Abstract
Bloodstream infection (BSI) is defined by positive blood cultures in a patient with systemic signs of infection and may be either secondary to a documented source or primary—that is, without identified origin. Community-acquired BSIs in immunocompetent adults usually involve drug-susceptible bacteria, while healthcare-associated BSIs are frequently due to multidrug-resistant (MDR) strains. Early adequate antimicrobial therapy is a key to improve patient outcomes, especially in those with criteria for sepsis or septic shock, and should be based on guidelines and direct examination of available samples. Local epidemiology, suspected source, immune status, previous antimicrobial exposure, and documented colonization with MDR bacteria must be considered for the choice of first-line antimicrobials in healthcare-associated and hospital-acquired BSIs. Early genotypic or phenotypic tests are now available for bacterial identification and early detection of resistance mechanisms and may help, though their clinical impact warrants further investigations. Initial antimicrobial dosing should take into account the pharmacokinetic alterations commonly observed in ICU patients, with a loading dose in case of sepsis or septic shock. Initial antimicrobial combination attempting to increase the antimicrobial spectrum should be discussed when MDR bacteria are suspected and/or in the most severely ill patients. Source identification and control should be performed as soon as the hemodynamic status is stabilized. De-escalation from a broad-spectrum to a narrow-spectrum antimicrobial may reduce antibiotic selection pressure without negative impact on mortality. The duration of therapy is usually 5–8 days though longer durations may be discussed depending on the underlying illness and the source of infection. This narrative review covers the epidemiology, diagnostic workflow and therapeutic aspects of BSI in ICU patients and proposed up-to-date expert statements.
Keywords: Sepsis, Bloodstream infections, Critically ill, Antibiotic, Antibiotic stewardship, Source control, Duration of therapy, Epidemiology, Multidrug-resistant pathogens, Rapid diagnosis
Take-home messages
| This expert statement covers the available evidence on the epidemiology, diagnosis and treatment of bloodstream infections in the ICU. Key elements are: knowledge of the local epidemiology and of the risk factors due to bacterial resistance and inadequate therapy; optimization of the antimicrobial dose and infection source control. The potential benefit of new rapid diagnostic tests, antibiotic de-escalation and short duration of antimicrobial is also discussed. |
Introduction
Bloodstream infection (BSI) is defined by positive blood cultures in a patient with systemic signs of infection and may be either secondary to a documented source or primary—that is, without identified origin (https://www.cdc.gov/nhsn/pdfs/pscmanual/4psc_clabscurrent.pdf accessed December 22th 2019). Bloodstream infections (BSI) represent 40% of cases of community-acquired (CA) and hospital-acquired (HA) sepsis and septic shock and approximately 20% of the ICU-acquired cases (Table 1). It is invariably associated with poor outcomes especially when adequate antimicrobial therapy and source control are delayed [1–3]. This expert statement proposes key elements for early diagnosis and adequate therapy of both primary and secondary BSI (Table 2).
Table 1.
Prevalence of bloodstream infections in selected recent randomized trials including adult patients with sepsis or septic shock
| RCT | Patient population, N | Patients with BSI, n (%) | Registration no./reference |
|---|---|---|---|
| SMART (saline versus balanced crystalloids in ICU patients—secondary analysis focused on patients with sepsis) | 1641 | 653 (39.8) |
Brown et al. [124] |
| EUPHRATES (targeted polymyxin B hemoperfusion for patients with septic shock and elevated endotoxin level) | 450 | 134 (29.8) |
Dellinger et al. [125] |
| APROCCHSS (hydrocortisone plus fludrocortisone versus placebo for patients with septic shock) | 1240 | 454 (36.6) |
Annane et al. [126] |
| ARISE (EGDT vs usual care for patients with septic shock) | 1591 | 601 (37.8) |
ANZICS. [127] |
| ProCESS (protocol-based vs usual care for patients with septic shock) | 1341 | 396 (29.5) |
ProCESS investigators. [128] |
RCT randomized controlled trial, BSI bloodstream infection, ICU intensive care unit, EGDT early goal-directed therapy
Table 2.
Twenty key points for the management of bloodstream infection in critically ill patients
| Statements | |
| 1. | The rising incidence of ESBLE is the most prominent matter of concern in community-acquired BSI |
| 2. | The rising incidence of CPE and XDR Acinetobacter baumannii in HA-BSI is a matter of serious concern |
| 3. | ICU-acquired BSI frequently occurs in critically ill patients, especially those with high severity indexes, immunosuppression, a surgical reason for admission, and the need for ECMO or other invasive procedures |
| 4. | Most of ICU-acquired BSIs are related to catheter infection, intra-abdominal infections, and ventilator-associated pneumonia though no definite source is identified for a substantial proportion of cases |
| 5. | Direct identification using Maldi-TOF or genotypic methods are accurate for bacterial identification especially for Gram-negative pathogens |
| 6. | Genotypic methods of bacterial detection and resistance mechanisms identification are accurate. These methods may positively impact the timing and adequacy of antimicrobial therapy in ICU patients with BSI though real-life clinical studies are still needed to appraise their input precisely |
| 7. | Choices about antimicrobials for treating critically ill patients with BSI should take into account several overlapped factors: (i) the empirical or targeted nature of the treatment; (ii) the presumed or proven origin site of the infection; (iii) the suspected or proven presence of antimicrobial resistance; (iv) immune status, and (v) the suspected or proven presence of candidemia |
| 8. | A reasoned choice of empirical agents should be based on the suspected pathogen/s and on the estimated individual and environmental risks of MDR infection |
| 9. | Recently approved, novel agents active against MDR organisms might be used, only if clearly, appropriate according to local epidemiology, for empirical treatment in critically ill patients |
| 10. | In critically ill patients with BSI and increased distribution volume, loading dosages of hydrophilic antibiotics should be increased compared to dosages usually prescribed in non-critically ill patients |
| 11. | Maintenance dosages should be adjusted according to fluctuations in the estimated renal function |
| 12. | TDM should be routinely performed for vancomycin and aminoglycosides, and whenever feasible for polymyxins. TDM of beta-lactams may be used, especially for preventing neurotoxicity, but further research and standardization are needed for clearly delineating advantages and impact on patients’ outcomes |
| 13. | Continuing combination therapy in BSI due to XDR Gram-negative bacteria may have an outcome benefit in the most severely ill patients with septic shock |
| 14. | Source control including immediate removal of suspected intravascular catheters is always urgent in patients with septic shock |
| 15. | In life-threatening surgical site infections, a “damage control” approach is the safest way to gain time and achieve stability |
| 16. | ADE describes the initial re-evaluation of antimicrobial therapy when it targets decreasing the exposure to broad-spectrum antimicrobials. For treatment of BSI, it consists in stopping companion antibiotics or narrowing the spectrum of a pivotal antibiotic |
| 17. | The antimicrobial regimen should be re-evaluated for its spectrum and effectiveness every day after the blood culture becomes positive and new information becomes available |
| 18. | In ICU patients with uncomplicated BSI, duration of treatment can be matched to that of the source and the causative pathogen. In the absence of specific risk factors, a duration of when clinical stability is reached, shorter (≤ 7 days) should be proposed In the absence of specific risk factors, septic shock and if the source control is appropriate. preferred to longer antibiotic courses |
| 19. | Specific pathogens at risk of septic metastasis or treatment failure require duration of 14 days in cases of uncomplicated infections and up to 4–8 weeks for Specific sources such as bone and joint infections, empyema, septic metastasis or sources not amenable to adequate source control |
| 20. | Ongoing instability should not be a reason to blindly increase the duration of antimicrobial, but rather lead to investigate for insufficient source control, superinfection, drug-resistant pathogens or non-infectious causes of fever and shock. Continuing, escalating or stopping the antimicrobials accordingly should always be preceded by new microbiological specimens including blood cultures |
ESBLE extended-spectrum beta-lactamase-producing Enterobacterales, BSI bloodstream infection, CPE carbapenemase-producing Enterobacterales, XDR extensively drug-resistant, ICU intensive care unit, ECMO extra-corporeal membrane oxygenation, MDR multidrug-resistant, TDM therapeutic drug monitoring, ADE antibiotic de-escalation
Epidemiological features of bloodstream infection in ICU patients
BSI may complicate the course of a myriad of severe CA infectious diseases (Fig. 1). Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae and Streptococcus pneumoniae account for more than 70% of all CA-BSI though pathogen distribution varies substantially depending on infection foci and patient characteristics [4, 5]. Of note, Pseudomonas aeruginosa causes up to 5% of community-onset BSI, essentially in patients with severe underlying conditions (e.g., immunosuppression) and/or recent healthcare exposure and suffering from urinary tract infection (UTI) or pneumonia—yet, causative strains remain usually susceptible to first-line antipseudomonal agents, with a restricted prevalence of multidrug-resistant (MDR) isolates [5, 6]. After a spectacular rise in the early 2000’s, the incidence of CA-BSI due to community-associated methicillin-resistant S. aureus (MRSA) now trends to plateau in the United States and most of other endemic regions [7]. Meanwhile, the global burden of CA-BSI due to extended-spectrum beta-lactamase-producing Enterobacterales (ESBL-PE) is amplifying steadily due to massive spread of these pathogens in the community [4, 8]. Nowadays, the prevalence of ESBL-producing isolates commonly exceeds 5% in E. coli and K. pneumoniae CA-BSI secondary to UTI or intra-abdominal infection and may reach 20% in certain geographical areas, thereby equalling the proportion reported in HA-BSI [9, 10].
Fig. 1.
Bloodstream infections in critically ill patients: main sources and leading pathogens. BSI bloodstream infection, CAP community-acquired pneumonia, HCAP healthcare-associated pneumonia, UTI urinary tract infection, ESBL-PE extended-spectrum beta-lactamase-producing Enterobacterales, SSTI skin and soft-tissue infection, HAP hospital-acquired pneumonia, VAP ventilator-associated pneumonia. Community-acquired BSI: BSI first identified [blood cultures sampling] less than 48 h following hospital admission in a patient without recent exposure to the healthcare system. Healthcare-associated BSI: community-onset BSI in patients requiring chronic haemodialysis, living in a nursing home, or recently exposed to antibiotics, in-home nursing care, or the hospital environment. Hospital-acquired BSI: BSI first identified more than 48 h after hospital admission. ICU-acquired BSI: BSI first identified more than 48 h after ICU admission. Primary BSI indicates BSI without identification of a definite source
HA-BSI in critically ill patients are imported (i.e., documented at ICU admission) and acquired in the ICU in roughly 25% and 75% of cases, respectively [2, 5]. Overall, ICU-acquired BSI occurs during 5–7% of admissions, corresponding to an average of 6–10 episodes per 1000 patient-days [1, 3, 11–14]. Main risk factors for ICU-acquired BSI include high severity indexes at admission, prolonged stay, immunosuppression, liver disease, surgical admission, and the requirement for invasive devices or procedures [11]. In the EUROBACT-1 international study (n = 1156), ICU-acquired BSI mostly ensued from catheter-related infections (21%), nosocomial pneumonia (21%), and intra-abdominal infections (12%)—strikingly, no definite source was identified for 24% of episodes [2].
In ICUs applying current prevention bundles for the insertion and maintenance of central venous catheter (CVC), CVC-related BSI occurs in 0.5–1.5% of exposed patients, with a median incidence density ranging from 0.5 to 2.5 episodes per 1000 catheter-days [15–17]. Defective asepsis, a jugular or femoral insertion (versus the subclavian site) and the duration of catheterisation remain the leading risk factors for CVC-related BSI [18–20]. The hazard of arterial catheter-related BSI appears similar to what is observed with CVCs (that is, around 1 episode per 1000 catheter-days), with a nearly two-fold risk increase with femoral accesses when compared to the radial site [21]. Lastly, patients under extra-corporeal membrane oxygenation (ECMO) are at major risk for ICU-acquired BSI with an incidence density reaching 20 episodes per 1000 ECMO-days [22]. Most of BSIs in this particular population with extended mechanical ventilation and ICU stay are related to ventilator-associated pneumonia or other infectious foci rather than cannula infection [23].
The main pathogens responsible for HA-BSI in critically ill patients are listed in Table 3. The epidemiology of MDR pathogens widely differs from one ICU to another according to case-mix, local policies for infection control and antimicrobial stewardship, and geographical location—BSI due to non-fermenting Gram-negative bacilli such as P. aeruginosa and Acinetobacter baumannii are notably more prevalent in warm countries or during warm periods in temperate areas [24]. However, and as for other ICU-acquired infections, the incidence of BSI due to ESBL-PE, carbapenemase-producing Enterobacterales, MDR P. aeruginosa, MDR Acinetobacter baumannii, MRSA and methicillin-resistant coagulase-negative staphylococci is high and even continues to increase in most parts of the world [25]. Table 4 indicates the current resistance rates in major pathogens responsible for hospital-acquired infections—including HA-BSI—in large surveillance networks.
Table 3.
Hospital-acquired bloodstream infection in ICU patients: pathogen distribution in selected multicentre studies published after 2010
| Study [ref] | Corona et al. [5] | Prowle et al. [11] | Climo et al.[13] a | Adrie et al. [1] | Tabah et al. [2] | Noto et al. [12] a | NHSN [129] | Wittekamp et al. [14] a | SENTRY Network [9] | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Inclusion period | 2002–2003 | 1998–2009 | 2007–2009 | 1998–2013 | 2009 | 2012–2013 | 2011–2014 | 2013–2017 | 1997–2016 | ||
| Geographical area | Worldwide | Australia | USA | Worldwide | USA | USA | Europe | Worldwide | |||
| Population | General ICU population | General ICU population | General ICU population | Outcomerea network, France (General ICU population) | General ICU population | General ICU population | Hospitalized patients | Mechanically ventilated patients | Hospitalized patients | ||
| Type and number of BSI events | HA-BSI (n = 351) (%) |
ICU-BSI (n = 915) (%) |
ICU-BSI (n = 330) (%) |
ICU-BSI (n = 131) (%) |
ICU-BSI (n = 571) (%) |
HA-BSI (n = 279) (%) |
ICU-BSI (n = 877) (%) |
ICU-BSI (n = 113) (%) |
HA-CLABSI (n = 85,994) (%) |
ICU-BSI (n = 305) (%) |
HA-BSI (n = 103,945) (%) |
| Escherichia coli | 10 | 6 | GNB (pooled), 28 | 5 | 10 | 19 | 5 | 4 | 5 | Enterobacterales (pooled), 32 | 16 |
| Klebsiella spp. | 9 | 8 | 4 | 4 | 8 | 15 | 6 | 8 | 9 | ||
| Enterobacter spp. | 5 | 6 | 6 | 6 | 7 | 8 | 1 | 4 | 4b | ||
| Pseudomonas aeruginosa | 10 | 10 | 2 | 10 | 10 | 12 | 2 | 4 | 7 | 7 | |
| Acinetobacter baumannii | 5 | 6 | 2 | 3 | 7 | 16 | 0 | NA | NA | 3 | |
| Staphylococcus aureus | 26 | 24 | 27 | 6 | 15 | 16 | 8 | 15 | 13 | 4 | 21 |
| CoNS | 20 | 30 | 24 | 26 | 19 | 10 | 13 | 39 | 16 | 32 | 5 |
| Enterococcus spp. | 9 | 11 | 17 | 19 | 8 | 10 | 13 | 9 | 17 | 9 | 11 |
| Candida spp. | 10 | 6 | 15 | 12 | 9 | 7 | 8 | 6 | 14 | 5 | NA |
| Others | 5 | 3 | 7 | 19 | 16 | < 5 | < 5 | 17 | 11 | 24 | |
Note that the sum for each column may exceed 100% due to polymicrobial BSI
NHSN National Healthcare Safety Network, HA-BSI hospital-acquired bloodstream infection, ICU-BSI ICU-acquired BSI, CLABSI central line-associated BSI, CoNS coagulase-negative staphylococci, NA non-available
aRCTs for the evaluation of preventive measures for ICU-BSI: only data from the control groups are exposed in the table
bReported for Enterobacter cloacae only
cReported for Staphylococcus epidermidis only
Table 4.
Current resistance rates in major pathogens responsible for hospital-acquired infections according to World Health Organization regions—available data from large surveillance networks
| Resistant isolates (%) among invasive isolates of a given species | WHO regions | ||||
|---|---|---|---|---|---|
| Americas | Europe | Eastern Mediterranean | South-East Asia | Western Pacific | |
| Escherichia coli/resistance to ESC | 16–22 | 28–36 | 11–41 | 20–61 | 0–77 |
| Klebsiella pneumoniae/resistance to ESC | 21–56 | 41–62 | 17–50 | 53–100 | 27–72 |
| Klebsiella pneumoniae/resistance to carbapenems | 9–11 | 0–4 | 0–54 | 0–52 | 0–8 |
| Pseudomonas aeruginosa/MDR phenotype | 18–20 | NA | 30–36 | 34–43 | 30–35 |
| Acinetobacter baumannii/resistance to carbapenems | 47–64 | 0–23 | 60–70 | 26–65 | 62–72 |
| Staphylococcus aureus/resistance to methicillin | 42–55 | 33–95 | 13–53 | 2–81 | 4–84 |
WHO World Health Organization, ESC extended-spectrum cephalosporins, MDR multidrug-resistant
Data were extracted from the WHO Antimicrobial Resistance Global Report 2019 (http://www.who.int/antimicrobial-resistance/publications/surveillancereport), National Healthcare Safety Network/Centers for Disease Control and Prevention Report 2015–2017 [130], European Antimicrobial Resistance Surveillance Network Annual Report 2016 (http://www.ecdc.europa.eu/en/healthtopics/antimicrobial_resistance), International Nosocomial Infection Control Consortium Report 2010–2015 [131], CHINET Surveillance Network Report 2014 [132], and other references [133, 134]. Available resistance rates in the specific context of ICU-acquired infections are in the upper ranges of reported values for all geographical areas. Note that similar large-scale surveillance data are not available for the Africa region
Early microbiological diagnosis in BSI
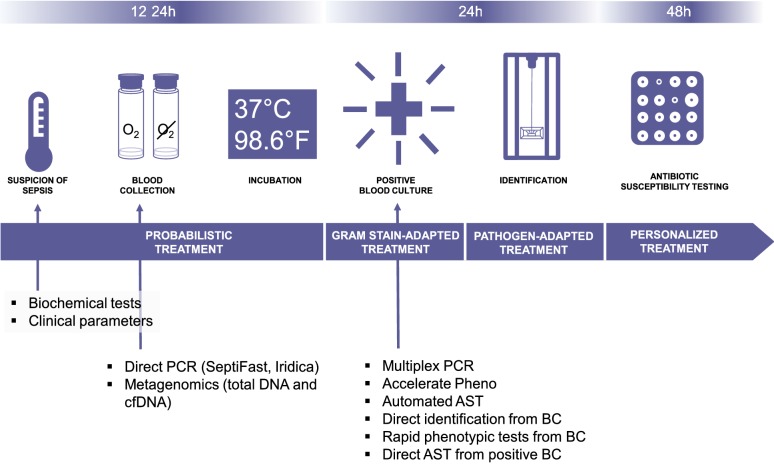
Culture-based methods remain the gold standard to identify the causative microorganism in sepsis, with a recommended sampling of at least two sets of aerobic and anaerobic blood cultures (10–20 mL per bottle) following rigorous skin disinfection [26]; yet the rhythm imposed by the growth time requirements of the latter is barely compatible with the ‘need for speed’ in the context of sepsis (Fig. 2). It should be kept in mind that the initiation of empirical antimicrobial therapy significantly reduces the sensitivity of blood cultures drawn shortly after treatment initiation [27].
Fig. 2.
Current workflow of microbiological diagnosis in bloodstream infection. PCR polymerase chain reaction, CfDNA cell-free DNA, AST antibiotic susceptibility testing, BC blood culture. Biochemical tests such as C-reactive protein of Procalcitonin is most of time elevated in case of BSI but not sufficiently accurate to discard the diagnosis. A significant decrease of these biomarkers should be used to shorten the duration of antimicrobial therapy
Molecular assays are increasingly deployed in bacteriology laboratories as rapid alternatives to culture-based methods. Attempts have been made to directly detect pathogens and resistance markers by PCR on blood samples without prior incubation (Roche LightCycler®SeptiFast, SeeGene MagicPlex® Sepsis Test, Abbott Iridica); however, these tests have not met a broad success because of their medium sensitivity and specificity (Table 5) and the lack of full automation. Furthermore, these tests only seek for a limited number of antibiotic resistance genes so that the probabilistic regimen can only be adapted according to the bacterial species. More recently, a magnetic resonance-based test (T2Bacteria Panel, T2Biosystems) was made available and showed a higher sensitivity (90%) than previous methods together with a shorter turn-around time (3.5 h vs. 5–8 h) [28].
Table 5.
Rapid diagnostic tools for early optimization of antimicrobial therapy in patients with bloodstream infections: methods, turn-around time and diagnostic performances
| Name | Manufacturer | Method | Input | TAT | Sensitivity | Specificity | References |
|---|---|---|---|---|---|---|---|
| MagicplexTM Sepsis Test | Seegene | RT-PCR | Blood sample | 5 h | 0.65 | 0.92 | Carrara et al. [135] |
| 0.29 | 0.95 | Zboromyrska et al. [136] | |||||
| 0.47 | 0.66 | Ziegler et al. [137] | |||||
| T2Bacteria® Panel | T2Biosystems | Magnetic resonance | Blood sample | 3.5 | 0.9% (95% CI, 0.76–0.96) | 0.9 (95% CI, 0.88–0.91) | Nguyen et al. [28] |
| FilmArray® Blood Culture | bioFire | PCR | Positive BC | 1 h | 0.92-1a | 0.76-1a | Altun et al. [138] |
| 0.95 | ND | Bhatti et al. [139] | |||||
| Verigene® Blood culture tests | Luminex | PCR | Positive BC | 2.5 h | 0.99 | ND | Bhatti et al. [139] |
| 0.95 | ND | Kim et al. [140] | |||||
| Accelerate Pheno™ | Accelerate Diagnostics | FISH and microscopy | Positive BC |
1.5 h (identification) 7 h (AST) |
0.95 | 0.99 | Lutgring et al. [141] |
| 0.96 | 0.99 | Charnot-Katsikas et al. [142] | |||||
|
VitekMS Biotyper |
bioMérieux Bruker |
MALDI-TOF | Positive BC | <1 h (identification), < 1 h-4 h (AST) |
Concordance for Gram-negativeb: 0.83–1 Concordance for Gram-positiveb: 0.32–0.89 |
Faron [31] | |
RT-PCR real-time polymerase chain reaction, ESI–MS electrospray ionization mass spectrometry, BC blood culture, FISH fluorescence in situ hybridization, AST antibiotic susceptibility testing, MALDI-TOF matrix-assisted laser desorption ionization–time of flight mass spectrometry
aAccording to the target
bIdentification at the species level
Since then, PCR-based tests have re-focused on positive blood cultures (BC) (such as the BioFire FilmArray Blood Culture Identification and the Luminex Verigene), meaning that the test comes after a first culture-based test. The multiplex PCR (mPCR) tests applied on positive blood culture have excellent performances and have been showed to decrease the time to an optimized antibiotic regimen (spectrum narrowing or broadening or even cessation when a contaminant was identified) but not the mortality or the length of stay [29]. One major limitation of these genotypic methods is the limitation of the number of PCR probes. A negative PCR should be interpreted in view of the overall findings, possible source of infection and other available bacteriological results. Consequently, a solid expertise and strong collaboration between microbiologists and clinicians are needed [30]. Besides PCR, matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF) can also be used to identify bacteria directly from positive BC after a purification protocol (not automatized yet), with good performances for Gram-negative bacteria (> 90% concordance with subsequent culture) but not for Gram-positive bacteria (~ 80% concordance) [31]. MALDI-TOF can also be used for antibiotic susceptibility testing, with the possibility to compare spectrum after a short incubation (1–4 h) with or without antibiotics, or to directly detect peaks matching the resistance mechanisms [31]. While the proof of concept has been established, direct AST from BC using MALDI-TOF still needs standardization to enter the routine workflow.
Recently, next-generation sequencing (NGS) methods and application of machine-learning methods have showed promising results in the diagnostic of BSI. Clinical metagenomics (CMg) refers to the sequencing of the nucleic acids present in a clinical sample to identify pathogens and to infer their susceptibility to antimicrobials [32]. A variant of CMg is cell-free DNA (cfDNA) sequencing, i.e. the sequencing of extracellular cell-free DNA in clinical samples. It has been showed that the absolute concentrations of plasmatic cfDNA in patients with sepsis were elevated when compared to healthy volunteers and that the cfDNA sequences could identify potential bacterial pathogens missed by conventional, culture-based methods [33]. A recent work including 348 patients reported a 93% sensitivity but only a 63% specificity for cfDNA sequencing [34]. Indeed, metagenomic sequencing identified much more bacteria than culture, with 62/166 samples negative with conventional methods but with microorganisms found in cfDNA sequencing. Of note, cfDNA sequencing results were delivered the day after the sample arrival. Another work based on metagenomic sequencing of plasma from immunosuppressed patients found similar results, with a 95% negative predictive value [35].
Finally, while molecular tests are interesting, the performance of old-fashioned culture methods may be improved to provide more timely results. AST performed from the morning positive BC can be read at the end of the working day [36], but this would not apply to BC found positive later. In this perspective, the continuous processing of samples through lab automation could break the barriers intrinsic to the lab workflow. Similarly, the Accelerate Pheno provides an automated solution to deliver identification and a phenotypic AST within 6–8 h [37].
Choice of antimicrobial therapy
Decisions on which antimicrobials should be employed for treating bloodstream infections (BSI) in critically ill patients depend on several, overlapped factors: (1) the empirical or targeted nature of the treatment; (2) the presumed or proven origin site of the infection; (3) the suspected or proven presence of antimicrobial resistance (notably in healthcare settings with endemic MDR pathogens and/or in patients with recent exposure to antimicrobial drugs); (4) the suspected or proven presence of candidemia [25, 38–41]. Immunosuppression (e.g., neutropenia, HIV infection, or current or recent immunosuppressive therapy) should also been taken into account since immunocompromised hosts are at increasing risk of both infection with MDR bacteria—due to more frequent antimicrobial and healthcare exposure—and non-bacterial sepsis, notably resulting from invasive fungal infection [26, 42, 43].
Considering that antimicrobials are mainly used empirically in critically ill patients [44], both a reasoned administration of empirical agents on the basis of the suspected pathogen/s and efforts to pursue a rapid etiological diagnosis for allowing de-escalation are essential measures for treatment optimization [25, 45–47]. In this scenario, there is certainly a need for a balanced use of recently approved agents active against MDR organisms, in order not to delay the administration of an effective therapy and, on the other hand, not to accelerate the selection of further resistance using them indiscriminately [48, 49]. In addition, the availability of novel beta-lactams/beta-lactamases inhibitor (BL-BLI) combinations, which express selected activity against MDR Gram-negative bacteria expressing different determinants of resistance, has already started to change clinical reasoning at the bedside of septic patients. For example, the type of locally prevalent carbapenemases should now be taken into account when prompting empirical therapies [50]. Trying to balance all the above-reported factors, possible choices for treating BSI in critically ill patients, together with their activity against MDR pathogens and dosage recommendations, are detailed in Table 6.
Table 6.
Characteristics of antibacterial drugs indicated (or used off-label in selected cases) for treating bloodstream infections (BSI) in critically ill patients
| Antibacterials | Activity against MDR pathogens | Class, PD index of choice Suggested dosage in critically–ill patients |
Status |
|---|---|---|---|
| Amikacin | Possibly active against MDR-GNB, although increased resistance to classical aminoglycosides has been reported [79, 143] |
Aminoglycosides, AUC/MIC 25-30 mg/kg q24h (modified according to TDM) |
Approved |
| Aztreonam | Active against MBL producers not expressing mechanisms of aztreonam resistance (e.g., other beta-lactamases, AmpC hyperexpression, efflux pumps) |
Monobactams, T > MIC 1–2 g q8h |
Approved |
|
Aztreonam/ Avibactam |
ESLBL-PE CPE (all classes of carbapenemases, including MBL) |
Monobactams plus BLI, T > MIC 6500 mg aztreonam/2167 mg avibactam q24h on day 1 followed by 6000 mg aztreonam/2000 mg avibactam q24h |
In clinical development; potential indications according to phase-3 RCT are cIAI, HAP/VAP (NCT03329092) and serious infections due to MBL-producing bacteria (NCT03580044) |
| Cefepime | Active against AmpC hyperproducer enterobacterales |
Cephalosporins, T > MIC 2 g q8h or continuous infusion |
Approved |
| Cefiderocol |
ESBL-PE CPE (all classes of carbapenemases, including MBL) MDR-PA CRAB |
Siderophore cephalosporins, T > MIC 2 g q8h |
FDA Approved for cUTI caused by susceptible Gram-negative microorganisms, who have limited or no alternative treatment options according to phase-3 RCT are infections due to carbapenem-resistant organisms in different sites (NCT02714595). Pivotal study on HAP/VAP finished (NCT03032380) |
| Ceftobiprole |
MRSA VISA hVISA VRSA |
Cephalosporins, T > MIC 500 mg q8 h |
Approved for CAP and HAP (excluding VAP) In vitro and/or limited clinical data reporting a possible use as salvage therapy in combination with vancomycin or daptomycin for MRSA bacteremia |
|
Ceftolozane/ Tazobactam |
ESBL-PE MDR-PA |
Cephalosporins plus BLI, T > MIC 1.5 g q8h (3 g q8h for pneumonia) |
Approved for cIAI (in combination with metronidazole) and cUTI Approved by FDA for VAP/HAP, with the CHMP of EMA also recently adopting a positive opinion recommending a change to the terms of the marketing authorization, including also VAP/HAP among approved indications |
| Ceftaroline |
MRSA VISA hVISA VRSA |
Cephalosporins, T > MIC 600 mg q12 h |
Approved for ABSSSI and CAP In vitro and/or limited clinical data reporting a possible use as salvage therapy in combination with vancomycin or daptomycin for MRSA bacteremia |
| Ceftazidime |
Cephalosporins, T > MIC 6 g q24h continuous infusion |
Approved | |
|
Ceftazidime/ Avibactam |
ESBL-PE CPE (class A and class D carbapenemases) MDR-PA |
Cephalosporins plus BLI, T > MIC 2.5 g q8h |
Approved for cIAI (in combination with metronidazole), cUTI, HABP/VABP, and infections due to aerobic Gram-negative organisms in adult patients with limited treatment options |
| Ceftriaxone |
Cephalosporins, T > MIC 1–2 g q24h |
Approved | |
| Colistin |
ESBL-PE CPE (all classes of carbapenemases, including MBL) MDR-PA CRAB |
Polymyxins, AUC/MIC 9 MU loading dose, 4.5 MU every 8–12 h (modified according to TDM where available; higher dosages to be possibly considered in patients with ARC [58]) |
Approved Recommended for serious infections due to susceptible bacteria when other treatment options are limited |
| Daptomycin |
MRSA VRE |
Lipopeptides, AUC/MIC 8–10 mg/kg q24h |
Approved for cSSTI and right-sided endocarditis |
| Eravacycline |
MRSA VRE ESBL-PE CPE CRAB |
Fluocyclines, AUC/MIC 1 mg/kg q12h |
Approved for cIAI To be possibly used for BSI due to MDR organisms in absence of dependable alternative options, in combination with other agents (expert opinion) |
| Ertapenem | ESBL-PE |
Carbapenems, T > MIC 1 g q12 h |
Approved for IAI, CAP, acute gynecological infections, and diabetic food infections |
| Fosfomycin |
ESBL-PE CPE (all classes of carbapenemases, including MBL) MDR-PA MRSA VRE |
PEP analogues, unclear [144] 4–6 g q6h continuous infusion |
Approved For BSI used in combination with other agents for the treatment of MDR infections with limited treatment options (also for CRAB), although in lack of high-level evidence |
| Gentamicin | Possibly active against MDR-GNB, although increased resistance to classical aminoglycosides has been reported [79, 143] |
Aminoglycosides, AUC/MIC 5–7 mg/kg q24h (modified according to TDM) |
Approved |
|
Imipenem/ Cilastatin |
ESBL-PE |
Carbapenems, T > MIC 0.5–1 g q6h |
Approved |
|
Imipenem/ Relebactam |
ESBL-PE CPE (class A carbapenemases) Some MDR-PA |
Carbapenems plus BLI, T > MIC 500 mg/250–125 mg q6h |
FDA approved for the treatment of cUTI and cIAI. The phase-3 RCT are HAP/VAP (NCT02493764) is ongoing. |
| Meropenem | ESBL-PE |
Carbapenems, T > MIC 1–2 g q8h or extended infusion (over 4 h) |
Approved |
|
Meropenem/ Vaborbactam |
ESBL-PE CPE (class A carbapenemases) |
Carbapenems plus BLI, T > MIC 4 g q8h |
Approved for cUTI, cIAI, HAP, VAP, and infections due to aerobic Gram-negative organisms in patients with limited treatment options |
|
Piperacillin/ Tazobactam |
Possibly active against ESBL-PE, although the results of the MERINO trial discourage the use of piperacillin/tazobactam for severe ESBL-PE infections [145] |
Penicillins plus BLI, T > MIC 4.5 g q6h continuous infusion |
Approved |
| Plazomicin |
ESBL-PE CPE (all classes of carbapenemases, including MBL, although resistance has been described in NDM-1 producing strains, owing to co-expression of plazomicin-inactivating methyltransferases [146]) MDR-PA CRAB |
Aminoglycosides, AUC/MIC 15 mg/kg q24h |
An application has been recently submitted to EMA for approval of plazomicin for cUTI and other severe infections (plazomicin is approved by FDA for cUTI) |
| Tigecycline |
MRSA VRE ESBL-PE CPE (all classes of carbapenemases, including MBL) CRAB |
Glycylcyclines, AUC/MIC 100–200 mg loading those, then 50–100 mg q12h |
Approved for cSSTI (excluding diabetic foot infections) and cIAI For BSI used only in combination with other agents for infections due to MDR organisms in presence of limited alternative therapeutic options |
| Vancomycin | MRSA |
Glycopeptides, AUC/MIC 15–30 mg/kg loading dose, 30–60 mg/kg q12h, or continuous infusion (modified according to TDM) |
Approved |
ABSSSI acute bacterial skin and skin-structure infections, ARC augmented renal clearance, AUC area under the concentration curve, BLI beta-lactamases inhibitors, BSI bloodstream infections, CAP community-acquired pneumonia, CHMP Committee for Medicinal Products for Human Use, cIAI complicated intra-abdominal infections, CPE carbapenemase-producing Enterobacterales, CRAB carbapenem-resistant Acinetobacter baumannii, cSSTI complicated skin and soft-tissue infections, cUTI complicated urinary tract infections, EMA European Medicines Agency, ESBL-PE extended-spectrum beta-lactamase-producing Enterobacterales, FDA Food and Drug Administration, HAP hospital-acquired pneumonia, MBL metallo-beta-lactamases, NDM New Delhi metallo-beta-lactamase, L-AmB liposomal amphotericin B, MDR multidrug-resistant, MIC minimum inhibitory concentration, MRSA methicillin-resistant Staphylococcus aureus, MU million units, PA Pseudomonas aeruginosa, PD pharmacodynamics, PEP phosphoenolpyruvate, RCT randomized controlled trials, TDM therapeutic drug monitoring, VAP ventilator-associated pneumonia, VRE vancomycin-resistant enterococci
Role of therapeutic drug monitoring (TDM)
Useful pharmacokinetic (PK) parameters for deciding antimicrobial dosages are not routinely measurable in critically ill patients. However, albeit imperfect, some practical and immediately available proxies exist that may help optimizing dosages. First, higher loading dosages of hydrophilic antimicrobials are required in critically ill patients with a positive fluid balance indicating a high volume of distribution (Vd) [51–53]. Second, the facts that most antimicrobials used in ICUs are excreted by the kidneys, that either augmented renal clearance (ARC) or acute kidney injury (AKI) can be present in critically ill patients with BSI, and that renal replacement therapies (RRT) are not infrequently used in these patients imply that careful attention should be devoted to the adjustment of maintenance dosages according to the fluctuations in renal function during the course of treatment [25, 52, 54–56]. With regard to pharmacodynamics (PD), knowledge of the different PD index of choice (T > minimum inhibitory concentration (MIC), Cmax/MIC, or area under the curve(AUC)/MIC) pertaining to the different antimicrobial classes is crucial both for selecting the most appropriate type of infusion (e.g., continuous vs. intermittent) and for measuring the impact of suspected/measured pathogens MIC on the probability of target attainment, taking into account possible variability in MIC measurements [25, 52, 57].
However, TDM remains desirable for antimicrobial treatments in critically ill patients, owing to the imperfect prediction of PK and PD in this population without measurement, even when carefully taking into account both patients chronic and acute characteristics and the expected drug behavior [52, 58, 59]. Practically, TDM appears beneficial for minimizing toxicity and/or improving clinical responses in patients treated with vancomycin or aminoglycosides [60, 61], while further evidence and standardization are needed to clearly delineate and maximize any possible clinical impact of TDM on the use of beta-lactams [25, 62, 63]. For some antimicrobial classes with inherent variable serum concentrations, technical difficulties and its frequent unavailability outside research laboratories prevent a widespread use of TDM (e.g., polymyxins, for which nonetheless TDM remains desirable whenever feasible) [58]. Detailed discussion on possible PK/PD targets (either for improving bacterial killing/clinical outcome or reducing toxicity) and sampling times for different antibiotic classes in critically ill patients undergoing TDM are available elsewhere [64, 65].
Single-drug or combination therapy for bloodstream infection in ICU patients
In an era of increasing resistance prevalence, the primary objective of an empirical combination regimen (usually a beta-lactam plus an aminoglycoside or a fluoroquinolone) is to maximize the likelihood of administering at least one drug with activity against the causative pathogen. Yet, once antimicrobial susceptibility results become available, the benefit of continuing with a dual regimen rather than a single active agent remains equivocal owing to fragmentary or conflicting evidence.
First, experimental models suggest that antimicrobial associations may synergistically prevent or postpone the selection of resistant mutants, especially in P. aeruginosa and other non-fermenting Gram-negative pathogens [66]. However, clinical data are lacking to appraise the relevance of these findings and whether combination therapy effectively protects from the emergence of resistance at the infection site is still unsettled. Interestingly, in a randomized controlled trial (RCT) including 551 patients with sepsis, receiving a meropenem–moxifloxacin combination was associated with a lower risk of persistent or subsequent infection with meropenem-resistant pathogens than meropenem alone (1.3% versus 9.1%, respectively, P = 0.04) [67]. This endpoint was unfortunately not addressed in the gut microbiota—that is, the main reservoir of MDR Gram-negative bacteria in ICU patients. Intuitively, adding a second drug to a broad-spectrum beta-lactam may amplify the ecological side-effects on commensal ecosystems and the routine use of combination therapy can probably not be justified on the sole basis of preventing resistance at the infection site.
Next, several meta-analyses failed to demonstrate that the use of a beta-lactam/aminoglycoside association reduces fatality rates in patients with BSI—including those with neutropenia or sepsis—when compared to a monotherapy with the same beta-lactam [68–70]. Besides, adding an aminoglycoside to a beta-lactam-based regimen has been consistently linked with an increased hazard of acute renal failure at the acute phase of infection [68, 69, 71]. On a pathogen-specific approach, there is currently no evidence that a double-active regimen impacts the outcome of patients with BSI due to methicillin-susceptible S. aureus (except in those with implanted devices) or Enterobacterales, including AmpC-hyperproducers and ESBL-PE [72–75]. Along this line, the benefit of a polymyxin-based combination has not been convincingly proven in patients infected with carbapenem-resistant A. baumannii though this strategy may perform better than polymyxin alone in patients with BSI and/or when high-dose colistin regimen are administered (i.e., ≥ 6 MUI per day) [76–79]. Controversies equally persist about the prognostic effect of combination therapy in P. aeruginosa BSI [73, 80, 81]; yet, no survival improvement was demonstrated in a meta-analysis of RCTs comparing beta-lactam plus aminoglycoside or fluoroquinolone versus beta-lactam alone in patients with this condition [82]. It should be emphasized, however, that most of available studies are relatively ancient, include a limited number of ICU patients, and display substantial heterogeneity in terms of antimicrobial administration schemes, sepsis definition, and severity indexes at BSI onset.
The benefit of combination therapy could actually be restricted to the most severely ill patients. Indeed, in a meta-regression analysis of observational studies and RCTs, combination therapy was associated with improved survival in patients with a baseline risk of death > 25% [odds ratio (OR) for death 0.51, 95% CI 0.41–0.64], while a harmful effect was strikingly observed in less severe patients (OR 1.53, 95% CI 1.16–2.03) [83], putatively due to toxic and/or ecological adverse events. Similar results were reported in a cohort of 437 patients with BSI due to carbapenemase-producing Enterobacterales (mostly KPC-producing K. pneumoniae), with a survival benefit of combination therapy in those with a high probability of death (OR 0.56, 95% CI 0.34–0.91) but not in the lower mortality stratum [84]. Pending for confirmatory studies to definitely solve this essential issue [85], combination therapy remains recommended in patients with septic shock but should not be routinely prescribed for the definite treatment of those with other severe infections, including sepsis without circulatory failure [26].
Early appropriate source control
The search for the source of BSI (that is, secondary BSI) should be guided by the patient clinical presentation. The most common conditions that may require a specific approach for source control are obstructive UTI, skin and soft-tissue infections and intra-abdominal infections for secondary CA-BSI, and vascular device and surgical site infection for secondary HA-BSI. Source control to eliminate infectious foci follows principles of damage control and should be limited to drainage, debridement, device removal and compartment decompression in case of hemodynamic instability and organ failures [86].
An important body of the literature argues for a systematic catheter removal in case of catheter-related BSI in critically ill patients [87–89]. However, the device is actually the source of sepsis in less than half of those with a suspected catheter-related infection [90]. The systematic removal should thus be balanced with a more conservative attitude in the absence of septic shock but remains the rule in case of septic shock, immune suppression, or persistent bacteremia under appropriate antimicrobial therapy.
For BSIs related to surgical site infection, source control is a major determinant of outcome [91]. However, the optimal delay for source control is debated—from less than 6 to less than 12 h [91–94]. Indeed, the cost–benefit ratio of an immediate drainage in unstabilized patients or a hemodynamic and physiological stabilization-first and secondary source control is a matter of debate. Immediate damage control using less risky, minimally invasive surgical debridement and/or percutaneous drainage (delaying the need for definitive surgery until the patient is stabilized) is probably the best option [95]. It should be discussed with anesthetists and surgeons on an individual basis.
Key elements of surgical source control include drainage, debridement, cleansing, irrigation, and control of the source of contamination [95]. Yet, the quality of source control is difficult to evaluate [96], somewhat subjective and depends on the surgeon’s perception. A standardized reporting file of the operative procedure may help. A closed collaboration between surgeons and intensivists during the patients’ follow-up is, therefore, fundamental.
De-escalation strategy
Antimicrobial de-escalation (ADE) is a component of antimicrobial stewardship strategies (AMS) aiming at both reducing the spectrum of antibiotic therapy and decreasing the emergence of antimicrobial resistance [97].
ADE is variably defined, and usually includes narrowing the spectrum of a pivotal antibiotic, often a β-lactam, and/or decreasing the number of agents [98, 99]. Those components should be scrutinized separately but in general ADE is part of the re-evaluation of the antimicrobial regimen that happens 2–3 days after the infection was diagnosed when results of microbiological specimens become available. BSI is very particular as it is the only kind of infection where the pathogen is always known (by definition), and as such a perfect candidate for this re-evaluation.
Where the source and the pathogen of the BSI are known, it can be safely recommended, even in immunocompromised patients [99, 100], to stop companion antibiotics intended to broaden the spectrum of therapy. Indeed, for a Gram-negative BSI, anti-MRSA or anti-fungal agents should not be continued longer than it is needed to know those are not in cause.
The case of the pivotal antibiotic is more complex because of multiple factors:
While resistance to carbapenems may increase after extended courses, a lot of the harm has already been done after 1–3 days of therapy [101].
Ranking the spectrum of antibiotics is complex and yields variable results depending on the method, the region of the world and the priorities that are considered [98, 102, 103].
Whether narrowing the clinical antimicrobial spectrum decreases the emergence of resistance has not been adequately studied, and while it has some rationale, in some cases, the opposite may be true [104].
Some ADE regimens such as switching a beta-lactam for a fluoroquinolone may be useful in the ward to allow for oral treatment and discharge from hospital. This potential benefit does not exist for ICU patients, and those regimens are likely to cause additional emergence of resistance.
Caution is important for sources that are frequently polymicrobial such as intra-abdominal infections. A positive BC may yield a single pathogen, while specimens from the source may identify multiple pathogens. Furthermore, there may be other important pathogens that were not cultured and require the broad-spectrum antimicrobial that was initially started.
In silico PK/PD modeling has warned that with conventional dosing strategies narrower spectrum beta-lactams may have higher risks of non-target attainment than their broad-spectrum alternatives [105].
Some narrower spectrum alternatives are more effective than broad-spectrum regimens. For instance, oxacillin or cephazolin are superior to piperacillin/tazobactam in S. aureus BSI [106].
Risk exists that ADE may cause an increase in the total duration of antimicrobial therapy [107]. Multiple studies on different sources of infection lead to recommend shorter rather than longer duration of antimicrobial therapy and this may be a more important target than changing molecules within a treatment [108, 109].
In ADE, it is particularly important to not increase the duration of treatment because of days where treatments overlap. We recommend that a stop date and time is calculated from the time of effective treatment and that this is maintained for the treatment after ADE.
We recommend consideration is given to all or a combination of those reasons before deciding if narrowing the pivotal antimicrobial is the appropriate course of action in critically ill patients with a BSI. ADE should be an integral part of the global AMS strategy, targeting the optimization of the treatment of patients with an infection. ADE is part of the clinical and microbiological re-evaluation that should happen for every patient with a BSI every time the laboratory provides new information. Those time points include the alert for positivity and Gram stain, the speciation and sensitivities of the pathogen.
Duration of therapy
Sufficient duration of antimicrobial therapy is required to prevent clinical failure and relapse. It should, however, not exceed what is required to achieve that target as longer courses may cause adverse events, toxicity, emergence of antimicrobial resistance, increase costs and resource use.
Duration of therapy should be defined as starting on the first day after an adequate antimicrobial is administered, and the source has been treated, and the blood cultures have become negative. To define clearance of the bacteremia, we require at least one set of negative blood cultures obtained 2–4 days after the infection [110]. Sampling more than one follow-up sets of blood cultures is preferable to avoid the skip phenomenon. This was described for S. aureus as when persisting bacteremia may be missed if insufficient follow-up cultures are performed [111].
From a recently published cohort study of 1202 ICU patients with BSI, we learn that current practice consists in extended duration of treatment for those patients with a median of 14 days (IQR, 9–17.5 days) [112]. After proper adjustment and excluding early deaths, duration of treatment showed no association with either survival or bacteremia relapse [15]. Most importantly, data extrapolated from observational studies on duration of treatment should be analyzed with extreme caution in populations with an inherently high risk of death. In survivor bias, the patients who have died early have had less days alive where the treatment could be given, hence received a shorter course of treatment. This artificially increases the risk of death associated with shorter courses and may have led clinicians to favor unnecessarily longer treatments.
A multicenter RCT involving 3598 ICU patients with BSI to 7 vs 14 days of antibiotics is ongoing (planned enrollment of 3598 patients). Results will not be available until 2022 (Balance trial-NCT03005145) and, until then, we will need to rely on a lower quality of evidence from uncontrolled or underpowered randomized trials that are described below.
The safety of a shorter antibiotic therapy for Gram-negative uncomplicated BSI was recently shown in a RCT including 604 patients across 3 centers. The authors enrolled hemodynamically stable patients without fever for at least 48 h at day 7 after the BSI onset They established non-inferiority of 7 against 14 days of treatment for a primary composite outcome of mortality, clinical failure, readmissions and extended hospitalization at day 90 [113]. The validity of these results in Pseudomonas aeruginosa BSI and in population with higher severity or prevalence of immunosuppression was suggested in a multicenter cohort of 249 patients included from 5 hospitals [114]. They used a causal inference model with adjustment on the inverse probability of treatment weighting. The composite outcome of recurrent P. aeruginosa infection at any site or mortality at 30 days was similar in both groups (OR 1.06; 95% CI 0.42–2.68; p = 0.91). Recurrent infections occurred in 7% of the short course and 11% of the prolonged course groups, thereby invalidating the reasoning to continue antibiotics beyond the recommended duration to prevent relapse [114].
This is in line with the findings of a meta-analysis of short versus long antibiotic treatments in patients with bacteremia caused by the most common infectious syndromes [108]. Only one trial conducted in children with acute nephronia—that is an intermediate stage between acute pyelonephritis and renal abscess—favored longer compared to shorter antibiotic courses [115]. For other trials and in pooled results, there was no difference in survival, clinical or microbiological cure with owing to treatment duration.
Trials investigating the infectious syndromes causing BSI in the ICU are important as in most cases, it may be the source that should guide our treatment rather than its consequence (the bacteremia). Short treatment should safely be used for ventilator-associated pneumonia (VAP) [116], or post-operative intra-abdominal infections provided source control was optimal [109].
When judging of duration of antibiotics, there is this second time point at 5–7 days. The decision of escalation/de-escalation/no change or dose adjustments should be taken after 2–3 days when microbiological specimens became available [98]. The effectiveness of therapy should be judged after 1 week of treatment on clinical and microbiological resolution of the infection. This will include defervescence and resolution of organ failures and shock, negative follow-up cultures, the absence of endocarditis or metastatic sites of infection and no implanted prosthesis which are all required to define an uncomplicated infection [110]. Problems with source control and/or superinfections at the source will also uncover around that time point. If those are resolved and the pathogen or the source is not specifically requiring extended treatments antibiotic regimen can be safely stopped.
For some pathogens, such as S. aureus or in cases of uncomplicated candidemia, treatment should be continued for 14 days after the first negative blood culture [110, 117]. Some particular source of infections where the pathogen is quiescent or living in biofilms, the presence of an untreatable source, septic metastasis or micro-abscesses also require prolonged therapy. Trans-thoracic/transoesophagal echocardiograph and funduscopy should be performed before deciding the duration of therapy. Indeed, short-term therapy (15 days) was shown to be effective only in selected cases of uncomplicated S. aureus right-sided infectious endocarditis or left-sided native valve infectious endocarditis due to highly susceptible streptococci. Most current recommendations emphasize prolonged antibiotic administration (4–6 weeks or even 8 weeks) for S. aureus prosthetic valve endocarditis. Valve cultures should be taken into account to decide how long to continue antimicrobial therapy after valve replacement [118]. Longer therapies are also needed for bone and joint infections (4–8 weeks), brain abscesses (8 weeks), empyema (4–6 weeks) or, in general, when the source control is impossible or incomplete. It is especially the case when infected devices or prosthesis are left in place.
The major limitation of systematically shortening the duration of therapy in uncomplicated infection is the lack of controlled trials confirming its safety. Importantly, the stabilization of the infection process may be difficult to define and often subjective. The use of individualized follow-up of procalcitonin (PCT) levels might be helpful in certain CA infections [119]—indeed, available data suggest that a PCT-driven reduction of treatment duration in patients with otherwise improving clinical status does not result in increased mortality, including in those with BSI [120, 121]. In case of incomplete source control, the duration of therapy might be guided by repeated morphological exams such as leucocyte scintigraphy, and PET scans.
The case of ongoing instability or clinical worsening is complex and may be due to multiple different causes. Often combined, interconnected and leading to diagnostic dilemma with a very high risk of death. Failure of treatment at the source, superinfection with a different or the same pathogen that has become resistant to the ongoing antimicrobial therapy, residual infected tissues or material at the source or at other sites via hematogenous dissemination will all require new specimens, possibly new percutaneous or surgical control, optimization and sometimes escalation of antibiotics. The duration will need to be recalculated from that point in time. Furthermore, it is always important to suspect infections related to the high intensity of care, such as VAP, CLABSI, or a CAUTI. Peripheral blood cultures, specimens of each clinically suspected source and changing the CVC, arterial line and any indwelling material with sending catheter tips for microbiology are most often necessary as part of the treatment and diagnostic workup. While in patients without shock, there are hints to a benefit for waiting for results of such investigations [122]. In cases with high severity, worsening shock and the risk of an untreated infection, it is often required to escalate the antimicrobial regimen in the meantime. The new regimen should take in account colonization and the most frequent pathogens and resistance patterns according to the source and patient category and always be preceded by new blood cultures and specimens of any potential source.
It is, however, important to always remember that non-infectious causes of fever may complicate the clinical picture, most prevalently drug reactions or antibiotic related fever and venous thromboembolism [123]. It is only with meticulous review of the history, available microbiology, clinical examination and targeted investigations that the decision can be taken to escalate the spectrum, extend the duration or sometimes stopping the antimicrobials altogether to allow for an effective microbiological workup.
Concluding remarks
Community-acquired and healthcare-associated BSIs are common situations to manage in ICU patients and are associated with impaired outcomes, especially in case of sepsis/septic shock, immune deficiency, and delayed adequate antimicrobial therapy and/or source control. The prevalence of MDR pathogens is high or even increasing in healthcare-associated BSI, thereby strengthening the need for prospective clinical evaluation of novel diagnostic tools that enable earlier identification of resistance markers. Pending for such data, the choice of empirical regimen depends on a variety of clinical parameters, with the patient’s individual risk of MDR pathogen being the leading one. Double-active regimen might improve survival in the most severely ill patients yet further studies should be focused on this essential issue. Antimicrobial de-escalation should be considered once culture results become available and the source of BSI is identified and controlled. Treatment duration longer than 5–8 days may be indicated only in certain clinical scenarios and/or in BSI due to particular pathogens such as S. aureus.
Compliance with ethical standards
Conflicts of interest
The authors declare no conflict of interest linked to the submitted work. Outside the submitted work: JFT declares research grants from Pfizer, Merck, 3 M, Astellas, Biomerieux; scientific Board participation with Maat Pharma, Merck, Bayer pharma, Medimune, Gilead, VenatoRx, Nabriva, Paratek; lecture fees for Merck, Pfizer, Biomerieux.ER declares research grants from bioMérieux; scientific board participation with MaaT Pharma, Pathoquest, DaVolterra and Illumina; lecture fees from MSD, Pfizer, Mobidiag and Correvio.FB declares lectures fees from Merck and BioMérieux, scientific board participation with Merck, and conference invitation from Pfizer and Merck. MB has participated in advisory boards and/or received speaker honoraria from Achaogen, Angelini, Astellas, Bayer, Basilea, Biomerieux, Cidara, Gilead, Menarini, MSD, Nabriva, Paratek, Pfizer, Roche, Melinta, Shionogi, Tetraphase, VenatoRx and Vifor and has received study grants from Angelini, Basilea, Astellas, Shionogi, Cidara, Melinta, Gilead, Pfizer and MSD.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Adrie C, Garrouste-Orgeas M, Ibn Essaied W, Schwebel C, Darmon M, Mourvillier B, Ruckly S, Dumenil AS, Kallel H, Argaud L, Marcotte G, Barbier F, Laurent V, Goldgran-Toledano D, Clec’h C, Azoulay E, Souweine B, Timsit JF. Attributable mortality of ICU-acquired bloodstream infections: impact of the source, causative micro-organism, resistance profile and antimicrobial therapy. J Infect. 2017;74:131–141. doi: 10.1016/j.jinf.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Tabah A, Koulenti D, Laupland K, Misset B, Valles J, Bruzzi de Carvalho F, Paiva JA, Cakar N, Ma X, Eggimann P, Antonelli M, Bonten MJ, Csomos A, Krueger WA, Mikstacki A, Lipman J, Depuydt P, Vesin A, Garrouste-Orgeas M, Zahar JR, Blot S, Carlet J, Brun-Buisson C, Martin C, Rello J, Dimopoulos G, Timsit JF. Characteristics and determinants of outcome of hospital-acquired bloodstream infections in intensive care units: the EUROBACT International Cohort Study. Intensive Care Med. 2012;38:1930–1945. doi: 10.1007/s00134-012-2695-9. [DOI] [PubMed] [Google Scholar]
- 3.Zahar JR, Timsit JF, Garrouste-Orgeas M, Francais A, Vesin A, Descorps-Declere A, Dubois Y, Souweine B, Haouache H, Goldgran-Toledano D, Allaouchiche B, Azoulay E, Adrie C. Outcomes in severe sepsis and patients with septic shock: pathogen species and infection sites are not associated with mortality. Crit Care Med. 2011;39:1886–1895. doi: 10.1097/CCM.0b013e31821b827c. [DOI] [PubMed] [Google Scholar]
- 4.Laupland KB, Church DL. Population-based epidemiology and microbiology of community-onset bloodstream infections. Clin Microbiol Rev. 2014;27:647–664. doi: 10.1128/CMR.00002-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corona A, Bertolini G, Lipman J, Wilson AP, Singer M. Antibiotic use and impact on outcome from bacteraemic critical illness: the BActeraemia Study in Intensive Care (BASIC) J Antimicrob Chemother. 2010;65:1276–1285. doi: 10.1093/jac/dkq088. [DOI] [PubMed] [Google Scholar]
- 6.McCarthy KL, Paterson DL. Community-acquired Pseudomonas aeruginosa bloodstream infection: a classification that should not falsely reassure the clinician. Eur J Clin Microbiol Infect Dis. 2017;36:703–711. doi: 10.1007/s10096-016-2852-0. [DOI] [PubMed] [Google Scholar]
- 7.See I, Mu Y, Albrecht V, Karlsson M, Dumyati G, Hardy DJ, Koeck M, Lynfield R, Nadle J, Ray SM, Schaffner W, Kallen AJ. Trends in incidence of methicillin-resistant Staphylococcus aureus bloodstream infections differ by strain type and healthcare exposure, United States, 2005–2013. Clin Infect Dis. 2019;70:19–25. doi: 10.1093/cid/ciz158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karanika S, Karantanos T, Arvanitis M, Grigoras C, Mylonakis E. Fecal colonization with extended-spectrum beta-lactamase-producing Enterobacteriaceae and risk factors among healthy individuals: a systematic review and metaanalysis. Clin Infect Dis. 2016;63:310–318. doi: 10.1093/cid/ciw283. [DOI] [PubMed] [Google Scholar]
- 9.Diekema DJ, Hsueh PR, Mendes RE, Pfaller MA, Rolston KV, Sader HS, Jones RN. The microbiology of bloodstream infection: 20-year trends from the SENTRY antimicrobial surveillance program. Antimicrob Agents Chemother. 2019;63:e00355. doi: 10.1128/AAC.00355-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Angelis G, Fiori B, Menchinelli G, D’Inzeo T, Liotti FM, Morandotti GA, Sanguinetti M, Posteraro B, Spanu T. Incidence and antimicrobial resistance trends in bloodstream infections caused by ESKAPE and Escherichia coli at a large teaching hospital in Rome, a 9-year analysis (2007-2015) Eur J Clin Microbiol Infect Dis. 2018;37:1627–1636. doi: 10.1007/s10096-018-3292-9. [DOI] [PubMed] [Google Scholar]
- 11.Prowle JR, Echeverri JE, Ligabo EV, Sherry N, Taori GC, Crozier TM, Hart GK, Korman TM, Mayall BC, Johnson PD, Bellomo R. Acquired bloodstream infection in the intensive care unit: incidence and attributable mortality. Crit Care (London, England) 2011;15:R100. doi: 10.1186/cc10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noto MJ, Domenico HJ, Byrne DW, Talbot T, Rice TW, Bernard GR, Wheeler AP. Chlorhexidine bathing and health care-associated infections: a randomized clinical trial. JAMA. 2015;313:369–378. doi: 10.1001/jama.2014.18400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Climo MW, Yokoe DS, Warren DK, Perl TM, Bolon M, Herwaldt LA, Weinstein RA, Sepkowitz KA, Jernigan JA, Sanogo K, Wong ES. Effect of daily chlorhexidine bathing on hospital-acquired infection. N Engl J Med. 2013;368:533–542. doi: 10.1056/NEJMoa1113849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wittekamp BH, Plantinga NL, Cooper BS, Lopez-Contreras J, Coll P, Mancebo J, Wise MP, Morgan MPG, Depuydt P, Boelens J, Dugernier T, Verbelen V, Jorens PG, Verbrugghe W, Malhotra-Kumar S, Damas P, Meex C, Leleu K, van den Abeele AM, Pimenta Gomes, de Matos AF, Fernandez Mendez S, Vergara Gomez A, Tomic V, Sifrer F, Villarreal Tello E, Ruiz Ramos J, Aragao I, Santos C, Sperning RHM, Coppadoro P, Nardi G, Brun-Buisson C, Bonten MJM. Decontamination strategies and bloodstream infections with antibiotic-resistant microorganisms in ventilated patients: a randomized clinical trial. JAMA. 2018;320:2087–2098. doi: 10.1001/jama.2018.13765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ista E, van der Hoven B, Kornelisse RF, van der Starre C, Vos MC, Boersma E, Helder OK. Effectiveness of insertion and maintenance bundles to prevent central-line-associated bloodstream infections in critically ill patients of all ages: a systematic review and meta-analysis. Lancet Infect Dis. 2016;16:724–734. doi: 10.1016/S1473-3099(15)00409-0. [DOI] [PubMed] [Google Scholar]
- 16.Parienti JJ, Mongardon N, Megarbane B, Mira JP, Kalfon P, Gros A, Marque S, Thuong M, Pottier V, Ramakers M, Savary B, Seguin A, Valette X, Terzi N, Sauneuf B, Cattoir V, Mermel LA, du Cheyron D. Intravascular complications of central venous catheterization by insertion site. N Engl J Med. 2015;373:1220–1229. doi: 10.1056/NEJMoa1500964. [DOI] [PubMed] [Google Scholar]
- 17.Gunther SC, Schwebel C, Hamidfar-Roy R, Bonadona A, Lugosi M, Ara-Somohano C, Minet C, Potton L, Cartier JC, Vesin A, Chautemps M, Styfalova L, Ruckly S, Timsit JF. Complications of intravascular catheters in ICU: definitions, incidence and severity. A randomized controlled trial comparing usual transparent dressings versus new-generation dressings (the ADVANCED study) Intensive Care Med. 2016;42:1753–1765. doi: 10.1007/s00134-016-4582-2. [DOI] [PubMed] [Google Scholar]
- 18.Parienti JJ, du Cheyron D, Timsit JF, Traore O, Kalfon P, Mimoz O, Mermel LA. Meta-analysis of subclavian insertion and nontunneled central venous catheter-associated infection risk reduction in critically ill adults. Crit Care Med. 2012;40:1627–1634. doi: 10.1097/CCM.0b013e31823e99cb. [DOI] [PubMed] [Google Scholar]
- 19.Timsit JF, Bouadma L, Ruckly S, Schwebel C, Garrouste-Orgeas M, Bronchard R, Calvino-Gunther S, Laupland K, Adrie C, Thuong M, Herault MC, Pease S, Arrault X, Lucet JC. Dressing disruption is a major risk factor for catheter-related infections. Crit Care Med. 2012;40:1707–1714. doi: 10.1097/CCM.0b013e31824e0d46. [DOI] [PubMed] [Google Scholar]
- 20.Timsit JF, L’Heriteau F, Lepape A, Francais A, Ruckly S, Venier AG, Jarno P, Boussat S, Coignard B, Savey A. A multicentre analysis of catheter-related infection based on a hierarchical model. Intensive Care Med. 2012;38:1662–1672. doi: 10.1007/s00134-012-2645-6. [DOI] [PubMed] [Google Scholar]
- 21.O’Horo JC, Maki DG, Krupp AE, Safdar N. Arterial catheters as a source of bloodstream infection: a systematic review and meta-analysis. Crit Care Med. 2014;42:1334–1339. doi: 10.1097/CCM.0000000000000166. [DOI] [PubMed] [Google Scholar]
- 22.Biffi S, Di Bella S, Scaravilli V, Peri AM, Grasselli G, Alagna L, Pesenti A, Gori A. Infections during extracorporeal membrane oxygenation: epidemiology, risk factors, pathogenesis and prevention. Int J Antimicrob Agents. 2017;50:9–16. doi: 10.1016/j.ijantimicag.2017.02.025. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt M, Bréchot N, Hariri S, Guiguet M, Luyt CE, Makri R, Leprince P, Trouillet JL, Pavie A, Chastre J, Combes A. Nosocomial infections in adult cardiogenic shock patients supported by venoarterial extracorporeal membrane oxygenation. Clin Infect Dis. 2012;55:1633–1641. doi: 10.1093/cid/cis783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richet H. Seasonality in Gram-negative and healthcare-associated infections. Clin Microbiol Infect. 2012;18:934–940. doi: 10.1111/j.1469-0691.2012.03954.x. [DOI] [PubMed] [Google Scholar]
- 25.Timsit JF, Bassetti M, Cremer O, Daikos G, de Waele J, Kallil A, Kipnis E, Kollef M, Laupland K, Paiva JA, Rodriguez-Bano J, Ruppe E, Salluh J, Taccone FS, Weiss E, Barbier F. Rationalizing antimicrobial therapy in the ICU: a narrative review. Intensive Care Med. 2019;45:172–189. doi: 10.1007/s00134-019-05520-5. [DOI] [PubMed] [Google Scholar]
- 26.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent JL, Wiersinga WJ, Zimmerman JL, Dellinger RP. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 27.Cheng MP, Stenstrom R, Paquette K, Stabler SN, Akhter M, Davidson AC, Gavric M, Lawandi A, Jinah R, Saeed Z, Demir K, Huang K, Mahpour A, Shamatutu C, Caya C, Troquet JM, Clark G, Yansouni CP, Sweet D. Blood culture results before and after antimicrobial administration in patients with severe manifestations of sepsis: a diagnostic study. Ann Internal Med. 2019 doi: 10.7326/M19-1696. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen MH, Clancy CJ, Pasculle AW, Pappas PG, Alangaden G, Pankey GA, Schmitt BH, Rasool A, Weinstein MP, Widen R, Hernandez DR, Wolk DM, Walsh TJ, Perfect JR, Wilson MN, Mylonakis E. Performance of the T2 bacteria panel for diagnosing bloodstream infections: a diagnostic accuracy study. Ann Intern Med. 2019;170:845–852. doi: 10.7326/M18-2772. [DOI] [PubMed] [Google Scholar]
- 29.Banerjee R, Teng CB, Cunningham SA, Ihde SM, Steckelberg JM, Moriarty JP, Shah ND, Mandrekar JN, Patel R. Randomized trial of rapid multiplex polymerase chain reaction-based blood culture identification and susceptibility testing. Clin Infect Dis. 2015;61:1071–1080. doi: 10.1093/cid/civ447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Timbrook TT, Morton JB, McConeghy KW, Caffrey AR, Mylonakis E, LaPlante KL. The effect of molecular rapid diagnostic testing on clinical outcomes in bloodstream infections: a systematic review and meta-analysis. Clin Infect Dis. 2017;64:15–23. doi: 10.1093/cid/ciw649. [DOI] [PubMed] [Google Scholar]
- 31.Faron ML, Buchan BW, Ledeboer NA. Matrix-assisted laser desorption ionization-time of flight mass spectrometry for use with positive blood cultures: methodology, performance, and optimization. J Clin Microbiol. 2017;55:3328–3338. doi: 10.1128/JCM.00868-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiu CY, Miller SA. Clinical metagenomics. Nat Rev Genet. 2019;20:341–355. doi: 10.1038/s41576-019-0113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grumaz S, Stevens P, Grumaz C, Decker SO, Weigand MA, Hofer S, Brenner T, von Haeseler A, Sohn K. Next-generation sequencing diagnostics of bacteremia in septic patients. Genome Med. 2016;8:73. doi: 10.1186/s13073-016-0326-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blauwkamp TA, Thair S, Rosen MJ, Blair L, Lindner MS, Vilfan ID, Kawli T, Christians FC, Venkatasubrahmanyam S, Wall GD, Cheung A, Rogers ZN, Meshulam-Simon G, Huijse L, Balakrishnan S, Quinn JV, Hollemon D, Hong DK, Vaughn ML, Kertesz M, Bercovici S, Wilber JC, Yang S. Analytical and clinical validation of a microbial cell-free DNA sequencing test for infectious disease. Nat Microbiol. 2019;4:663–674. doi: 10.1038/s41564-018-0349-6. [DOI] [PubMed] [Google Scholar]
- 35.Parize P, Muth E, Richaud C, Gratigny M, Pilmis B, Lamamy A, Mainardi JL, Cheval J, de Visser L, Jagorel F, Ben Yahia L, Bamba G, Dubois M, Join-Lambert O, Leruez-Ville M, Nassif X, Lefort A, Lanternier F, Suarez F, Lortholary O, Lecuit M, Eloit M. Untargeted next-generation sequencing-based first-line diagnosis of infection in immunocompromised adults: a multicentre, blinded, prospective study. Clin Microbiol Infect. 2017;23(574):e571–e574. doi: 10.1016/j.cmi.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 36.Hogan CA, Watz N, Budvytiene I, Banaei N. Rapid antimicrobial susceptibility testing by VITEK(R)2 directly from blood cultures in patients with Gram-negative rod bacteremia. Diagn Microbiol Infect Dis. 2019;94:116–121. doi: 10.1016/j.diagmicrobio.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 37.Marschal M, Bachmaier J, Autenrieth I, Oberhettinger P, Willmann M, Peter S. Evaluation of the accelerate pheno system for fast identification and antimicrobial susceptibility testing from positive blood cultures in bloodstream infections caused by Gram-negative pathogens. J Clin Microbiol. 2017;55:2116–2126. doi: 10.1128/JCM.00181-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bassetti M, Peghin M, Vena A, Giacobbe DR. Treatment of infections due to MDR Gram-negative bacteria. Front Med. 2019;6:74. doi: 10.3389/fmed.2019.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Calandra T, Roberts JA, Antonelli M, Bassetti M, Vincent JL. Diagnosis and management of invasive candidiasis in the ICU: an updated approach to an old enemy. Crit Care. 2016;20:125. doi: 10.1186/s13054-016-1313-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bassetti M, Righi E, Montravers P, Cornely OA. What has changed in the treatment of invasive candidiasis? A look at the past 10 years and ahead. J Antimicrob Chemother. 2018;73:i14–i25. doi: 10.1093/jac/dkx445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abbas M, Paul M, Huttner A. New and improved? A review of novel antibiotics for Gram-positive bacteria. Clin Microbiol Infect. 2017;23:697–703. doi: 10.1016/j.cmi.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 42.Timsit JF, Sonneville R, Kalil AC, Bassetti M, Ferrer R, Jaber S, Lanternier F, Luyt CE, Machado F, Mikulska M, Papazian L, Pene F, Poulakou G, Viscoli C, Wolff M, Zafrani L, Van Delden C. Diagnostic and therapeutic approach to infectious diseases in solid organ transplant recipients. Intensive Care Med. 2019;45:573–591. doi: 10.1007/s00134-019-05597-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schnell D, Montlahuc C, Bruneel F, Resche-Rigon M, Kouatchet A, Zahar JR, Darmon M, Pene F, Lemiale V, Rabbat A, Vincent F, Azoulay E, Mokart D. De-escalation of antimicrobial therapy in critically ill hematology patients: a prospective cohort study. Intensive Care Med. 2019;45:743–745. doi: 10.1007/s00134-019-05554-9. [DOI] [PubMed] [Google Scholar]
- 44.Klein Klouwenberg PM, Cremer OL, van Vught LA, Ong DS, Frencken JF, Schultz MJ, Bonten MJ, van der Poll T. Likelihood of infection in patients with presumed sepsis at the time of intensive care unit admission: a cohort study. Crit Care. 2015;19:319. doi: 10.1186/s13054-015-1035-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buehler SS, Madison B, Snyder SR, Derzon JH, Cornish NE, Saubolle MA, Weissfeld AS, Weinstein MP, Liebow EB, Wolk DM. Effectiveness of practices to increase timeliness of providing targeted therapy for inpatients with bloodstream infections: a laboratory medicine best practices systematic review and meta-analysis. Clin Microbiol Rev. 2016;29:59–103. doi: 10.1128/CMR.00053-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mangioni D, Viaggi B, Giani T, Arena F, D’Arienzo S, Forni S, Tulli G, Rossolini GM. Diagnostic stewardship for sepsis: the need for risk stratification to triage patients for fast microbiology workflows. Future Microbiol. 2019;14:169–174. doi: 10.2217/fmb-2018-0329. [DOI] [PubMed] [Google Scholar]
- 47.Patel R, Tsalik EL, Petzold E, Fowler VG, Jr, Klausner JD, Evans S, Antibacterial Resistance Leadership G MASTERMIND: bringing microbial diagnostics to the clinic. Clin Infect Dis. 2017;64:355–360. doi: 10.1093/cid/ciw788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bassetti M, Poulakou G, Ruppe E, Bouza E, Van Hal SJ, Brink A. Antimicrobial resistance in the next 30 years, humankind, bugs and drugs: a visionary approach. Intensive Care Med. 2017;43:1464–1475. doi: 10.1007/s00134-017-4878-x. [DOI] [PubMed] [Google Scholar]
- 49.Tacconelli E, Gorska A, De Angelis G, Lammens C, Restuccia G, Schrenzel J, Huson DH, Carevic B, Preotescu L, Carmeli Y, Kazma M, Spanu T, Carrara E, Malhotra-Kumar S, Gladstone BP. Estimating the association between antibiotic exposure and colonization with extended-spectrum beta-lactamase-producing Gram-negative bacteria using machine learning methods: a multicentre, prospective cohort study. Clin Microbiol Infect. 2019;26:87–94. doi: 10.1016/j.cmi.2019.05.013. [DOI] [PubMed] [Google Scholar]
- 50.Giacobbe DR, Mikulska M, Viscoli C. Recent advances in the pharmacological management of infections due to multidrug-resistant Gram-negative bacteria. Expert Rev Clin Pharmacol. 2018;11:1219–1236. doi: 10.1080/17512433.2018.1549487. [DOI] [PubMed] [Google Scholar]
- 51.De Waele JJ, Lipman J, Carlier M, Roberts JA. Subtleties in practical application of prolonged infusion of beta-lactam antibiotics. Int J Antimicrob Agents. 2015;45:461–463. doi: 10.1016/j.ijantimicag.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 52.Roberts JA, Taccone FS, Lipman J. Understanding PK/PD. Intensive Care Med. 2016;42:1797–1800. doi: 10.1007/s00134-015-4032-6. [DOI] [PubMed] [Google Scholar]
- 53.Roberts JA, Paul SK, Akova M, Bassetti M, De Waele JJ, Dimopoulos G, Kaukonen KM, Koulenti D, Martin C, Montravers P, Rello J, Rhodes A, Starr T, Wallis SC, Lipman J, Study D DALI: defining antibiotic levels in intensive care unit patients: are current beta-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis. 2014;58:1072–1083. doi: 10.1093/cid/ciu027. [DOI] [PubMed] [Google Scholar]
- 54.Blot S, Lipman J, Roberts DM, Roberts JA. The influence of acute kidney injury on antimicrobial dosing in critically ill patients: are dose reductions always necessary? Diagn Microbiol Infect Dis. 2014;79:77–84. doi: 10.1016/j.diagmicrobio.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 55.Pistolesi V, Morabito S, Di Mario F, Regolisti G, Cantarelli C, Fiaccadori E. A guide to understanding antimicrobial drug dosing in critically ill patients on renal replacement therapy. Antimicrob Agents Chemother. 2019;63:e00583. doi: 10.1128/AAC.00583-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Udy AA, Varghese JM, Altukroni M, Briscoe S, McWhinney BC, Ungerer JP, Lipman J, Roberts JA. Subtherapeutic initial beta-lactam concentrations in select critically ill patients: association between augmented renal clearance and low trough drug concentrations. Chest. 2012;142:30–39. doi: 10.1378/chest.11-1671. [DOI] [PubMed] [Google Scholar]
- 57.Mouton JW, Muller AE, Canton R, Giske CG, Kahlmeter G, Turnidge J. MIC-based dose adjustment: facts and fables. J Antimicrob Chemother. 2018;73:564–568. doi: 10.1093/jac/dkx427. [DOI] [PubMed] [Google Scholar]
- 58.Tsuji BT, Pogue JM, Zavascki AP, Paul M, Daikos GL, Forrest A, Giacobbe DR, Viscoli C, Giamarellou H, Karaiskos I, Kaye D, Mouton JW, Tam VH, Thamlikitkul V, Wunderink RG, Li J, Nation RL, Kaye KS. International consensus guidelines for the optimal use of the polymyxins: endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP) Pharmacotherapy. 2019;39:10–39. doi: 10.1002/phar.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tangden T, Ramos Martin V, Felton TW, Nielsen EI, Marchand S, Bruggemann RJ, Bulitta JB, Bassetti M, Theuretzbacher U, Tsuji BT, Wareham DW, Friberg LE, De Waele JJ, Tam VH, Roberts JA, Infection Section for the European Society of Intensive Care Medicine tP, Pharmacodynamics Study Group of the European Society of Clinical M, Infectious Diseases tISoA-IP, the Critically Ill Patients Study Group of European Society of Clinical M, Infectious D The role of infection models and PK/PD modelling for optimising care of critically ill patients with severe infections. Intensive Care Med. 2017;43:1021–1032. doi: 10.1007/s00134-017-4780-6. [DOI] [PubMed] [Google Scholar]
- 60.Duszynska W, Taccone FS, Hurkacz M, Kowalska-Krochmal B, Wiela-Hojenska A, Kubler A. Therapeutic drug monitoring of amikacin in septic patients. Crit Care. 2013;17:R165. doi: 10.1186/cc12844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Prybylski JP. Vancomycin trough concentration as a predictor of clinical outcomes in patients with Staphylococcus aureus bacteremia: a meta-analysis of observational studies. Pharmacotherapy. 2015;35:889–898. doi: 10.1002/phar.1638. [DOI] [PubMed] [Google Scholar]
- 62.Huttner A, Harbarth S, Hope WW, Lipman J, Roberts JA. Therapeutic drug monitoring of the beta-lactam antibiotics: what is the evidence and which patients should we be using it for? J Antimicrob Chemother. 2015;70:3178–3183. doi: 10.1093/jac/dkv201. [DOI] [PubMed] [Google Scholar]
- 63.Wong G, Briscoe S, McWhinney B, Ally M, Ungerer J, Lipman J, Roberts JA. Therapeutic drug monitoring of beta-lactam antibiotics in the critically ill: direct measurement of unbound drug concentrations to achieve appropriate drug exposures. J Antimicrob Chemother. 2018;73:3087–3094. doi: 10.1093/jac/dky314. [DOI] [PubMed] [Google Scholar]
- 64.Wong G, Sime FB, Lipman J, Roberts JA. How do we use therapeutic drug monitoring to improve outcomes from severe infections in critically ill patients? BMC Infect Dis. 2014;14:288. doi: 10.1186/1471-2334-14-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jager NG, van Hest RM, Lipman J, Taccone FS, Roberts JA. Therapeutic drug monitoring of anti-infective agents in critically ill patients. Expert Rev Clin Pharmacol. 2016;9:961–979. doi: 10.1586/17512433.2016.1172209. [DOI] [PubMed] [Google Scholar]
- 66.Tamma PD, Cosgrove SE, Maragakis LL. Combination therapy for treatment of infections with gram-negative bacteria. Clin Microbiol Rev. 2012;25:450–470. doi: 10.1128/CMR.05041-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brunkhorst FM, Oppert M, Marx G, Bloos F, Ludewig K, Putensen C, Nierhaus A, Jaschinski U, Meier-Hellmann A, Weyland A, Grundling M, Moerer O, Riessen R, Seibel A, Ragaller M, Buchler MW, John S, Bach F, Spies C, Reill L, Fritz H, Kiehntopf M, Kuhnt E, Bogatsch H, Engel C, Loeffler M, Kollef MH, Reinhart K, Welte T. Effect of empirical treatment with moxifloxacin and meropenem vs meropenem on sepsis-related organ dysfunction in patients with severe sepsis: a randomized trial. JAMA. 2012;307:2390–2399. doi: 10.1001/jama.2012.5833. [DOI] [PubMed] [Google Scholar]
- 68.Paul M, Lador A, Grozinsky-Glasberg S, Leibovici L. Beta lactam antibiotic monotherapy versus beta lactam-aminoglycoside antibiotic combination therapy for sepsis. Cochrane Database Syst Rev. 2014;1:CD003344. doi: 10.1002/14651858.CD003344.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Paul M, Dickstein Y, Schlesinger A, Grozinsky-Glasberg S, Soares-Weiser K, Leibovici L. Beta-lactam versus beta-lactam-aminoglycoside combination therapy in cancer patients with neutropenia. Cochrane Database Syst Rev. 2013;6:CD003038. doi: 10.1002/14651858.CD003038.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sjovall F, Perner A, Hylander Moller M. Empirical mono- versus combination antibiotic therapy in adult intensive care patients with severe sepsis—a systematic review with meta-analysis and trial sequential analysis. J Infect. 2017;74:331–344. doi: 10.1016/j.jinf.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 71.Ong DSY, Frencken JF, Klein Klouwenberg PMC, Juffermans N, van der Poll T, Bonten MJM, Cremer OL. Short-course adjunctive gentamicin as empirical therapy in patients with severe sepsis and septic shock: a prospective observational cohort study. Clin Infect Dis. 2017;64:1731–1736. doi: 10.1093/cid/cix186. [DOI] [PubMed] [Google Scholar]
- 72.Rieg S, Joost I, Weiss V, Peyerl-Hoffmann G, Schneider C, Hellmich M, Seifert H, Kern WV, Kaasch A. Combination antimicrobial therapy in patients with Staphylococcus aureus bacteraemia-a post hoc analysis in 964 prospectively evaluated patients. Clin Microbiol Infect. 2017;23:406e401–406e408. doi: 10.1016/j.cmi.2016.08.026. [DOI] [PubMed] [Google Scholar]
- 73.Ripa M, Rodriguez-Nunez O, Cardozo C, Naharro-Abellan A, Almela M, Marco F, Morata L, De La Calle C, Del Rio A, Garcia-Vidal C, Ortega MDM, Guerrero-Leon MLA, Feher C, Torres B, Puerta-Alcalde P, Mensa J, Soriano A, Martinez JA. Influence of empirical double-active combination antimicrobial therapy compared with active monotherapy on mortality in patients with septic shock: a propensity score-adjusted and matched analysis. J Antimicrob Chemother. 2017;72:3443–3452. doi: 10.1093/jac/dkx315. [DOI] [PubMed] [Google Scholar]
- 74.Justo JA, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. Combination therapy vs. monotherapy for Gram-negative bloodstream infection: matching by predicted prognosis. Int J Antimicrob Agents. 2018;51:488–492. doi: 10.1016/j.ijantimicag.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 75.Russo A, Falcone M, Gutierrez-Gutierrez B, Calbo E, Almirante B, Viale PL, Oliver A, Ruiz-Garbajosa P, Gasch O, Gozalo M, Pitout J, Akova M, Pena C, Cisneros JM, Hernandez-Torres A, Farcomeni A, Prim N, Origuen J, Bou G, Tacconelli E, Tumbarello M, Hamprecht A, Karaiskos I, de la Calle C, Perez F, Schwaber MJ, Bermejo J, Lowman W, Hsueh PR, Mora-Rillo M, Rodriguez-Gomez J, Souli M, Bonomo RA, Paterson DL, Carmeli Y, Pascual A, Rodriguez-Bano J, Venditti M. Predictors of outcome in patients with severe sepsis or septic shock due to extended-spectrum beta-lactamase-producing Enterobacteriaceae. Int J Antimicrob Agents. 2018;52:577–585. doi: 10.1016/j.ijantimicag.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 76.Paul M, Daikos GL, Durante-Mangoni E, Yahav D, Carmeli Y, Benattar YD, Skiada A, Andini R, Eliakim-Raz N, Nutman A, Zusman O, Antoniadou A, Pafundi PC, Adler A, Dickstein Y, Pavleas I, Zampino R, Daitch V, Bitterman R, Zayyad H, Koppel F, Levi I, Babich T, Friberg LE, Mouton JW, Theuretzbacher U, Leibovici L. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria: an open-label, randomised controlled trial. Lancet Infect Dis. 2018;18:391–400. doi: 10.1016/S1473-3099(18)30099-9. [DOI] [PubMed] [Google Scholar]
- 77.Vardakas KZ, Mavroudis AD, Georgiou M, Falagas ME. Intravenous colistin combination antimicrobial treatment vs. monotherapy: a systematic review and meta-analysis. Int J Antimicrob Agents. 2018;51:535–547. doi: 10.1016/j.ijantimicag.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 78.Zusman O, Altunin S, Koppel F, Dishon Benattar Y, Gedik H, Paul M. Polymyxin monotherapy or in combination against carbapenem-resistant bacteria: systematic review and meta-analysis. J Antimicrob Chemother. 2017;72:29–39. doi: 10.1093/jac/dkw377. [DOI] [PubMed] [Google Scholar]
- 79.Russo A, Bassetti M, Ceccarelli G, Carannante N, Losito AR, Bartoletti M, Corcione S, Granata G, Santoro A, Giacobbe DR, Peghin M, Vena A, Amadori F, Segala FV, Giannella M, Di Caprio G, Menichetti F, Del Bono V, Mussini C, Petrosillo N, De Rosa FG, Viale P, Tumbarello M, Tascini C, Viscoli C, Venditti M. Bloodstream infections caused by carbapenem-resistant Acinetobacter baumannii: clinical features, therapy and outcome from a multicenter study. J Infect. 2019;79:130–138. doi: 10.1016/j.jinf.2019.05.017. [DOI] [PubMed] [Google Scholar]
- 80.Tschudin-Sutter S, Fosse N, Frei R, Widmer AF. Combination therapy for treatment of Pseudomonas aeruginosa bloodstream infections. PLoS One. 2018;13:e0203295. doi: 10.1371/journal.pone.0203295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pena C, Suarez C, Ocampo-Sosa A, Murillas J, Almirante B, Pomar V, Aguilar M, Granados A, Calbo E, Rodriguez-Bano J, Rodriguez F, Tubau F, Oliver A, Martinez-Martinez L. Effect of adequate single-drug vs combination antimicrobial therapy on mortality in Pseudomonas aeruginosa bloodstream infections: a post hoc analysis of a prospective cohort. Clin Infect Dis. 2013;57:208–216. doi: 10.1093/cid/cit223. [DOI] [PubMed] [Google Scholar]
- 82.Vardakas KZ, Tansarli GS, Bliziotis IA, Falagas ME. Beta-Lactam plus aminoglycoside or fluoroquinolone combination versus beta-lactam monotherapy for Pseudomonas aeruginosa infections: a meta-analysis. Int J Antimicrob Agents. 2013;41:301–310. doi: 10.1016/j.ijantimicag.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 83.Kumar A, Safdar N, Kethireddy S, Chateau D. A survival benefit of combination antibiotic therapy for serious infections associated with sepsis and septic shock is contingent only on the risk of death: a meta-analytic/meta-regression study. Crit Care Med. 2010;38:1651–1664. doi: 10.1097/CCM.0b013e3181e96b91. [DOI] [PubMed] [Google Scholar]
- 84.Gutierrez-Gutierrez B, Salamanca E, de Cueto M, Hsueh PR, Viale P, Pano-Pardo JR, Venditti M, Tumbarello M, Daikos G, Canton R, Doi Y, Tuon FF, Karaiskos I, Perez-Nadales E, Schwaber MJ, Azap OK, Souli M, Roilides E, Pournaras S, Akova M, Perez F, Bermejo J, Oliver A, Almela M, Lowman W, Almirante B, Bonomo RA, Carmeli Y, Paterson DL, Pascual A, Rodriguez-Bano J. Effect of appropriate combination therapy on mortality of patients with bloodstream infections due to carbapenemase-producing Enterobacteriaceae (INCREMENT): a retrospective cohort study. Lancet Infect Dis. 2017;17:726–734. doi: 10.1016/S1473-3099(17)30228-1. [DOI] [PubMed] [Google Scholar]
- 85.Coopersmith CM, De Backer D, Deutschman CS, Ferrer R, Lat I, Machado FR, Martin GS, Martin-Loeches I, Nunnally ME, Antonelli M, Evans LE, Hellman J, Jog S, Kesecioglu J, Levy MM, Rhodes A. Surviving sepsis campaign: research priorities for sepsis and septic shock. Intensive Care Med. 2018;44:1400–1426. doi: 10.1007/s00134-018-5175-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weber DG, Bendinelli C, Balogh ZJ. Damage control surgery for abdominal emergencies. Br J Surg. 2014;101:e109–e118. doi: 10.1002/bjs.9360. [DOI] [PubMed] [Google Scholar]
- 87.Timsit JF, Rupp M, Bouza E, Chopra V, Karpanen T, Laupland K, Lisboa T, Mermel L, Mimoz O, Parienti JJ, Poulakou G, Souweine B, Zingg W. A state of the art review on optimal practices to prevent, recognize, and manage complications associated with intravascular devices in the critically ill. Intensive Care Med. 2018;44:742–759. doi: 10.1007/s00134-018-5212-y. [DOI] [PubMed] [Google Scholar]
- 88.Burnham JP, Rojek RP, Kollef MH. Catheter removal and outcomes of multidrug-resistant central-line-associated bloodstream infection. Medicine. 2018;97:e12782. doi: 10.1097/MD.0000000000012782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Andes DR, Safdar N, Baddley JW, Playford G, Reboli AC, Rex JH, Sobel JD, Pappas PG, Kullberg BJ. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: a patient-level quantitative review of randomized trials. Clin Infect Dis. 2012;54:1110–1122. doi: 10.1093/cid/cis021. [DOI] [PubMed] [Google Scholar]
- 90.Lecronier M, Valade S, Bige N, de Prost N, Roux D, Lebeaux D, Maury E, Azoulay E, Demoule A, Dres M. Removal of totally implanted venous access ports for suspected infection in the intensive care unit: a multicenter observational study. Ann Intensive Care. 2018;8:41. doi: 10.1186/s13613-018-0383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Martinez ML, Ferrer R, Torrents E, Guillamat-Prats R, Goma G, Suarez D, Alvarez-Rocha L, Pozo Laderas JC, Martin-Loeches I, Levy MM, Artigas A. Impact of source control in patients with severe sepsis and septic shock. Crit Care Med. 2017;45:11–19. doi: 10.1097/CCM.0000000000002011. [DOI] [PubMed] [Google Scholar]
- 92.Bloos F, Thomas-Ruddel D, Ruddel H, Engel C, Schwarzkopf D, Marshall JC, Harbarth S, Simon P, Riessen R, Keh D, Dey K, Weiss M, Toussaint S, Schadler D, Weyland A, Ragaller M, Schwarzkopf K, Eiche J, Kuhnle G, Hoyer H, Hartog C, Kaisers U, Reinhart K. Impact of compliance with infection management guidelines on outcome in patients with severe sepsis: a prospective observational multi-center study. Crit Care. 2014;18:R42. doi: 10.1186/cc13755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Boyer A, Vargas F, Coste F, Saubusse E, Castaing Y, Gbikpi-Benissan G, Hilbert G, Gruson D. Influence of surgical treatment timing on mortality from necrotizing soft tissue infections requiring intensive care management. Intensive Care Med. 2009;35:847–853. doi: 10.1007/s00134-008-1373-4. [DOI] [PubMed] [Google Scholar]
- 94.Rausei S, Pappalardo V, Ruspi L, Colella A, Giudici S, Ardita V, Frattini F, Rovera F, Boni L, Dionigi G. Early versus delayed source control in open abdomen management for severe intra-abdominal infections: a retrospective analysis on 111 cases. World J Surg. 2018;42:707–712. doi: 10.1007/s00268-017-4233-y. [DOI] [PubMed] [Google Scholar]
- 95.Montravers P, Blot S, Dimopoulos G, Eckmann C, Eggimann P, Guirao X, Paiva JA, Sganga G, De Waele J. Therapeutic management of peritonitis: a comprehensive guide for intensivists. Intensive Care Med. 2016;42:1234–1247. doi: 10.1007/s00134-016-4307-6. [DOI] [PubMed] [Google Scholar]
- 96.Solomkin JS, Ristagno RL, Das AF, Cone JB, Wilson SE, Rotstein OD, Murphy BS, Severin KS, Bruss JB. Source control review in clinical trials of anti-infective agents in complicated intra-abdominal infections. Clin Infect Dis. 2013;56:1765–1773. doi: 10.1093/cid/cit128. [DOI] [PubMed] [Google Scholar]
- 97.De Waele JJ, Akova M, Antonelli M, Canton R, Carlet J, De Backer D, Dimopoulos G, Garnacho-Montero J, Kesecioglu J, Lipman J, Mer M, Paiva JA, Poljak M, Roberts JA, Rodriguez Bano J, Timsit JF, Zahar JR, Bassetti M. Antimicrobial resistance and antibiotic stewardship programs in the ICU: insistence and persistence in the fight against resistance. A position statement from ESICM/ESCMID/WAAAR round table on multi-drug resistance. Intensive Care Med. 2018;44:189–196. doi: 10.1007/s00134-017-5036-1. [DOI] [PubMed] [Google Scholar]
- 98.Tabah A, Cotta MO, Garnacho-Montero J, Schouten J, Roberts JA, Lipman J, Tacey M, Timsit JF, Leone M, Zahar JR, De Waele JJ. A systematic review of the definitions, determinants, and clinical outcomes of antimicrobial de-escalation in the intensive care unit. Clin Infect Dis. 2016;62:1009–1017. doi: 10.1093/cid/civ1199. [DOI] [PubMed] [Google Scholar]
- 99.Tabah A, Bassetti M, Kollef MH, Zahar JR, Paiva JA, Timsit JF, Roberts JA, Schouten J, Giamarellou H, Rello J, De Waele J, Shorr AF, Leone M, Poulakou G, Depuydt P, Garnacho-Montero J. Antimicrobial de-escalation in critically ill patients: a position statement from a task force of the European Society of Intensive Care Medicine (ESICM) and European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Critically Ill Patients Study Group (ESGCIP) Intensive Care Med. 2019 doi: 10.7326/M19-1696. [DOI] [PubMed] [Google Scholar]
- 100.Mokart D, Slehofer G, Lambert J, Sannini A, Chow-Chine L, Brun JP, Berger P, Duran S, Faucher M, Blache JL, Saillard C, Vey N, Leone M. De-escalation of antimicrobial treatment in neutropenic patients with severe sepsis: results from an observational study. Intensive Care Med. 2014;40:41–49. doi: 10.1007/s00134-013-3148-9. [DOI] [PubMed] [Google Scholar]
- 101.Armand-Lefevre L, Angebault C, Barbier F, Hamelet E, Defrance G, Ruppe E, Bronchard R, Lepeule R, Lucet JC, El Mniai A, Wolff M, Montravers P, Plesiat P, Andremont A. Emergence of imipenem-resistant gram-negative bacilli in intestinal flora of intensive care patients. Antimicrob Agents Chemother. 2013;57:1488–1495. doi: 10.1128/AAC.01823-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Madaras-Kelly K, Jones M, Remington R, Hill N, Huttner B, Samore M. Development of an antibiotic spectrum score based on veterans affairs culture and susceptibility data for the purpose of measuring antibiotic de-escalation: a modified Delphi approach. Infect Control Hosp Epidemiol. 2014;35:1103–1113. doi: 10.1086/677633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Weiss E, Zahar JR, Garrouste-Orgeas M, Ruckly S, Essaied W, Schwebel C, Timsit JF. De-escalation of pivotal beta-lactam in ventilator-associated pneumonia does not impact outcome and marginally affects MDR acquisition. Intensive Care Med. 2016;42:2098–2100. doi: 10.1007/s00134-016-4448-7. [DOI] [PubMed] [Google Scholar]
- 104.Woerther PL, Lepeule R, Burdet C, Decousser JW, Ruppe E, Barbier F. Carbapenems and alternative beta-lactams for the treatment of infections due to extended-spectrum beta-lactamase-producing Enterobacteriaceae: what impact on intestinal colonisation resistance? Int J Antimicrob Agents. 2018;52:762–770. doi: 10.1016/j.ijantimicag.2018.08.026. [DOI] [PubMed] [Google Scholar]
- 105.Carlier M, Roberts JA, Stove V, Verstraete AG, Lipman J, De Waele JJ. A simulation study reveals lack of pharmacokinetic/pharmacodynamic target attainment in de-escalated antibiotic therapy in critically ill patients. Antimicrob Agents Chemother. 2015;59:4689–4694. doi: 10.1128/AAC.00409-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Beganovic M, Cusumano JA, Lopes V, LaPlante KL, Caffrey AR. Comparative effectiveness of exclusive exposure to nafcillin or oxacillin, cefazolin, piperacillin/tazobactam, and fluoroquinolones among a national cohort of veterans with methicillin-susceptible Staphylococcus aureus bloodstream infection. Open Forum Infect Dis. 2019;6:270. doi: 10.1093/ofid/ofz270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Leone M, Bechis C, Baumstarck K, Lefrant JY, Albanese J, Jaber S, Lepape A, Constantin JM, Papazian L, Bruder N, Allaouchiche B, Bezulier K, Antonini F, Textoris J, Martin C. De-escalation versus continuation of empirical antimicrobial treatment in severe sepsis: a multicenter non-blinded randomized noninferiority trial. Intensive Care Med. 2014;40:1399–1408. doi: 10.1007/s00134-014-3411-8. [DOI] [PubMed] [Google Scholar]
- 108.Havey TC, Fowler RA, Daneman N. Duration of antibiotic therapy for bacteremia: a systematic review and meta-analysis. Crit Care. 2011;15:R267. doi: 10.1186/cc10545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Montravers P, Tubach F, Lescot T, Veber B, Esposito-Farese M, Seguin P, Paugam C, Lepape A, Meistelman C, Cousson J, Tesniere A, Plantefeve G, Blasco G, Asehnoune K, Jaber S, Lasocki S, Dupont H. Short-course antibiotic therapy for critically ill patients treated for postoperative intra-abdominal infection: the DURAPOP randomised clinical trial. Intensive Care Med. 2018;44:300–310. doi: 10.1007/s00134-018-5088-x. [DOI] [PubMed] [Google Scholar]
- 110.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, Talan DA, Chambers HF. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52:e18–e55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 111.Fiala J, Palraj BR, Sohail MR, Lahr B, Baddour LM. Is a single set of negative blood cultures sufficient to ensure clearance of bloodstream infection in patients with Staphylococcus aureus bacteremia? The skip phenomenon. Infection. 2019;47:1047–1053. doi: 10.1007/s15010-019-01339-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Daneman N, Rishu AH, Xiong W, Bagshaw SM, Dodek P, Hall R, Kumar A, Lamontagne F, Lauzier F, Marshall J, Martin CM, McIntyre L, Muscedere J, Reynolds S, Stelfox HT, Cook DJ, Fowler RA. Duration of antimicrobial treatment for bacteremia in canadian critically ill patients. Crit Care Med. 2016;44:256–264. doi: 10.1097/CCM.0000000000001393. [DOI] [PubMed] [Google Scholar]
- 113.Yahav D, Franceschini E, Koppel F, Turjeman A, Babich T, Bitterman R, Neuberger A, Ghanem-Zoubi N, Santoro A, Eliakim-Raz N, Pertzov B, Steinmetz T, Stern A, Dickstein Y, Maroun E, Zayyad H, Bishara J, Alon D, Edel Y, Goldberg E, Venturelli C, Mussini C, Leibovici L, Paul M. Seven versus fourteen days of antibiotic therapy for uncomplicated gram-negative bacteremia: a non-inferiority randomized controlled trial. Clin Infect Dis. 2018;69:1091–1098. doi: 10.1093/cid/ciy1054. [DOI] [PubMed] [Google Scholar]
- 114.Fabre V, Amoah J, Cosgrove SE, Tamma PD. Antibiotic therapy for Pseudomonas aeruginosa bloodstream infections: how long is long enough? Clin Infect Dis. 2019;69:2011–2014. doi: 10.1093/cid/ciz223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cheng CH, Tsau YK, Lin TY. Effective duration of antimicrobial therapy for the treatment of acute lobar nephronia. Pediatrics. 2006;117:e84–e89. doi: 10.1542/peds.2005-0917. [DOI] [PubMed] [Google Scholar]
- 116.Chastre J, Wolff M, Fagon JY, Chevret S, Thomas F, Wermert D, Clementi E, Gonzalez J, Jusserand D, Asfar P, Perrin D, Fieux F, Aubas S. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. JAMA. 2003;290:2588–2598. doi: 10.1001/jama.290.19.2588. [DOI] [PubMed] [Google Scholar]
- 117.Martin-Loeches I, Antonelli M, Cuenca-Estrella M, Dimopoulos G, Einav S, De Waele JJ, Garnacho-Montero J, Kanj SS, Machado FR, Montravers P, Sakr Y, Sanguinetti M, Timsit JF, Bassetti M. ESICM/ESCMID task force on practical management of invasive candidiasis in critically ill patients. Intensive Care Med. 2019;45:789–805. doi: 10.1007/s00134-019-05599-w. [DOI] [PubMed] [Google Scholar]
- 118.Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F, Dulgheru R, El Khoury G, Erba PA, Iung B, Miro JM, Mulder BJ, Plonska-Gosciniak E, Price S, Roos-Hesselink J, Snygg-Martin U, Thuny F, Tornos Mas P, Vilacosta I, Zamorano JL. 2015 ESC Guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM) Eur Heart J. 2015;36:3075–3128. doi: 10.1093/eurheartj/ehv319. [DOI] [PubMed] [Google Scholar]
- 119.Bouadma L, Luyt CE, Tubach F, Cracco C, Alvarez A, Schwebel C, Schortgen F, Lasocki S, Veber B, Dehoux M, Bernard M, Pasquet B, Regnier B, Brun-Buisson C, Chastre J, Wolff M. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet. 2010;375:463–474. doi: 10.1016/S0140-6736(09)61879-1. [DOI] [PubMed] [Google Scholar]
- 120.Pepper DJ, Sun J, Rhee C, Welsh J, Powers JH, 3rd, Danner RL, Kadri SS. Procalcitonin-guided antibiotic discontinuation and mortality in critically ill adults: a systematic review and meta-analysis. Chest. 2019;155:1109–1118. doi: 10.1016/j.chest.2018.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Meier MA, Branche A, Neeser OL, Wirz Y, Haubitz S, Bouadma L, Wolff M, Luyt CE, Chastre J, Tubach F, Christ-Crain M, Corti C, Jensen JS, Deliberato RO, Kristoffersen KB, Damas P, Nobre V, Oliveira CF, Shehabi Y, Stolz D, Tamm M, Mueller B, Schuetz P. Procalcitonin-guided antibiotic treatment in patients with positive blood cultures: a patient-level meta-analysis of randomized trials. Clin Infect Dis. 2018;69:388–396. doi: 10.1093/cid/ciy917. [DOI] [PubMed] [Google Scholar]
- 122.Hranjec T, Rosenberger LH, Swenson B, Metzger R, Flohr TR, Politano AD, Riccio LM, Popovsky KA, Sawyer RG. Aggressive versus conservative initiation of antimicrobial treatment in critically ill surgical patients with suspected intensive-care-unit-acquired infection: a quasi-experimental, before and after observational cohort study. Lancet Infect Dis. 2012;12:774–780. doi: 10.1016/S1473-3099(12)70151-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Minet C, Potton L, Bonadona A, Hamidfar-Roy R, Somohano CA, Lugosi M, Cartier JC, Ferretti G, Schwebel C, Timsit JF. Venous thromboembolism in the ICU: main characteristics, diagnosis and thromboprophylaxis. Crit Care. 2015;19:287. doi: 10.1186/s13054-015-1003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Brown RM, Wang L, Coston TD, Krishnan NI, Casey JD, Wanderer JP, Ehrenfeld JM, Byrne DW, Stollings JL, Siew ED, Bernard GR, Self WH, Rice TW, Semler MW. Balanced crystalloids versus saline in sepsis. A secondary analysis of the SMART clinical trial. Am J Respir Crit Care Med. 2019;200:1487–1495. doi: 10.1164/rccm.201903-0557OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dellinger RP, Bagshaw SM, Antonelli M, Foster DM, Klein DJ, Marshall JC, Palevsky PM, Weisberg LS, Schorr CA, Trzeciak S, Walker PM. Effect of targeted polymyxin b hemoperfusion on 28-day mortality in patients with septic shock and elevated endotoxin level: the EUPHRATES randomized clinical trial. JAMA. 2018;320:1455–1463. doi: 10.1001/jama.2018.14618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Annane D, Renault A, Brun-Buisson C, Megarbane B, Quenot JP, Siami S, Cariou A, Forceville X, Schwebel C, Martin C, Timsit JF, Misset B, Ali Benali M, Colin G, Souweine B, Asehnoune K, Mercier E, Chimot L, Charpentier C, Francois B, Boulain T, Petitpas F, Constantin JM, Dhonneur G, Baudin F, Combes A, Bohe J, Loriferne JF, Amathieu R, Cook F, Slama M, Leroy O, Capellier G, Dargent A, Hissem T, Maxime V, Bellissant E. Hydrocortisone plus fludrocortisone for adults with septic shock. N Engl J Med. 2018;378:809–818. doi: 10.1056/NEJMoa1705716. [DOI] [PubMed] [Google Scholar]
- 127.Peake SL, Delaney A, Bailey M, Bellomo R, Cameron PA, Cooper DJ, Higgins AM, Holdgate A, Howe BD, Webb SA, Williams P. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371:1496–1506. doi: 10.1056/NEJMoa1404380. [DOI] [PubMed] [Google Scholar]
- 128.Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, Pike F, Terndrup T, Wang HE, Hou PC, LoVecchio F, Filbin MR, Shapiro NI, Angus DC. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370:1683–1693. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Weiner LM, Webb AK, Limbago B, Dudeck MA, Patel J, Kallen AJ, Edwards JR, Sievert DM. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the national healthcare safety network at the centers for disease control and prevention, 2011–2014. Infect Control Hosp Epidemiol. 2016;37:1288–1301. doi: 10.1017/ice.2016.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Weiner-Lastinger LM, Abner S, Edwards JR, Kallen AJ, Karlsson M, Magill SS, Pollock D, See I, Soe MM, Walters MS, Dudeck MA. Antimicrobial-resistant pathogens associated with adult healthcare-associated infections: summary of data reported to the National Healthcare Safety Network, 2015–2017. Infect Control Hosp Epidemiol. 2019;41:1–18. doi: 10.1017/ice.2019.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rosenthal VD, Al-Abdely HM, El-Kholy AA, AlKhawaja SAA, Leblebicioglu H, Mehta Y, Rai V, Hung NV, Kanj SS, Salama MF, Salgado-Yepez E, Elahi N, Morfin Otero R, Apisarnthanarak A, De Carvalho BM, Ider BE, Fisher D, Buenaflor M, Petrov MM, Quesada-Mora AM, Zand F, Gurskis V, Anguseva T, Ikram A, Aguilar de Moros D, Duszynska W, Mejia N, Horhat FG, Belskiy V, Mioljevic V, Di Silvestre G, Furova K, Ramos-Ortiz GY, Gamar Elanbya MO, Satari HI, Gupta U, Dendane T, Raka L, Guanche-Garcell H, Hu B, Padgett D, Jayatilleke K, Ben Jaballah N, Apostolopoulou E, Prudencio Leon WE, Sepulveda-Chavez A, Telechea HM, Trotter A, Alvarez-Moreno C, Kushner-Davalos L. International Nosocomial Infection Control Consortium report, data summary of 50 countries for 2010–2015: device-associated module. Am J Infect Control. 2016;44:1495–1504. doi: 10.1016/j.ajic.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 132.Hu FP, Guo Y, Zhu DM, Wang F, Jiang XF, Xu YC, Zhang XJ, Zhang CX, Ji P, Xie Y, Kang M, Wang CQ, Wang AM, Xu YH, Shen JL, Sun ZY, Chen ZJ, Ni YX, Sun JY, Chu YZ, Tian SF, Hu ZD, Li J, Yu YS, Lin J, Shan B, Du Y, Han Y, Guo S, Wei LH, Wu L, Zhang H, Kong J, Hu YJ, Ai XM, Zhuo C, Su DH, Yang Q, Jia B, Huang W. Resistance trends among clinical isolates in China reported from CHINET surveillance of bacterial resistance, 2005–2014. Clin Microbiol Infect. 2016;22(Suppl 1):S9–14. doi: 10.1016/j.cmi.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 133.Hsu LY, Apisarnthanarak A, Khan E, Suwantarat N, Ghafur A, Tambyah PA. Carbapenem-resistant Acinetobacter baumannii and Enterobacteriaceae in South and Southeast Asia. Clin Microbiol Rev. 2017;30:1–22. doi: 10.1128/CMR.00042-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bonell A, Azarrafiy R, Huong VTL, Viet TL, Phu VD, Dat VQ, Wertheim H, van Doorn HR, Lewycka S, Nadjm B. A systematic review and meta-analysis of ventilator-associated pneumonia in adults in Asia: an analysis of national income level on incidence and etiology. Clin Infect Dis. 2019;68:511–518. doi: 10.1093/cid/ciy543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Carrara L, Navarro F, Turbau M, Seres M, Moran I, Quintana I, Martino R, Gonzalez Y, Brell A, Cordon O, Diestra K, Mata C, Mirelis B, Coll P. Molecular diagnosis of bloodstream infections with a new dual-priming oligonucleotide-based multiplex PCR assay. J Med Microbiol. 2013;62:1673–1679. doi: 10.1099/jmm.0.064758-0. [DOI] [PubMed] [Google Scholar]
- 136.Zboromyrska Y, Cilloniz C, Cobos-Trigueros N, Almela M, Hurtado JC, Vergara A, Mata C, Soriano A, Mensa J, Marco F, Vila J. Evaluation of the magicplex sepsis real-time test for the rapid diagnosis of bloodstream infections in adults. Front Cell Infect Microbiol. 2019;9:56. doi: 10.3389/fcimb.2019.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ziegler I, Fagerstrom A, Stralin K, Molling P. Evaluation of a commercial multiplex PCR assay for detection of pathogen DNA in blood from patients with suspected sepsis. PLoS One. 2016;11:e0167883. doi: 10.1371/journal.pone.0167883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Altun O, Almuhayawi M, Ullberg M, Ozenci V. Clinical evaluation of the FilmArray blood culture identification panel in identification of bacteria and yeasts from positive blood culture bottles. J Clin Microbiol. 2013;51:4130–4136. doi: 10.1128/JCM.01835-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bhatti MM, Boonlayangoor S, Beavis KG, Tesic V. Evaluation of FilmArray and Verigene systems for rapid identification of positive blood cultures. J Clin Microbiol. 2014;52:3433–3436. doi: 10.1128/JCM.01417-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kim JS, Kang GE, Kim HS, Song W, Lee KM. Evaluation of verigene blood culture test systems for rapid identification of positive blood cultures. Biomed Res Int. 2016;2016:1081536. doi: 10.1155/2016/1081536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lutgring JD, Bittencourt C, McElvania TeKippe E, Cavuoti D, Hollaway R, Burd EM. Evaluation of the accelerate pheno system: results from two academic medical centers. J Clin Microbiol. 2018;56:e01672. doi: 10.1128/JCM.01672-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Charnot-Katsikas A, Tesic V, Love N, Hill B, Bethel C, Boonlayangoor S, Beavis KG. Use of the accelerate pheno system for identification and antimicrobial susceptibility testing of pathogens in positive blood cultures and impact on time to results and workflow. J Clin Microbiol. 2018;56:e01166. doi: 10.1128/JCM.01166-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kadri SS, Adjemian J, Lai YL, Spaulding AB, Ricotta E, Prevots DR, Palmore TN, Rhee C, Klompas M, Dekker JP, Powers JH, 3rd, Suffredini AF, Hooper DC, Fridkin S, Danner RL, National Institutes of Health Antimicrobial Resistance Outcomes Research I Difficult-to-treat resistance in Gram-negative bacteremia at 173 US hospitals: retrospective cohort analysis of prevalence, predictors, and outcome of resistance to all first-line agents. Clin Infect Dis. 2018;67:1803–1814. doi: 10.1093/cid/ciy378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dijkmans AC, Zacarias NVO, Burggraaf J, Mouton JW, Wilms EB, van Nieuwkoop C, Touw DJ, Stevens J, Kamerling IMC. Fosfomycin: pharmacological, clinical and future perspectives. Antibiotics (Basel) 2017;6:24. doi: 10.3390/antibiotics6040024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Harris PNA, Tambyah PA, Lye DC, Mo Y, Lee TH, Yilmaz M, Alenazi TH, Arabi Y, Falcone M, Bassetti M, Righi E, Rogers BA, Kanj S, Bhally H, Iredell J, Mendelson M, Boyles TH, Looke D, Miyakis S, Walls G, Al Khamis M, Zikri A, Crowe A, Ingram P, Daneman N, Griffin P, Athan E, Lorenc P, Baker P, Roberts L, Beatson SA, Peleg AY, Harris-Brown T, Paterson DL, Investigators MT, the Australasian Society for Infectious Disease Clinical Research N Effect of piperacillin-tazobactam vs meropenem on 30-day mortality for patients with E coli or Klebsiella pneumoniae bloodstream infection and ceftriaxone resistance: a randomized clinical trial. JAMA. 2018;320:984–994. doi: 10.1001/jama.2018.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Livermore DM, Mushtaq S, Warner M, Zhang JC, Maharjan S, Doumith M, Woodford N. Activity of aminoglycosides, including ACHN-490, against carbapenem-resistant Enterobacteriaceae isolates. J Antimicrob Chemother. 2011;66:48–53. doi: 10.1093/jac/dkq408. [DOI] [PubMed] [Google Scholar]