Abstract
Ventilator-associated pneumonia (VAP) is one of the most severe complications in patients with traumatic brain injury (TBI) and is considered a risk factor for poor outcomes. However, the incidence of VAP among patients with TBI reported in studies varies widely. What is more, the risk factors and outcomes of VAP are controversial. This study estimates the incidence, risk factors, and outcomes of VAP in patients with TBI and provides evidence for prevention and treatment. PubMed, EMBASE, Cochrane Library, and Web of Science databases were searched from the earliest records to May 2018. Data involving the incidence, risk factors, and outcomes were extracted for meta-analysis. The results showed that the incidence of VAP was 36% (95% confidence interval (CI) 31–41%); risk factors analyses showed that smoking [odds ratio (OR) 2.13; 95% CI 1.16–3.92], tracheostomy (OR 9.55; 95% CI 3.24–28.17), blood transfusion on admission (OR 2.54; 95% CI 1.24–5.18), barbiturate infusion (OR 3.52; 95% CI 1.68–7.40), injury severity score (OR 4.65; 95% CI 1.96–7.34), and head abbreviated injury scale (OR 2.99; 95% CI 1.66–5.37) were related to the occurrence of VAP. When patients developed VAP, mechanical ventilation time (OR 5.45; 95% CI 3.78–7.12), ICU length of stay (OR 6.85; 95% CI 4.90–8.79), and hospital length of stay (OR 10.92; 95% CI 9.12–12.72) were significantly increased. However, VAP was not associated with an increased risk of mortality (OR 1.28; 95% CI 0.74–2.21). VAP is common in patients with TBI. It is affected by a series of factors and has a poor prognosis.
Electronic supplementary material
The online version of this article (10.1007/s12028-019-00773-w) contains supplementary material, which is available to authorized users.
Keywords: Ventilator-associated pneumonia, Traumatic brain injury, Incidence, Risk factors, Outcome
Introduction
Traumatic brain injury (TBI) is brain damage caused by external force. It is the main cause of death and disability among trauma patients [1]. It is estimated that 53,000 people die each year from TBI in the United States and more than 5.3 million Americans are currently disabled for TBI [2]. Patients who have undergone TBI often suffer from profound suppression of the cellular immune system and impaired consciousness [3], and they usually require tracheal intubation and ventilator support [4], both of which increased the incidence of ventilator-associated pneumonia (VAP).
VAP is a type of nosocomial pneumonia developing 48 h or more after receiving mechanical ventilation. Studies have shown that VAP can lead to increased mortality and morbidity [5]. In addition, when patients suffered from VAP, the medical expenses increase [6].
The incidence of VAP among patients with TBI reported in studies varies widely, ranging from 23 to 60% [7–9]. In addition, previous studies have demonstrated that the risk factors for VAP among patients with TBI include smoking, higher injury severity score (ISS), tracheostomy, diabetes, and so on [8–12]. However, these studies did not provide consistent evidence for prevention and treatment due to many reasons, such as small sample sizes, different population sources, and the use of different study designs [11, 12]. What is more, some studies indicated that VAP increased the risk of mortality [13, 14], but other studies showed that VAP was not associated with mortality [9, 15]. Identification of risk factors and outcomes associated with VAP could permit clinical staff to provide close infection surveillance and timely care for TBI patients at high risk for pneumonia. Therefore, a systematic summary is urgently needed.
In the present study, we performed a meta-analysis of the incidence, risk factors, and outcomes of VAP in patients with TBI, so as to provide evidence for the prevention and treatment of VAP among patients with TBI.
Methods
Search Strategy
A literature search was performed using PubMed, EMBASE, Cochrane Library, and Web of Science databases up to May 2018. We used Medical Subject Headings and keywords as follows: (traumatic brain injury OR brain injuries, traumatic OR brain injury OR TBI OR craniocerebral trauma OR craniocerebral injury OR head injury OR head trauma) AND (ventilator-associated pneumonia OR VAP OR pneumonia, ventilator associated OR ventilator associated pneumonia). The specific searching strategy is described in Table S1. In the initial selection phase, two reviewers (Y.L. and C.L.) screened the title and abstract of studies independently. Studies that obviously did not meet the inclusion criteria were excluded, and those included articles were read in full text to confirm their eligibility. Any disagreement was resolved by discussion with a third reviewer (S.W.). Reference lists of relevant studies were also manually searched for further studies. The publication language of studies was not limited. Our search strategy was in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines [16], The PRISMA checklist is described in Table S2.
Inclusion Criteria
The inclusion criteria for our study were: (1) All enrolled patients had undergone TBI and had mechanical ventilation for more than 48 h. (2) A clear definition and diagnosis of VAP. (3) Articles must provide one or more index for the incidence, risk factors, or outcomes of VAP. (4) Cohort study or case–control study. (5) Full text available. (6) When multiple publications contain duplicate results, the study with the largest population was included.
Data Extraction and Data Transformation
Two people extract data from eligible articles independently, including study characteristics (first author, publication time, country, number of patients, number of VAP, gender, age, study design, the definition of VAP, the severity of TBI), incidence, risk factors, and indexes representing clinical outcomes (mortality, mechanical ventilation time, intensive care unit (ICU) length of stay, hospital length of stay), so as to provide a comprehensive description of VAP among patients with TBI.
Some studies were presenting continuous data (ventilator days, ICU length of stay, hospital length of stay, ISS [17]) using the mean and standard deviation or using the median and the first and third quartiles. To be able to use these data in our meta-analysis, we converted the median and the first and third quartiles to the mean and standard deviation using the method described by Wan et al. [18].
Quality Assessment
The quality of the studies was evaluated by two independent reviewers (Y.L. and C.L.) using the Newcastle–Ottawa Scale (NOS) [19], which was used for cohort designs and case–control designs. The quality of the studies was evaluated based on three perspectives: selection (four items), comparability (one item), and exposure or outcome (three items). The NOS rating system with a score ranging from 0 to 9 stars. High-quality studies should reach seven stars or more, 4–6 stars are of medium quality, and less than four stars are of poor quality.
Statistical Analysis
The incidence of VAP among patients with TBI was calculated using Stata 12.0 (Stata Corp, College Station, TX); subgroup analyses in terms of study design, region, the definition of VAP, and the severity of TBI were also performed. Risk factors and outcomes of VAP in patients with TBI were analyzed using the Review Manager Software (RevMan5.3; Cochrane Collaboration, Oxford, UK). For measurement data, the weighted mean difference and 95% confidence intervals (CIs) were calculated to compare the mean difference. For count data, odds ratios (ORs) and 95% CIs were used. They were considered significantly associated with VAP when P < 0.05. Heterogeneity was calculated using I2 statistic. Heterogeneity was considered to be present if I2 > 50% and random effects models were used; otherwise, a fixed effects model was used. Publication bias was performed by Begg’s and Egger’s test using Stata 12.0 [20, 21]; a p value > 0.05 indicating the lack of publication bias.
Results
Literature Search
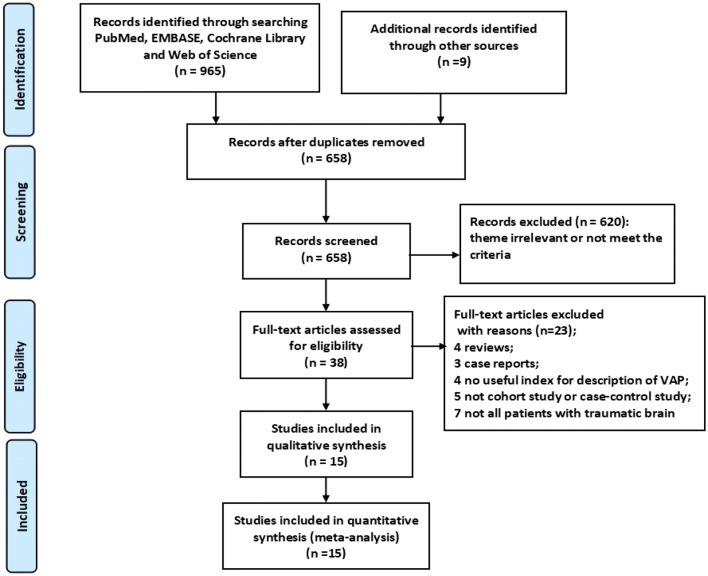
A total of 974 records were identified from the databases (PubMed: 182, EMBASE: 255, Cochrane Library: 135, Web of Science 393) and manually searched. After duplicates were removed, 658 records were retained. After initial screening of article titles and abstracts, 620 records were excluded. Then, 38 records were retained for further screening, among which, there were four reviews and three case reports; four studies were not having a useful index for the description of VAP, and five studies were not cohort studies or case–control studies. Not all the patients enrolled in seven studies were with TBI. Finally, 15 articles were included in this meta-analysis (Fig. 1).
Fig. 1.
Flow diagram of the study selection process
Study Characteristics and Quality Assessment
A total of 15 articles were included in this meta-analysis. The characteristics of the included studies are summarized in Table 1. Six studies were cohort studies, and nine studies were case–control studies. According to the NOS, two articles got eight stars, five articles got seven stars, five articles got six stars, and three articles got five stars (Table 2).
Table 1.
Characteristics of the studies included in the meta-analysis
| Study | Country | Number of patients | Number of VAP | Gender (male/female) | Age | The severity of TBI | ||
|---|---|---|---|---|---|---|---|---|
| VAP | Non-VAP | VAP | Non-VAP | |||||
| Sirvent [22] | Spain | 100 | 26 | 22/4 | 52/22 | 37 ± 19*,a | Moderate-to-severe TBI | |
| Leone [10] | France | 324 | 120 | – | – | – | – | All TBI |
| Rincón-Ferrari [9] | Spain | 310 | 72 | 65/7 | – | 28 (20–39.75)† | – | Severe TBI |
| Bronchard [23] | France | 109 | 55 | – | – | 34 ± 15*,a | All TBI | |
| Kallel [14] | Tunisia | 241 | 77 | – | – | 35.8 ± 14.9* | – | All TBI |
| Zygun [15] | Canada | 134 | 60 | 49/11 | 55/19 | 36 ± 16* | 40 ± 20* | Severe TBI |
| Wu [24] | China | 220 | 53 | 30/23 | – | 33.8 ± 19.07* | – | Severe TBI |
| Lepelletier [25] | France | 161 | 65 | 101/60b | 39.8 ± 11.1*,a | Severe TBI | ||
| Marjanović [26] | Serbia | 72 | 31 | 22/9 | 31/10 | 40.97 ± 16.84* | 50.51 ± 18.92* | Severe TBI |
| Ma [12] | China | 162 | 40 | 27/13 | 86/36 | 36.6 ± 10.3* | 37.5 ± 11.8* | Severe TBI |
| Plurad [11] | USA | 94 | 33 | 27/6 | 42/19 | 30.4 ± 21.1*,a | Severe TBI | |
| Guo [13] | China | 137 | 52 | 78/59b | 41 ± 18* | 44 ± 19* | Severe TBI | |
| Jovanovic [8] | Serbia | 144 | 73 | 56/17 | 59/12 | – | – | Severe TBI |
| Hamele [27] | USA | 119 | 42 | 29/13 | 56/21 | 9 (6–13)† | 6 (1.5–13)† | Moderate-to-severe TBI |
| Esnault [7] | France | 175 | 106 | 90/16 | 49/20 | 36 (23–53)† | 39 (23–59)† | Severe TBI |
| Study | Incidence of VAP | EOVAP | LOVAP | Study design | Definition of VAP |
|---|---|---|---|---|---|
| (Time/incidence) | (Time/incidence) | ||||
| Sirvent [22] | – | ≤ 5 days/26% | – | Prospective/cohort | Clinical, radiographical, microbiological criteria |
| Leone [10] | 37% | – | – | Prospective/case–control | Clinical, radiographical, microbiological criteria |
| Rincón-Ferrari [9] | 23.2% | ≤ 4 days/9.7% | > days/13.5% | Prospective/case–control | CDC criteria |
| Bronchard [23] | 50.5% | ≤ 7 days/41.3% | > 7 days/9.2% | Prospective/cohort | Clinical, radiographical, microbiological criteria |
| Kallel [14] | 31.9% | – | – | Retrospective/case–control | Clinical, radiographical, microbiological criteria |
| Zygun [15] | 45% | – | – | Prospective/cohort | CDC criteria |
| Wu [24] | 24% | ≤ 4 days/9.5% | > 4 days/14.5% | Retrospective/case–control | Clinical, radiographical, microbiological criteria |
| Lepelletier [25] | 40.4% | ≤ 4 days/21.1% | > 4 days/19.3% | Retrospective/cohort | American thoracic society |
| Marjanović 26] | 43% | – | – | Retrospective/case–control | Clinical, radiographical, microbiological criteria |
| Ma [12] | 25% | – | – | Retrospective/case–control | American thoracic society |
| Plurad [11] | 35% | ≤ 3 days/16% | > 3 days/19% | Retrospective/case–control | Clinical, radiographical, microbiological criteria |
| Guo [13] | 38% | – | – | Retrospective/case–control | Chinese Respiratory Society |
| Jovanovic [8] | 50% | ≤ 4 days/24% | > 4 days/26% | Prospective/cohort | Clinical, radiographical, microbiological criteria |
| Hamele [27] | 36% | – | – | Retrospective/cohort | CDC criteria |
| Esnault [7] | – | ≤ 7 days/60.6% | – | Retrospective/case–control | Clinical, radiographical, microbiological criteria |
CDC Centers for Disease Control and Prevention, EOVAP early-onset ventilator-associated pneumonia, LOVAP late-onset ventilator-associated pneumonia, TBI traumatic brain injury, VAP ventilator-associated pneumonia
*Values are represented as the mean ± standard derivation; †values are represented as the median (interquartile range); aage distribution of patients in both groups (VAP and non-VAP); bgender distribution of patients in both groups (VAP and non-VAP)
Table 2.
Quality assessment of the studies using the Newcastle–Ottawa scale
| Study | Selection | Comparability | Exposure | Total scores | |||||
|---|---|---|---|---|---|---|---|---|---|
| Is case definition adequate? | Representativeness of cases | Selection of controls | Definition of controls | Comparability on the basis of designs or analysis | Ascertainment of exposure | Same ascertainment method for cases and controls | Nonresponse rate | ||
| Case–control studies | |||||||||
| Leone [10] | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 8 | |
| Rincón-Ferrari [9] | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 8 | |
| Kallel [14] | ★ | ★ | ★★ | ★ | ★ | ★ | 7 | ||
| Wu [24] | ★ | ★ | ★ | ★ | ★ | 5 | |||
| Marjanović [26] | ★ | ★ | ★ | ★ | ★ | 5 | |||
| Ma [12] | ★ | ★ | ★ | ★ | ★ | ★ | 6 | ||
| Plurad [11] | ★ | ★ | ★ | ★ | ★ | ★ | 6 | ||
| Guo [13] | ★ | ★ | ★ | ★ | ★ | 5 | |||
| Esnault [7] | ★ | ★ | ★ | ★ | ★ | ★ | 6 | ||
| Selection | Comparability | Outcome | Total scores | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness of exposed cohort | Selection of the nonexposed cohort | Ascertainment of exposure | Demonstration that outcome was not present at start of study | Comparability on the basis of design or analysis | Assessment of outcome | Was follow-up long enough for outcomes to occur? | Adequacy of follow-up of cohorts | ||
| Cohort studies | |||||||||
| Sirvent [22] | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 7 | |
| Bronchard [23] | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 7 | |
| Zygun [15] | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 7 | |
| Lepelletier [25] | ★ | ★ | ★ | ★ | ★ | ★ | 6 | ||
| Jovanovic [8] | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 7 | |
| Hamele [27] | ★ | ★ | ★ | ★ | ★ | ★ | 6 | ||
Incidence
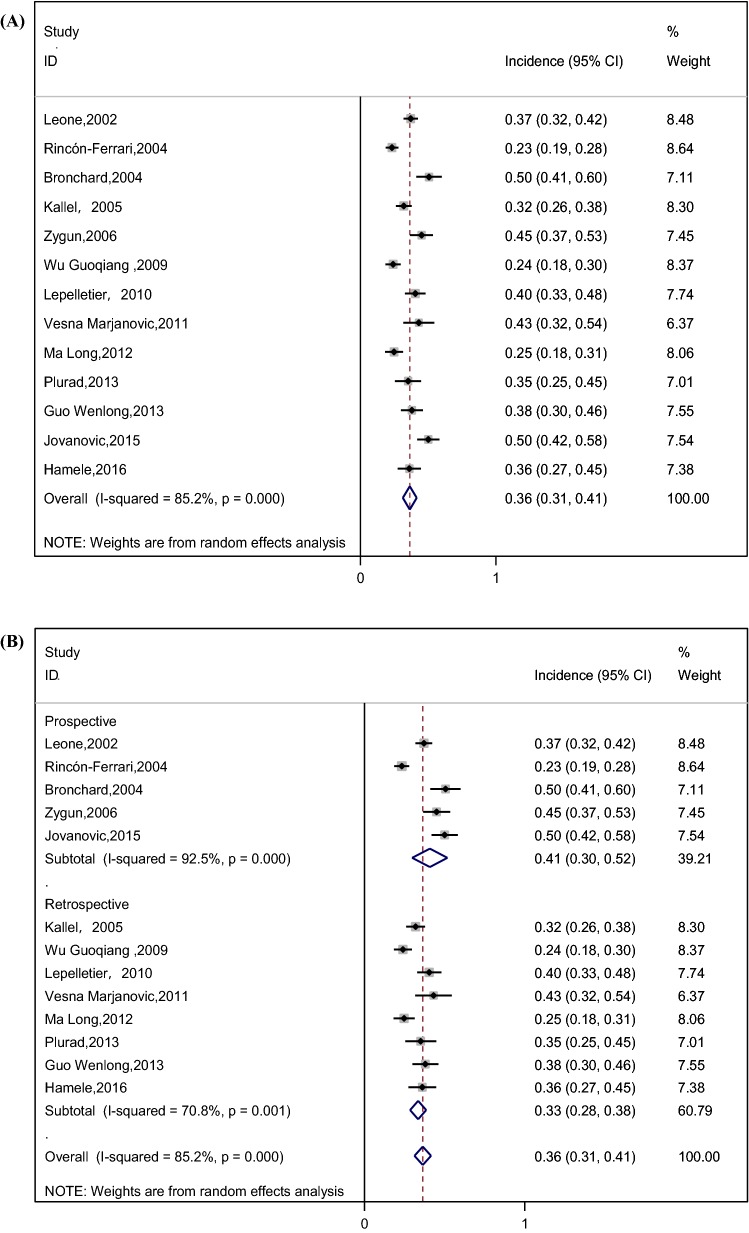
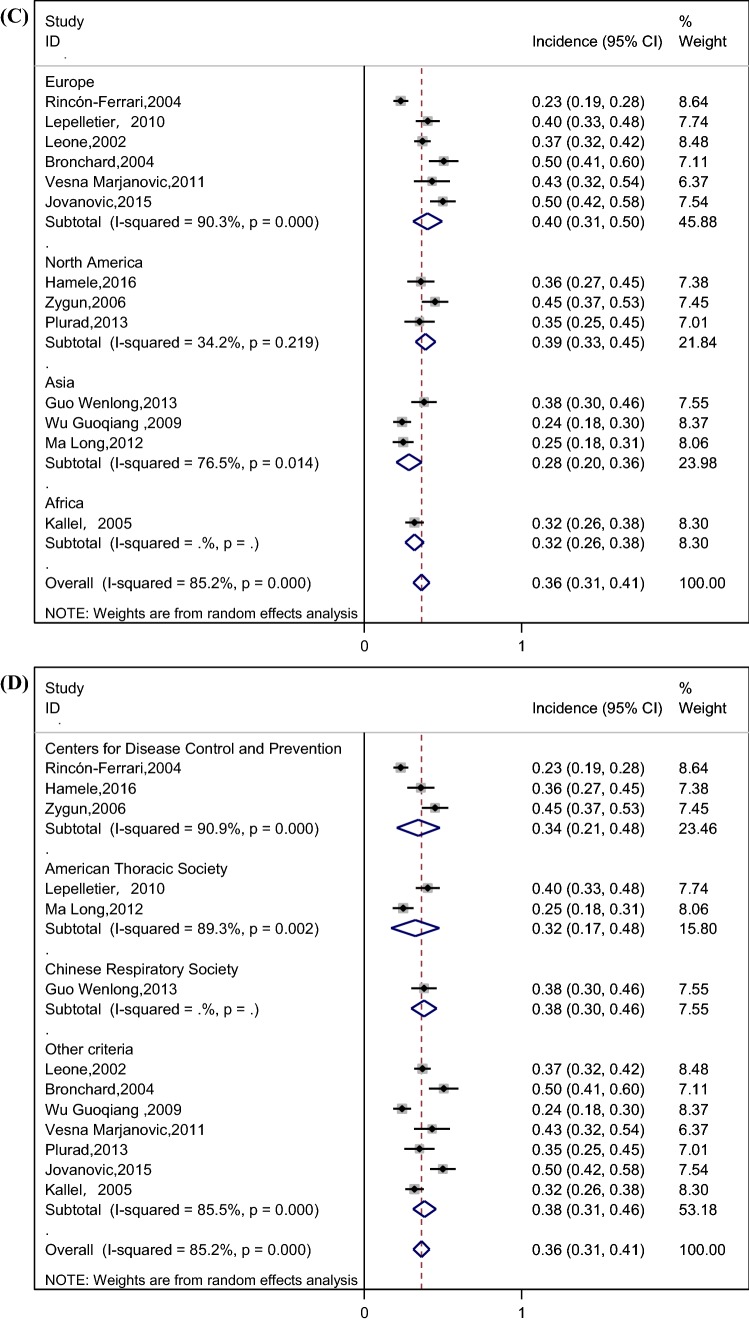
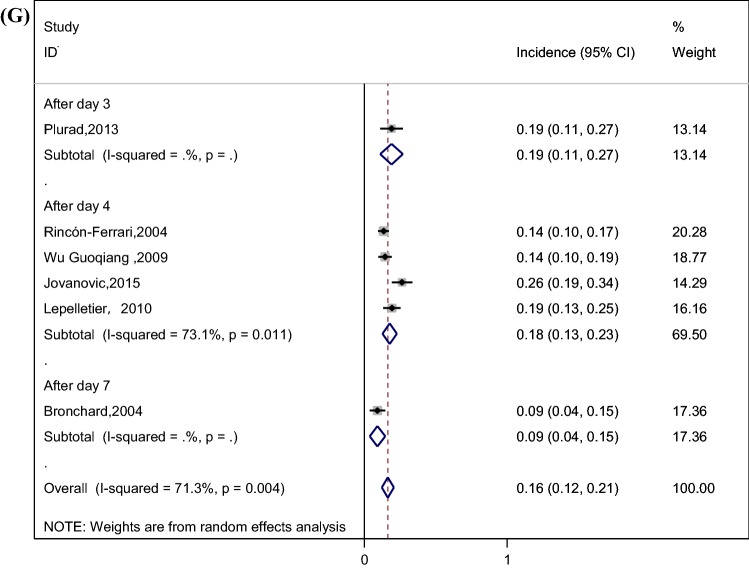
The pooled incidence of VAP was 36% (95% CI 31–41%) among patients with TBI, as shown in Fig. 2a. We performed a subgroup analysis by study design (prospective and retrospective) to explore its impact on VAP incidence (Fig. 2b); The forest plot illustrated that the incidence of VAP in prospective studies (41%) was relatively higher than their counterparts in retrospective studies (33%). Stratified analysis based on variation in the region was applied because of its potential effect on the incidence of VAP (Fig. 2c), and the results showed that the incidence of VAP in North America (39%) and Europe (40%) was relatively higher than Asia (28%). We also performed a subgroup analysis by definition of VAP (Fig. 2d), and the results showed the incidence of VAP in Centers for Disease Control (CDC) criteria [28], American Thoracic Society criteria [29], Chinese Respiratory Society criteria [30] with 34%, 32%, 38%, respectively. Considering the impact of TBI severity on the incidence of VAP, we performed a subgroup analysis based on the severity of TBI (Fig. 2e), and the result showed that the incidences of VAP in severe TBI patients (defined according to the standards used in the original study, such as Glasgow coma score (GCS) ≤ 8, the presence of clinical or radiographic herniation, etc.) and all TBI patients were similar, with 35% and 39%, respectively. In addition to the overall incidence of VAP, some studies divided VAP into early-onset ventilator-associated pneumonia (EOVAP) and late-onset ventilator-associated pneumonia (LOVAP). The results showed that the incidences of EOVAP (occurring in the first 3–7 days of mechanical ventilation) and LOVAP (occurring after 3–7 days of mechanical ventilation) were 26% and 16%, respectively (Fig. 2f, g).
Fig. 2.
a Incidence of VAP in patients with TBI (CI confidence interval, ID identification). b Incidence of VAP by subgroup analysis of study design (prospective and retrospective) (CI confidence interval, ID identification). c Incidence of VAP by subgroup analysis of the region (CI confidence interval, ID identification). d Incidence of VAP by subgroup analysis of the definition of VAP (CI confidence interval, ID identification). e Incidence of VAP by subgroup analysis of the severity of TBI (CI confidence interval, ID identification, TBI traumatic brain injury). f Incidence of EOVAP in patients with TBI (CI confidence interval, ID identification). g Incidence of LOVAP in patients with TBI (CI confidence interval, ID identification)
Risk Factors
There are 18 potential risk factors (reported in at least two studies) summarized in this meta-analysis. We found a significant relationship between VAP and the following risk factors: smoking, tracheostomy, blood transfusion on admission, barbiturate infusion, ISS, head abbreviated injury scale (AIS), as noted in Table 3. The data demonstrated that smoking increased the risk for VAP among patients with TBI (OR 2.13; 95% CI 1.16–3.92; P < 0.05)**. Additionally patients with tracheostomy were more vulnerable to VAP (OR 9.55; 95% CI 3.24–28.17; P < 0.05). What is more, blood transfusion on admission also increased the risk for VAP (OR 2.54; 95% CI 1.24–5.18; P < 0.05). Additionally, barbiturate infusion increased the risk for VAP among patients with TBI (OR 3.52; 95% CI 1.68–7.40; P < 0.05). After data integration of three studies, pooled results showed that patients with higher ISS were more likely to be diagnosed with VAP compared to those with lower ISS (OR 4.65; 95% CI 1.96–7.34; P < 0.05). Moreover, patients with head AIS ≥ 3 were more vulnerable to VAP (OR 2.99; 95% CI 1.66–5.37; P < 0.05). No significant differences were detected based on gender (male), age, diabetes, shock, prophylactic antibiotics, corticosteroids, abdomen AIS, thorax AIS, thorax trauma, spinal trauma, facial trauma, abdomen trauma; therefore, these factors were determined not to be risk factors for VAP. The forest plots of risk factors are described in Figure S1.
Table 3.
Risk factors of ventilator-associated pneumonia in patients with TBI
| Risk factors | Combination studies | Analysis model | Heterogeneity of studies (I2) (%) | Meta-analysis | Begg’s test (p) | Egger’s test (p) | |
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P value | ||||||
| Gender (male) | 9 | Fixed | 0 | 1.02 (0.75–1.38) | 0.91 | 0.602 | 0.304 |
| Age | 5 | Random | 65 | − 1.89 (− 5.57 to 1.80) | 0.32 | 0.086 | 0.005 |
| Smoking | 2 | Fixed | 0 | 2.13 (1.16–3.92) | 0.01 | 1 | NA |
| Shock | 4 | Fixed | 25 | 1.27 (0.79–2.04) | 0.33 | 0.308 | 0.531 |
| Diabetes | 3 | Fixed | 0 | 1.47 (0.72–3.01) | 0.30 | 1 | 0.779 |
| Tracheostomy | 5 | Random | 85 | 9.55 (3.24–28.17) | 0.001 | 1 | 0.675 |
| Blood transfusion on admission | 2 | Fixed | 2 | 2.54 (1.24–5.18) | 0.01 | 1 | NA |
| Prophylactic antibiotics | 3 | Random | 51 | 0.97 (0.50–1.90) | 0.93 | 0.296 | 0.234 |
| Barbiturate infusion | 2 | Fixed | 0 | 3.52 (1.68–7.40) | 0.001 | 1 | NA |
| Corticosteroids | 2 | Fixed | 0 | 1.47 (0.59–3.66) | 0.40 | 1 | NA |
| ISS | 3 | Fixed | 0 | 4.65 (1.96–7.34) | 0.001 | 1 | 0.245 |
| AIS head ≥ 3 | 2 | Fixed | 0 | 2.99 (1.66–5.37) | 0.001 | 1 | NA |
| AIS abdomen ≥ 3 | 3 | Fixed | 24 | 1.32 (0.71–2.47) | 0.38 | 1 | 0.452 |
| AIS thorax ≥ 3 | 4 | Random | 73 | 1.56 (0.65–3.76) | 0.32 | 1 | 0.942 |
| Thorax trauma | 3 | Random | 82 | 2.04 (0.67–6.16) | 0.21 | 1 | 0.794 |
| Spinal trauma | 2 | Random | 69 | 1.20 (0.30–4.77) | 0.80 | 1 | NA |
| Facial trauma | 2 | Fixed | 0 | 0.83 (0.51–1.34) | 0.44 | 1 | NA |
| Abdomen trauma | 2 | Random | 59 | 1.35 (0.45–4.07) | 0.59 | 1 | NA |
AIS abbreviated injury scale, CI confidence interval, ISS injury severity score, NA not available, OR odds ratio, TBI traumatic brain injury
Clinical Outcomes
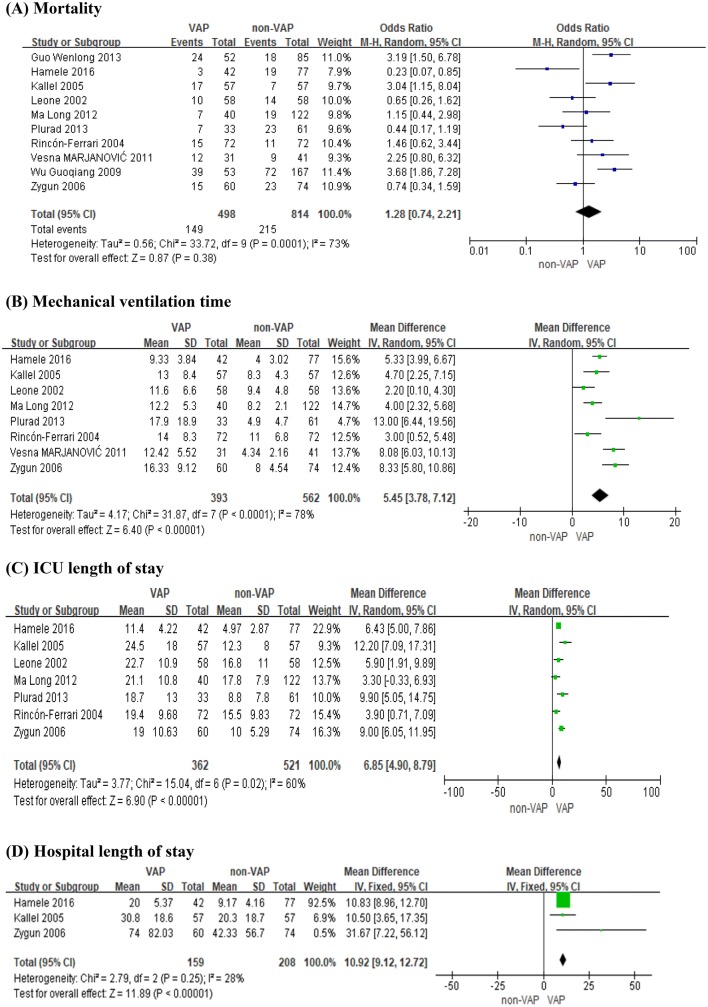
After data integration of ten studies, pooled results showed that VAP was not associated with mortality (OR 1.28; 95% CI 0.74–2.21; P = 0.38), as shown in Fig. 3a. However, VAP increased the mechanical ventilation time (OR 5.45; 95% CI 3.78–7.12; P<0.05), as shown in Fig. 3b. In addition, our pooled results also showed that ICU length of stay was increased significantly in patients infected with VAP (OR 6.85; 95% CI 4.90–8.79; P<0.05), as shown in Fig. 3c. Patients who developed VAP also had a longer duration of hospital length of stay (OR 10.92; 95% CI 9.12–12.72; P<0.05), as shown in Fig. 3d.
Fig. 3.
Outcomes of VAP among patients with TBI. a Mortality; b mechanical ventilation time; c ICU length of stay; d hospital length of stay (CI confidence interval, IV inverse variance, M–H Mantel–Haenszel, SD standard deviation, VAP ventilator-associated pneumonia)
Publication Bias
There was no significant publication bias in the analysis of the incidence of VAP by Egger’s test (P = 0.196) and Begg’s test (P = 0.161). The results of publication bias for risk factor analysis are shown in Table 3. Moreover, the mortality (Egger’s test P = 0.076; Begg’s test P = 0.107), mechanical ventilation time (Egger’s test P = 0.404; Begg’s test P = 0.902), ICU length of stay (Egger’s test P = 0.593; Begg’s test P = 0.548), and hospital length of stay (Egger’s test P = 0.454; Begg’s test P = 0.296) also had no significant publication bias.
Discussion
To our best knowledge, this study was the first meta-analysis exploring the incidence, risk factors, and outcomes of VAP in patients with TBI.
This meta-analysis showed that the incidence of VAP in patients with TBI was 36% (95% CI 31–41%) by pooling data from the included studies. However, the spectacular disparity in the incidence of VAP was shown in the previous studies. Bronchard et al. [23] and Jovanovic et al. [8] reported that the incidence of VAP was 50%, while Rincón-Ferrari et al. [9] found that the incidence of VAP was about 23%. Differences in the study design, region, definition of VAP, and the severity of TBI may explain this wide range, leading to subgroup analyses based on these factors. The result demonstrated that the incidence of VAP in prospective studies was significantly higher than retrospective studies (41% vs. 33%). A study performed by J Tobias et al. showed that data obtained retrospectively from the medical records were less complete and accurate compared to data obtained from the patients prospectively [31]. Retrospective information is fraught with the problems of missing data, conflicting data, and illegibility [32]. We recommend that more prospective studies be carried out in the future so as to obtain more rigorous results about VAP. In addition, there was a significant difference in the incidence of VAP between North America, Europe and Asia (39% vs. 40% vs. 28%) by subgroup analysis; this difference may be due to the lack of active infection monitoring systems in Asian countries compared to European and North American countries, which led to the inadequate feedback of hospital infection surveillance data and underestimate of the incidence of VAP [33–35]. What is more, the result showed that the incidences of VAP in severe TBI patients and all TBI patients were similar, with 35% and 39%, respectively. In this meta-analysis, three studies included all TBI patients, rather than only those with severe TBI [10, 14, 23]. Most of the patients in these three studies had severe neurologic injury. The mean GCS score of patients was 7.38 in the study conducted by Kallel et al. [14], and 7.2 in the study conducted by Bronchard et al. [23]. The median GCS score of patients was 6 in the study conducted by Leone et al. [10]. This may explain why the incidence of VAP in these three studies is similar to the studies including patients with severe TBI. Additionally, as for EOVAP and LOVAP, the incidence of EOVAP and LOVAP is related to the cutoff point used for definition [36]. However, the cutoff point in the literature is usually inconsistent [37]. Unifying EOVAP and LOVAP cutoff point is of great significance to the treatment of patients and clinical research. Further research is needed to accurately assess the cutoff point.
Identification of risk factors associated with VAP could permit clinical staff to provide close infection surveillance and timely care for TBI patients at high risk for pneumonia so as to optimize clinical outcomes. In the present meta-analysis, we integrated all eligible studies of VAP in patients with TBI. By integrating the results of previous studies and expanding the sample size, we found a significant relationship between VAP and the following risk factors: smoking, tracheostomy, blood transfusion on admission, barbiturate infusion, ISS, and head AIS. We identified that smoking increased the risk for VAP, probably because smoking impairs mucociliary clearance, making it easier for pathogens to colonize in the respiratory tract [38]. Smoking remains an important contributor to pneumonia, and all patients with TBI should be encouraged to quit. Additionally, tracheotomy increased the risk for VAP; tracheotomy bypasses normal respiratory defense mechanisms, such as oropharynx and cilia, which contribute to the occurrence of VAP [39]. It has been reported that use of suction-above-the-cuff tracheotomy tubes reduces the incidence of VAP [40]. More attention in the details of tracheotomy care such as the use of suction-above-the-cuff tracheotomy tubes [40] may be helpful in decreasing the associated risk when tracheotomy is performed. Furthermore, blood transfusion on admission heightens the risk for VAP, which may be because the early period of acute severe trauma is associated with an immunosuppressive inflammatory response, and this response was increased in magnitude when patients received a transfusion of blood products [41, 42]. In addition, our results showed that barbiturate use increased the risk of VAP in TBI patients, which may be related to the immunosuppressive effect of barbiturates given they; promote reversible bone marrow suppression in patients with TBI [43] and increased susceptibility to develop VAP [27]. Although some treatments such as the use of barbiturates or blood transfusions are frequently indicated, they should be administered appropriately. Moreover, the result of this meta-analysis demonstrated that patients with higher ISS or head AIS represent a specific target population for the prevention of VAP. The AIS provides a method for ranking and comparing injuries by severity in body regions [44]. The ISS is derived from the AIS and provides a description of the overall severity of injury for patients with trauma [17]. A study by Teixeira Lopes et al. [45] analyzed the risk factors associated with post-traumatic complications in hospitalized trauma patients. The study revealed that the higher the severity of trauma, the greater the number of hospitalization complications. Furthermore, the main complications presented by trauma patients were infections, especially VAP and sepsis [45, 46]. The results suggest that ISS and AIS should be considered in therapeutic decisions to help healthcare providers properly identify patients at high risk for VAP and modify patient care to minimize the risk of VAP. VAP should be closely monitored to detect infections in the target population early and to take measures to achieve optimal results.
There is much discussion about whether to use prophylactic antibiotics for TBI patients. Our meta-analysis showed that there was no association between prophylactic antibiotics and the occurrence of VAP. A study by Reizine et al. [47] showed that prophylactic antibiotics decreased the incidence of EOVAP but had no influence on LOVAP; additionally, they found that prophylactic antibiotics delayed the occurrence of VAP. Nevertheless, they suggested that antibiotic prophylaxis should not be routinely used in clinical practice because antibiotic prophylaxis has no effect on length of stay and mortality. In addition, a study by Hoth et al. [48] showed that trauma patients receiving antibiotic prophylaxis showed an increase in multi-drug-resistant bacteria. For the reasons mentioned above, we do not recommend prophylactic antibiotics in patients with TBI.
The main finding of this study was that VAP did not increase the mortality in patients with TBI. This was consistent with the results of Bregeon’s [49] study, which ultimately showed that VAP did not seem to be an independent risk factor for death. Josephson’s [50] study also showed that VAP does not lead to increased mortality in patients with neurovascular diseases, and he pointed out that death in patients with VAP was due mainly to neurologic injury and withdrawal of care. Additionally, VAP increased the mechanical ventilation time and prolonged the ICU length of stay and hospital length of stay. This is in agreement with the findings of many studies [51, 52], indicating that VAP imposed a great burden on patients [52].
This meta-analysis has many advantages. First of all, we conducted a systematic literature search. By integrating the results of previous studies, expanding the sample size and performing quantitative analysis, the accurate incidence of VAP in patients with TBI was provided from a global perspective. At the same time, subgroup analyses were performed to identify potential factors responsible for inconsistencies in previous studies, and we found that the incidence of VAP in North America (39%) and Europe (40%) was relatively higher than Asia (28%) and the incidence of VAP in prospective studies (41%) was relatively higher than that in retrospective studies (33%). In addition, we analyzed many aspects of VAP in patients with TBI including the incidence, risk factors, and outcomes so as to provide a comprehensive understanding of VAP.
Limitation
However, some limitations of this meta-analysis should be mentioned. First, the criteria used to diagnose VAP in the included studies were inconsistent and the analyzed studies used the following methods for VAP ascertainment: Three studies used the CDC criteria [28], two studies used the American Thoracic Society criteria [29], one study used the Chinese Respiratory Society criteria [30], nine studies were based on clinical, radiographical and microbiological criteria to provide references for doctors’ diagnosis. In addition, the cutoff points used to define EOVAP and LOVAP in the included studies were inconsistent. For example, some studies defined that VAP occurring in the first 4 days after mechanical ventilation was EOVAP [9], while some studies defined that VAP occurring in the first 7 days after mechanical ventilation was EOVAP [23]. We recommend that more research about EOVAP and LOVAP be conducted in the future, so as to get more accurate knowledge about it. There are still many uncertainties in the diagnosis of VAP, which needs further research.
Conclusions
In the present study, we integrated all eligible studies of VAP in patients with TBI, demonstrating that patients with TBI were at very high risk of developing VAP, with a pooled incidence of 36%. We also found that VAP does not increase the mortality in patients with TBI, but VAP increases the mechanical ventilation time, ICU length of stay, and hospital length of stay. Moreover, this meta-analysis demonstrated that smoking, tracheostomy, blood transfusion on admission, barbiturate infusion, ISS, and head AIS are risk factors for VAP, providing specific target population for the prevention of VAP.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
YTL was responsible for study design, literature search, data extraction, data analysis, and manuscript drafting. CXL was responsible for literature search, data extraction, and manuscript drafting. WX was responsible for data analysis and manuscript drafting. TTS was responsible for manuscript drafting and manuscript revision. SHW was responsible for study design, study supervision, and manuscript revision.
Source of support
This study was funded by the Natural Science Foundation of Shandong Province (Grant No. ZR2018 MG015).
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cristofori I, Levin HS. Traumatic brain injury and cognition. Handb Clin Neurol. 2015;128:579–611. doi: 10.1016/B978-0-444-63521-1.00037-6. [DOI] [PubMed] [Google Scholar]
- 2.Alali AS, Burton K, Fowler RA, et al. Economic evaluations in the diagnosis and management of traumatic brain injury: a systematic review and analysis of quality. Value Health. 2015;18(5):721–734. doi: 10.1016/j.jval.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 3.Griffin GD. The injured brain: TBI, mTBI, the immune system, and infection: connecting the dots. Mil Med. 2011;176(4):364–368. doi: 10.7205/MILMED-D-10-00021. [DOI] [PubMed] [Google Scholar]
- 4.Ngubane T. Mechanical ventilation and the injured brain. South Afr J Anaesth Analg. 2011;17(1):76–80. [Google Scholar]
- 5.Pileggi C, Bianco A, Flotta D, Nobile CGA, Pavia M. Prevention of ventilator-associated pneumonia, mortality and all intensive care unit acquired infections by topically applied antimicrobial or antiseptic agents: a meta-analysis of randomized controlled trials in intensive care units. Crit Care. 2011;15(3):R155. doi: 10.1186/cc10285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rello J, Ollendorf DA, Oster G, et al. Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest. 2002;122(6):2115–2121. doi: 10.1378/chest.122.6.2115. [DOI] [PubMed] [Google Scholar]
- 7.Esnault P, Nguyen C, Bordes J, et al. Early-onset ventilator-associated pneumonia in patients with severe traumatic brain injury: incidence, risk factors, and consequences in cerebral oxygenation and outcome. Neurocrit Care. 2017;27(2):187–198. doi: 10.1007/s12028-017-0397-4. [DOI] [PubMed] [Google Scholar]
- 8.Jovanovic B, Milan Z, Markovic-Denic L, et al. Risk factors for ventilator-associated pneumonia in patients with severe traumatic brain injury in a Serbian trauma centre. Int J Infect Dis. 2015;38:46–51. doi: 10.1016/j.ijid.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Rincon-Ferrari MD, Flores-Cordero JM, Leal-Noval SRN, Murillo-Cabezas F, Cayuelas A. Impact of ventilator-associated pneumonia in patients with severe head injury. J Trauma Inj Infect Crit Care. 2004;57(6):1234–1240. doi: 10.1097/01.TA.0000119200.70853.23. [DOI] [PubMed] [Google Scholar]
- 10.Leone M, Bourgoin A, Giuly E, et al. Influence on outcome of ventilator-associated pneumonia in multiple trauma patients with head trauma treated with selected digestive decontamination. Crit Care Med. 2002;30(8):1741–1746. doi: 10.1097/00003246-200208000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Plurad DS, Kim D, Bricker S, et al. Ventilator-associated pneumonia in severe traumatic brain injury: the clinical significance of admission chest computed tomography findings. J Surg Res. 2013;183(1):371–376. doi: 10.1016/j.jss.2013.01.036. [DOI] [PubMed] [Google Scholar]
- 12.Ma L, LI N, Wu K, An YY, Zhang TR. Risk factors for ventilator-associated pneumonia in severe head trauma patients and pathogen analysis. Chin J Nosocomiol. 2012;22(2):261–263. [Google Scholar]
- 13.Guo WL, Zhou Y, Wang SQ, Nie W, Jiang QX, Chen Q. Risk factors analysis of ventilator-associated pneumonia in severe brain injury patients. China J Mod Med. 2013;23(24):90–92. [Google Scholar]
- 14.Kallel H, Chelly H, Bahloul M, et al. The effect of ventilator-associated pneumonia on the prognosis of head trauma patients. J Trauma. 2005;59(3):705–710. [PubMed] [Google Scholar]
- 15.Zygun DA, Zuege DJ, Boiteau PJE, et al. Ventilator-associated pneumonia in severe traumatic brain injury. Neurocrit Care. 2006;5(2):108–114. doi: 10.1385/NCC:5:2:108. [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baker SP, O’Neill B, Haddon W, Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14(3):187. doi: 10.1097/00005373-197403000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 20.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 22.Sirvent JM, Torres A, Vidaur L, Armengol J, de Batlle J, Bonet A. Tracheal colonisation within 24 h of intubation in patients with head trauma: risk factor for developing early-onset ventilator-associated pneumonia. Intensive Care Med. 2000;26(9):1369–1372. doi: 10.1007/s001340000611. [DOI] [PubMed] [Google Scholar]
- 23.Bronchard R, Albaladejo P, Brezac G, et al. Early onset pneumonia. Anesthesiology. 2004;100(2):234–239. doi: 10.1097/00000542-200402000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Wu GQ, Li ZY, Lv WX. Ventilator-associated pneumonia following severe craniocerebral trauma and its clinical characteristics. Chin J Nosocomiol. 2009;19(13):1649–1651. [Google Scholar]
- 25.Lepelletier D, Roquilly A, Latte DDD, et al. Retrospective analysis of the risk factors and pathogens associated with early-onset ventilator-associated pneumonia in surgical-ICU head-trauma patients. J Neurosurg Anesthesiol. 2010;22(1):32–37. doi: 10.1097/ANA.0b013e3181bdf52f. [DOI] [PubMed] [Google Scholar]
- 26.Marjanović V, Novak V, Velicković L, Marjanović G. The incidence and risk factors of ventilator-associated pneumonia in patients with severe traumatic brain injury. Med Pregl. 2011;64(7–8):403. doi: 10.2298/MPNS1108403M. [DOI] [PubMed] [Google Scholar]
- 27.Hamele M, Stockmann C, Cirulis M, et al. Ventilator-associated pneumonia in pediatric traumatic brain injury. J Neurotrauma. 2016;33(9):832–839. doi: 10.1089/neu.2015.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16:128–140. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 29.Niederman M, Craven D, Bonten MJ. American Thoracic Society and the Infectious Diseases Society of America Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 30.Chinese Society of Respiratory Medicine Guidelines for the diagnosis and treatment of hospital acquired pneumonia (draft) Chin J Tuberculol Respir Dis. 1999;14(4):160–161. [Google Scholar]
- 31.Tobias JN, Brown DFM, Swati S, Weiner JB, Wang AC, Yuchiao C. The accuracy and completeness of data collected by prospective and retrospective methods. Acad Emerg Med. 2005;12(9):884–895. doi: 10.1197/j.aem.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz RJ, Panacek EA. Basics of research (part 7): archival data research. Air Med J. 1996;15(3):119–124. doi: 10.1016/S1067-991X(96)90037-1. [DOI] [PubMed] [Google Scholar]
- 33.Benedetta A, Didier P. Healthcare-associated infection in developing countries: simple solutions to meet complex challenges. Infect Control Hosp Epidemiol. 2007;28(12):1323–1327. doi: 10.1086/521656. [DOI] [PubMed] [Google Scholar]
- 34.Kaier K, Lambert ML, Frank UK, et al. Impact of availability of guidelines and active surveillance in reducing the incidence of ventilator-associated pneumonia in Europe and worldwide. BMC Infect Dis. 2014;14(1):199. doi: 10.1186/1471-2334-14-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Machi S, Katsumi Y, Jun T. Epidemiological approach to nosocomial infection surveillance data: the Japanese Nosocomial Infection Surveillance System. Environ Health Prev Med. 2008;13(1):30–35. doi: 10.1007/s12199-007-0004-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giard M, Lepape A, Allaouchiche B, et al. Early- and late-onset ventilator-associated pneumonia acquired in the intensive care unit: comparison of risk factors. J Crit Care. 2008;23(1):27–33. doi: 10.1016/j.jcrc.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 37.Ibn SW, Souweine B, Garrouste-Orgeas M, et al. Respective impact of implementation of prevention strategies, colonization with multiresistant bacteria and antimicrobial use on the risk of early- and late-onset VAP: an analysis of the OUTCOMEREA network. PLoS ONE. 2017;12(11):e0187791. doi: 10.1371/journal.pone.0187791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chapman C, Morgan P, Cadilhac DA, Purvis T, Andrew NE. Risk factors for the development of chest infections in acute stroke: a systematic review. Top Stroke Rehabil. 2018;2:1–14. doi: 10.1080/10749357.2018.1481567. [DOI] [PubMed] [Google Scholar]
- 39.Joanne Elliot Z, Charlton ElliotS. An overview of mechanical ventilation in the intensive care unit. Nurs Stand. 2018;32(28):41–49. doi: 10.7748/ns.2018.e10710. [DOI] [PubMed] [Google Scholar]
- 40.Ledgerwood LG, Salgado MD, Hugh B, Ken Y, Ann S, Belafsky PC. Tracheotomy tubes with suction above the cuff reduce the rate of ventilator-associated pneumonia in intensive care unit patients. Ann Otol Rhinol Laryngol. 2013;122(1):3–8. doi: 10.1177/000348941312200102. [DOI] [PubMed] [Google Scholar]
- 41.Torrance HD, Brohi K, Pearse RM, et al. Association between gene expression biomarkers of immunosuppression and blood transfusion in severely injured polytrauma patients. Scand J Trauma Resusc Emerg Med. 2014;22(S1):O7. doi: 10.1186/1757-7241-22-S1-O7. [DOI] [PubMed] [Google Scholar]
- 42.Xu G, Hu B, Chen G, et al. Analysis of blood trace elements and biochemical indexes levels in severe craniocerebral trauma adults with Glasgow Coma Scale and Injury Severity Score. Biol Trace Elem Res. 2015;164(2):192–197. doi: 10.1007/s12011-014-0225-z. [DOI] [PubMed] [Google Scholar]
- 43.Stover JF, Stocker R. Barbiturate coma may promote reversible bone marrow suppression in patients with severe isolated traumatic brain injury. Eur J Clin Pharmacol. 1998;54(7):529–534. doi: 10.1007/s002280050508. [DOI] [PubMed] [Google Scholar]
- 44.Foreman BP, Ruth RC, Jennifer P, et al. Usefulness of the abbreviated injury score and the injury severity score in comparison to the Glasgow Coma Scale in predicting outcome after traumatic brain injury. J Trauma. 2007;62(4):946–950. doi: 10.1097/01.ta.0000229796.14717.3a. [DOI] [PubMed] [Google Scholar]
- 45.Teixeira Lopes MCB, de Aguiar WJ, Yamaguchi WhitakerI. In-hospital complications in trauma patients according to injury severity. J Trauma Nurs. 2019;26(1):10–16. doi: 10.1097/JTN.0000000000000411. [DOI] [PubMed] [Google Scholar]
- 46.De AJW, Saleh CM, Whitaker IY. Risk factors for complications of traumatic injuries. J Trauma Nurs Off J Soc Trauma Nurses. 2016;23(5):275. doi: 10.1097/JTN.0000000000000233. [DOI] [PubMed] [Google Scholar]
- 47.Reizine F, Asehnoune K, Roquilly A, et al. Effects of antibiotic prophylaxis on ventilator-associated pneumonia in severe traumatic brain injury. A post hoc analysis of two trials. J Crit Care. 2019;50:221–226. doi: 10.1016/j.jcrc.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 48.Jason JH, Franklin GA, Stassen NA, Girard SM, Rodriguez RJ, Rodriguez JL. Prophylactic antibiotics adversely affect nosocomial pneumonia in trauma patients. J Trauma Acute Care Surg. 2003;55(2):249–254. doi: 10.1097/01.TA.0000083334.93868.65. [DOI] [PubMed] [Google Scholar]
- 49.Bregeon F, Ciais V, Carret V, et al. Is ventilator-associated pneumonia an independent risk factor for death? Anesthesiology. 2001;94(4):554–560. doi: 10.1097/00000542-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 50.Josephson SA, Moheet AM, Gropper MA, Nichols AD, Smith WS. Ventilator-associated pneumonia in a neurologic intensive care unit does not lead to increased mortality. Neurocrit Care. 2010;12(2):155–158. doi: 10.1007/s12028-009-9285-x. [DOI] [PubMed] [Google Scholar]
- 51.Gautam A, Ganu SS, Tegg OJ, Andresen DN, Wilkins BH, Schell DN. Ventilator-associated pneumonia in a tertiary paediatric intensive care unit: a 1-year prospective observational study. Crit Care Resusc. 2012;14(4):283–289. [PubMed] [Google Scholar]
- 52.Warren DK, Shukla SJ, Olsen MA, et al. Outcome and attributable cost of ventilator-associated pneumonia among intensive care unit patients in a suburban medical center. Crit Care Med. 2003;31(5):1312–1317. doi: 10.1097/01.CCM.0000063087.93157.06. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.