] [PubMed] [Google Scholar]
- 8.Dunmire SK, Verghese PS, Balfour HH., Jr Primary Epstein–Barr virus infection. J Clin Virol. 2018;102:84–92. doi: 10.1016/j.jcv.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Al-Samkari H, Berliner N. Hemophagocytic lymphohistiocytosis. Annu Rev Pathol. 2018;13:27–49. doi: 10.1146/annurev-pathol-020117-043625. [DOI] [PubMed] [Google Scholar]
- 10.Ascherio A, Munger KL, Lunemann JD. The initiation and prevention of multiple sclerosis. Nat Rev Neurol. 2012;8(11):602–612. doi: 10.1038/nrneurol.2012.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson DB, McDonnell WJ, Gonzalez-Ericsson PI, Al-Rohil RN, Mobley BC, Salem JE, et al. A case report of clonal EBV-like memory CD4+ T cell activation in fatal checkpoint inhibitor-induced encephalitis. Nat Med. 2019;25(8):1243–1250. doi: 10.1038/s41591-019-0523-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chatterjee B, Deng Y, Holler A, Nunez N, Azzi T, Vanoaica LD, et al. CD8+ T cells retain protective functions despite sustained inhibitory receptor expression during Epstein–Barr virus infection in vivo. PLoS Pathog. 2019;15:e1007748. doi: 10.1371/journal.ppat.1007748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tugizov SM, Berline JW, Palefsky JM. Epstein–Barr virus infection of polarized tongue and nasopharyngeal epithelial cells. Nat Med. 2003;9(3):307–314. doi: 10.1038/nm830. [DOI] [PubMed] [Google Scholar]
- 14.Tugizov SM, Herrera R, Palefsky JM. Epstein–Barr virus transcytosis through polarized oral epithelial cells. J Virol. 2013;87(14):8179–8194. doi: 10.1128/JVI.00443-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frost TC, Gewurz BE. Epigenetic crossroads of the Epstein–Barr virus B-cell relationship. Curr Opin Virol. 2018;32:15–23. doi: 10.1016/j.coviro.2018.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thorley-Lawson DA. EBV persistence—introducing the virus. Curr Top Microbiol Immunol. 2015;390(Pt 1):151–209. doi: 10.1007/978-3-319-22822-8_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hochberg D, Middeldorp JM, Catalina M, Sullivan JL, Luzuriaga K, Thorley-Lawson DA. Demonstration of the Burkitt's lymphoma Epstein–Barr virus phenotype in dividing latently infected memory cells in vivo. Proc Natl Acad Sci USA. 2004;101(1):239–244. doi: 10.1073/pnas.2237267100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laichalk LL, Thorley-Lawson DA. Terminal differentiation into plasma cells initiates the replicative cycle of Epstein–Barr virus in vivo. J Virol. 2005;79(2):1296–1307. doi: 10.1128/JVI.79.2.1296-1307.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hutt-Fletcher LM. The long and complicated relationship between Epstein–Barr virus and epithelial cells. J Virol. 2017;91:1. doi: 10.1128/JVI.01677-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Latour S, Fischer A. Signaling pathways involved in the T-cell-mediated immunity against Epstein–Barr virus: lessons from genetic diseases. Immunol Rev. 2019;291(1):174–189. doi: 10.1111/imr.12791. [DOI] [PubMed] [Google Scholar]
- 21.Damania B, Münz C. Immunodeficiencies that predispose to pathologies by human oncogenic gamma-herpesviruses. FEMS Microbiol Rev. 2019;43:181–192. doi: 10.1093/femsre/fuy044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katano H, Ali MA, Patera AC, Catalfamo M, Jaffe ES, Kimura H, et al. Chronic active Epstein–Barr virus infection associated with mutations in perforin that impair its maturation. Blood. 2004;103(4):1244–1252. doi: 10.1182/blood-2003-06-2171. [DOI] [PubMed] [Google Scholar]
- 23.Rohr J, Beutel K, Maul-Pavicic A, Vraetz T, Thiel J, Warnatz K, et al. Atypical familial hemophagocytic lymphohistiocytosis due to mutations in UNC13D and STXBP2 overlaps with primary immunodeficiency diseases. Haematologica. 2010;95(12):2080–2087. doi: 10.3324/haematol.2010.029389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen JI, Niemela JE, Stoddard JL, Pittaluga S, Heslop H, Jaffe ES, et al. Late-onset severe chronic active EBV in a patient for five years with mutations in STXBP2 (MUNC18-2) and PRF1 (perforin 1) J Clin Immunol. 2015;35(5):445–448. doi: 10.1007/s10875-015-0168-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salzer E, Daschkey S, Choo S, Gombert M, Santos-Valente E, Ginzel S, et al (2012) Combined immunodeficiency with life-threatening EBV-associatedlymphoproliferative disorder in patients lacking functional CD27. Haematologica [DOI] [PMC free article] [PubMed]
- 26.Schwab C, Gabrysch A, Olbrich P, Patino V, Warnatz K, Wolff D, et al (2018) Phenotype, penetrance, and treatment of 133 CTLA-4-insufficient individuals. J Allergy Clin Immunol [DOI] [PMC free article] [PubMed]
- 27.van Montfrans JM, Hoepelman AI, Otto S, van Gijn M, van de Corput L, de Weger RA, et al. CD27 deficiency is associated with combined immunodeficiency and persistent symptomatic EBV viremia. J Allergy Clin Immunol. 2012;129(3):787e6–793e6. doi: 10.1016/j.jaci.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coffey AJ, Brooksbank RA, Brandau O, Oohashi T, Howell GR, Bye JM, et al. Host response to EBV infection in X-linked lymphoproliferative disease results from mutations in an SH2-domain encoding gene. Nat Genet. 1998;20(2):129–135. doi: 10.1038/2424. [DOI] [PubMed] [Google Scholar]
- 29.Nichols KE, Harkin DP, Levitz S, Krainer M, Kolquist KA, Genovese C, et al. Inactivating mutations in an SH2 domain-encoding gene in X-linked lymphoproliferative syndrome. Proc Natl Acad Sci U S A. 1998;95(23):13765–13770. doi: 10.1073/pnas.95.23.13765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sayos J, Wu C, Morra M, Wang N, Zhang X, Allen D, et al. The X-linked lymphoproliferative-disease gene product SAP regulates signals induced through the co-receptor SLAM. Nature. 1998;395(6701):462–469. doi: 10.1038/26683. [DOI] [PubMed] [Google Scholar]
- 31.Alosaimi MF, Hoenig M, Jaber F, Platt CD, Jones J, Wallace J, et al. Immunodeficiency and EBV-induced lymphoproliferation caused by 4–1BB deficiency. J Allergy Clin Immunol. 2019;144(2):574 e5–583 e5. doi: 10.1016/j.jaci.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Somekh I, Thian M, Medgyesi D, Gulez N, Magg T, Gallon Duque A, et al (2019) CD137 deficiency causes immune dysregulation with predisposition to lymphomagenesis. Blood [DOI] [PMC free article] [PubMed]
- 33.Chaigne-Delalande B, Li FY, O'Connor GM, Lukacs MJ, Jiang P, Zheng L, et al. Mg2+ regulates cytotoxic functions of NK and CD8 T cells in chronic EBV infection through NKG2D. Science. 2013;341(6142):186–191. doi: 10.1126/science.1240094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Vries E, Koene HR, Vossen JM, Gratama JW, von dem Borne AE, Waaijer JL, et al. Identification of an unusual Fc gamma receptor IIIa (CD16) on natural killer cells in a patient with recurrent infections. Blood. 1996;88(8):3022–3027. [PubMed] [Google Scholar]
- 35.Huck K, Feyen O, Niehues T, Ruschendorf F, Hubner N, Laws HJ, et al. Girls homozygous for an IL-2-inducible T cell kinase mutation that leads to protein deficiency develop fatal EBV-associated lymphoproliferation. J Clin Invest. 2009;119(5):1350–1358. doi: 10.1172/JCI37901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin E, Palmic N, Sanquer S, Lenoir C, Hauck F, Mongellaz C, et al. CTP synthase 1 deficiency in humans reveals its central role in lymphocyte proliferation. Nature. 2014;510(7504):288–292. doi: 10.1038/nature13386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rigaud S, Fondaneche MC, Lambert N, Pasquier B, Mateo V, Soulas P, et al. XIAP deficiency in humans causes an X-linked lymphoproliferative syndrome. Nature. 2006;444(7115):110–114. doi: 10.1038/nature05257. [DOI] [PubMed] [Google Scholar]
- 38.Biron CA, Byron KS, Sullivan JL. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med. 1989;320(26):1731–1735. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- 39.Eidenschenk C, Dunne J, Jouanguy E, Fourlinnie C, Gineau L, Bacq D, et al. A novel primary immunodeficiency with specific natural-killer cell deficiency maps to the centromeric region of chromosome 8. Am J Hum Genet. 2006;78(4):721–727. doi: 10.1086/503269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Durandy A, Kracker S, Fischer A. Primary antibody deficiencies. Nat Rev Immunol. 2013;13(7):519–533. doi: 10.1038/nri3466. [DOI] [PubMed] [Google Scholar]
- 41.Verma N, Grimbacher B, Hurst JR. Lung disease in primary antibody deficiency. Lancet Respir Med. 2015;3(8):651–660. doi: 10.1016/S2213-2600(15)00202-7. [DOI] [PubMed] [Google Scholar]
- 42.El Hawary RE, Mauracher AA, Meshaal SS, Eldash A, Abd Elaziz DS, Alkady R, et al. MHC-II deficiency among egyptians: novel mutations and unique phenotypes. J Allergy Clin Immunol Pract. 2019;7(3):856–863. doi: 10.1016/j.jaip.2018.07.046. [DOI] [PubMed] [Google Scholar]
- 43.Ouederni M, Vincent QB, Frange P, Touzot F, Scerra S, Bejaoui M, et al. Major histocompatibility complex class II expression deficiency caused by a RFXANK founder mutation: a survey of 35 patients. Blood. 2011;118(19):5108–5118. doi: 10.1182/blood-2011-05-352716. [DOI] [PubMed] [Google Scholar]
- 44.Strowig T, Gurer C, Ploss A, Liu YF, Arrey F, Sashihara J, et al. Priming of protective T cell responses against virus-induced tumors in mice with human immune system components. J Exp Med. 2009;206(6):1423–1434. doi: 10.1084/jem.20081720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chijioke O, Muller A, Feederle R, Barros MH, Krieg C, Emmel V, et al. Human natural killer cells prevent infectious mononucleosis features by targeting lytic Epstein–Barr virus infection. Cell Rep. 2013;5(6):1489–1498. doi: 10.1016/j.celrep.2013.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yuling H, Ruijing X, Li L, Xiang J, Rui Z, Yujuan W, et al. EBV-induced human CD8+ NKT cells suppress tumorigenesis by EBV-associated malignancies. Cancer Res. 2009;69(20):7935–7944. doi: 10.1158/0008-5472.CAN-09-0828. [DOI] [PubMed] [Google Scholar]
- 47.Xiang Z, Liu Y, Zheng J, Liu M, Lv A, Gao Y, et al. Targeted activation of human Vgamma9Vdelta2-T cells controls Epstein–Barr virus-induced B cell lymphoproliferative disease. Cancer Cell. 2014;26(4):565–576. doi: 10.1016/j.ccr.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 48.Zumwalde NA, Sharma A, Xu X, Ma S, Schneider CL, Romero-Masters JC, et al. Adoptively transferred Vgamma9Vdelta2 T cells show potent antitumor effects in a preclinical B cell lymphomagenesis model. JCI Insight. 2017;2:13. doi: 10.1172/jci.insight.93179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McHugh D, Caduff N, Murer A, Engelmann C, Deng Y, Zdimerova H, et al. Infection and immune control of human oncogenic gamma-herpesviruses in humanized mice. Philos Trans R Soc Lond B Biol Sci. 2019;374(1773):20180296. doi: 10.1098/rstb.2018.0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.He R, Hou S, Liu C, Zhang A, Bai Q, Han M, et al. Follicular CXCR5- expressing CD8+ T cells curtail chronic viral infection. Nature. 2016;537(7620):412–428. doi: 10.1038/nature19317. [DOI] [PubMed] [Google Scholar]
- 51.Leong YA, Chen Y, Ong HS, Wu D, Man K, Deleage C, et al. CXCR5+ follicular cytotoxic T cells control viral infection in B cell follicles. Nat Immunol. 2016;17(10):1187–1196. doi: 10.1038/ni.3543. [DOI] [PubMed] [Google Scholar]
- 52.Gottschalk S, Rooney CM. Adoptive T-cell immunotherapy. Curr Top Microbiol Immunol. 2015;391:427–454. doi: 10.1007/978-3-319-22834-1_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Icheva V, Kayser S, Wolff D, Tuve S, Kyzirakos C, Bethge W, et al. Adoptive transfer of Epstein–Barr virus (EBV) nuclear antigen 1-specific t cells as treatment for EBV reactivation and lymphoproliferative disorders after allogeneic stem-cell transplantation. J Clin Oncol. 2013;31(1):39–48. doi: 10.1200/JCO.2011.39.8495. [DOI] [PubMed] [Google Scholar]
- 54.Bollard CM, Tripic T, Cruz CR, Dotti G, Gottschalk S, Torrano V, et al. Tumor-specific T-cells engineered to overcome tumor immune evasion induce clinical responses in patients with relapsed Hodgkin lymphoma. J Clin Oncol. 2018;36(11):1128–1139. doi: 10.1200/JCO.2017.74.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith C, Tsang J, Beagley L, Chua D, Lee V, Li V, et al. Effective treatment of metastatic forms of Epstein–Barr virus-associated nasopharyngeal carcinoma with a novel adenovirus-based adoptive immunotherapy. Cancer Res. 2012;72(5):1116–1125. doi: 10.1158/0008-5472.CAN-11-3399. [DOI] [PubMed] [Google Scholar]
- 56.Pender MP, Csurhes PA, Smith C, Beagley L, Hooper KD, Raj M, et al. Epstein–Barr virus-specific adoptive immunotherapy for progressive multiple sclerosis. Mult Scler. 2014;20(11):1541–1544. doi: 10.1177/1352458514521888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pender MP, Csurhes PA, Smith C, Douglas NL, Neller MA, Matthews KK, et al. Epstein–Barr virus-specific T cell therapy for progressive multiple sclerosis. JCI Insight. 2018;3:22. doi: 10.1172/jci.insight.124714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pappworth IY, Wang EC, Rowe M. The switch from latent to productive infection in Epstein–Barr virus-infected B cells is associated with sensitization to NK cell killing. J Virol. 2007;81(2):474–482. doi: 10.1128/JVI.01777-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Azzi T, Lunemann A, Murer A, Ueda S, Beziat V, Malmberg KJ, et al. Role for early-differentiated natural killer cells in infectious mononucleosis. Blood. 2014;124(16):2533–2543. doi: 10.1182/blood-2014-01-553024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dunmire SK, Grimm JM, Schmeling DO, Balfour HH, Jr, Hogquist KA. The incubation period of primary Epstein–Barr virus infection: viral dynamics and immunologic events. PLoS Pathog. 2015;11(12):e1005286. doi: 10.1371/journal.ppat.1005286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Williams H, McAulay K, Macsween KF, Gallacher NJ, Higgins CD, Harrison N, et al. The immune response to primary EBV infection: a role for natural killer cells. Br J Haematol. 2005;129(2):266–274. doi: 10.1111/j.1365-2141.2005.05452.x. [DOI] [PubMed] [Google Scholar]
- 62.Hendricks DW, Balfour HH, Jr, Dunmire SK, Schmeling DO, Hogquist KA, Lanier LL. Cutting edge: NKG2ChiCD57+ NK cells respond specifically to acute infection with cytomegalovirus and not Epstein–Barr virus. J Immunol. 2014;192(10):4492–4496. doi: 10.4049/jimmunol.1303211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Djaoud Z, Guethlein LA, Horowitz A, Azzi T, Nemat-Gorgani N, Olive D, et al. Two alternate strategies for innate immunity to Epstein–Barr virus: one using NK cells and the other NK cells and gammadelta T cells. J Exp Med. 2017;214(6):1827–1841. doi: 10.1084/jem.20161017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chung BK, Tsai K, Allan LL, Zheng DJ, Nie JC, Biggs CM, et al. Innate immune control of EBV-infected B cells by invariant natural killer T cells. Blood. 2013;122(15):2600–2608. doi: 10.1182/blood-2013-01-480665. [DOI] [PubMed] [Google Scholar]
- 65.Ewer KJ, Lambe T, Rollier CS, Spencer AJ, Hill AV, Dorrell L. Viral vectors as vaccine platforms: from immunogenicity to impact. Curr Opin Immunol. 2016;41:47–54. doi: 10.1016/j.coi.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 66.Small JC, Ertl HC. Viruses—from pathogens to vaccine carriers. Curr Opin Virol. 2011;1(4):241–245. doi: 10.1016/j.coviro.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ura T, Okuda K, Shimada M. Developments in viral vector-based vaccines. Vaccines (Basel) 2014;2(3):624–641. doi: 10.3390/vaccines2030624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kallel H, Kamen AA. Large-scale adenovirus and poxvirus-vectored vaccine manufacturing to enable clinical trials. Biotechnol J. 2015;10(5):741–747. doi: 10.1002/biot.201400390. [DOI] [PubMed] [Google Scholar]
- 69.Nasar F, Matassov D, Seymour RL, Latham T, Gorchakov RV, Nowak RM, et al. Recombinant Isfahan virus and vesicular stomatitis virus vaccine vectors provide durable, multivalent, single-dose protection against lethal alphavirus challenge. J Virol. 2017;91:8. doi: 10.1128/JVI.01729-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gu SY, Huang TM, Ruan L, Miao YH, Lu H, Chu CM, et al. First EBV vaccine trial in humans using recombinant vaccinia virus expressing the major membrane antigen. Dev Biol Stand. 1995;84:171–177. [PubMed] [Google Scholar]
- 71.Casey CG, Iskander JK, Roper MH, Mast EE, Wen XJ, Torok TJ, et al. Adverse events associated with smallpox vaccination in the United States, January–October 2003. JAMA. 2005;294(21):2734–2743. doi: 10.1001/jama.294.21.2734. [DOI] [PubMed] [Google Scholar]
- 72.Sanchez-Sampedro L, Perdiguero B, Mejias-Perez E, Garcia-Arriaza J, Di Pilato M, Esteban M. The evolution of poxvirus vaccines. Viruses. 2015;7(4):1726–1803. doi: 10.3390/v7041726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Parrino J, McCurdy LH, Larkin BD, Gordon IJ, Rucker SE, Enama ME, et al. Safety, immunogenicity and efficacy of modified vaccinia Ankara (MVA) against Dryvax challenge in vaccinia-naive and vaccinia-immune individuals. Vaccine. 2007;25(8):1513–1525. doi: 10.1016/j.vaccine.2006.10.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Taylor GS, Jia H, Harrington K, Lee LW, Turner J, Ladell K, et al. A recombinant modified vaccinia ankara vaccine encoding Epstein–Barr virus (EBV) target antigens: a phase I trial in UK patients with EBV-positive cancer. Clin Cancer Res. 2014;20(19):5009–5022. doi: 10.1158/1078-0432.CCR-14-1122-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hui EP, Taylor GS, Jia H, Ma BB, Chan SL, Ho R, et al. Phase I trial of recombinant modified vaccinia ankara encoding Epstein–Barr viral tumor antigens in nasopharyngeal carcinoma patients. Cancer Res. 2013;73(6):1676–1688. doi: 10.1158/0008-5472.CAN-12-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chia WK, Wang WW, Teo M, Tai WM, Lim WT, Tan EH, et al. A phase II study evaluating the safety and efficacy of an adenovirus-DeltaLMP1-LMP2 transduced dendritic cell vaccine in patients with advanced metastatic nasopharyngeal carcinoma. Ann Oncol. 2012;23(4):997–1005. doi: 10.1093/annonc/mdr341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bollard CM, Gottschalk S, Torrano V, Diouf O, Ku S, Hazrat Y, et al. Sustained complete responses in patients with lymphoma receiving autologous cytotoxic T lymphocytes targeting Epstein–Barr virus latent membrane proteins. J Clin Oncol. 2014;32(8):798–808. doi: 10.1200/JCO.2013.51.5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Smith C, Cooper L, Burgess M, Rist M, Webb N, Lambley E, et al. Functional reversion of antigen-specific CD8+ T cells from patients with Hodgkin lymphoma following in vitro stimulation with recombinant polyepitope. J Immunol. 2006;177(7):4897–4906. doi: 10.4049/jimmunol.177.7.4897. [DOI] [PubMed] [Google Scholar]
- 79.Capone S, D'Alise AM, Ammendola V, Colloca S, Cortese R, Nicosia A, et al. Development of chimpanzee adenoviruses as vaccine vectors: challenges and successes emerging from clinical trials. Expert Rev Vaccines. 2013;12(4):379–393. doi: 10.1586/erv.13.15. [DOI] [PubMed] [Google Scholar]
- 80.Dicks MD, Spencer AJ, Edwards NJ, Wadell G, Bojang K, Gilbert SC, et al. A novel chimpanzee adenovirus vector with low human seroprevalence: improved systems for vector derivation and comparative immunogenicity. PLoS ONE. 2012;7(7):e40385. doi: 10.1371/journal.pone.0040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lu S. Heterologous prime-boost vaccination. Curr Opin Immunol. 2009;21(3):346–351. doi: 10.1016/j.coi.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kardani K, Bolhassani A, Shahbazi S. Prime-boost vaccine strategy against viral infections: mechanisms and benefits. Vaccine. 2016;34(4):413–423. doi: 10.1016/j.vaccine.2015.11.062. [DOI] [PubMed] [Google Scholar]
- 83.Paris RM, Kim JH, Robb ML, Michael NL. Prime-boost immunization with poxvirus or adenovirus vectors as a strategy to develop a protective vaccine for HIV-1. Expert Rev Vaccines. 2010;9(9):1055–1069. doi: 10.1586/erv.10.106. [DOI] [PubMed] [Google Scholar]
- 84.Musich T, Robert-Guroff M. New developments in an old strategy: heterologous vector primes and envelope protein boosts in HIV vaccine design. Expert Rev Vaccines. 2016;15(8):1015–1027. doi: 10.1586/14760584.2016.1158108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ewer KJ, O'Hara GA, Duncan CJ, Collins KA, Sheehy SH, Reyes-Sandoval A, et al. Protective CD8+ T-cell immunity to human malaria induced by chimpanzee adenovirus-MVA immunisation. Nat Commun. 2013;4:2836. doi: 10.1038/ncomms3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Swadling L, Capone S, Antrobus RD, Brown A, Richardson R, Newell EW, et al. A human vaccine strategy based on chimpanzee adenoviral and MVA vectors that primes, boosts, and sustains functional HCV-specific T cell memory. Sci Transl Med. 2014;6(261):261ra153. doi: 10.1126/scitranslmed.3009185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Venkatraman N, Ndiaye BP, Bowyer G, Wade D, Sridhar S, Wright D, et al. Safety and immunogenicity of a heterologous prime-boost ebola virus vaccine regimen in healthy adults in the United Kingdom and Senegal. J Infect Dis. 2019;219(8):1187–1197. doi: 10.1093/infdis/jiy639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cappuccini F, Pollock E, Stribbling S, Hill AVS, Redchenko I. 5T4 oncofoetal glycoprotein: an old target for a novel prostate cancer immunotherapy. Oncotarget. 2017;8(29):47474–47489. doi: 10.18632/oncotarget.17666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ruhl J, Citterio C, Engelmann C, Haigh TA, Dzionek A, Dreyer JH, et al. Heterologous prime-boost vaccination protects from EBV antigen expressing lymphomas. J Clin Invest. 2019;129:2071–2087. doi: 10.1172/JCI125364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gurer C, Strowig T, Brilot F, Pack M, Trumpfheller C, Arrey F, et al. Targeting the nuclear antigen 1 of Epstein Barr virus to the human endocytic receptor DEC-205 stimulates protective T-cell responses. Blood. 2008;112:1231–1239. doi: 10.1182/blood-2008-03-148072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Leung CS, Maurer MA, Meixlsperger S, Lippmann A, Cheong C, Zuo J, et al. Robust T-cell stimulation by Epstein–Barr virus-transformed B cells after antigen targeting to DEC-205. Blood. 2013;121(9):1584–1594. doi: 10.1182/blood-2012-08-450775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Meixlsperger S, Leung CS, Ramer PC, Pack M, Vanoaica LD, Breton G, et al. CD141+ dendritic cells produce prominent amounts of IFN-alpha after dsRNA recognition and can be targeted via DEC-205 in humanized mice. Blood. 2013;121(25):5034–5044. doi: 10.1182/blood-2012-12-473413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Flynn BJ, Kastenmuller K, Wille-Reece U, Tomaras GD, Alam M, Lindsay RW, et al. Immunization with HIV Gag targeted to dendritic cells followed by recombinant New York vaccinia virus induces robust T-cell immunity in nonhuman primates. Proc Natl Acad Sci USA. 2011;108(17):7131–7136. doi: 10.1073/pnas.1103869108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Paavonen J, Jenkins D, Bosch FX, Naud P, Salmeron J, Wheeler CM, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369(9580):2161–2170. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- 95.Chichester JA, Green BJ, Jones RM, Shoji Y, Miura K, Long CA, et al. Safety and immunogenicity of a plant-produced Pfs25 virus-like particle as a transmission blocking vaccine against malaria: a Phase 1 dose-escalation study in healthy adults. Vaccine. 2018;36(39):5865–5871. doi: 10.1016/j.vaccine.2018.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ogembo JG, Muraswki MR, McGinnes LW, Parcharidou A, Sutiwisesak R, Tison T, et al. A chimeric EBV gp350/220-based VLP replicates the virion B-cell attachment mechanism and elicits long-lasting neutralizing antibodies in mice. J Transl Med. 2015;13:50. doi: 10.1186/s12967-015-0415-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Perez EM, Foley J, Tison T, Silva R, Ogembo JG. Novel Epstein–Barr virus-like particles incorporating gH/gL-EBNA1 or gB-LMP2 induce high neutralizing antibody titers and EBV-specific T-cell responses in immunized mice. Oncotarget. 2017;8(12):19255–19273. doi: 10.18632/oncotarget.13770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Adhikary D, Behrends U, Feederle R, Delecluse HJ, Mautner J. Standardized and highly efficient expansion of Epstein–Barr virus-specific CD4+ T cells by using virus-like particles. J Virol. 2008;82(8):3903–3911. doi: 10.1128/JVI.02227-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zimmermann J, Hammerschmidt W. Structure and role of the terminal repeats of Epstein–Barr virus in processing and packaging of virion DNA. J Virol. 1995;69(5):3147–3155. doi: 10.1128/jvi.69.5.3147-3155.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Delecluse HJ, Pich D, Hilsendegen T, Baum C, Hammerschmidt W. A first-generation packaging cell line for Epstein–Barr virus-derived vectors. Proc Natl Acad Sci USA. 1999;96(9):5188–5193. doi: 10.1073/pnas.96.9.5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Feederle R, Shannon-Lowe C, Baldwin G, Delecluse HJ. Defective infectious particles and rare packaged genomes produced by cells carrying terminal-repeat-negative Epstein–Barr virus. J Virol. 2005;79(12):7641–7647. doi: 10.1128/JVI.79.12.7641-7647.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ruiss R, Jochum S, Wanner G, Reisbach G, Hammerschmidt W, Zeidler R. A virus-like particle-based Epstein–Barr virus vaccine. J Virol. 2011;85(24):13105–13113. doi: 10.1128/JVI.05598-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pavlova S, Feederle R, Gartner K, Fuchs W, Granzow H, Delecluse HJ. An Epstein–Barr virus mutant produces immunogenic defective particles devoid of viral DNA. J Virol. 2013;87(4):2011–2022. doi: 10.1128/JVI.02533-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.van Zyl DG, Tsai MH, Shumilov A, Schneidt V, Poirey R, Schlehe B, et al. Immunogenic particles with a broad antigenic spectrum stimulate cytolytic T cells and offer increased protection against EBV infection ex vivo and in mice. PLoS Pathog. 2018;14(12):e1007464. doi: 10.1371/journal.ppat.1007464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ogembo JG, Kannan L, Ghiran I, Nicholson-Weller A, Finberg RW, Tsokos GC, et al. Human complement receptor type 1/CD35 is an Epstein–Barr Virus receptor. Cell Rep. 2013;3(2):371–385. doi: 10.1016/j.celrep.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li Q, Spriggs MK, Kovats S, Turk SM, Comeau MR, Nepom B, et al. Epstein–Barr virus uses HLA class II as a cofactor for infection of B lymphocytes. J Virol. 1997;71(6):4657–4662. doi: 10.1128/jvi.71.6.4657-4662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chesnokova LS, Jiang R, Hutt-Fletcher LM. Viral entry. Curr Top Microbiol Immunol. 2015;391:221–235. doi: 10.1007/978-3-319-22834-1_7. [DOI] [PubMed] [Google Scholar]
- 108.Mutsvunguma LZ, Rodriguez E, Escalante GM, Muniraju M, Williams JC, Warden C, et al. Identification of multiple potent neutralizing and non-neutralizing antibodies against Epstein–Barr virus gp350 protein with potential for clinical application and as reagents for mapping immunodominant epitopes. Virology. 2019;536:1–15. doi: 10.1016/j.virol.2019.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tanner JE, Hu J, Alfieri C. Construction and Characterization of a humanized anti-Epstein–Barr virus gp350 antibody with neutralizing activity in cell culture. Cancers (Basel) 2018;10:4. doi: 10.3390/cancers10040112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sokal EM, Hoppenbrouwers K, Vandermeulen C, Moutschen M, Leonard P, Moreels A, et al. Recombinant gp350 vaccine for infectious mononucleosis: a phase 2, randomized, double-blind, placebo-controlled trial to evaluate the safety, immunogenicity, and efficacy of an Epstein–Barr virus vaccine in healthy young adults. J Infect Dis. 2007;196(12):1749–1753. doi: 10.1086/523813. [DOI] [PubMed] [Google Scholar]
- 111.Moutschen M, Leonard P, Sokal EM, Smets F, Haumont M, Mazzu P, et al. Phase I/II studies to evaluate safety and immunogenicity of a recombinant gp350 Epstein–Barr virus vaccine in healthy adults. Vaccine. 2007;25(24):4697–4705. doi: 10.1016/j.vaccine.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 112.Servat E, Ro BW, Cayatte C, Gemmell L, Barton C, Rao E, et al. Identification of the critical attribute(s) of EBV gp350 antigen required for elicitation of a neutralizing antibody response in vivo. Vaccine. 2015;33(48):6771–6777. doi: 10.1016/j.vaccine.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 113.Cui X, Cao Z, Sen G, Chattopadhyay G, Fuller DH, Fuller JT, et al. A novel tetrameric gp350 1–470 as a potential Epstein–Barr virus vaccine. Vaccine. 2013;31(30):3039–3045. doi: 10.1016/j.vaccine.2013.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhao B, Zhang X, Krummenacher C, Song S, Gao L, Zhang H, et al. Immunization with Fc-based recombinant Epstein–Barr virus gp350 elicits potent neutralizing humoral immune response in a BALB/c mice model. Front Immunol. 2018;9:932. doi: 10.3389/fimmu.2018.00932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Heeke DS, Lin R, Rao E, Woo JC, McCarthy MP, Marshall JD. Identification of GLA/SE as an effective adjuvant for the induction of robust humoral and cell-mediated immune responses to EBV-gp350 in mice and rabbits. Vaccine. 2016;34(23):2562–2569. doi: 10.1016/j.vaccine.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 116.Hoffman GJ, Lazarowitz SG, Hayward SD. Monoclonal antibody against a 250,000-dalton glycoprotein of Epstein–Barr virus identifies a membrane antigen and a neutralizing antigen. Proc Natl Acad Sci USA. 1980;77(5):2979–2983. doi: 10.1073/pnas.77.5.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tanner JE, Coincon M, Leblond V, Hu J, Fang JM, Sygusch J, et al. Peptides designed to spatially depict the Epstein–Barr virus major virion glycoprotein gp350 neutralization epitope elicit antibodies that block virus-neutralizing antibody 72A1 interaction with the native gp350 molecule. J Virol. 2015;89(9):4932–4941. doi: 10.1128/JVI.03269-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cui X, Cao Z, Chen Q, Arjunaraja S, Snow AL, Snapper CM. Rabbits immunized with Epstein–Barr virus gH/gL or gB recombinant proteins elicit higher serum virus neutralizing activity than gp350. Vaccine. 2016;34(34):4050–4055. doi: 10.1016/j.vaccine.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 119.Snijder J, Ortego MS, Weidle C, Stuart AB, Gray MD, McElrath MJ, et al. An antibody targeting the fusion machinery neutralizes dual-tropic infection and defines a site of vulnerability on Epstein–Barr virus. Immunity. 2018;48(4):799 e9–811 e9. doi: 10.1016/j.immuni.2018.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kanekiyo M, Bu W, Joyce MG, Meng G, Whittle JR, Baxa U, et al. Rational design of an Epstein–Barr virus vaccine targeting the receptor-binding site. Cell. 2015;162(5):1090–1100. doi: 10.1016/j.cell.2015.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bu W, Joyce MG, Nguyen H, Banh DV, Aguilar F, Tariq Z, et al. Immunization with components of the viral fusion apparatus elicits antibodies that neutralize Epstein–Barr virus in B cells and epithelial cells. Immunity. 2019;50(5):130 5 e6–1316 e6. doi: 10.1016/j.immuni.2019.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sitki-Green D, Covington M, Raab-Traub N. Compartmentalization and transmission of multiple Epstein–Barr virus strains in asymptomatic carriers. J Virol. 2003;77(3):1840–1847. doi: 10.1128/JVI.77.3.1840-1847.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Walling DM, Brown AL, Etienne W, Keitel WA, Ling PD. Multiple Epstein–Barr virus infections in healthy individuals. J Virol. 2003;77(11):6546–6550. doi: 10.1128/JVI.77.11.6546-6550.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hjalgrim H, Askling J, Rostgaard K, Hamilton-Dutoit S, Frisch M, Zhang JS, et al. Characteristics of Hodgkin's lymphoma after infectious mononucleosis. N Engl J Med. 2003;349(14):1324–1332. doi: 10.1056/NEJMoa023141. [DOI] [PubMed] [Google Scholar]
- 125.Thacker EL, Mirzaei F, Ascherio A. Infectious mononucleosis and risk for multiple sclerosis: a meta-analysis. Ann Neurol. 2006;59(3):499–503. doi: 10.1002/ana.20820. [DOI] [PubMed] [Google Scholar]