Abstract
Immunoinformatics is a science that helps to create significant immunological information using bioinformatics softwares and applications. One of the most important applications of immunoinformatics is the prediction of a variety of specific epitopes for B cell recognition and T cell through MHC class I and II molecules. This method reduces costs and time compared to laboratory tests. In this state-of-the-art review, we review about 50 papers to find the latest and most used immunoinformatic tools as well as their applications for predicting the viral, bacterial and tumoral structural and linear epitopes of B and T cells. In the clinic, the main application of prediction of epitopes is for designing peptide-based vaccines. Peptide-based vaccines are a considerably potential alternative to low-cost vaccines that may reduce the risks related to the production of common vaccines.
Keywords: Epitope prediction, Immunoinformatics approach, Epitopes, Antigenic peptide, Vaccine design, Peptide-based vaccines
Introduction
The immune system consists of a complex network of thousands of molecules that are interconnected. Application of high-throughput techniques can provide much invaluable information about the immune system and immune component interactions. Therefore, there is a need for a computational approach to store and analyze this information. Immunology has recently been influenced by resources and software providing insight into the features of the immune system and developed a new approach called immunoinformatics (Brusic and Petrovsky 2005; Gardy et al. 2009; Tomar and De 2010).
Immunoinformatics includes studies and design of algorithms for potential mapping of B cell and T-cell epitopes. With the help of this knowledge, antigenic regions of the target proteins can be detected (Davies and Flower 2007). The research conducted on the basis of in silico immunoinformatics methods demonstrating the high potential of this approach in reducing the time and cost of immunogenicity studies and the reliable results of these methods in the past decade has attracted researchers to this approach. Antigenic regions called epitopes are regions of antigens that are identified by the immune system cells and have the immunogenic property for the immune cells (Chen et al. 2012).
B and T cells are the main components of the acquired immune system that bind to the antigen epitopes. In order to the T cells identify the antigens, antigenic peptides should be attached to clefts of MHC (major histocompatibility complex) molecules, also known as HLA (human leukocyte antigen) in humans. MHC molecules expressed on antigen presenting cells (APCs) represent epitope peptides to T cells. These peptide epitopes should be linear that can attach to the cleft of MHCs. T cells are phenotypically classified into CD8+ and CD4+ lymphocytes. (Martin et al. 2006) Activated CD8+ cells are called cytotoxic T cells (CTLs) bound to peptides presented by class I MHC molecules on APCs and are specific to intracellular antigens. Activated CD4+ cells are called T helper (TH) cells bound to peptides presented by class II MHC molecules and are specific to extracellular antigens (Raoufi et al. 2015; Moise and De Groot 2006). HLA system coded with 21 genes on chromosome 6 is highly polymorphic. HLA Class I has three HLA-A, HLA-B, and HLA-C loci, which detect peptide sequences of 8–12 amino acids long. HLA Class II contains HLA-DR, HLA-DP and HLA-DQ, and is bound to larger sequences of 15–24 amino acids. Identifying different alleles of HLAs is very important for predicting T cell epitopes (Sette et al. 1990).
Epitopes for B cells can be discontinuous (conformational) and/or continuous (linear). In linear epitopes, the amino acid sequence of the protein is used as an epitope and there are various criteria to choose an optimal epitope. The three-dimensional antigen structure plays an important role, and for predicting these epitopes, their three-dimensional structure should be used. Therefore, the peptide sequences of these epitopes can be adjacent to each other in the three-dimensional structure, but in the linear sequence, they are distant from each other (Sharon et al. 2014). Nuclear magnetic resonance (NMR) and X-ray crystallographic methods are two methods for determining the number of epitopes for B cells that are very costly and time-consuming. Today, with the advancement of immunoinformatics tools, it is possible to predict B cell epitopes more accurately. The most antigenic epitopes are not linear sequences of amino acids; they are structures composed of different protein sections in the form of three-dimensional structure. Therefore, their bioinformatics modelling is required for the proper development of antigenic regions in predicting the epitopes of B cells (Potocnakova et al. 2016).
Identification and prediction of antigenic regions for epitopes of T and B cells are the most important applications of immunoinformatics. Immunoinformatics appears to be a useful tool for the identification of new antigenic epitopes that can be used in new vaccine design for various infectious diseases caused by different pathogens including bacteria, viruses, fungi, and parasites. In addition to the microbial vaccine, immunoinformatics can be used for different types of malignancies with the pathogenic origin (i.e. hepatocellular carcinoma, cervical carcinoma, head and neck carcinoma, etc.) and non-pathogen based cancers including lung cancer, breast cancer, prostate cancer colon cancer etc. In this review, the major immunoinformatics software tools used in recent years are reviewed for introducing discontinuous and continuous viral, bacterial and tumor-specific epitopes for B and T cells based on their frequency in applied and valid results.
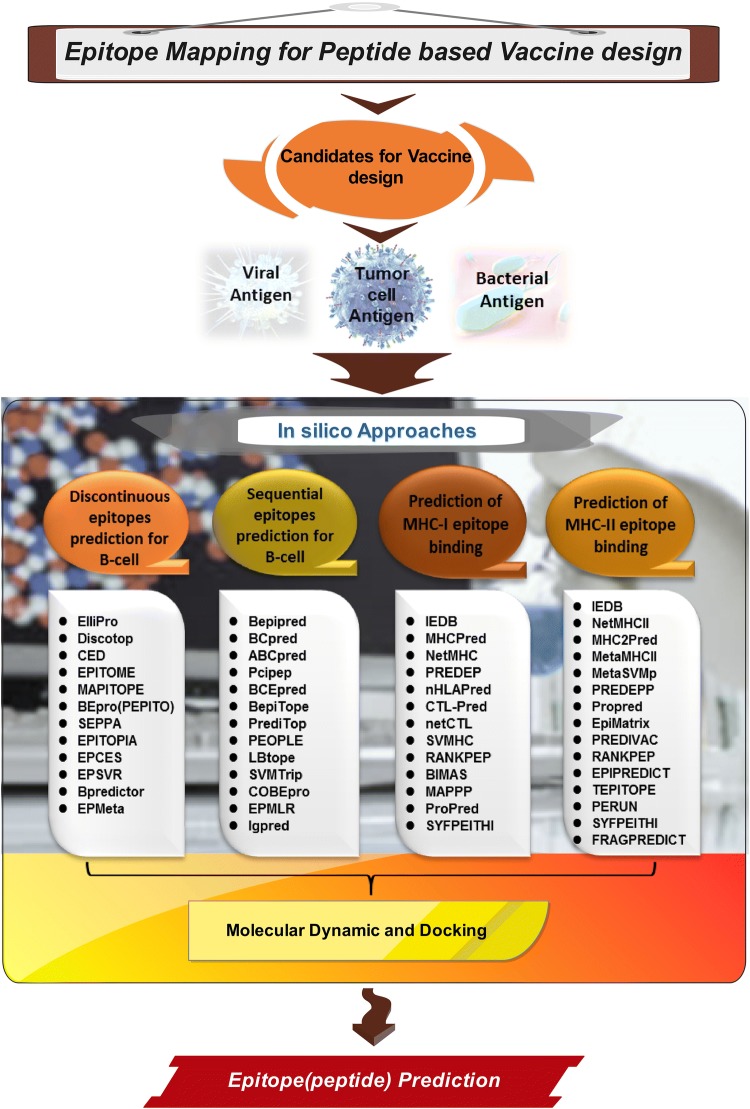
Schematic diagram of the stages of the peptide-based vaccine development, and an overview of the process of epitope mapping for peptide-based vaccine design against three types of viral, bacterial and tumor-specific antigens have been shown in Fig. 1.
Fig. 1.
Schematic diagram of the stages of peptide-based vaccine development using immunoinformatics. At the first stage, candidate antigens are selected. These antigens can be viral, bacterial or tumor-specific antigens. At the next stage (with in silico approach), immunoinformatics software and tools are used to find the epitopes of MHC I, MHC II and B cells. At this stage, in order to evaluate the top-level epitope connection to the favourable binding site, molecular dynamics, molecular docking studies are used. Finally, high binding affinity epitopes for MHCs and B-cells are presented as predicted epitopes for the design of peptide-based vaccines
Methodology
We conducted a state-of-the-art review aimed to study and evaluate the latest software and their applications for epitope mapping used in the recent publisher. Google Scholar and Scopus databases were used for searching. All papers matching the immunogenicity search strategy in silico prediction and epitope prediction approaches and immunoinformatics tools were collected. Among these papers, 50 papers covering the years 2006 through 2018 were used as sources of this review. These papers have been fully studied and categorized. The classification was based on the softwares and applications to predict epitopes of MHC Class 1 and MHC class 2 for T cells as well as linear and conformational B cell epitopes. Also, the classification of the papers and their results based on the nature of the target antigen were divided into three sections: bacterial, viral and tumor-specific antigens.
Finally, the quality of the results of the reviewed papers was evaluated, and the frequency of the application of each software in the specific sections was determined and discussed in separate sections of this study.
Prediction of T Cell Epitopes
Prediction of MHC-I-Binding Epitopes
Various bioinformatics tools are available to predict MHC-I epitopes. Among the papers reviewed, 37 cases have studied these epitopes; and the tools used in these studies are as follows:
IEDB, NetCTL, MHCPred, NetMHC, nHLAPred, CTL-Pred, SVMHC, RANKPEP, BIMAS, MAPPP, ProPred, SYFPEITHI, PREDEP, MHCPEP.
The result of qualitative evaluations and frequency of software application in the papers confirms the frequent application of IEDB, but it is noteworthy that in many studies, first, using one of the tools listed above, epitopes are predicted, and then IEDB tool is used to verify and ensure the result. Of course, IEDB can also be used alone for predictions using Stabilized Matrix Method (SMM) method.
The results of our studies for the frequency of each software in the prediction of MHC-I bound epitopes by the nature of the antigen in three sections of viral, bacterial and tumor-specific antigens have been shown in Tables 1, 2 and 3. As shown in Tables 1, 2 and 3, NetCTL and IEDB softwares were mostly used for the prediction of MHC-I bound viral epitopes, SVMHC software for the prediction of bacterial epitopes bound to the MHC-I and ProPred-I, SVMHC and SYFPEITHI softwares for the prediction of MHC-I bound tumor-specific epitopes.
Table 1.
The assortment of articles for epitope prediction of viral antigen (38 articles out of 50 articles)
| Viral antigen | |||
|---|---|---|---|
| In silico softwares | fi | fi/N × 100 | |
| MHC-I epitope binding prediction (total articles = 28)a |
NetCTL IEDB ProPred-I SYFPEITHI NetMHCpan BIMAS CTLpred RANKPEP nHLAPred MHCpred |
10 10 9 9 5 5 3 2 2 1 |
35.71 35.71 32.14 32.14 17.85 17.85 10.71 7.14 7.14 3.57 |
| MHC-II epitope binding prediction (total articles = 20)b |
IEDB Propred-II NetMHCII SYFPEITHI MHCpred PREDIVAC MetaMHCII MetaSVMP RANKPEP BIMAS |
8 6 4 3 2 1 1 1 1 1 |
40 30 20 15 10 5 5 5 5 5 |
| Discontinuous B-cell epitope prediction (total articles = 6)c |
Discotope ElliPro |
4 4 |
66.6 66.6 |
| Continuous B-cell epitope prediction (total articles = 21)d |
Bepipred BCpred IEDB ABCpred Ellipro LBtope |
16 5 1 1 1 1 |
76.19 23.8 4.76 4.76 4.76 4.76 |
| References (total articles = 38) | Raoufi et al. (2015), Oany et al. (2014, 2015) Zheng et al. (2017), Alam et al. (2016), Mohammed et al. (2017), Dash et al. (2017), Negahdaripour et al. (2018), Saha et al. (2017), Osman et al. (2016), Badawi et al. (2016), Manijeh et al. (2013), Shi et al. (2015a, b), Farhadi et al. (2015), Yang et al. (2015), Sakib et al. (2014), Shehzadi et al. (2012), Verma et al. (2015), Kamthania and Sharma (2015), Huang et al. (2015), Ali and Islam (2015), Khan et al. (2015), Sundar et al. (2007), Wu et al. (2012), Dutta et al. (2015), Chakraborty (2014), Hemmati et al. (2017), Singh et al. (2013), Kumar et al. (2013), Shekhar et al. (2012), Saraswat et al. (2012), Sharmin and Islam (2014), Hasan et al. (2013), Ali et al. (2017) and Mirza et al. (2016) | ||
Their related web tools have been shown in the second column. In the third and fourth column, the frequency and percentage of the regularity of each web tool in articles are shown
aTwenty-eight cases of 50 articles was studied for prediction of MHC class I epitopes
bTwenty cases of 50 articles were studied for prediction of MHC class II epitopes
cSix articles were studied on discontinuous B-cell epitope prediction
dTwenty-one articles were studied continuous B-cell epitope prediction
Table 2.
The assortment of articles for epitope prediction of bacterial antigens (8 articles out of 50 articles)
| Bacterial antigen | |||
|---|---|---|---|
| In silico softwares | fi | fi/N × 100 | |
| MHC-I epitope binding prediction (total articles = 6)a |
SVMHC TepiTope ProPred-I NetMHCpan CTLpred RANKPEP nHLAPred MHCpred |
2 1 1 1 1 1 1 1 |
33.3 16.6 16.6 16.6 16.6 16.6 16.6 16.6 |
| MHC-II epitope binding prediction (total articles = 5)b |
Propred-II NetMHCII MetaMHCII RANKPEP MetaSVMP MHCpred |
4 2 2 2 1 1 |
80 40 40 40 20 20 |
| Discontinuous B-cell epitope prediction (total articles = 5)c |
Discotope ElliPro CBtope |
5 2 1 |
100 40 20 |
| Continuous B-cell epitope prediction (total articles = 5)d |
BCpred ABCpred Bepipred IEDB Ellipro |
3 2 1 1 1 |
60 40 20 20 20 |
| References (total articles = 8) | Saadi et al. (2017), Orbai et al. (2017), Meza et al. (2017), Farhadi et al. (2015), Raoufi et al. (2015, 2017), Nezafat et al. (2016), Liu et al. (2017) and Coscolla et al. (2015) | ||
Their related web tools have been shown in the second column. In the third and fourth column, the frequency and percentage of the regularity of each web tool in articles are shown
aSix cases of 50 articles was studied for prediction of MHC Class I epitopes
bFive cases of 50 articles were studied for prediction of MHC Class II epitopes
cFive articles were studied on discontinuous B-cell epitope prediction
dFive articles were studied on continuous B-cell epitope prediction
Table 3.
The assortment of articles for epitope prediction of bacterial antigens (4 articles out of 50 articles)
| Tumor cell antigen | |||
|---|---|---|---|
| In silico softwares | fi | fi/N × 100 | |
| MHC-I epitope binding prediction (total articles = 3)a |
ProPred-I SVMHC SYFPEITHI MHCPEP MHCpred NetMHCpan |
2 2 2 1 1 1 |
66.6 66.6 66.6 33.3 33.3 33.3 |
| MHC-II epitope binding prediction (total articles = 3)b |
MHCpred Propred-II MetaMHCII IEDB RANKPEP |
2 1 1 1 1 |
66.6 33.3 33.3 33.3 33.3 |
| Discontinuous B-cell epitope prediction (total articles = 4)c |
Discotope PEPOP EliPro CBtope CEP |
3 2 1 1 1 |
75 50 25 25 25 |
| Continuous B-cell epitope prediction (total articles = 3)d |
ABCpred Bepipred Ellipro BCpred IEDB |
3 2 2 1 1 |
100 66.6 66.6 33.3 33.3 |
| References (total articles = 4) | Nezafat et al. (2014), Manijeh et al. (2013), Mahdavi et al. (2012) and Mahdavi and Moreau (2016) | ||
Their related web tools have been shown in the second column. In the third and fourth columns, the frequency and percentage of the regularity of each web tool in articles are shown
aThree cases of 50 articles were studied for prediction of MHC Class I epitopes
bThree cases of 50 articles were studied for prediction of MHC Class II epitopes
cFour articles were studied on discontinuous B-cell epitope prediction
dThree articles were studied on continuous B-cell epitope prediction
Prediction of MHC-II-Binding Epitopes
According to quantitative investigations in this study, approximately 50% of the papers have been devoted to predicting MHC-II epitopes, frequently studying viruses.
Each of the papers used a series of bioinformatics tools to predict MHC-II epitopes, where the most widely used list in this study is as follows:
IEDB, NetMHC-II, MHCpred-II, MetaMHC-II, MetaSVMP, Propred-II, RANKPEP, PREDIVAC, SYFPEITH, BIMAS, CTL-Pred, EpiTOP, MHCPEP, EpiVax, PREDEPP, TEPITOPE, EPIPREDICT, EpiDOCK, Concensus, and EpiMatrix.
The qualitative studies and frequency of software application in these studies have shown that IEDB is among the most widely used and most available tools.
As shown in Table 1, 2 and 3, the results of our studies revealed the frequency of each software in the prediction of MHC-II attached epitopes according to the nature of antigens evaluated in the three sections of viral, bacterial and tumor-specific antigens. According to these Tables, IEDB software was mostly used for the prediction of MHC-II attached virus-based epitopes, Propred-II software for the prediction of MHC-II attached bacterial epitopes and MHCpred software for the prediction of MHC-II attached tumor-specific epitopes.
Prediction of B Cell Epitopes
Prediction of B Cell Linear Epitopes
Prediction of linear epitopes testing due to the continuity of amino acids unlike conformational epitopes had fewer complexities and problems. Therefore, 58% of the papers reviewed had addressed these epitopes.
According to studied papers, bioinformatics tools to predict linear epitopes are as follows:
Bepipred, BCpred, ABCpred, Pcipep, BCEpred, BepiTope, PrediTop, PEOPLE, LBtope, SVMTrip, COBEpro, EPMLR and Igpred.
The qualitative studies and frequency of using software programs in 29 papers showed that Bepipred has the most application for predicting epitopes.
The prediction of linear epitopes is not limited to using an informatics tool, but it requires the evaluation of side features of methods used simultaneously for this purpose, that includes:
Koloaskar-Tongankar antigenicity, Emini surface accessibility prediction, Chou-fasman beta-turn prediction, prediction of floppy-prediction and Parker hydrophilicity prediction.
Tables 1, 2 and 3 show the results of our studies using software programs in the prediction of linear epitopes of B cell according to the nature of the antigens evaluated in the three sections of viral, bacterial, and tumor-specific antigens. Based on our review, Bepipred software had the highest frequency for predicting linear viral B cell epitopes and BCpred software for predicting linear bacterial B cell epitopes. Also, ABCpred software had the highest frequency for predicting tumor-specific B cell epitopes.
Prediction of Conformational B Cell Epitopes
Among the 50 papers reviewed, 15 cases for conformational epitopes were found.
In previous studies, various bioinformatics tools have been introduced to assist in the process of prediction of discontinuous epitopes, each which has its own features and is selected depending on the type of work and purpose of the user as follows:
Discotope, Ellipro, CBTope, Epitope, BEPro, CEP, SEPPA, CED, EPITOME, MAPOTOPE, EPCES, EPSVR and EPMETA.
With qualitative reviews and frequency of applications in the previous studies conducted on the results of the papers, we found that among the software programs introduced, Discotope and Ellipro were the most widely utilized.
Tables 1, 2 and 3 show the results of our studies in the frequency of each software for prediction of conformational epitopes of B cell according to the nature of the antigen evaluated in the three sections of viral, bacterial and tumor-specific antigens. Based on our investigations, Discotope and Ellipro softwares are most widely used for predicting conformational viral B cell epitopes and Discotope software is most widely used for predicting bacterial conformational B cell epitopes and predicting tumor-specific B cell epitopes.
Molecular Docking
Molecular docking is a method for predicting how different formations of a small molecule or combination of molecules connected to a suitable target site. This prediction is performed through a computerized program, including regeneration of all possible formations of ligand structure and placement of all these ligand formations in a cavity of the active target protein position and then scoring of the examined conditions based on free energy or binding energy.
In the field of epitope mapping, after predicting and evaluating the candidate epitopes, the molecular docking approach allows us to study the predicted epitopes’ connection to the binding packet (cavity binding site) of the antibody molecules, MHC-I and MHC-II. Then evaluated in terms of the number of hydrogen bindings and free energy or binding energy with a strong binding potential for screening in silico candidate epitopes (Friesner et al. 2004).
Among the 50 papers studied, we reviewed 19 papers on docking.
The following are softwares and tools required for molecular docking used in the previous studies:
Autodock Vina, autodock 4, Patch dock, Molegro Virtual Docker, Mti auto dock, Cluspro 2.0 and Python Prescription.
It has been shown that the most widely used of these software programs in previous studies was Autodock including autodock vina and autodock 4.
Conclusions
Epitope identification using high-performance immuno-analytic tools can be very useful in various applications in the field of epitope mapping, including the design of peptide-based vaccines, identification of immunological processes, prediction of epitopes used in the diagnosis of diseases, determination of features of antibodies in various diseases, etc. (Hemmati et al. 2017; Mohsenzadegan et al. 2015, 2018).
The proper and accurate prediction of epitopes can be the basis for the development of therapeutic methods, including the production and design of peptide-based vaccines. Peptide-based vaccines are one of the attractive and alternative strategies for the design of vaccines, which only contain peptide fragments with a length of 20–30 amino acids of the pathogen proteins that have the highest antigenicity and can stimulate and activate the immune response. In fact, the immune response is developed only against immunogenic epitopes, and additional portions of antigens causing allergic reactions that can be removed (Mohsenzadegan et al. 2018).
Therefore, a possible alternative method for immunization is the identification of peptide epitopes that stimulate the immune responses and the use of their fully synthetic copies as a vaccine. These peptides are a good candidate for vaccine production, because they are easily produced, not mutated, are chemically stable and potentially do not cause infection. These sequences can be also manipulated. In this case, their chemical stability can be increased, and unwanted side effects seen in normal sequences can be minimized through these manipulations. The identification of peptide epitopes can be useful in the production of vaccines against microorganisms that have very little growth in the medium, as well as microorganisms whose antigenic regions are not detected during the normal infection of the immune system. These methods are used in the production of the vaccine against anti-cancer antigens (Mohsenzadegan et al. 2015; Perrie et al. 2007; Petrovsky and Aguilar 2004; Thompson and Staats 2011; Li et al. 2014).
The advancement of next-generation sequencing (NGS) techniques that provide the complete and accurate sequences of genomes of pathogens, and even humans, contribute to the advancement of epitope predictive techniques as well as the facility of designing and developing peptide-based vaccines. These vaccines can be universal and work against all types of microorganisms strains as well as can be designed to work for all individuals, given the presence of different HLA alleles per person (Toussaint et al. 2011; Backert and Kohlbacher 2015). In addition, B and T cell epitopes can be attached together and create an immunogen molecule. The use of immunoinformatics methods to predict epitopes and subsequent development of peptide-based vaccines reduces the costs and the time as well as increases accuracy compared to pure laboratory tests (Raoufi et al. 2015).
As shown in Tables 1, 2, and 3, our investigations identified the frequency and application of each of the novel software used for epitope mapping. The results obtained will be very useful and valuable for future studies in research on epitope regions’ for viral, bacterial and tumor antigens. We hope that the relationship between research centers and companies producing immunoinformatics softwares will be facilitated by scientists and researchers in this field in the near future.
Acknowledgements
This study was supported by Iran University of medical sciences.
Abbreviations
- MHC
The major histocompatibility complex
- HLA
The human leukocyte antigen
- NMR
Nuclear magnetic resonance
- IEDB
The immune epitope database
- SVMHC
Server for prediction of MHC-binding peptides
- MAPPP
MHC-I antigenic peptide processing prediction
- EPSVR
Epitopes prediction with support vector regression
Funding
The funding source is a college institute and has sponsored the project financially and approved.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alam A, Ali S, Ahamad S, Malik MZ, Ishrat R. From ZikV genome to vaccine: in silico approach for the epitope-based peptide vaccine against Zika virus envelope glycoprotein. Immunology. 2016;149(4):386–399. doi: 10.1111/imm.12656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali MT, Islam MO. A highly conserved GEQYQQLR epitope has been identified in the nucleoprotein of Ebola virus by using an in silico approach. Adv Bioinform. 2015;2015:278197. doi: 10.1155/2015/278197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M, Pandey RK, Khatoon N, Narula A, Mishra A, Prajapati VK. Exploring dengue genome to construct a multi-epitope based subunit vaccine by utilizing immunoinformatics approach to battle against dengue infection. Sci Rep. 2017;7(1):9232. doi: 10.1038/s41598-017-09199-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backert L, Kohlbacher O. Immunoinformatics and epitope prediction in the age of genomic medicine. Genome Med. 2015;7(1):119. doi: 10.1186/s13073-015-0245-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawi MM, Alla AAF, Alam SS, Mohamed WA, Nasr-Eldin Osman DA, Ali SAAA, Ahmed EM, Adam AA, Abdullah RO, Salih MA. Immunoinformatics predication and in silico modeling of epitope-based peptide vaccine against virulent Newcastle disease viruses. Am J Infect Dis Microbiol. 2016;4(3):61–71. [Google Scholar]
- Brusic V, Petrovsky N. Immunoinformatics and its relevance to understanding human immune disease. Expert Rev Clin Immunol. 2005;1(1):145–157. doi: 10.1586/1744666X.1.1.145. [DOI] [PubMed] [Google Scholar]
- Chakraborty SU. Ebola vaccine: multiple peptide-epitope loaded vaccine formulation from proteome using reverse vaccinology approach. Int J Pharm Pharm Sci. 2014;6:407–412. [Google Scholar]
- Chen J, Wang Y, Guo D, Shen B. A systems biology perspective on rational design of peptide vaccine against virus infections. Curr Top Med Chem. 2012;12(12):1310–1319. doi: 10.2174/156802612801319043. [DOI] [PubMed] [Google Scholar]
- Coscolla M, Copin R, Sutherland J, Gehre F, de Jong B, Owolabi O, Mbayo G, Giardina F, Ernst JD, Gagneux SM. tuberculosis T cell epitope analysis reveals paucity of antigenic variation and identifies rare variable TB antigens. Cell Host Microbe. 2015;18(5):538–548. doi: 10.1016/j.chom.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash R, Das R, Junaid M, Akash MF, Islam A, Hosen SZ. In silico-based vaccine design against Ebola virus glycoprotein. Adv Appl Bioinform Chem. 2017;10:11. doi: 10.2147/AABC.S115859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies MN, Flower DR. Harnessing bioinformatics to discover new vaccines. Drug Discov Today. 2007;12(9–10):389–395. doi: 10.1016/j.drudis.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Dutta DK, Rhodes K, Wood SC. In silico prediction of Ebola Zaire GP 1, 2 immuno-dominant epitopes for the Balb/c mouse. BMC Immunol. 2015;16(1):59. doi: 10.1186/s12865-015-0126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhadi T, Nezafat N, Ghasemi Y, Karimi Z, Hemmati S, Erfani N. Designing of complex multi-epitope peptide vaccine based on omps of Klebsiella pneumoniae: an in silico approach. Int J Pept Res Ther. 2015;21(3):325–341. [Google Scholar]
- Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, Repasky MP, Knoll EH, Shelley M, Perry JK, Shaw DE. Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem. 2004;47(7):1739–1749. doi: 10.1021/jm0306430. [DOI] [PubMed] [Google Scholar]
- Gardy JL, Lynn DJ, Brinkman FS, Hancock RE. Enabling a systems biology approach to immunology: focus on innate immunity. Trends Immunol. 2009;30(6):249–262. doi: 10.1016/j.it.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Hasan A, Hossain M, Alam J. A computational assay to design an epitope-based peptide vaccine against Saint Louis encephalitis virus. Bioinform Biol Insights. 2013;7:BBI-S13402. doi: 10.4137/BBI.S13402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmati M, Raoufi E, Fallahi H (2017) Predicting candidate epitopes on ebola virus for possible vaccine development. In: Advances Ebola control. IntechOpen
- Huang WL, Tsai MJ, Hsu KT, Wang JR, Chen YH, Ho SY. Prediction of linear B-cell epitopes of hepatitis C virus for vaccine development. BMC Med Genomics. 2015;8(4):S3. doi: 10.1186/1755-8794-8-S4-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamthania M, Sharma DK. Screening and structure-based modeling of T-cell epitopes of Nipah virus proteome: an immunoinformatic approach for designing peptide-based vaccine. 3 Biotech. 2015;5(6):877–882. doi: 10.1007/s13205-015-0303-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MA, Hossain MU, Rakib-Uz-Zaman SM, Morshed MN. Epitope-based peptide vaccine design and target site depiction against Ebola viruses: an immunoinformatics study. Scand J Immunol. 2015;82(1):25–34. doi: 10.1111/sji.12302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Jain A, Verma SK. Screening and structure-based modeling of T-cell epitopes of Marburg virus NP, GP and VP40: an immunoinformatic approach for designing peptide-based vaccine. Trends Bioinform. 2013;6(1):10. [Google Scholar]
- Li W, Joshi M, Singhania S, Ramsey K, Murthy A. Peptide vaccine: progress and challenges. Vaccines. 2014;2(3):515–536. doi: 10.3390/vaccines2030515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Chen C, Chen C, Ding R, Marslin G. Construction of a recombinant OmpC dominant epitope-based vaccine against escherichia coli and evaluation of its immunogenicity and protective immunity. Jundishapur J Microbiol. 2017;10(11):e55652. [Google Scholar]
- Mahdavi M, Moreau V. In silico designing breast cancer peptide vaccine for binding to MHC class I and II: a molecular docking study. Comput Biol Chem. 2016;1(65):110–116. doi: 10.1016/j.compbiolchem.2016.10.007. [DOI] [PubMed] [Google Scholar]
- Mahdavi M, Mohabatkar H, Keyhanfar M, Dehkordi AJ, Rabbani M. Linear and conformational B cell epitope prediction of the HER 2 ECD-subdomain III by in silico methods. Asian Pac J Cancer Prev. 2012;13(7):3053–3059. doi: 10.7314/apjcp.2012.13.7.3053. [DOI] [PubMed] [Google Scholar]
- Manijeh M, Mehrnaz K, Violaine M, Hassan M, Abbas J, Mohammad R. In silico design of discontinuous peptides representative of B and T-cell epitopes from HER2-ECD as potential novel cancer peptide vaccines. Asian Pac J Cancer Prev. 2013;14(10):5973–5981. doi: 10.7314/apjcp.2013.14.10.5973. [DOI] [PubMed] [Google Scholar]
- Martin JE, Sullivan NJ, Enama ME, Gordon IJ, Roederer M, Koup RA, Bailer RT, Chakrabarti BK, Bailey MA, Gomez PL, Andrews CA. A DNA vaccine for Ebola virus is safe and immunogenic in a phase I clinical trial. Clin Vaccine Immunol. 2006;13(11):1267–1277. doi: 10.1128/CVI.00162-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meza B, Ascencio F, Sierra-Beltrán AP, Torres J, Angulo C. A novel design of a multi-antigenic, multistage and multi-epitope vaccine against Helicobacter pylori: an in silico approach. Infect Genet Evolut. 2017;1(49):309–317. doi: 10.1016/j.meegid.2017.02.007. [DOI] [PubMed] [Google Scholar]
- Mirza MU, Rafique S, Ali A, Munir M, Ikram N, Manan A, Salo-Ahen OM, Idrees M. Towards peptide vaccines against Zika virus: immunoinformatics combined with molecular dynamics simulations to predict antigenic epitopes of Zika viral proteins. Sci Rep. 2016;9(6):37313. doi: 10.1038/srep37313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed AA, Hashim O, Elrahman KA, Hamdi A, Hassan MA. Epitope-based peptide vaccine design against mokola rabies virus glycoprotein G utilizing in silico approaches. Immunome Res. 2017;13(144):2. [Google Scholar]
- Mohsenzadegan M, Shekarabi M, Madjd Z, Asgari M, Abolhasani M, Tajik N, Farajollahi MM. Study of NGEP expression pattern in cancerous tissues provides novel insights into prognostic marker in prostate cancer. Biomark Med. 2015;9(4):391–401. doi: 10.2217/bmm.14.106. [DOI] [PubMed] [Google Scholar]
- Mohsenzadegan M, Saebi F, Yazdani M, Abolhasani M, Saemi N, Jahanbani F, Farajollahi MM. Autoantibody against new gene expressed in prostate protein is traceable in prostate cancer patients. Biomark Med. 2018;12(10):1125–1138. doi: 10.2217/bmm-2018-0069. [DOI] [PubMed] [Google Scholar]
- Moise L, De Groot AS. Putting immunoinformatics to the test. Nat Biotechnol. 2006;24(7):791. doi: 10.1038/nbt0706-791. [DOI] [PubMed] [Google Scholar]
- Negahdaripour M, Nezafat N, Eslami M, Ghoshoon MB, Shoolian E, Najafipour S, Morowvat MH, Dehshahri A, Erfani N, Ghasemi Y. Structural vaccinology considerations for in silico designing of a multi-epitope vaccine. Infect Genet Evolut. 2018;1(58):96–109. doi: 10.1016/j.meegid.2017.12.008. [DOI] [PubMed] [Google Scholar]
- Nezafat N, Ghasemi Y, Javadi G, Khoshnoud MJ, Omidinia E. A novel multi-epitope peptide vaccine against cancer: an in silico approach. J Theor Biol. 2014;21(349):121–134. doi: 10.1016/j.jtbi.2014.01.018. [DOI] [PubMed] [Google Scholar]
- Nezafat N, Karimi Z, Eslami M, Mohkam M, Zandian S, Ghasemi Y. Designing an efficient multi-epitope peptide vaccine against Vibrio cholerae via combined immunoinformatics and protein interaction based approaches. Comput Biol Chem. 2016;1(62):82–95. doi: 10.1016/j.compbiolchem.2016.04.006. [DOI] [PubMed] [Google Scholar]
- Oany AR, Emran AA, Jyoti TP. Design of an epitope-based peptide vaccine against spike protein of human coronavirus: an in silico approach. Drug Des Dev Ther. 2014;8:1139. doi: 10.2147/DDDT.S67861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oany AR, Ahmad SA, Hossain MU, Jyoti TP. Identification of highly conserved regions in L-segment of Crimean-Congo hemorrhagic fever virus and immunoinformatic prediction about potential novel vaccine. Adv Appl Bioinform Chem. 2015;8:1. doi: 10.2147/AABC.S75250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orbai AM, De Wit M, Mease PJ, Duffin KC, Elmamoun M, Tillett W, Campbell W, FitzGerald O, Gladman DD, Goel N, Gossec L. Updating the Psoriatic Arthritis (PsA) core domain set: a report from the PsA workshop at OMERACT 2016. J Rheumatol. 2017;44(10):1522–1528. doi: 10.3899/jrheum.160904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman MM, ElAmin EE, Al-Nour MY, Alam SS, Adam RS, Ahmed AA, Elsayed AA, Abdalla MH, Salih MA. In silico design of epitope based peptide vaccine against virulent strains of HN-newcastle disease virus (NDV) in poultry species. Int J Multidiscip Curr Res. 2016;4:868–878. [Google Scholar]
- Perrie Y, Kirby D, Bramwell VW, Mohammed AR. Recent developments in particulate-based vaccines. Recent Pat Drug Deliv Formul. 2007;1(2):117–129. doi: 10.2174/187221107780831897. [DOI] [PubMed] [Google Scholar]
- Petrovsky N, Aguilar JC. Vaccine adjuvants: current state and future trends. Immunol Cell Biol. 2004;82(5):488–496. doi: 10.1111/j.0818-9641.2004.01272.x. [DOI] [PubMed] [Google Scholar]
- Potocnakova L, Bhide M, Pulzova LB. An introduction to B-cell epitope mapping and in silico epitope prediction. J Immunol Res. 2016;2016:6760830. doi: 10.1155/2016/6760830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raoufi E, Hemmati M, EinAbadi H, Fallahi H (2015) Predicting candidate epitopes on Ebolaviruse for possible vaccine development. In: Proceedings of the 2015 IEEE/ACM international conference on advances in social networks analysis and mining, pp 1083–1088. ACM
- Raoufi E, Akrami H, Khansarinejad B, Abtahi H (2015) Epitope mapping and antigenic evaluation of Helicobacter pylori Urease subunit beta fragment. In: IEEE/ACM international conference on advances in social networks analysis and mining (ASONAM), pp 1076–1082. IEEE
- Raoufi E, Akrami H, Khansarinejad B, Abtahi H. Expression and antigenic evaluation of Helicobacter pylori UreB fragment. Jundishapur J Microbiol. 2017;10(5):e41645. [Google Scholar]
- Saadi M, Karkhah A, Nouri HR. Development of a multi-epitope peptide vaccine inducing robust T cell responses against brucellosis using immunoinformatics based approaches. Infect Genet Evolut. 2017;1(51):227–234. doi: 10.1016/j.meegid.2017.04.009. [DOI] [PubMed] [Google Scholar]
- Saha CK, Hasan MM, Hossain MS, Jahan MA, Azad AK. In silico identification and characterization of common epitope-based peptide vaccine for Nipah and Hendra viruses. Asian Pac J Trop Med. 2017;10(6):529–538. doi: 10.1016/j.apjtm.2017.06.016. [DOI] [PubMed] [Google Scholar]
- Sakib MS, Islam M, Hasan AK, Nabi AH. Prediction of epitope-based peptides for the utility of vaccine development from fusion and glycoprotein of nipah virus using in silico approach. Adv Bioinform. 2014;2014:402492. doi: 10.1155/2014/402492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraswat A, Shraddha JA, Pathak A, Verma SK, Kumar A. Immuno-informatic speculation and computational modeling of novel MHC-II human leukocyte antigenic alleles to elicit vaccine for ebola virus. J Vaccines Vaccin. 2012;3(141):2. [Google Scholar]
- Sette A, Buus S, Appella E, Adorini L, Grey HM. Structural requirements for the interaction between class II MHC molecules and peptide antigens. Immunol Res. 1990;9(1):2–7. doi: 10.1007/BF02918474. [DOI] [PubMed] [Google Scholar]
- Sharmin R, Islam AB. A highly conserved WDYPKCDRA epitope in the RNA directed RNA polymerase of human coronaviruses can be used as epitope-based universal vaccine design. BMC Bioinform. 2014;15(1):161. doi: 10.1186/1471-2105-15-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon J, Rynkiewicz MJ, Lu Z, Yang CY. Discovery of protective B-cell epitopes for development of antimicrobial vaccines and antibody therapeutics. Immunology. 2014;142(1):1–23. doi: 10.1111/imm.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehzadi A, Rehman SU, Husnain T. Selection of epitope-based vaccine targets of HCV genotype 1 of Asian origin: a systematic in silico approach. Bioinformation. 2012;8(20):957. doi: 10.6026/97320630008957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar C, Dev K, Verma SK, Kumar A. In-silico: screening and modeling of CTL binding epitopes of Crimean congo hemorrhagic fever virus. Trends Bioinform. 2012;5:14–24. [Google Scholar]
- Shi J, Zhang J, Li S, Sun J, Teng Y, Wu M, Li J, Li Y, Hu N, Wang H, Hu Y. Epitope-based vaccine target screening against highly pathogenic MERS-CoV: an in silico approach applied to emerging infectious diseases. PLoS ONE. 2015;10(12):e0144475. doi: 10.1371/journal.pone.0144475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Sun J, Wu M, Hu N, Li J, Li Y, Wang H, Hu Y. Inferring protective CD8 + T-cell epitopes for NS5 protein of four serotypes of dengue virus Chinese isolates based on HLA-a,-b and-c allelic distribution: implications for epitope-based universal vaccine design. PLoS ONE. 2015;10(9):e0138729. doi: 10.1371/journal.pone.0138729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H, Ansari HR, Raghava GP. Improved method for linear B-cell epitope prediction using antigen’s primary sequence. PLoS ONE. 2013;8(5):e62216. [Google Scholar]
- Sundar K, Boesen A, Coico R. Computational prediction and identification of HLA-A2. 1-specific Ebola virus CTL epitopes. Virology. 2007;360(2):257–263. doi: 10.1016/j.virol.2006.09.042. [DOI] [PubMed] [Google Scholar]
- Thompson AL, Staats HF. Cytokines: the future of intranasal vaccine adjuvants. Clin Dev Immunol. 2011;31:2011. doi: 10.1155/2011/289597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomar N, De RK. Immunoinformatics: an integrated scenario. Immunology. 2010;131(2):153–168. doi: 10.1111/j.1365-2567.2010.03330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toussaint NC, Maman Y, Kohlbacher O, Louzoun Y. Universal peptide vaccines–optimal peptide vaccine design based on viral sequence conservation. Vaccine. 2011;29(47):8745–8753. doi: 10.1016/j.vaccine.2011.07.132. [DOI] [PubMed] [Google Scholar]
- Verma SK, Yadav S, Kumar A. In silico prediction of B-and T-cell epitope on Lassa virus proteins for peptide based subunit vaccine design. Adv Biomed Res. 2015;4:201. doi: 10.4103/2277-9175.166137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Yu T, Song X, Yi S, Hou L, Chen W. Prediction and identification of mouse cytotoxic T lymphocyte epitopes in Ebola virus glycoproteins. Virol J. 2012;9(1):111. doi: 10.1186/1743-422X-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Sun W, Guo J, Zhao G, Sun S, Yu H, Guo Y, Li J, Jin X, Du L, Jiang S. In silico design of a DNA-based HIV-1 multi-epitope vaccine for Chinese populations. Hum Vaccines Immunother. 2015;11(3):795–805. doi: 10.1080/21645515.2015.1012017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Lin X, Wang X, Zheng L, Lan S, Jin S, Ou Z, Wu J. In silico analysis of epitope-based vaccine candidates against hepatitis B virus polymerase protein. Viruses. 2017;9(5):112. doi: 10.3390/v9050112. [DOI] [PMC free article] [PubMed] [Google Scholar]