Abstract
This article evaluates the epidemiological evidence for a relationship between vaccination and neurological disease, specifically multiple sclerosis, Guillain–Barré syndrome and narcolepsy. The statistical methods used to test vaccine safety hypotheses are described and the merits of different study designs evaluated; these include the cohort, case-control, case-coverage and the self-controlled case-series methods. For multiple sclerosis, the evidence does not support the hypothesized relationship with hepatitis B vaccine. For Guillain−Barré syndrome, the evidence suggests a small elevated risk after influenza vaccines, though considerably lower than after natural influenza infection, with no elevated risk after human papilloma virus vaccine. For narcolepsy, there is strong evidence of a causal association with one adjuvanted vaccine used in the 2009/10 influenza pandemic. Rapid investigation of vaccine safety concerns, however biologically implausible, is essential to maintain public and professional confidence in vaccination programmes.
Key Points
| The assumption of a causal association with a vaccine based on a temporal association is often incorrect as unrelated events will occur by chance irrespective of vaccination. |
| When many studies are performed to answer the same question, the key to demonstrating causality is consistency of results from well-designed studies. |
| Robust epidemiolocal methods should be in place to rapidly respond to scares because once confidence is lost in a vaccine it is hard to restore. |
| Not all vaccine safety concerns can be anticipated based on biological plausibility |
Introduction
Vaccination is one of the most effective public health interventions successfully controlling many serious infectious diseases and saving millions of lives globally each year [1]. However, as with any medical treatment or drug, vaccination can never be entirely risk free in terms of unwanted side effects. An important feature of vaccination is that unlike most therapeutic drugs, vaccines are given prophylactically to healthy individuals, often young children. When an event occurs shortly after vaccination in an otherwise healthy individual without an obvious cause, it is tempting to attribute its occurrence to the preceding vaccination. The assumption of a causal association with a vaccine from purely a temporal association is often incorrect as unrelated events will occur by chance irrespective of vaccination. It can be hard to disentangle these temporal associations when there is a strong perception that a temporal association is necessarily evidence of a causal association and the onset of the condition is insidious and its timing relies on patient or parental recall [2]. Even if only based on a temporal sequence of events, it is important that such safety concerns are rapidly investigated with robust epidemiological studies to allow mitigation procedures to be put in place if an association is confirmed or, if unfounded, to have the necessary evidence to sustain public confidence in the vaccination programme without which coverage drops and disease control is lost.
In this article, which focusses on the evaluation of the relationship between vaccination and neurological diseases, the statistical approaches to causality assessment are first discussed and their relative merits evaluated, followed by an overview of a selection of vaccine safety studies involving neurological disease with differing conclusions; some of the included studies have shown a small elevated risk, others none, two lack evidence to draw any definitive conclusion and one provides robust evidence of causal association.
Epidemiological Statistical Methods
To establish whether the signal seen is associated with the vaccine and to quantify the risk, a formal epidemiological study is usually needed. This requires a pre-specified protocol detailing the population under study, the period after vaccination for which an elevated risk is suspected, and the methods for case identification and statistical analysis. Most importantly, the ascertainment of the condition of interest must be unbiased with respect to vaccination history [3]. The following statistical methods have been used most commonly to address vaccine safety questions and to control for the inherent biases in the population and data under study. Although these methods aim to address confounding, it can be difficult to fully control for this in an observational study. An assessment of the likelihood of residual confounding/bias and its potential extent is an important consideration when weighing up the strength of a study and drawing a conclusion with regard to causality.
Cohort
In a cohort study, the risk of developing the condition is compared in the vaccinated and unvaccinated individuals in the study population. Cohort studies need to be very large to detect rare vaccine adverse events and this often makes them impractical for a prospective study. Retrospective cohort designs can use routinely collected data and cases identified by clinical coding but this study design may be disadvantaged by the need to collect a large number of confounding variables. Factors such as underlying illnesses, sociodemographic characteristics, and propensity to consult may differ between unvaccinated and vaccinated individuals and would therefore need to be adjusted for in the analysis as they can independently determine the likelihood of the adverse event under study.
The advantage is that an entire population is studied and relative and absolute incidence estimates can be reported. In addition, once the cohort is defined, several outcomes can be assessed within the same study design. When studying a vaccine that is given as part of a national schedule and high coverage is achieved, the small unvaccinated group may differ from the vaccinated group in ways that are difficult to capture and control for in an adjusted analyses. Additionally, care must be taken to ensure unvaccinated cases are indeed unvaccinated and the data are not missing. This can occur when regional vaccine datasets are used and the transfer and sharing of data are not comprehensive.
Cohort studies are feasible for vaccine safety studies when data from a whole country or region can be used. An example of this is in Denmark where Danish residents contribute to a large linked dataset consisting of demographic factors that are linked to health information including potential confounding variables [4].
Self-Controlled Case Series
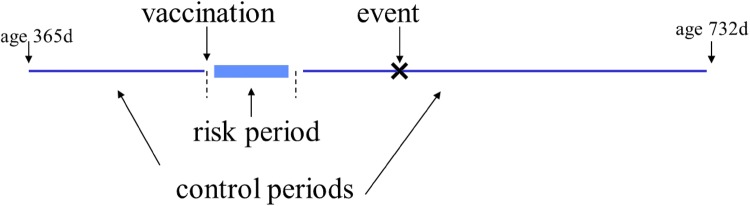
The self-controlled case-series method (SCCS) was designed for rapid unbiased assessment in vaccine safety studies using available disease surveillance data that may not be amenable to cohort analysis. The method only requires information on the timing of cases during a defined observation period and their vaccination status [5]. The cases act as their own controls as the incidence of the event in pre-defined risk periods following vaccination is compared to the incidence outside the risk period generating a relative incidence (RI) measure (Fig. 1). A significant advantage of the method is that confounding factors that do not vary over the observation period, such as co-morbidities or sociodemographic status, are automatically controlled for. Adjustment for time-varying confounders such as age is also possible by dividing up the observation period further into age categories. It has been demonstrated that the power of the SCCS method is nearly as good as a cohort study when uptake is high and risk intervals are short, and it is superior to that of a case-control study [6].
Fig. 1.
Self-controlled case-series method showing the contribution of one case. d days
The self-controlled case-series method has been used by Public Health England to address many pertinent vaccine safety concerns [7–10]. This design has been chosen both because of its simplicity and ability to control for individual level confounding and also because a national cohort of cases cannot be easily defined using the national hospital data as no national immunisation register is available. Unlike a cohort study, the SCCS method does not provide absolute risk estimates. However, if the number of doses given to the population from which the cases are derived is known and if ascertainment is complete, then absolute risks can be estimated and the cases attributable to vaccination estimated from the magnitude of the RI.
Case Control
A case-control study requires smaller numbers than a cohort study but the same confounding and bias can occur and it also has the added difficulty of selecting the correct controls for comparison. For vaccinations given in the short age range in the first and second year of life or during a short calendar period to target ages, close matching of the controls on date of birth is required. Prior vaccination status is then compared between cases and controls using the date of onset in cases as a reference date. To obtain enough power to assess the required risk, multiple controls per case are often needed and defining appropriate criteria for the selection of controls can be problematic. While it is important to ensure that controls are similar to cases on characteristics such as age and geographical location that can independently affect vaccination status, over matching is a risk if too many extraneous variables are included in the matching, resulting in loss of efficiency and potentially introducing bias. A case-control study does not provide absolute risk estimates, rather it measures the odds of vaccination in cases compared to controls. However, as with the SCCS method, if the number of doses given to the population from which the cases are derived is known and if ascertainment is complete, then absolute risks can be estimated and the cases attributable to vaccination estimated from the magnitude of the odds ratio.
The case-control design has been used where controls can be selected from the same population as cases and can be readily matched on the relevant variables. As a case-control approach is more efficient than the cohort approach, it is often used on large databases that could be used for a cohort analysis. Examples include the Vaccine Safety Datalink in the USA, which accesses complete patient records from Health Maintenance Organisations, or studies using hospital admission data bases linked to national immunisation registers such as the Australian Childhood Immunisation Register [11–13].
Case-Coverage Design
The case-coverage design has recently been used in vaccine safety studies [14, 15]. It is similar to the screening method, which until recently has been primarily used for vaccine effectiveness assessment [16], although it is more limited in terms of adjustment for possible confounders than the SCCS method. Each case is matched to a population coverage estimate and this is then used to see if the number of cases vaccinated is greater than expected. The method uses logistic regression on the odds of vaccination with an offset for the log-odds of the matched population coverage, thus it is similar to a case-control study with thousands of controls per individual.
This design has been used by Public Health England to assess the risk between AS03 adjuvanted H1N1 pandemic vaccine Pandemrix™ and narcolepsy. Because Pandemrix™ was rolled out over a short period of time in the winter season of 2009/10 targeting children of different ages according to whether they had certain co-morbid conditions, it was necessary to have detailed information on dates of vaccination and dates of birth to estimate the population coverage for each narcolepsy case by age and time period. This was available from a representative subset of general practices in England, which also provided information on co-morbidities, the only other variable considered as a potential confounder [14]. In the first study assessing the risk of narcolepsy in children [14], both the SCCS method and the case-coverage design was used. The results from the SCCS method were found not to be clear as this method requires the incidence in a pre-specified risk period after vaccination relative to the baseline incidence to be compared. Because the duration of the risk period had not been defined at the time, the post-vaccination interval was found to be too short and resulted in the inclusion in the baseline period of four patients with symptoms more than 6 months after vaccination.
The choice of study design to answer a vaccine safety question will depend on the hypothesis to be tested, the available data sources and the extent to which confounding variables are likely to bias the results. The SCCS method has now become the gold standard design in vaccine safety studies, owing to the benefits highlighted above, but for each study question the methods should be adapted and potential biases considered in the context of the population under study, the dataset being utilised and the hypothesis being tested. It will inevitably be a trade-off between the ideal and the practical and the best designs will vary according to setting. When many studies are performed to answer the same question, the key to demonstrating causality is consistency in the results from well-designed studies [17].
Neurological Disease and Vaccines
Neurological conditions have a long history of causal associations with vaccination being inferred from temporally related onsets. An example is the damage that was made to the UK whole-cell pertussis vaccination programme in the late 1970s when neurological damage was wrongly attributed to the vaccine based on case reports of infants with onset of encephalopathy shortly after vaccination. These reports of permanent brain damage following vaccination attracted intense and sustained professional and media interest causing vaccination rates to fall from 79% in 1973 to 31% in 1978. Following this, three national epidemics of pertussis occurred with an estimated 5000 hospital admissions, 200 cases of pneumonia, 83 cases of convulsions and 38 deaths [18, 19]. Neurological vaccine safety concerns can be broadly assigned to either being biologically plausible or unsubstantiated and unexpected. The biologically plausible group are often a direct effect from a component of the vaccine. For example, in the case of a live attenuated vaccine, the adverse reaction could mimic, at a lower frequency, what the non-attenuated wild virus would do. This is demonstrated in the rare risk of acute flaccid paralysis following the oral polio vaccine after a reversion to virulence or with the risk of aseptic meningitis after the attenuated Urabe mumps strain in the measles-mumps-rubella vaccine due to retention of some neurovirulent characteristics [20, 21]. The unsubstantiated and unexpected group occurs usually because of the timing of the vaccine, which coincides with the diagnosis of the condition and has no immediate biologically plausible explanation. Examples of this are measles-mumps-rubella and autism [22], gait disturbance and measles-mumps-rubella [23], and thiomersal and developmental delay [24]. Although a signal may not have a clear biological basis for its causation, it still needs to be fully investigated using robust epidemiological methods.
Neurological diseases for which a causal association with vaccination has been suspected have some common features. First, they are often serious conditions that are rare, second, their aetiology and pathophysiology are poorly understood, and third, immune stimulation is thought to play a role in the pathogenesis of the condition. Because vaccines provoke an immune response, albeit targeted to a specific antigen, it can be tempting to invoke a superficially plausible causal pathway when adverse events with a suspected immune aetiology arise shortly after vaccination.
Multiple Sclerosis and Hepatitis B Vaccine
Universal hepatitis B vaccine was recommended by the World Health Organization in the early 1990s to protect against the hepatitis B virus, which can cause chronic liver damage and cancer. Following this recommendation, France carried out a mass vaccine campaign in 1994. Shortly after, reports of cases of multiple sclerosis (MS) with onset or relapse after vaccination were reported, leading to the hypothesis that the vaccine could cause an acute autoimmune reaction in susceptible persons soon after administration. With a lack of adequate background rates of MS in the vaccinated population to put the reported cases into perspective, mistrust in the vaccine soon grew and the vaccine programme was subsequently suspended.
A systematic review and meta-analysis by Mouchet et al. published in 2018 that included 13 studies with a control group found no evidence of an increased risk. The overall adjusted risk ratios for MS was 1.19 (95% confidence interval [CI] 0.93–1.52) and for central demyelination was 1.25 (95% CI 0.97–1.62) [25]. Within the systematic review, there was one study that found a significant association using a primary care database from England [26]. This study was unable to adjust for all risk factors and additionally no routine hepatitis B vaccination programme was in place at the time with most of the vaccine delivered via occupational health departments whose records may not be routinely transferred to primary care databases.
France continues to have suboptimal vaccine coverage [27, 28] and has the lowest level of confidence in vaccine safety in Europe [29, 30]. This demonstrates the need to have robust methods in place to rapidly respond to such scares because once confidence is lost in a vaccine it is difficult to restore and may generate a more general lack of confidence in vaccine safety.
Guillain–Barré Syndrome and Vaccines
Guillain–Barré syndrome (GBS) is the most common cause of acute neuromuscular paralysis in the developed world resulting in muscle weakness and sometimes paralysis, which can lead to respiratory failure and a death rate in up to 13% [31]. The strongest evidence of a causal link with a vaccine was obtained during the 1976 US swine influenza vaccine programme in military personnel, which was found to be associated with a risk of one case per 100,000 and resulted in the suspension of the vaccine programme [32]. Since then, GBS has been a potential vaccine-associated adverse event of interest particularly for vaccines given in adolescence, an age coinciding with the age at which autoimmune diseases are often diagnosed.
Guillain–Barré Syndrome and Influenza Vaccine
A meta-analysis in 2015 by Martin Arias et al. included 39 studies published between 1981 and 2014 and found that the receipt of any influenza vaccine carried a small increased risk of GBS of 1.41 (95% CI 1.20–1.66). The overall relative risk for GBS after seasonal vaccine was marginally increased at 1.22 (95% CI 1.01–1.48), with a somewhat larger relative risk of 1.84 (1.36–2.50) for the 2009 H1N1 pandemic vaccine but this was not significantly higher than the relative risk for seasonal vaccine [33]. The authors did not find any statistically significant differences by geographical region nor between adjuvanted and unadjuvanted vaccines. An earlier meta-analysis of studies using the SCCS method also found a small elevated risk of GBS after the monovalent H1N1 pandemic vaccine, with an RI of 2.42 (95% CI 1.58–3.72) in the 42 days following vaccination [34]. Similarly, Salmon et al. found an RI of 2.35 (95% CI 1.42–4.01) in a large study in the USA [35].
In contrast, a strong association between GBS and a preceding influenza-like-illness was shown in a study in England using primary care data and the SCCS method. No association was seen with influenza vaccine in the 0–90 days after administration (RI 0.76 [95% CI 0.41–1.40]) but a significantly increased risk was found in the 90 days after influenza-like illness (RI 7.35 [95% CI 4.36–12.38]) [36].
These studies show that a small overall risk of GBS after influenza vaccine probably does exist with a slightly larger risk after the 2009 monovalent pandemic vaccine. The mechanism may be multi-factored with the risk varying with the vaccine used, co-circulation of other infections and the inherent susceptibility to developing GBS. However, the small risk that exists does not outweigh the risk of developing GBS after influenza itself.
Guillain-Barré Syndrome and Human Papilloma Virus Vaccine
Human papilloma virus vaccine is given at an age when autoimmune disorders are often diagnosed. Following a French study reporting a signal for GBS after human papilloma virus vaccination, a study was conducted in England identifying GBS cases in a national hospital discharge database (Hospital Episode Statistics) [37]. Primary care practitioners were then contacted for the vaccination history and asked to confirm GBS diagnosis and provide an onset date and send supporting documentation. In a self-controlled case-series analysis of 101 cases with a record of human papilloma virus vaccination, there were episodes in the 0- to 91-day risk period after any dose with no significant increased risk, RI 1.04 (95% CI 0.47–2.28). The analysis was also stratified by manufacturer (of either the quadrivalent or bivalent product); there was no difference in the RI between products and no significant increased risk for either manufacturer.
Narcolepsy and Pandemrix™
The pandemic influenza vaccine, Pandemrix™, was the most widely used vaccine in Europe during the 2009 pandemic. It was a monovalent H1N1 pdm 09 vaccine containing AS03, a powerful oil-in-water adjuvant. Uptake of the vaccine varied between countries with high coverage of 75% in children in Finland [38] and lower coverage in England where children in a risk group eligible for the seasonal influenza vaccine and later all children under 5 years of age were targeted, with uptake being 37% and 24%, respectively. In England, Pandemrix™ was also used in the influenza season 2010/11 because of a shortage of seasonal influenza vaccine.
In August 2010, concerns were raised in Finland and Sweden, where vaccine coverage was high, about a possible association between narcolepsy and Pandemrix™ when a large increase in cases of narcolepsy in vaccinated cases was reported by sleep centres [38, 39]. A subsequent cohort study in Finland reported a 13-fold increased risk of narcolepsy following Pandemrix™ in children aged 4–19 years, the majority of whom had onset within 3 months of vaccination and almost all within 6 months [38, 40].
Narcolepsy was a totally unexpected adverse event and the early reports were met with initial scepticism in the global vaccine community. The World Health Organization Global Advisory Committee in Vaccine Safety issued a statement in April 2011 stating “no excess of narcolepsy has been reported from several other European states where Pandemrix was used” and “It seems likely that some as yet unidentified additional factor was operating in Sweden and Finland”. However, it was unlikely that narcolepsy would be identified by passive surveillance systems in other countries where Pandemrix™ coverage was low given the low background incidence of the condition and the complexity and frequent delays in diagnosis.
To assess this risk identified in Finland, the Health Protection Agency (now Public Health England) performed a study in sleep centres in England where the majority of children with sleep disorders are seen. This study identified a 14-fold increased risk in those vaccinated with Pandemrix™ [14] with the attributable risk estimated to be 1.9 per 100,000 doses. This demonstrated that even in a country were vaccine coverage was low, the association can be demonstrated using robust epidemiological methods.
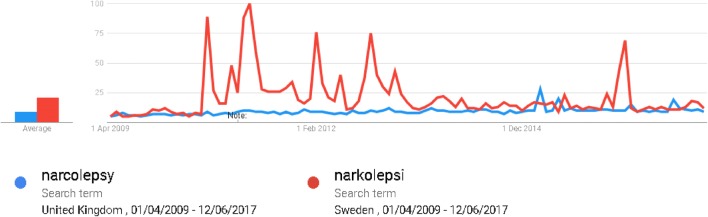
The study of the relationship between narcolepsy and Pandemrix™ has been an epidemiological challenge in terms of identifying the cases and their vaccine histories in a non-biased manner. Not only can the diagnosis be lengthy and complex, but admitted patient care databases, which are widely used for a non-biased ascertainment of cases in vaccine safety studies, are incomplete as patients experiencing narcolepsy may not be admitted and if they are admitted, the admission date is not an accurate reflection of the onset of the narcolepsy symptoms leading to misclassification bias. An important consideration when selecting cases is the awareness of the hypothesised association. This awareness may lead to an increased reporting of cases known to be vaccinated and has two aspects; public awareness and professional awareness. First, this heightened awareness may lead to vaccinated individuals presenting to healthcare institutions and being diagnosed earlier than unvaccinated cases leading to ascertainment bias. If a condition has an insidious onset making the recall of the first symptom difficult to determine, media attention may lead to a differential recall of the symptom-onset date in the vaccinated cases. Using source documents, which were created prior to any media attention in the country of study, can address this potential recall bias. Professional awareness is likely to occur even if media attention is low, as health professionals in the specialty will be aware of current topics of interest through professional bodies and literature. Differential misclassification bias will occur if cases known to have been vaccinated are more likely to be assigned a diagnosis of narcolepsy than unvaccinated cases. In the study from England, public awareness of the association was assessed by analysing Google searches for “narcolepsy” in the period of interest and found there was little activity in the UK compared to Sweden (Fig. 2) [14].
Fig. 2.
Google searches for “narcolepsy” or “narkolepsi” from UK and Sweden for the period 01/04/2009 to 12/06/2017. Relative scaling is based on the average traffic
Even with these practical challenges, there has now been a consistent strong association demonstrated in countries that used Pandemrix™ but no association has been seen with other pandemic or seasonal vaccines [17]. As with all vaccine safety studies, but particularly in the case of narcolepsy and Pandemrix™ where the association was completely unexpected, the key to demonstrating causality was consistency of results from well-designed studies in different settings.
Conclusions
The answer to the question of whether vaccination can cause neurological disease is multifaceted. The evidence does not support an association between MS and the hepatitis vaccine, while for GBS and influenza vaccines the evidence suggests a small increased risk though it is much smaller than the risk from a natural influenza virus infection. The now established association between narcolepsy and Pandemrix™ should act as a lesson for the vaccine safety community that sometimes unexpected but serious conditions can arise and need to be investigated rapidly however biologically implausible. The neurological vaccine safety issues outlined here demonstrate that rapid assessments of safety signals are needed to ensure that public confidence is maintained in national immunisation programmes. The confirmation of a signal and estimation of the magnitude of vaccine-attributable risk will require consistent results from a number of well-designed epidemiological studies, preferably conducted in different settings. As the experience with narcolepsy has shown, not all vaccine safety concerns can be anticipated on the basis of biologically plausible and thus predictable effects. As new vaccines are introduced, the basis of discussions on vaccine safety should be the acceptance that vaccination can carry a small risk but that this risk needs to be balanced against the enormous individual and public health benefits.
Compliance with Ethical Standards
Funding
Public Health England, National Infection Service, Immunisation and Countermeasures Division has provided vaccine manufacturers with post-marketing surveillance reports, which the Marketing Authorisation Holders are required to submit to the UK licensing authority in compliance with their Risk Management Strategy. A cost recovery charge is made for these reports.
Conflict of interest
Julia Stowe, Nick Andrews and Elizabeth Miller have no conflicts of interest that are directly relevant to the content of this article.
References
- 1.World Health Organization. The power of vaccines: still not fully utilized. 2019. https://www.who.int/publications/10-year-review/vaccines/en/. Accessed 27 Sep 2019.
- 2.Andrews N, Miller E, Taylor B, Lingam R, Simmons A, Stowe J, et al. Recall bias, MMR, and autism. Arch Dis Child. 2002;87(6):493–494. doi: 10.1136/adc.87.6.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller E, Stowe J. Vaccine safety surveillance. In: Talbot J, Aronson JK (eds) Stephens’ detection and evaluation of adverse drug reactions. 6th ed. Wiley-Blackwell. 2012.
- 4.Hviid A. Postlicensure epidemiology of childhood vaccination: the Danish experience. Expert Rev Vaccines. 2006;5(5):641–649. doi: 10.1586/14760584.5.5.641. [DOI] [PubMed] [Google Scholar]
- 5.Farrington CP. Control without separate controls: evaluation of vaccine safety using case-only methods. Vaccine. 2004;22(15–16):2064–2070. doi: 10.1016/j.vaccine.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 6.Andrews N.J. Statistical assessment of the association between vaccination and rare adverse events post-licensure. Vaccine. 2001;20:S49–S53. doi: 10.1016/S0264-410X(01)00280-8. [DOI] [PubMed] [Google Scholar]
- 7.Taylor B, Miller E, Farrington C, Petropoulos M-C, Favot-Mayaud I, Li J, et al. Autism and measles, mumps, and rubella vaccine: no epidemiological evidence for a causal association. Lancet. 1999;353(9169):2026–2029. doi: 10.1016/S0140-6736(99)01239-8. [DOI] [PubMed] [Google Scholar]
- 8.Andrews N, Stowe J, Al-Shahi Salman R, Miller E. Guillain-Barre syndrome and H1N1 (2009) pandemic influenza vaccination using an AS03 adjuvanted vaccine in the United Kingdom: self-controlled case series. Vaccine. 2011;29(45):7878–7882. doi: 10.1016/j.vaccine.2011.08.069. [DOI] [PubMed] [Google Scholar]
- 9.Miller E, Waight P, Farrington CP, Andrews N, Stowe J, Taylor B. Idiopathic thrombocytopenic purpura and MMR vaccine. Arch Dis Child. 2001;84(3):227–229. doi: 10.1136/adc.84.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stowe J, Andrews N, Ladhani S, Miller E. The risk of intussusception following monovalent rotavirus vaccination in England: a self-controlled case-series evaluation. Vaccine. 2016;34(32):3684–3689. doi: 10.1016/j.vaccine.2016.04.050. [DOI] [PubMed] [Google Scholar]
- 11.Kramarz P, France EK, Destefano F, Black SB, Shinefield H, Ward JI, et al. Population-based study of rotavirus vaccination and intussusception. Pediatr Infect Dis J. 2001;20(4):410–416. doi: 10.1097/00006454-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Carlin JB, Macartney KK, Lee KJ, Quinn HE, Buttery J, Lopert R, et al. Intussusception risk and disease prevention associated with rotavirus vaccines in Australia’s National Immunization Program. Clin Infect Dis. 2013;57(10):1427–1434. doi: 10.1093/cid/cit520. [DOI] [PubMed] [Google Scholar]
- 13.Black C, Kaye JA, Jick H. MMR vaccine and idiopathic thrombocytopaenic purpura. Br J Clin Pharmacol. 2003;55(1):107–111. doi: 10.1046/j.1365-2125.2003.01790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller E, Andrews N, Stellitano L, Stowe J, Winstone AM, Shneerson J, et al. Risk of narcolepsy in children and young people receiving AS03 adjuvanted pandemic A/H1N1 2009 influenza vaccine: retrospective analysis. BMJ. 2013;346:f794. doi: 10.1136/bmj.f794. [DOI] [PubMed] [Google Scholar]
- 15.Stowe J, Andrews N, Kosky C, Dennis G, Eriksson S, Hall A, et al. Risk of narcolepsy after AS03 adjuvanted pandemic A/H1N1 2009 influenza vaccine in adults: a case-coverage study in England. Sleep. 2016;39(5):1051–1057. doi: 10.5665/sleep.5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farrington CP. Estimation of vaccine effectiveness using the screening method. Int J Epidemiol. 1993;22(4):742–746. doi: 10.1093/ije/22.4.742. [DOI] [PubMed] [Google Scholar]
- 17.Sarkanen TO, Alakuijala APE, Dauvilliers YA, Partinen MM. Incidence of narcolepsy after H1N1 influenza and vaccinations: systematic review and meta-analysis. Sleep Med Rev. 2018;38:177–186. doi: 10.1016/j.smrv.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Amirthalingam G, Gupta S, Campbell H. Pertussis immunisation and control in England and Wales, 1957 to 2012: a historical review. Euro Surveill. 2013;18:38. doi: 10.2807/1560-7917.ES2013.18.38.20587. [DOI] [PubMed] [Google Scholar]
- 19.Baker JP. The pertussis vaccine controversy in Great Britain, 1974-1986. Vaccine. 2003;21(25–26):4003–4010. doi: 10.1016/S0264-410X(03)00302-5. [DOI] [PubMed] [Google Scholar]
- 20.Miller E, Goldacre M, Pugh S, Colville A, Farrington P, Flower A, et al. Risk of aseptic meningitis after measles, mumps, and rubella vaccine in UK children. Lancet. 1993;341(8851):979–982. doi: 10.1016/0140-6736(93)91069-X. [DOI] [PubMed] [Google Scholar]
- 21.Miller E, Andrews N, Stowe J, Grant A, Waight P, Taylor B. Risks of convulsion and aseptic meningitis following measles-mumps-rubella vaccination in the United Kingdom. Am J Epidemiol. 2007;165(6):704–709. doi: 10.1093/aje/kwk045. [DOI] [PubMed] [Google Scholar]
- 22.Taylor B, Lingam R, Simmons A, Stowe J, Miller E, Andrews N. Autism and MMR vaccination in North London; no causal relationship. Mol Psychiatry. 2002;7(Suppl. 2):S7–S8. doi: 10.1038/sj.mp.4001163. [DOI] [PubMed] [Google Scholar]
- 23.Miller E, Andrews N, Grant A, Stowe J, Taylor B. No evidence of an association between MMR vaccine and gait disturbance. Arch Dis Child. 2005;90(3):292–296. doi: 10.1136/adc.2003.048165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andrews N, Miller E, Grant A, Stowe J, Osborne V, Taylor B. Thiomerosal exposure in infants and developmental disorders: a retrospective cohort study in the United Kingdom does not support a causal association. Pediatrics. 2004;114(3):584–591. doi: 10.1542/peds.2003-1177-L. [DOI] [PubMed] [Google Scholar]
- 25.Mouchet J, Salvo F, Raschi E, Poluzzi E, Antonazzo IC, De Ponti F, et al. Hepatitis B vaccination and the putative risk of central demyelinating diseases: a systematic review and meta-analysis. Vaccine. 2018;36(12):1548–1555. doi: 10.1016/j.vaccine.2018.02.036. [DOI] [PubMed] [Google Scholar]
- 26.Hernan MA, Jick SS, Olek MJ, Jick H. Recombinant hepatitis B vaccine and the risk of multiple sclerosis: a prospective study. Neurology. 2004;63(5):838–842. doi: 10.1212/01.WNL.0000138433.61870.82. [DOI] [PubMed] [Google Scholar]
- 27.WHO and UNICEF. Estimates of national immunization coverage. France. https://www.who.int/immunization/monitoring_surveillance/data/fra.pdf. Accessed 30 Sept 2019.
- 28.European Centre for Disease Prevention and Control. Measles vaccination coverage (second dose, 2014–2016). 2017. https://ecdc.europa.eu/en/publications-data/vaccination-coverage-second-dose-measles-containing-vaccine-eueea-2017. Accessed 30 Sept 2019.
- 29.Larson HJ, de Figueiredo A, Xiahong Z, Schulz WS, Verger P, Johnston IG, et al. The state of vaccine confidence 2016: global insights through a 67-country survey. EBioMedicine. 2016;12:295–301. doi: 10.1016/j.ebiom.2016.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verger P, Fressard L, Collange F, Gautier A, Jestin C, Launay O, et al. Vaccine hesitancy among general practitioners and its determinants during controversies: a national cross-sectional survey in France. EBioMedicine. 2015;2(8):891–897. doi: 10.1016/j.ebiom.2015.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seneviratne U. Guillain-Barre syndrome. Postgrad Med J. 2000;76(902):774–782. doi: 10.1136/pmj.76.902.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, Keenlyside RA, Ziegler DW, Retailliau HF, et al. Guillain-Barre syndrome following vaccination in the National Influenza Immunization Program, United States, 1976–1977. Am J Epidemiol. 1979;110(2):105–123. doi: 10.1093/oxfordjournals.aje.a112795. [DOI] [PubMed] [Google Scholar]
- 33.Martin Arias LH, Sanz R, Sainz M, Treceno C, Carvajal A. Guillain-Barre syndrome and influenza vaccines: a meta-analysis. Vaccine. 2015;33(31):3773–3778. doi: 10.1016/j.vaccine.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 34.Dodd CN, Romio SA, Black S, Vellozzi C, Andrews N, Sturkenboom M, et al. International collaboration to assess the risk of Guillain Barre syndrome following influenza A (H1N1) 2009 monovalent vaccines. Vaccine. 2013;31(40):4448–4458. doi: 10.1016/j.vaccine.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 35.Salmon DA, Proschan M, Forshee R, Gargiullo P, Bleser W, Burwen DR, et al. Association between Guillain-Barre syndrome and influenza A (H1N1) 2009 monovalent inactivated vaccines in the USA: a meta-analysis. Lancet. 2013;381(9876):1461–1468. doi: 10.1016/S0140-6736(12)62189-8. [DOI] [PubMed] [Google Scholar]
- 36.Stowe J, Andrews N, Wise L, Miller E. Investigation of the temporal association of Guillain-Barre syndrome with influenza vaccine and influenzalike illness using the United Kingdom General Practice Research Database. Am J Epidemiol. 2009;169(3):382–388. doi: 10.1093/aje/kwn310. [DOI] [PubMed] [Google Scholar]
- 37.Andrews N, Stowe J, Miller E. No increased risk of Guillain-Barre syndrome after human papilloma virus vaccine: a self-controlled case-series study in England. Vaccine. 2017;35(13):1729–1732. doi: 10.1016/j.vaccine.2017.01.076. [DOI] [PubMed] [Google Scholar]
- 38.Partinen M, Saarenpaa-Heikkila O, Ilveskoski I, Hublin C, Linna M, Olsen P, et al. Increased incidence and clinical picture of childhood narcolepsy following the 2009 H1N1 pandemic vaccination campaign in Finland. PloS One. 2012;7(3):e33723. doi: 10.1371/journal.pone.0033723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Persson I, Granath F, Askling J, Ludvigsson JF, Olsson T, Feltelius N. Risks of neurological and immune-related diseases, including narcolepsy, after vaccination with Pandemrix: a population- and registry-based cohort study with over 2 years of follow-up. J Intern Med. 2014;275(2):172–190. doi: 10.1111/joim.12150. [DOI] [PubMed] [Google Scholar]
- 40.Nohynek H, Jokinen J, Partinen M, Vaarala O, Kirjavainen T, Sundman J, et al. AS03 adjuvanted AH1N1 vaccine associated with an abrupt increase in the incidence of childhood narcolepsy in Finland. PloS One. 2012;7(3):e33536. doi: 10.1371/journal.pone.0033536. [DOI] [PMC free article] [PubMed] [Google Scholar]