Abstract
Microaspiration of bacteriologically contaminated oropharyngeal secretions alongside the cuff of an endotracheal tube (ETT) is a key mechanism for development of ventilator-associated pneumonia. We have constructed a prototype double-cuffed ETT equipped with a supplemental port in-between the cuffs through which continuous positive airway pressure (CPAP) is delivered. Pressure in the intercuff space propels secretions upwards and produces 100% tracheal sealing in an in vitro model. We conducted a 24 h study to investigate the sealing effect of this ETT in 12 critically ill mechanically ventilated patients. Methylene blue, instilled through a bronchoscope on top of the proximal cuff, was used as leakage tracer. Fiberoptic visualisation of the trachea was performed 1 h and 24 h thereafter. Leakage was confirmed if blue dye was detected on the tracheal mucosa beyond the tip of the ETT. In no patient, dye passed by the cuffs during the study period. Presence of the ETT did not interfere with ventilator settings, patient mobilization, physiotherapy, and technical acts. Overall, pressures in the intercuff space remained between 10 and 15 cmH2O. Excessive pressure swings were swiftly corrected by the CPAP system. A double-cuffed ETT, offering “pressurized sealing” of the trachea, safely and effectively prevented leakage during 24 h mechanical ventilation.
Keywords: Endotracheal tube, Cuff, Double-cuffed, Mechanical ventilation, Ventilator-associated pneumonia, Microaspiration, Leakage, Critically ill
Introduction
Leakage of contaminated subglottic secretions alongside the cuff of an endotracheal tube (ETT) is the main path taken by pathogenic micro-organisms to invade the lungs of mechanically ventilated patients. Endobronchial bacterial colonization may subsequently evolve into ventilator-associated tracheobronchitis or ventilator-associated pneumonia (VAP) [1]. Cuff pressure control, semi-recumbent patient positioning, oral hygiene, and continuous application of positive end-expiratory pressure (PEEP) have already proven beneficial as VAP prevention measures [2]. However, designing a “leakage-free” ETT still remains an important challenge [3].
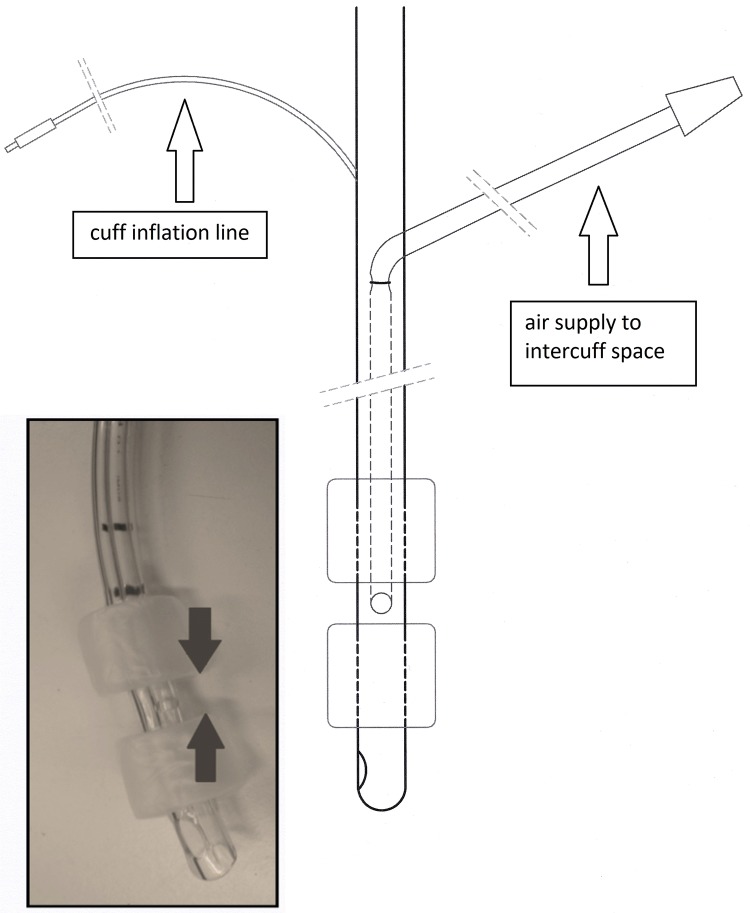
We have developed a prototype ETT (UK patent 1015078.7 and US patent 61/381,792) which prevents secretion inflow based upon a concept of "pressurized tracheal sealing". Briefly, this novel ETT is double-cuffed and equipped with a supplemental port in the space between the cuffs (Fig. 1). ETT and cuffs are made of polyvinylchloride. Both cuffs are cylindrical and have a high volume–low pressure design. A positive pressure, created and regulated by an external source, is continuously presented to the intercuff space (ICS) and propels secretions upwards towards the oropharynx. We previously demonstrated superiority of this ETT above currently commercialized ETTs in preventing leakage of secretions in a vertical syringe model and in an artificial trachea [4]. The current study intends to investigate this novel “pressurized sealing” concept in a cohort of mechanically ventilated critically ill patients.
Fig. 1.
Schematic representation of the prototype endotracheal tube. The inflated cuffs delineate an intercuff chamber or space (arrows). Total length from tip to proximal cuff is 70 mm
Materials and methods
We conducted an interventional pilot study in the intensive care unit (ICU) of the University Hospital Brussels. The study was registered at https://www.trialregister.nl (NTR 4428). The study protocol was approved by the Institutional Review Board of the University Hospital (file no. BUN 143201318818) and performed in accordance with the Declaration of Helsinki. Informed consent was obtained from the patient’s next of kin.
12 patients were consecutively enrolled, electively intubated, and continued on pressure-controlled or -assisted (continuous positive airway pressure (CPAP) plus pressure support (PS) ventilation. Correct placement of the ETT was confirmed and repeatedly checked on chest X-ray. Cuff pressures were permanently kept at 25 cmH2O by an automated digital cuff pressure manometer (VBN cuff controller, VBM Medizintechnik GmbH, Sulz am Neckar, Germany). An external device (Respironics REMstar, Phillips, Herrsching, Germany) delivered CPAP to the ICS via the port in-between cuffs. The flow generated by the CPAP source was continuously measured (Mass Flow Meter 4040, TSI, Minnesota, USA). Ventilator settings were not changed during the study. At zero flow, ICS pressure equalled the working pressure (16 cmH2O) of the external CPAP device. An increase in flow was associated with lower ICS pressure due to resistance at the entrance of the flow conduit into the tube. When ICS pressure exceeded working pressure, air flow reversed and left the circuit through the CPAP device. Overall, ICS pressures were maintained between 10 and 15 cmH2O.
Following thorough aspiration of upper and lower airways, 2 mL methylene blue diluted in normal saline was instilled through a bronchoscope above the vocal cords. Bronchoscopy was again performed 1 and 24 h after instillation. Leakage was confirmed if, on any occasion, blue dye stained the tracheal mucosa caudal to the tip of the ETT. All patients were in semi-recumbent position with the head of the bed elevated to 30°, except when being turned, washed, or undergoing bronchoscopy. Closed tracheal aspiration was performed whenever required. Oral mouth wash was administered two times daily. Recruitment manoeuvres (either by increasing PEEP or augmenting tidal volume) were not routinely performed but randomly assessed in all patients on controlled ventilation. In- and expiratory tidal volume, airway pressures, and effect of common manipulations (tracheal aspiration, recruitment manoeuvres, physiotherapy, catheter placement, …) were recorded. At the end of the study, patients were continued with the investigational tube but without “pressure sealing” and followed until extubation.
Results
Throughout the study, seven patients remained ventilated in pressure control mode and five subjects received CPAP/PS. No ETT displacement or disconnection was observed. In no patient, blue dye was seen beyond the ETT tip. Moreover, no dye leakage along the proximal cuff was observed.
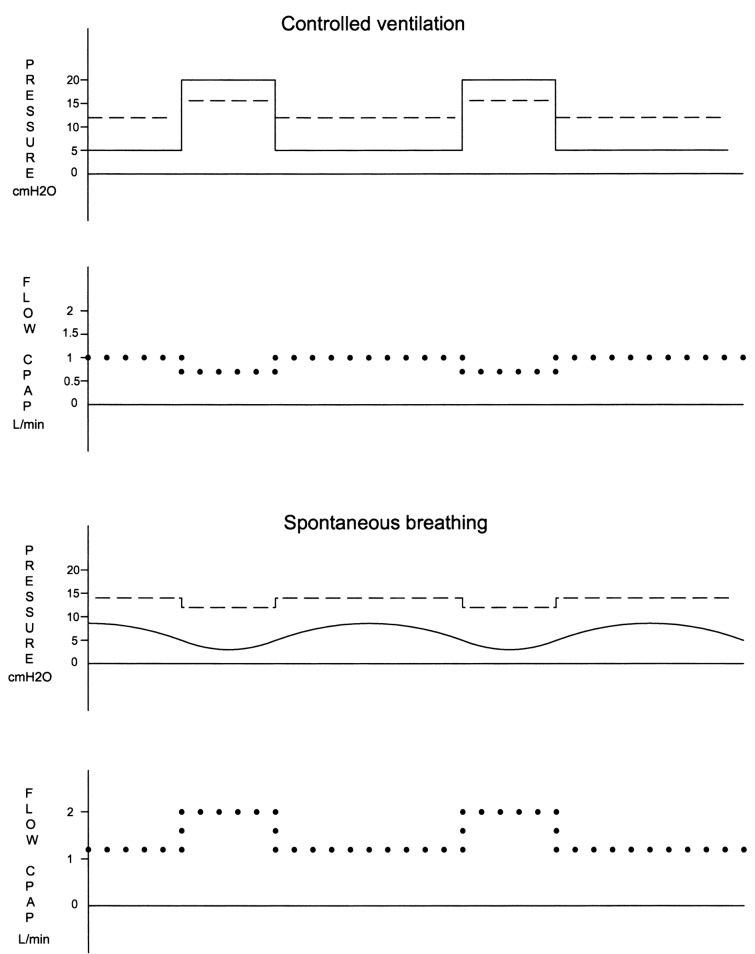
During controlled ventilation, ICS pressure was supported by PEEP during expiration and by the peak inspiratory pressure (PIP) during inspiration. The lowest ICS pressure was recorded at end-expiration. PIPs above 20 cmH2O increased ICS pressure above the working pressure of the CPAP device. During spontaneous breathing, inspiration created a slightly negative pressure below the tube which sucked air from the ICS. The resulting fall in ICS pressure was immediately compensated by an increased flow from the CPAP device. Expiration increased pressure under the tube tip and subsequently in the ICS. Flow from the CPAP device decreased accordingly.
PS levels up to 5 cmH2O were associated with a negative pressure under the tube at inspiration which attracted air from the ICS even in the presence of high PEEP levels. A PS above 5 cmH2O created sufficient pressure below the tube with lowest ICS pressure values recorded at end-expiration. Interaction between airway pressure, ICS pressure, and CPAP flow is depicted in Fig. 2.
Fig. 2.
Schematic representation of evolution and interaction of airway pressure (continuous line), intercuff space (ICS) pressure (straight dotted line) and flow from the CPAP device (spherical dotted line) during controlled and spontaneous ventilation. During controlled ventilation, ICS pressure rises with peak inspiratory pressure and CPAP flow decreases. During (PEEP-supported) expiration, ICS pressure decreases and CPAP flow increases accordingly. During spontaneous breathing (sustained by a pressure support up to 5 cmH2O), inspiration draws air from the ICS. The resulting decrease in ICS pressure is promptly restored by an increase in CPAP flow. Expiration produces the opposite effect. At pressure support levels above 5 cmH2O, pressures and CPAP flow follow the same pattern as in controlled ventilation
In controlled ventilation mode, pressure under the tube became lower than ICS pressure during expiration. This caused more air flow to the lungs as reflected by a slightly higher expiratory tidal volume. During spontaneous and assisted ventilation, ICS pressure exceeded airway pressure. Air moved during inspiration from the ICS to the lungs. This provided support for spontaneous breathing but was not added to the inspiratory tidal volume. All documented changes in pressure and flow had no impact on ventilator settings. Tracheal aspiration and recruitment raised ICS pressure but this was immediately compensated by a reduction or reversal of airflow. Catheter management, patient positioning, and physiotherapy did not influence ICS. No adverse or unwarranted effects related to the ETT were noticed.
Discussion
The present study adds clinical proof to previous experimental findings demonstrating a 100% sealing effect of this prototype ETT. During a 24 h-period, no leakage of dye was observed past the cuffs in patients receiving controlled or assisted ventilation. Patients were easily intubated and the tube remained well in place during patient mobilization, nursing manoeuvres, physiotherapy, and technical acts. The pressurized sealing effect did not significantly interfere with airway pressures and only slightly increased tidal volume. Pressure swings in the ICS were rapidly anticipated and corrected by the CPAP system.
The presence of an ETT increases susceptibility to VAP because bacteriologically contaminated secretions leak around an inflated ETT cuff [5] and/or biofilms infested with bacteria are formed inside the ETT [6]. In addition, patient disconnection from the ventilator, either accidentally or preparing extubation, or cuff deflation may cause inflow of secretions. Measures to avoid leakage and aspiration include the use of specific cuff materials (e.g., polyurethane), cuff shapes (e.g., conical), continuous automatic maintenance of cuff pressure, and ETTs equipped with a suction lumen for aspiration of subglottic secretions [7]. Silver-coated ETTs may limit bacterial colonization and biofilm formation in the lumen of the ETT [8]. Comparative trials assessing these specifically designed ETTs have shown a decreased aspiration risk, a lower VAP incidence, a decreased ventilation time and a delay in VAP onset [9-11] but no effect on duration of mechanical ventilation, ICU stay, or hospital stay [12, 13]. A main issue is that, regardless of the type of inflow prevention incorporated in an ETT, some leakage still occurs along the cuff folds [14]. Moreover, important safety concerns remain. Subglottic suctioning may cause pharyngeal and laryngeal injury [15] and condensation in polyurethane-cuffed ETTs leads to erratic manometric cuff pressure readings [16].
Double-cuffed tubes are sporadically used to prevent tracheal mucosal pressure lesions by alternating cuff inflation during prolonged intubation [17], to enhance airway protection by occupying the spaces above and below an open larynx (“anchoring” effect), or to allow specific endobronchial treatment (e.g., laser therapy) [18]. Hwang et al. designed a double-cuffed tracheal tube similar to ours but filled the intercuff space with a water-soluble gel. Applying this interrupting gel layer prevented fluid leakage for 48 h in a bench-top model [19]. This ETT was never tested in vivo because prolonged exposure to the gel components might harm the tracheal mucosa. In a later study, the same investigators connected a pressure system to the intercuff port. Application of appropriate negative suction pressures in the intercuff space completely inhibited saline leakage past the cuffs [20]. However, cuff length of this ETT prototype was too long to allow safe clinical use.
Our study has some important limitations. First, normal saline mixed with methylene blue was used as surrogate for subglottic accumulation of secretions. This allowed adequate visualisation but findings might have differed if liquids with heterogeneous visco-elastic properties or higher viscosity were used. Second, occurrence of VAP is tightly correlated with bacterial load. Methylene blue as marker of leakage does not allow quantitative evaluation of microaspiration. Other markers (e.g., pepsin or amylase) present in tracheal secretions can overcome this limitation in a clinical setting [21]. Third, the sealing efficacy of the ETT was studied for only 24 h. It remains to be proven whether complete sealing persists when patients remain longer ventilated.
In conclusion, a prototype double-cuffed ETT, offering “pressurized sealing” of the trachea, safely and effectively prevented leakage during 24 h mechanical ventilation. The current findings justify further evaluation of this novel ETT in patients ventilated for longer time periods with occurrence of VAP, safety, and cost-efficacy as primary endpoints.
Acknowledgements
The authors are indebted to Sumi Company Poland for manufacturing the endotracheal tube and to Michel Timmermans and Michel Vervoort for technical support.
Author contributions
HS and ES designed the study. JDR, JT, JJ and EDW enrolled patients and assisted in technical acts related to the study protocol. ES collected data. All authors participated in interpretation of data, literature review, and writing of the manuscript. All authors read and approved the final version of the manuscript.
Funding
The study received a research grant from the “Wetenschappelijk Fonds Willy Gepts” of the University Hospital, Vrije Universiteit Brussels.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zolfaghari PS, Wyncoll DLA. The tracheal tube gateway to ventilator-associated pneumonia. Crit Care. 2011;15:310. doi: 10.1186/cc10352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blot SI, Poelaert J, Kollef M. How to avoid microaspiration? A key element for the prevention of ventilator-associated pneumonia in intubated ICU patients. BMC Infect Dis. 2014;14:119. doi: 10.1186/1471-2334-14-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haas CF, Eakin RM, Konkle MA, Blank R. Endotracheal tubes: old and new. Respir Care. 2014;59:933–952. doi: 10.4187/respcare.02868. [DOI] [PubMed] [Google Scholar]
- 4.Spapen HD, Suys E, Diltoer M, Stiers W, Desmet G, Honoré PM. A newly developed tracheal tube offering 'pressurised sealing' outperforms currently available tubes in preventing cuff leakage: a benchtop study. Eur J Anaesthesiol. 2017;34:411–416. doi: 10.1097/EJA.0000000000000493. [DOI] [PubMed] [Google Scholar]
- 5.Pneumatikos IA, Dragoumanis CK, Bouros DE. Ventilator-associated pneumonia or endotracheal tube-associated pneumonia? An approach to the pathogenesis and preventive strategies emphasizing the importance of endotracheal tube. Anesthesiology. 2009;110:673–680. doi: 10.1097/ALN.0b013e31819868e0. [DOI] [PubMed] [Google Scholar]
- 6.Adair CG, Gorman SP, Feron BM, Byers LM, Jones DS, Goldsmith CE, Moore JE, Kerr JR, Curran MD, Hogg G, Webb CH, McCarthy GJ, Milligan KR. Implications of endotracheal tube biofilm for ventilator-associated pneumonia. Intensive Care Med. 1999;25:1072–1076. doi: 10.1007/s001340051014. [DOI] [PubMed] [Google Scholar]
- 7.Rouzé A, Jaillette E, Poissy J, Préau S, Nseir S. Tracheal tube design and ventilator-associated pneumonia. Respir Care. 2017;62:1316–1323. doi: 10.4187/respcare.05492. [DOI] [PubMed] [Google Scholar]
- 8.Li X, Yuan Q, Wang L, Du L, Deng L. Silver-coated endotracheal tube versus non-coated endotracheal tube for preventing ventilator-associated pneumonia among adults: a systematic review of randomized controlled trials. J Evid Based Med. 2012;5:25–30. doi: 10.1111/j.1756-5391.2012.01165.x. [DOI] [PubMed] [Google Scholar]
- 9.Tokmaji G, Vermeulen H, Müller MC, Kwakman PH, Schultz MJ, Zaat SA. Silver-coated endotracheal tubes for prevention of ventilator-associated pneumonia in critically ill patients. Cochrane Database Syst Rev. 2015;8:CD009201. [DOI] [PMC free article] [PubMed]
- 10.Muscedere J, Rewa O, McKechnie K, Jiang X, Laporta D, Heyland DK. Subglottic secretion drainage for the prevention of ventilator-associated pneumonia: a systematic review and meta-analysis. Crit Care Med. 2011;39:1985–1991. doi: 10.1097/CCM.0b013e318218a4d9. [DOI] [PubMed] [Google Scholar]
- 11.Maertens B, Blot K, Blot S. Prevention of ventilator-associated and early postoperative pneumonia through tapered endotracheal tube cuffs: a systematic review and meta-analysis of randomized controlled trials. Crit Care Med. 2018;46:316–323. doi: 10.1097/CCM.0000000000002889. [DOI] [PubMed] [Google Scholar]
- 12.Caroff DA, Li L, Muscedere J, Klompas M. Subglottic secretion drainage and objective outcomes: a systematic review and meta-analysis. Crit Care Med. 2016;44:830–840. doi: 10.1097/CCM.0000000000001414. [DOI] [PubMed] [Google Scholar]
- 13.Mao Z, Gao L, Wang G, Liu C, Zhao Y, Gu W, Kang H, Zhou F. Subglottic secretion suction for preventing ventilator-associated pneumonia: an updated meta-analysis and trial sequential analysis. Crit Care. 2016;20:353. doi: 10.1186/s13054-016-1527-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lucangelo U, Zin WA, Antonaglia V, Petrucci L, Viviani M, Buscema G, Borelli M, Berlot G. Effect of positive expiratory pressure and type of tracheal cuff on the incidence of aspiration in mechanically ventilated patients in an intensive care unit. Crit Care Med. 2008;36:409–413. doi: 10.1097/01.CCM.0000297888.82492.31. [DOI] [PubMed] [Google Scholar]
- 15.Suys E, Nieboer K, Stiers W, De Regt J, Huyghens L, Spapen H. Intermittent subglottic secretion drainage may cause tracheal damage in patients with few oropharyngeal secretions. Intensive Crit Care Nurs. 2013;29:317–320. doi: 10.1016/j.iccn.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Spapen H, Moeyersons W, Stiers W, Desmet G, Suys E. Condensation of humidified air in the inflation line of a polyurethane cuff precludes correct continuous pressure monitoring during mechanical ventilation. J Anesth. 2014;28:949–951. doi: 10.1007/s00540-014-1849-z. [DOI] [PubMed] [Google Scholar]
- 17.Salpekar PD. Double-cuff endotracheal tube. Br Med J. 1971;3(5773):525. doi: 10.1136/bmj.3.5773.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fried MP, Mallampati SR, Liu FC, Kaplan S, Caminear DS, Samonte BR. Laser resistant stainless steel endotracheal tube: experimental and clinical evaluation. Lasers Surg Med. 1991;11:301–306. doi: 10.1002/lsm.1900110315. [DOI] [PubMed] [Google Scholar]
- 19.Hwang JY, Han SH, Park SH, Park SJ, Park S, Oh SH, Kim JH. Interrupting gel layer between double cuffs prevents fluid leakage past tracheal tube cuffs. Br J Anaesth. 2013;111:496–504. doi: 10.1093/bja/aet152. [DOI] [PubMed] [Google Scholar]
- 20.Sohn HM, Baik JS, Hwang JY, Kim SY, Han SH, Kim JH. Devising negative pressure within intercuff space reduces microaspiration. BMC Anesthesiol. 2018;18:181. doi: 10.1186/s12871-018-0643-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaillette Emmanuelle, Girault Christophe, Brunin Guillaume, Zerimech Farid, Behal Hélène, Chiche Arnaud, Broucqsault-Dedrie Céline, Fayolle Cyril, Minacori Franck, Alves Isabelle, Barrailler Stéphanie, Labreuche Julien, Robriquet Laurent, Tamion Fabienne, Delaporte Emmanuel, Thellier Damien, Delcourte Claire, Duhamel Alain, Nseir Saad. Impact of tapered-cuff tracheal tube on microaspiration of gastric contents in intubated critically ill patients: a multicenter cluster-randomized cross-over controlled trial. Intensive Care Medicine. 2017;43(11):1562–1571. doi: 10.1007/s00134-017-4736-x. [DOI] [PubMed] [Google Scholar]