Abstract
Background
Initial trials of lung-targeted budesonide (0.25 mg/kg) in surfactant to prevent bronchopulmonary dysplasia (BPD) in premature infants have shown benefit; however, the optimal safe dose is unknown.
Methods
Dose-escalation study of budesonide (0.025, 0.05, 0.10 mg/kg) in calfactatant in extremely low gestational age neonates (ELGANs) requiring intubation at 3−14 days. Tracheal aspirate (TA) cytokines, blood budesonide concentrations, and untargeted blood metabolomics were measured. Outcomes were compared with matched infants receiving surfactant in the Trial Of Late SURFactant (TOLSURF).
Results
Twenty-four infants with mean gestational age 25.0 weeks and 743 g birth weight requiring mechanical ventilation were enrolled at mean age 6 days. Budesonide was detected in the blood of all infants with a half-life of 3.4 h. Of 11 infants with elevated TA cytokine levels at baseline, treatment was associated with sustained decrease (mean 65%) at all three dosing levels. There were time- and dose-dependent decreases in blood cortisol concentrations and changes in total blood metabolites. Respiratory outcomes did not differ from the historic controls.
Conclusions
Budesonide/surfactant had no clinical respiratory benefit at any dosing levels for intubated ELGANs. One-tenth the dose used in previous trials had minimal systemic metabolic effects and appeared effective for lung-targeted anti-inflammatory action.
Introduction
The survival of extremely low gestational age newborns (ELGANs) has dramatically improved; however, bronchopulmonary dysplasia (BPD) remains the most common morbidity of preterm birth.1 There are few effective and safe therapies to prevent BPD, which is associated with significant complications in the neonatal intensive care unit (NICU) and increased respiratory exacerbations, altered pulmonary function, and neurodevelopmental abnormalities after discharge.2–4 The pathogenesis for BPD is multifactorial, but an important component is inflammation that results from intrauterine events and postnatal hypoxia, hyperoxia, ventilator trauma and/or infection in susceptible infants with immature lungs.5–7 One of the most consistent clinical factors associated with the development of BPD is the need for mechanical ventilation, with BPD occurring in >70% of ELGANs who require ventilation at 7−14 days of age.8
Systemic postnatal corticosteroids (PCS) are potent anti-inflammatory agents and are one of the few therapies shown to decrease the severity of BPD.9,10 Unfortunately, the heterogeneous PCS treatment regimens that have been studied have not established the optimal type of corticosteroid, optimal dosage, or the optimal timing of initiation for the safe prevention of BPD.11 Early steroid treatment in conjunction with indomethacin treatment for the patent ductous arteriosus (PDA) has been associated with intestinal perforation.12,13 Systemic PCS, particularly dexamethasone, started at <8 days of life has been associated with increased cerebral palsy.14,15
There is interest in the potential to deliver a postnatal steroid topically to the lungs to improve the benefit:risk ratio. A trial of tapering inhaled budesonide started at <12 h of life in infants <28 weeks postmenstrual age (PMA) on any positive pressure support showed a significant decrease in BPD at 36 weeks PMA, but there was increased death in the treated group and no information on systemic levels of budesonide.16 Yeh et al. have conducted two unblinded pilot randomized trials of budesonide (0.25 mg/kg) mixed in surfactant (Survanta) versus surfactant alone administered soon after delivery to infants <1500 g with severe respiratory distress syndrome (RDS). Both trials demonstrated a significant decrease in the primary outcome of death or BPD at 36 weeks PMA (p < 0.001) without adverse neurodevelopmental effects at 2 years of age.17–19 While these surfactant-based trials are encouraging, further investigation is required to assure efficacy and safety and to establish the lowest effective dose. There are preclinical data supporting the use of a much lower dose of budesonide. For example, budesonide is five times more potent than dexamethasone for suppression of chemokines in fetal human lung explants;20 thus, treatment with 0.25 mg/kg budesonide in surfactant would expose the lung to much higher glucocorticoid levels than i.v. 0.25 mg/kg dexamethasone, an effective systemic dose for prevention of BPD.
The objective of our study was to investigate the optimal dose of budesonide suspended in surfactant and given intratracheally to decrease the short-term respiratory support and pulmonary inflammatory response of intubated ELGANs at risk for BPD. We hypothesized that a lower dose than the previously administered 0.25 mg/kg of budesonide would be equally effective in decreasing short-term respiratory support and the inflammatory response that contributes to BPD in intubated preterm infants.
Methods
Study design and participants
The Steroids And Surfactant In ELGANs study (SASSIE) was a phase I/II open label dose-escalation trial conducted at four US hospitals (Clinicaltrials.gov: NCT 02907593). We treated eight intubated ELGANs at risk for BPD with budesonide in surfactant at each of three dosing levels: 0.025, 0.05, 0.10 mg/kg. The first eight enrolled infants received the dose of 0.025 mg/kg, the second eight received 0.05 mg/kg, and the third group of eight received the dose of 0.10 mg/kg. Inclusion criteria were infants with a gestational age of 230/7 to 276/7 weeks who were mechanically ventilated between postnatal days 3−14. Infants who had received systemic steroids, or indomethacin, ibuprofen, or acetominophen to treat a PDA ≤96 h before the enrollment window ended, or had congenital malformations or chromosomal anomalies were excluded. Case-matched infants who received calfactant in the Trial of Late SURFactant (TOLSURF) served as the control group.8 Controls were matched 2 to 1 for birth weight, gestational age, sex, race, and initial respiratory severity score (RSS = mean airway pressure × fraction of inspired oxygen).
The trial was conducted under the IND # 128102 for the combined use of budesonide with the standard dose of calfactant. The research protocol was approved by the Institutional Review Boards of the participating institutions, and a parent of each infant provided written informed consent. An independent data safety monitoring board (DSMB) approved the protocol and met after each group of eight patients reached 28 days of age to review study progress and adverse events prior to approving progress to the next dosing level.
The primary objective of the study was to determine the clinical and anti-inflammatory efficacy by monitoring clinical outcome up to 28 days of age, including RSS, and measuring tracheal aspirate cytokine levels. By protocol, dose escalation was to be stopped if the DSMB had safety concerns or after two dose levels that met predefined efficacy criteria. Efficacy criteria included both clinical criteria (five of eight infants extubated within 72 h of the first study dose or after ≤3 daily treatments or one of several other predefined clinical respiratory outcomes at 28 days of age (see supplement) and evidence of anti-inflammation (≥50% suppression of baseline tracheal aspirate interleukin-8 (IL-8) or monocyte chemoattractant protein 1 (MCP1) at 24−72 h after the initial dose of study medication).
We chose to start the dose escalation at 0.025 mg/kg of budesonide mixed in the standard dose of calfactant because data from our fetal lung explant model20 had suggested this dose should effectively suppress lung chemokine levels. The subsequent dosing levels of budesonide were 0.05 mg/kg and 0.10 mg/kg. The research pharmacy at each institution prepared the study medication as described in Methods Supplement. Infants were dosed every 24 h if still requiring ventilation and could receive up to five doses. Clinical guidelines for management of ventilation and other patient care practices were developed and approved by investigators.8
Materials and procedures
Tracheal aspirate (TA) samples were collected for markers of inflammation. Dried blood spot (DBS) samples were collected for the measurement of budesonide and untargeted metabolomics to assess time- and dose-dependent changes in cortisol and other biochemicals. TA were collected from the infant’s endotracheal tube prior to and 12 h after the first study dose, just before each of the subsequent doses, and 48 h after the fifth dose if the infant remained intubated.8 DBS samples were collected at 15 min and at 1, 4, and 24 h after the initial dose if the infant had a central line, or 1, 4, and 24 h after the first dose if obtained by heel stick, and at 24 h after subsequent doses.21,22
Human chemokines CXCL-8/IL-8 and MCP-1/CCL2 were quantified in TA samples by enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN) that have intraassay variability of 5.8% each and interassay variability of 7.7% and 5.7%, respectively. Both assays have a sensitivity range of 31−2000 pg/ml. Secretory human IgA (sIgA) was used to normalize cytokine/chemokine data and was measured by ELISA (Lifespan Biosciences, Inc., Seattle, WA); intraassay variability of 5.8% and interassay variability of 6.0%, with a sensitivity range of 0.3−20 ng/µl). All samples were assayed in duplicate as twofold dilutions. We used the Victor3 multichannel plate reader (Perkin Elmer, Waltham, MA) for absorbance measurements.
Nine additional inflammatory mediators (IL-10, IL-12p70, IL-13, IL-1β, IL-2, IL-4, TNF-α, INF-γ) were measured from TA samples collected during the 0.1 mg/kg dose of budesonide; analysis was by a multiplex assay as previously described.23,24
Budesonide concentrations were measured in DBS using a validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) as previously described.22 The assay was linear between 1 and 50 ng/ml. Pharmacokinetic (PK) analysis of blood budesonide was conducted using the noncompartmental analysis module in Phoenix WinNonLin v6.4 (Certara, Princeton, NJ). An extravascular dosing model was used to represent budesonide administration via intratracheal instillation. The elimination slope was fit to the 1 and 4 h samples, and the linear up-log down calculation method was used. Data below the limit of quantitation for the LC-MS/MS assay (i.e. <1 ng/ml) were set as missing values.
Global metabolomic analysis using DBS was performed by Metabolon Inc. using ultrahigh performance liquid chromatography-tandem mass spectroscopy (UPLC-MS/MS). The processing, assay and analysis procedures have been described in detail.25
Statistical analysis
Data are presented as mean ± SD unless otherwise indicated. Cytokine and chemokine data were analyzed using Mann−Whitney test and analysis of variance (ANOVA) as appropriate. Dose-dependent differences in systemic budesonide exposure were assessed using ANOVA with Tukey post-hoc tests in GraphPad Prism v8.1 (GraphPad Software, San Diego, CA). ANOVA contrasts and Welch’s two-sample t test were used to identify biochemicals that differed significantly between the dosing levels of budesonide. Analysis by two-way ANOVA identified biochemicals exhibiting significant interaction and main effects for experimental parameters of dose and time point.
Results
Patients were enrolled between 3/11/2017 and 3/29/2018. A total of 280 ELGANs were screened with 63 eligible and 25 consented into the study (Fig. 1). One infant in the 0.1 mg/kg group had consent withdrawn prior to treatment. An additional infant was enrolled at that dosing level to provide eight infants per dosing level. Three dosing levels of budesonide in calfactant were administered. Enrollment into the study was stopped after completion of the 0.1 mg/kg dosing as no patient at any of the three dosing levels had fulfilled the predefined efficacy criteria.
Fig. 1. Enrollment of study participants.

A total of 25 infants consented into the study (one set of twins). One parent withdrew consent in dosing level 3; therefore, eight infants were dosed at each dosing level. Dosing levels of budesonide in calfactant were: 0.025 mg/kg; 0.05 mg/kg; 0.10 mg/kg. ELGANs extremely low gestational age newborns.
The mean gestational age, birth weight, and age at study dosing of enrolled infants at the respective dosing levels are presented in Table 1 along with the demographics of the 48 matched TOLSURF infants. All of the infants at the first two dosing levels and six of eight at the third dosing level had received surfactant within the first 24 h as per standard of care. All SASSIE infants remained intubated after the first dose of study drug and received two to five doses.
Table 1.
Demographics and acute dosing data of the SASSIE and TOLSURF Reference Group.
| Budesonide dose (mg/kg) | n | ASa (#) | Gestational age (weeks) | Birth weight (g) | Male (#) | Race/ethnicityb | Age at first dose (days) | Doses given (mean, range) | RSS Predosing | RSS 24 h after first dose | RSS 48 h after first dose |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.025 | 8 | 6 | 25.3 ± 1.6 | 885 ± 239 | 6 | 7/0/0/1 | 5.8 ± 3.2 | 3.9 (2−5) | 3.9 ± 2.7 | 2.7 ± 1.5c | 3.1 ± 2.1 |
| 0.05 | 8 | 7 | 25.3 ± 1.3 | 704 ± 196 | 2 | 6/1/0/1 | 8.0 ± 2.5 | 4.3 (3−5) | 3.7 ± 0.9 | 3.6 ± 1.4 | 3.0 ± 1.9 |
| 0.10 | 8 | 7 | 24.4 ± 0.9 | 651 ± 100 | 4 | 7/1/0/0 | 5.6 ± 3.2 | 3.9 (2−5) | 3.2 ± 2.3 | 2.5 ± 1.4 | 2.7 ± 1.4 |
| All SASSIE patients | 24 | 20 | 25.0 ± 1.3 | 743 ± 204 | 12 | 20/2/0/2 | 6.5 ± 2.9 | 4.0 (2−5) | 3.6 ± 2.0 | 2.9 ± 1.5d | 2.9 ± 1.8 |
| TOLSURF controls | 48 | 41 | 25.1 ± 1.2 | 742 ± 190 | 24 | 31/7/7/3 | 9.4 ± 2.7 | 3.9 ± 2.4 | 3.8 ± 1.8 |
TOLSURF controls were matched to surfactant-treated SASSIE infants by birth weight, gestational age, sex, race/ethnicity and initial RSS, in that order.
Mean ± SD unless otherwise noted.
AS antenatal steroids, RSS respiratory severity score (FiO2 × MAP).
aAS, any exposure to antenatal steroids.
bCaucasian/African American/Hispanic/other.
cp = 0.03 vs. predosing.
dp = 0.006 vs. predosing.
No infant at any of the three dosing levels achieved the primary outcome of meeting both the predefined clinical respiratory criteria and a >50% suppression of IL-8 or CCL2. There was a significant decrease in RSS at 24 h after initial dosing for the 0.025 mg/kg group and for the entire cohort (Table 1).
Table 2 shows the respiratory outcomes of SASSIE and TOLSURF infants at 28 days and 36 and 40 weeks of PMA. All infants required respiratory support at 28 days. Survival without BPD at 36 and 40 weeks was higher in the 0.025 mg/kg dosing group than the 0.05 or 0.10 mg/kg groups; however, the mean birth weight of the 0.025 mg/kg group was 885 g compared to 704 and 651 g in the other two groups. Overall, 3 of 24 (12.5%) in SASSIE vs. 2 of 48 (4.2%) TOLSURF infants died and fewer infants in SASSIE survived without BPD at 36 and 40 weeks (17% vs. 31 and 29% vs. 60%). No death or serious adverse events were considered to be related to treatment.
Table 2.
Infant outcomes of the SASSIE and TOLSURF Reference Group.
| Budesonide dose (mg/kg) | n | Death at <28 days | Respiratory support at 28 days (%) | Survival without BPD at 36 weeks (%) | Survival without BPD at 40 weeks (%) |
|---|---|---|---|---|---|
| 0.025 | 8 | 0 | 100 | 38 | 63 |
| 0.05 | 8 | 0 | 100 | 0 | 13 |
| 0.10 | 8 | 3 | 100 | 13 | 13 |
| All SASSIE patients | 24 | 3 | 100 | 17 | 29 |
| TOLSURF patients | 48 | 2 | 100 | 31 | 60 |
BPD bronchopulmonary dysplasia.
Budesonide was detected in the circulation of all 24 subjects; however, two (one in the 0.025 mg/kg and one in the 0.05 mg/kg dose cohorts) had insufficient data to calculate an elimination slope based on 1 and 4 h data. All but two of the samples collected at 24 h after the prior dose were below the limit of quantitation (n = 71 of 73 collected samples). Figure 2a shows the observed concentrations of budesonide in DBS at 0.25, 1, and 4 h following the first dose for all three dosing cohorts.
Fig. 2. Budesonide pharmokinetics in dried blood spots (DBS).
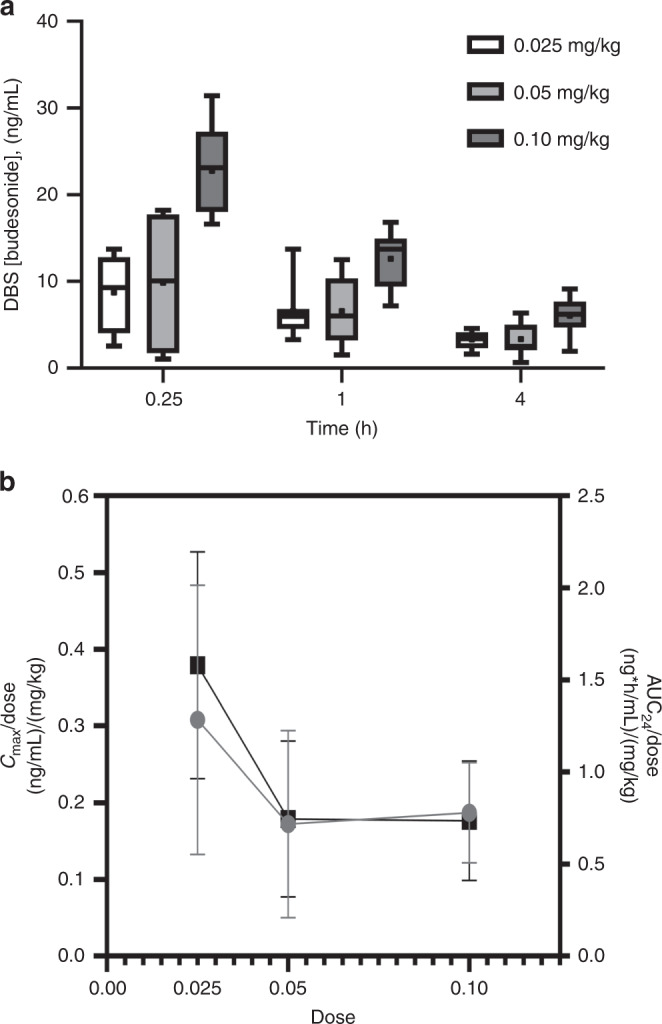
a Concentrations at 0.25, 1, and 4 h following the first dose in the 0.025 mg/kg (unfilled), 0.05 mg/kg (light gray filled), and 0.10 mg/kg (dark gray filled) dose cohorts. Whiskers represent minimum and maximum concentrations, boxes represent the 25th and 75th percentile, the central line represents the median, and the point represents the mean value. b Dose-normalized Cmax (gray) and AUC24 (black) for the three dosing cohorts. Relative to the administered dose, systemic budesonide exposure at the 0.025 mg/kg dose is greater than that observed for the other two dosing cohorts.
The half-life of blood budesonide was short (3.4 h) and similar between all dosing cohorts with mean peak concentrations for the 0.025, 0.05, and 0.10 mg/kg dose cohorts of 7.7, 8.6, and 18.7 ng/ml, respectively. When normalized for dose, the 0.05 mg/kg and 0.10 mg/kg cohorts had similar areas under the 24 h concentration-time curve (AUC24), but both were significantly different from the 0.025 mg/kg dose cohort (Fig. 2b, Table 3). Combined, these data suggest that while systemic budesonide is eliminated at the same rate independent of dose, the systemic absorption from the intratracheal dose was not dose-linear in this cohort.
Table 3.
Pharmacokinetics of the budesonide dosing cohorts.
| Budesonide dose (mg/kg) | ke | Half-life | Cmax | Cmax/Dose | AUC24 | AUC24/Dose |
|---|---|---|---|---|---|---|
| 1/h | h | ng/ml | (ng/ml)/(mg/kg) | (ng × h/m) | (ng × h/ml)/(mg/kg) | |
| 0.025 | 0.224 (40%) | 3.78 (57%) | 7.70 (57%) | 0.308 (57%) | 39.4 (39%) | 1.58 (39%) |
| 0.05 | 0.242 (26%) | 3.20 (37%) | 8.60 (71%) | 0.172 (71%) | 37.2 (57%) | 0.744 (57%) |
| 0.10 | 0.268 (43%) | 3.20 (56%) | 18.7 (35%) | 0.187 (35%) | 73.5 (44%) | 0.735 (44%) |
| ANOVA p value | 0.71 | 0.77 | 0.001 | 0.08 | 0.01 | 0.002 |
| Tukey p value | 0.025 vs. 0.05 | 0.95 | 0.11 | 0.99 | 0.007 | |
| 0.025 vs. 0.10 | 0.002 | 0.15 | 0.03 | 0.004 | ||
| 0.05 vs. 0.10 | 0.004 | 0.97 | 0.02 | 0.99 |
Data are calculated mean (%CV) pharmacokinetic parameter values for the three dose cohorts, as well as the p values from the ANOVA and Tukey post-hoc analyses.
ke elimination rate, Cmax maximum concentration, AUC24 area under the concentration 24-h time curve.
We assessed anti-inflammatory effects of budesonide in infant lungs by the assay of TA levels of two chemokines.20 There was adequate recovery of lung fluid for assay of IL-8, MCP1 and sIgA in TA of 22 of the 24 enrolled infants. The ratio of the mean chemokine value during treatment (from 12 h after the first dose to 24 h after the last dose) was determined, and infants designated as apparent responders if there was >20% decrease compared to predosing. The time course for IL-8 in two infants is shown in Fig. 3a; infant 1 had a sustained decrease in IL-8 beginning 12 h after the first dose with an increase at 48 h after the last dose, whereas infant 2 had no consistent change in IL-8 levels until after 6 days of therapy. Infant 1 in this figure represented a responder, while infant 2 was a nonresponder (Fig. 3a). Overall for the 22 infants, there were 11 apparent responders for IL-8 and 8 for MCP-1 (Fig. 3b), with a similar mean level of suppression noted for IL-8 (69 ± 13%) vs. MCP1 (60 ± 21%, NS).
Fig. 3. Levels of IL-8 and MCP1 with budesonide treatment.
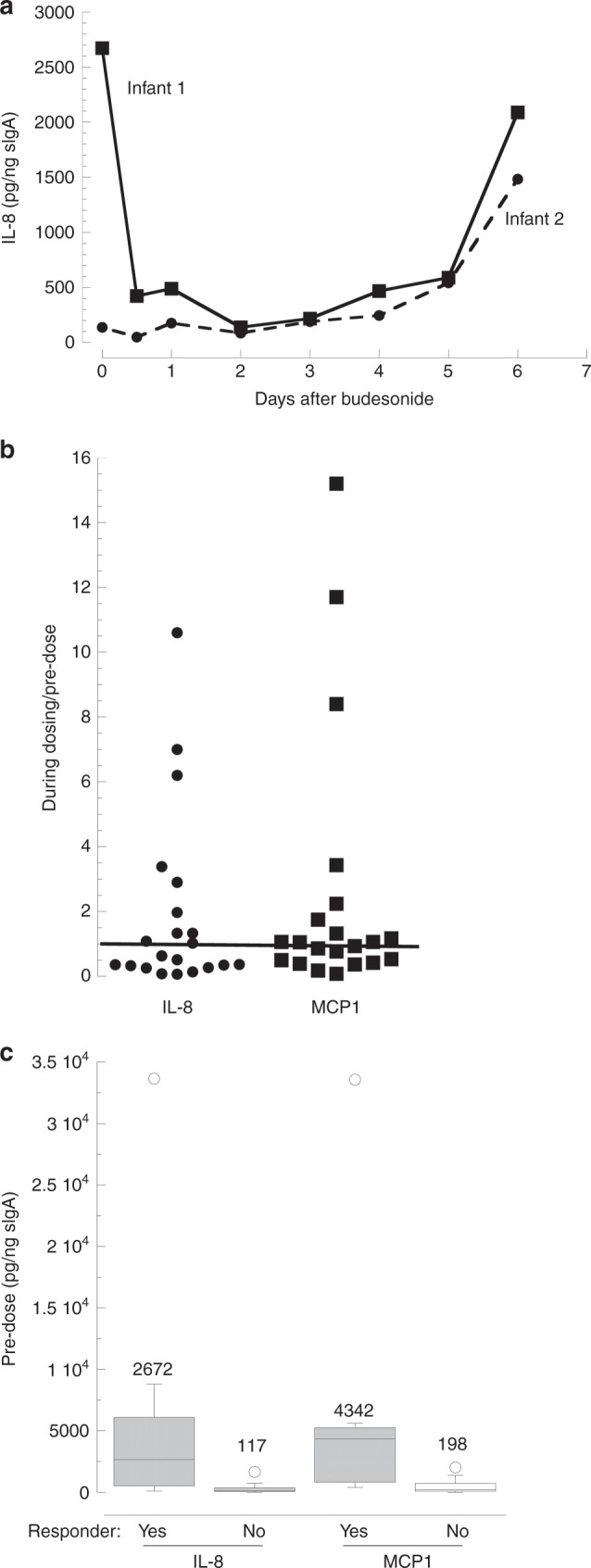
a Time course for tracheal aspirate (TA) IL-8 in two infants receiving five daily doses of 0.1 mg/kg budesonide. b Response of IL-8 and MCP1 to budesonide comparing TA levels during and before treatment. The post-treatment value is the mean for levels at 12 to 24 h after last dosing. The median values for all infants are 0.63 for IL-8 and 1.05 for MCP1 with 11/22 and 8/21 apparent responders (mean ratio <0.8), respectively. The line shows a ratio of 1.0. c Apparent responsiveness related to predosing levels of IL-8 and MCP1. Box plots with median values shown for each group of infants; p < 0.01 for yes vs. no for both chemokines by Mann−Whitney.
We explored possible factors related to the chemokine response to budesonide. Of interest, the predose levels of IL-8 and MCP1 varied widely (1096-fold and 3323-fold, respectively) and the levels of the two chemokines were strongly correlated (r = 0.98, p = 8−16). There was a strong association between predose levels and the decrease during treatment, with higher baseline levels for both IL-8 and MCP (~22-fold, p < 0.01) for responders compared to nonresponders (Fig. 3c). There was a weak correlation between blood budesonide level (at 4 h) and the change in TA IL-8 at 24 h (r = 0.46, p = 0.04, n = 20, Fig. 4), indicating that responsiveness was not strongly related to systemic budesonide levels. No significant differences were observed in clinical parameters comparing infants with suppression of IL-8 vs. those without suppression, although more of the responders (8/11) than nonresponders (4/11, p = 0.08) had a decrease in RSS with budesonide treatment (Table 4).
Fig. 4. IL-8 response and blood levels of budesonide.
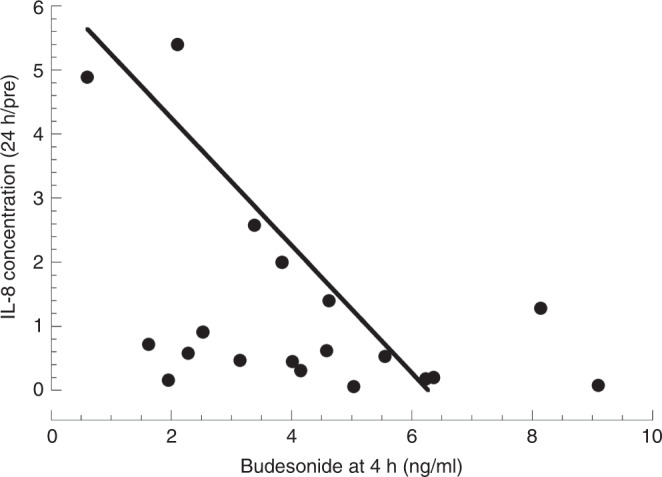
The change in IL-8 at 24 h compared to the predose level is plotted against the blood level of budesonide at 4 h for 20 infants. R = 0.46, p = 0.04.
Table 4.
Characteristics of infants based on apparent suppression of IL-8 by budesonide treatment.
| Responders (n = 11) | Nonresponders (n = 11) | p | |
|---|---|---|---|
| Gestational age (wk) | 25.0 ± 1.3 | 25.2 ± 1.3 | 0.62 |
| Birth weight (g) | 740 ± 192 | 788 ± 247 | 0.62 |
| Sex (m/f) | 5/6 | 7/4 | NS |
| Race (c/aa/h/o)a | 6/1/3/1 | 7/1/3/0 | NS |
| Doses budesonide | 4.5 ± 1.1 | 3.8 ± 1.4 | 0.23 |
| Budesonide dose (mg/kg) | 0.06 ± 0.04 | 0.06 ± 0.03 | 1.0 |
| Blood budesonide at 4 h (ng/ml) | 4.9 ± 2.1 | 3.2 ± 2.5 | 0.10 |
| RSS at enroll | 3.6 ± 1.9 | 3.0 ± 1.3 | 0.38 |
| RSS decrease >0.2 at 24 h | 8 | 4 | 0.08 |
Data are mean ± SD and p by unpaired t test.
RSS respiratory severity score (mean airway pressure × FiO2).
aCaucasian/African American/Hispanic/other.
Neither the number of responders, nor the level of suppression of IL-8 among responders, showed apparent dose-dependency: 5 of 8 with 56 ± 13% suppression at 0.025 mg/kg, 2 of 7 and 84 ± 13% suppression at 0.05 mg/kg, and 4 of 7 and 79 ± 12% suppression at 0.1 mg/kg. Corresponding numbers for MCP1 were 3/8, 2/7, and 3/7 with suppression of 46 ± 21%, 73 ± 13%, and 54 ± 35%, respectively. We used a multiplex approach to assay an additional nine inflammatory mediators in TA from infants receiving the 0.1 mg/kg dose (Table 5).23 Consistent decreases during budesonide treatment were observed for all mediators (mean % suppression values ranging from 51 to 79%) in three of the four infants with suppression of IL-8. Baseline levels were higher for responders than nonresponding infants for each mediator (range 1.8- to 5.0-fold) as observed for IL-8 and MCP1 (5.8- and 5.7-fold) at this budesonide dose. Overall, our results provide evidence for a strong anti-inflammatory response in the lung for some, but not all, infants that is independent of the budesonide dose between 0.025 and 0.1 mg/kg and is related to elevated inflammatory status in the lung at baseline.
Table 5.
Tracheal aspirate levels of additional inflammatory mediators for infants with apparent response to 0.1 mg/kg budesonide.
| Cyto/chemokine | Number of infants with suppression (n = 7) | % suppression (mean ± SD) | Predose level (suppress/no suppress) |
|---|---|---|---|
| IFN-γ | 3 | 68 ± 18 | 1.9 |
| IL-10 | 3 | 72 ± 21 | 3.7 |
| IL-12p70 | 3 | 69 ± 13 | 3.0 |
| IL-13 | 3 | 69 ± 3 | 5.0 |
| IL-1β | 3 | 64 ± 19 | 4.9 |
| IL-2 | 3 | 72 ± 19 | 2.0 |
| IL-4 | 3 | 65 ± 18 | 1.8 |
| IL-6 | 3 | 56 ± 18 | 4.3 |
| TNF-α | 2 | 51 ± 42 | 3.6 |
| IL-8 | 4 | 79 ± 12 | 5.8 |
| MCP1 | 3 | 54 ± 35 | 5.7 |
| Total | 65 ± 9 | 3.8 ± 1.5 |
Data are by multiplex assay for the first nine mediators and by ELISA for IL-8 and MCP1; suppression occurred in the same infants for each mediator. Apparent suppression was assigned for infants with consistently lower values during treatment compared to predosing with a mean value of >20%.
We identified eight infants in the TOLSURF cohort who received late surfactant, but not systemic PCS, and who had five TA samples collected over a 9 ± 2-day period. In this group, comparing mean levels for samples 2−5 with sample 1, there was a consistent suppression of IL-8 and MCP1 in 3 and 1 infants, respectively, with suppression ranging between 54 and 69%. For the value for the group. To examine systemic responses to budesonide, we performed untargeted metabolomics on SASSIE DBS.25 A total of 829 named biochemicals were detected in 121 dried blood spots from 23 infants over 7 time points (15 min−96 h post 3 infants with declining chemokine levels over time, baseline values (sample 1) were near the median treatment initiation). There was a statistically significant, time-dependent decrease in cortisol for infants receiving 0.05 and 0.1 mg/kg budesonide, with decreased levels occurring at 4 h and mean suppression of 52% and 75%, respectively, at 24 h (Fig. 5a). By contrast, blood cortisol did not change significantly for the group of infants treated with 0.025 mg/kg budesonide.
Fig. 5. Systemic effects of intratracheal budesonide treatment by MS:MS.
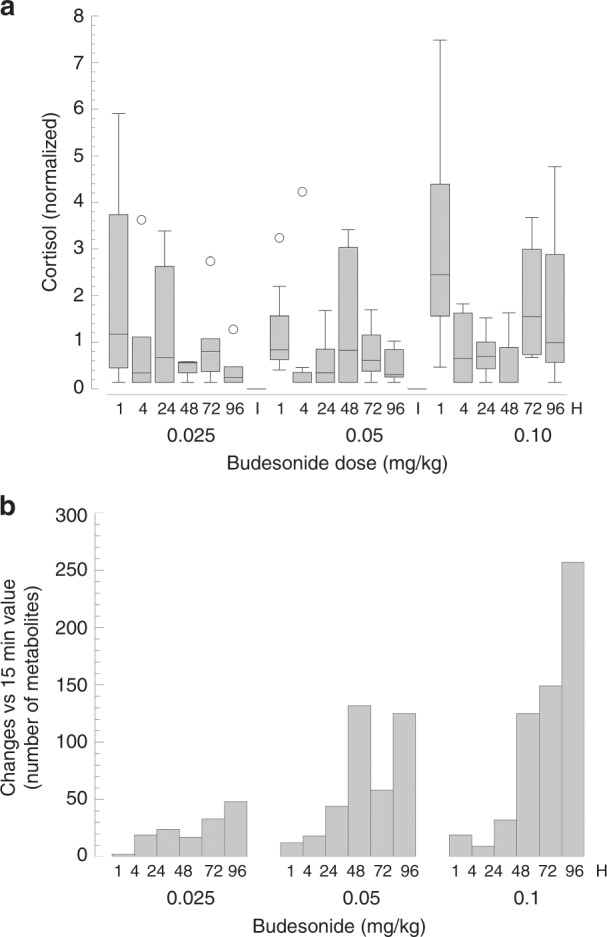
a Effect on blood cortisol by budesonide dose. Values at “1 h” combine results for both 15 min and 1 h, which were similar. Decreasing cortisol values with time was significant (p < 0.05 by two-way repeated measure ANOVA) for infants receiving 0.05 and 0.1 mg/kg budesonide. b Total number of significant changes in blood metabolites by budesonide dose over time. Data reflect increase or decrease with p < 0.05 compared to levels at 15 min using ANOVA. Total number of named metabolites was 829.
To evaluate global systemic metabolic responses, the number of significant (p < 0.05) changes (decreased or increased) were determined for each time point compared to the 15-min value (Fig. 5b). There was a time-dependent increase in number of changes at all three doses, with a similar relatively low level of changes (<40) in the first 24 h, and a dose-dependent response at later time points; at 96 h, the number of metabolite changes were 48, 127, and 260 at 0.025, 0.05, and 0.1 mg/kg, respectively, representing 5.8%, 15.3%, and 31.4% of the known biochemicals detected. These results indicate that intratracheal budesonide:surfactant treatment, at the doses and frequency used, has an approximately linear dose−response impact on blood biochemicals, consistent with extra-pulmonary effects of circulating steroid.
Discussion
We performed a dose-escalation study of budesonide in calfactant given intratracheally to ELGANs still requiring mechanical ventilation at 3−14 days of age starting at one-tenth the dose used in previous studies.18,19 Although most infants had a decrease in RSS following treatment, there were no differences between dosing groups in clinical respiratory outcomes at 28th day. BPD at 36 or 40 weeks PMA was less common in infants in the 0.025 mg/kg group; however, these infants had a higher mean birth weight than the other two groups. Only half of the infants had evidence of increased inflammatory markers in TA at initiation of the study, and these infants had a similar decrease in levels of the two cytokines with treatment at all three dosing levels. There was a dose-dependent increase in both suppression of blood cortisol and in the number of alterations in levels of other blood metabolites indicating systemic absorption and effects of the corticosteroid. These results suggest that intratracheal budesonide reduces lung inflammation only in infants who have elevated inflammatory status, and that this effect occurs at the lowest dose tested (0.025 mg/kg), which has minimal systemic metabolic effects and can be safely repeated if the infant remains intubated.
Survival without BPD for SASSIE infants was lower than for the historical matched TOLSURF cohort, which may reflect in part benefit from inhaled nitric oxide in nonwhite infants.26 The apparent lack of clinical benefit is also in contrast to the two pilot RCTs of Yeh et al., in which significantly fewer infants in the treated group (single dose of 0.25 mg/kg budesonide in Survanta) had BPD at 36 weeks PMA.18,19 There are several possible explanations for this difference. Our study did not have concurrent controls nor was it powered to detect significant differences in clinical outcomes. Importantly, in the Yeh studies,18,19 infants were enrolled within the first 24 h of receiving FiO2 > 0.6 and were mechanically ventilated whereas infants in SASSIE were only enrolled if they required mechanical ventilation between postnatal days 3 and 14 and were therefore in the highest risk group (>70%) for BPD.8 The lack of apparent clinical response may also reflect differences in the patient populations. The infants in Yeh’s studies were more mature and larger than the infants in SASSIE. In SASSIE, infants treated within 96 h of dosing for a PDA were excluded to minimize any potential interactions with study medication and this may have biased results.
Yeh et al. previously described the pharmacokinetics of budesonide in ten preterm neonates (<1500 g at birth) following intratracheal instillation of 0.25 mg/kg budesonide.18 The observed elimination half-life (3.4 h) in our study was similar to the Yeh study (4.1 h). The AUC reported in Yeh’s study (AUC0−8 = 115.7 ng × h/ml) extrapolates to an AUC24 of approximately 200 ng × h/ml. When normalized for dose, this value is similar to the AUC24 observed for the 0.10 mg/kg cohort in our study. These kinetics support daily treatment to sustain elevated blood budesonide. Concentrations of budesonide in human lung are not known, but in lambs levels in lung tissue decrease to 0.4% of the initial dose at 24 h.27
The Yeh study observed a peak concentration of approximately 20 ng/ml at 30 min after dose. This peak concentration is similar to the Cmax of 18.7 ng/ml at 15 min post dose from a lower dose (0.10 mg/kg) in our study, suggesting a ~2.5-fold difference in observed Cmax between the studies.18 From the data collected, it is unclear whether the difference in Cmax is due to a difference in absorption secondary to the surfactant used (Survanta in Yeh’s trial, Calfactant in our study), the gestational weight and/or age differences between study populations, the difference between samples collected at 15 and 30 min post dose, and/or methodologic differences.
The plasma concentrations of budesonide in infants can be compared to those achieved with antenatal betamethasone for enhancing fetal maturity and postnatal dexamethasone given for prevention of BPD. In this calculation, we convert corticosteroid levels to cortisol equivalents using factors of 7-fold for betamethasone and dexamethasone and 50-fold for budesonide based on receptor affinity and dose−response data from cultured human fetal lung.20,28 Mean peak concentrations as cortisol equivalents are: 4 µg/dl for normal 25-week human fetus, 15 µg/dl in cord blood after intramuscular antenatal betamethasone, 140 µg/dl with 0.25 mg/kg postnatal dexamethasone, and 72 µg/dl after 0.1 mg/kg intratracheal budesonide.29–31 Thus, both postnatal dexamethasone and budesonide at current doses transiently expose the infant to plasma concentrations considerably greater than those occurring with physiologic stress in the newborn (e.g., RDS ~15 µg/dl). Risks for the infant associated with these higher exposures may depend on both the concentration of corticosteroid and duration of exposure.
Treatment with budesonide in surfactant primarily targets lung inflammation, although it is possible that local effects are modulated by systemic exposure to budesonide. To assess local effects of budesonide, we measured two chemokines in lung fluid that are chemoattractants for neutrophils (IL-8) and lymphocytes (MCP1). Both mediators are produced in human fetal lung and are suppressed by glucocorticoids in explant culture.20 Levels of the two chemokines were strongly correlated at both baseline and after treatment, which is consistent with a previous observation for multiple cyto/chemokine levels in TA32 and indicate that our findings likely reflect the overall inflammatory status in the lung. Apparent suppression of IL-8 and MCP1 was observed in half of the SASSIE infants and was strongly associated with higher baseline levels, suggesting that inflammation is not a universal feature of lung injury in the first week and that benefit from budesonide (or any glucocorticoid) will only occur in selected infants with elevated inflammatory status.
The global blood metabolomic data demonstrated a clear dose−response association with budesonide. At 0.025 mg/kg budesonide, there was a small suppression of cortisol, and limited changes in other biochemicals, and both responses increased progressively with higher doses. This pattern contrasts with suppression of TA chemokines where no differences were apparent between doses, and is consistent with elevated levels of budesonide within the lung, compared to systemic levels, with intratracheal delivery. Thus, our results suggest that the best benefit:risk ratio of budesonide:surfactant therapy occurs at doses of 0.025/0.05 mg/kg, which are five- to tenfold lower than used currently in clinical studies.
Strengths of our study include the study population of intubated ELGANs who are at high risk for BPD, the dose-escalation study design, and measurement of multiple biomarkers for both lung anti-inflammatory and systemic effects of budesonide. Previous studies33,34 using surfactant-antibiotic mixtures had demonstrated interactions between these agents; however, we recently reported no apparent adverse effect of budesonide on calfactant activity in vitro nor an effect of calfactant on budesonide action.20 An important limitation of our chemokine data is the possibility that, in some infants, the lower chemokine levels during budesonide treatment reflected decreasing inflammation independent of glucocorticoid exposure as observed in some of the TOLSURF infants. However, the pattern of suppression observed with budesonide, beginning in most infants 12 h after the first dose and sustained during the treatment period, is consistent with suppression in response to budesonide. Also, we used sIgA to normalize chemokine levels to volume of lung fluid in the TA sample; it is possible that sIgA production in vivo varied during the collection period, which would introduce variability in the chemokine data. A limitation of our pharmacokinetic analysis is the absence of data after the 4 h time point; however, our half-life value was similar to that reported by Yeh et al., supporting the accuracy of our PK results.18
The dose-escalation study design limited the number of infants studied at each dosing level. Also, concurrent controls were not included in the study design, for ethical reasons, and the matched historical controls received inhaled NO and were not evaluated for other potential confounding factors such as chorioamnionitis. Earlier dosing, longer dosing, or the use of noninvasive approaches to surfactant delivery may result in different clinical outcomes. The original study design included a fourth dosing level (which would have been either 0.20 mg/kg or 0.25 mg/kg contingent on the response to dosing level 3), which may have provided more detail regarding clinical and anti-inflammatory response to the combination of the study medications. However, we decided not to continue enrolling the infants in this study after the first three dosage levels failed to show clinical benefit. Given that aggressive use of noninvasive mechanical ventilation has not reduced the incidence of BPD, that BPD may develop in ELGAN babies with little clinical initial evidence of lung disease, and that inflammation associated with BPD worsens over the first weeks after birth, the question of whether serial instillation of budesonide suspended in surfactant reduces BPD remains an unanswered question. Future randomized trials in ELGANs at high risk for BPD should require treatment over the biological period of risk to assess efficacy and safety in a fair way.
Our results do not support the “late” treatment of intubated ELGANs with a regimen of budesonide in surfactant at the three dosages we tested, but do provide important insights into blood budesonide concentrations, lung anti-inflammatory effects and potentially global metabolomics effects that are critical to the consideration of treatment with this combination of medications.
We were not powered to investigate association of inflammation with near-term pulmonary outcome, but we suggest that measurement of TA inflammatory mediators would be informative in future trials of PCS therapy.
In conclusion, assuming that the respiratory benefits of PCS therapy primarily relate to anti-inflammatory effects in the lung, our results suggest that one-tenth the budesonide dose in surfactant used in previous studies is sufficient to reduce inflammation with minimal systemic effects even with repeated treatment. Additional trials of this promising lung-targeted approach to preventing BPD are needed using lower doses of budesonide with further study of efficacy, safety, inflammatory markers and endocrine and metabolic responses.
Supplementary information
Acknowledgements
The authors thank the parents and all NICU personnel for their support. The authors appreciate the assistance of Namasivayam Ambalavanan, MD at the University of Alabama for performance of the multiplex cytokine analyses and the study coordination of: Kristin Milner, CMA, BS, CCRP at Oregon Health & Science University; Debbie Ott, BSN, RNC-NIC at AdventHealth for Children; Kim Barnette, RRT-NPS at University of Florida; Theresa Rogers, RN and Steven Steele, RN at Vanderbilt University Medical Center; David Anderson, Dr. Diana Wilkins, and Dr. Chris Reilly within the Center for Human Toxicology at the University of Utah in quantifying budesonide concentrations. The authors appreciate the time of Dr. Patricia Spitale, Dr. Thuy Nguyen, and Dr. Eric Chen of the DSMB. This study was supported by Thrasher Research Fund. ONY, Inc provided the Infasurf but was not involved in study design, collection, analysis, or interpretation of data, writing the manuscript or the decision to submit for publication.
Author contributions
Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data: All authors. Drafting the article or revising it critically for important intellectual content: All authors. Final approval of the version to be published: All authors.
Competing interests
The authors declare no competing interests.
Footnotes
Prior Presentation: Western Society of Pediatric Research 2019 and Pediatric Academic Society 2019
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version of this article (10.1038/s41390-020-0792-y) contains supplementary material, which is available to authorized users.
References
- 1.Higgins RD, et al. Bronchopulmonary dysplasia: executive summary of a workshop. J. Pediatr. 2018;197:300–308. doi: 10.1016/j.jpeds.2018.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McEvoy CT, et al. Bronchopulmonary dysplasia: NHLBI Workshop on the Primary Prevention of Chronic Lung Diseases. Ann. Am. Thorac. Soc. 2014;11(Suppl 3):S146–S153. doi: 10.1513/AnnalsATS.201312-424LD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keller RL, et al. Bronchopulmonary dysplasia and perinatal characteristics predict 1-year respiratory outcomes in newborns born at extremely low gestational age: a prospective cohort study. J. Pediatr. 2017;187:89–97. doi: 10.1016/j.jpeds.2017.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheong JLY, Doyle LW. An update on pulmonary and neurodevelopmental outcomes of bronchopulmonary dysplasia. Semin. Perinatol. 2018;42:478–484. doi: 10.1053/j.semperi.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 2001;163:1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 6.Stoll BJ, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110(2 Pt 1):285–291. doi: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]
- 7.Morrow LA, et al. Antenatal determinants of bronchopulmonary dysplasia and late respiratory disease in preterm infants. Am. J. Respir. Crit. Care Med. 2017;196:364–374. doi: 10.1164/rccm.201612-2414OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ballard RA, et al. Randomized trial of late surfactant treatment in ventilated preterm infants receiving inhaled nitric oxide. J. Pediatr. 2016;168:23–29. doi: 10.1016/j.jpeds.2015.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doyle LW, Cheong JL, Ehrenkranz RA, Halliday HL. Late (>7 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst. Rev. 2017;10:CD001145. doi: 10.1002/14651858.CD001145.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baud O, Watterberg KL. Prophylactic postnatal corticosteroids: early hydrocortisone. Semin. Fetal Neonatal Med. 2019;24:202–206. doi: 10.1016/j.siny.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Onland W, De Jaegere AP, Offringa M, van Kaam A. Systemic corticosteroid regimens for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst. Rev. 2017;1:CD010941. doi: 10.1002/14651858.CD010941.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doyle LW, Ehrenkranz RA, Halliday HL. Dexamethasone treatment in the first week of life for preventing bronchopulmonary dysplasia in preterm infants: a systematic review. Neonatology. 2010;98:217–224. doi: 10.1159/000286210. [DOI] [PubMed] [Google Scholar]
- 13.Stark AR, et al. Adverse effects of early dexamethasone in extremely-low-birth-weight infants. National Institute of Child Health and Human Development Neonatal Research Network. N. Engl. J. Med. 2001;344:95–101. doi: 10.1056/NEJM200101113440203. [DOI] [PubMed] [Google Scholar]
- 14.Doyle LW, Cheong JL, Ehrenkranz RA, Halliday HL. Early (<8 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst. Rev. 2017;10:CD001146. doi: 10.1002/14651858.CD001146.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheong JLY, Doyle LW. Long-term effects of postnatal corticosteroids to prevent or treat bronchopulmonary dysplasia: balancing the risks and benefits. Semin. Fetal Neonatal Med. 2019;24:197–201. doi: 10.1016/j.siny.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Bassler D, et al. Early inhaled budesonide for the prevention of bronchopulmonary dysplasia. N. Engl. J. Med. 2015;373:1497–1506. doi: 10.1056/NEJMoa1501917. [DOI] [PubMed] [Google Scholar]
- 17.Kuo HT, Lin HC, Tsai CH, Chouc IC, Yeh TF. A follow-up study of preterm infants given budesonide using surfactant as a vehicle to prevent chronic lung disease in preterm infants. J. Pediatr. 2010;156:537–541. doi: 10.1016/j.jpeds.2009.10.049. [DOI] [PubMed] [Google Scholar]
- 18.Yeh TF, et al. Early intratracheal instillation of budesonide using surfactant as a vehicle to prevent chronic lung disease in preterm infants: a pilot study. Pediatrics. 2008;121:e1310–e1318. doi: 10.1542/peds.2007-1973. [DOI] [PubMed] [Google Scholar]
- 19.Yeh TF, et al. Intra-tracheal administration of budesonide/surfactant to prevent bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 2016;193:86–95. doi: 10.1164/rccm.201505-0861OC. [DOI] [PubMed] [Google Scholar]
- 20.Barrette AM, et al. Antiinflammatory effects of budesonide in human fetal lung. Am. J. Respir. Cell Mol. Biol. 2016;55:623–632. doi: 10.1165/rcmb.2016-0068OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castillo-Mancilla JR, et al. Tenofovir, emtricitabine, and tenofovir diphosphate in dried blood spots for determining recent and cumulative drug exposure. AIDS Res. Hum. Retroviruses. 2013;29:384–390. doi: 10.1089/aid.2012.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rower JE, et al. Development and validation of an assay for quantifying budesonide in dried blood spots collected from extremely low gestational age neonates. J. Pharm. Biomed. Anal. 2019;167:7–14. doi: 10.1016/j.jpba.2019.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D’Angio CT, et al. Blood cytokine profiles associated with distinct patterns of bronchopulmonary dysplasia among extremely low birth weight infants. J. Pediatr. 2016;174:45–51.e5. doi: 10.1016/j.jpeds.2016.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skogstrand K, et al. Simultaneous measurement of 25 inflammatory markers and neurotrophins in neonatal dried blood spots by immunoassay with xMAP technology. Clin. Chem. 2005;51:1854–1866. doi: 10.1373/clinchem.2005.052241. [DOI] [PubMed] [Google Scholar]
- 25.Long T, et al. Whole-genome sequencing identifies common-to-rare variants associated with human blood metabolites. Nat. Genet. 2017;49:568–578. doi: 10.1038/ng.3809. [DOI] [PubMed] [Google Scholar]
- 26.Askie LM, et al. Race effects of inhaled nitric oxide in preterm infants: an individual participant data meta-analysis. J. Pediatr. 2018;193:34–39. doi: 10.1016/j.jpeds.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 27.Kothe TB, et al. Effects of budesonide and surfactant in preterm fetal sheep. Am. J. Physiol. Lung Cell Mol. Physiol. 2018;315:L193–L201. doi: 10.1152/ajplung.00528.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ballard PL, Carter JP, Graham BS, Baxter JD. A radioreceptor assay for evaluation of the plasma glucocorticoid activity of natural and synthetic steroids in man. J. Clin. Endocrinol. Metab. 1975;41:290–304. doi: 10.1210/jcem-41-2-290. [DOI] [PubMed] [Google Scholar]
- 29.Donaldson A, Nicolini U, Symes EK, Rodeck CH, Tannirandorn Y. Changes in concentrations of cortisol, dehydroepiandrosterone sulphate and progesterone in fetal and maternal serum during pregnancy. Clin. Endocrinol. 1991;35:447–451. doi: 10.1111/j.1365-2265.1991.tb03564.x. [DOI] [PubMed] [Google Scholar]
- 30.Lugo RA, Nahata MC, Menke JA, McClead RE., Jr. Pharmacokinetics of dexamethasone in premature neonates. Eur. J. Clin. Pharm. 1996;49:477–483. doi: 10.1007/BF00195934. [DOI] [PubMed] [Google Scholar]
- 31.Ballard PL, Granberg P, Ballard RA. Glucocorticoid levels in maternal and cord serum after prenatal betamethasone therapy to prevent respiratory distress syndrome. J. Clin. Invest. 1975;56:1548–1554. doi: 10.1172/JCI108236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merrill JD, et al. Pilot trial of late booster doses of surfactant for ventilated premature infants. J. Perinatol. 2011;31:599–606. doi: 10.1038/jp.2010.210. [DOI] [PubMed] [Google Scholar]
- 33.van’t Veen A, et al. Exogenous pulmonary surfactant as a drug delivering agent: influence of antibiotics on surfactant activity. Br. J. Pharm. 1996;118:593–598. doi: 10.1111/j.1476-5381.1996.tb15442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van’t Veen, A. et al. Influence of pulmonary surfactant on in vitro bactericidal activities of amoxicillin, ceftazidime, and tobramycin. Antimicrob., Agent Chemother. 329–333 (1995). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


