Abstract
Aflatoxin B1 (AFB1) contamination in foods is an important health challenge for low-and middle-income countries in subtropical regions. AFB1 has been detected in a variety of foodsin Guangzhou, while the risk of dietary exposure is unknown. This study aimed to assess the probabilistic risk of dietary exposure to AFB1 contamination in food stuffs in Guangzhou by using margin of exposure (MOE) and quantitative liver cancer risk approaches. A total of1854 AFB1-contaminated foodstuffs were sampled in supermarkets, agricultural markets, retail shops, and family workshops from 11 districts of Guangzhou, and AFB1 content was determined by HPLC-fluorescence detector. In total, 9.9% (184/1854) of the test samples had AFB1 concentrations above the limit of detection. Home-made peanut oil had the highest AFB1 concentration, with a mean value of 38.74 ± 47.45 μg kg−1. The average MOE levels of Guangzhou residents ranged from 100 to 1000. The risk of liver cancer was 0.0264 cancers (100,000 population year)−1. The health risks of suburban people were higher than those of urban people, and home-made peanut oil was the main contributorto dietary exposure to AFB1 among suburban residents in Guangzhou. The production of home-made peanut oil should be supervised to reduce the risk of AFB1 exposure.
Subject terms: Environmental monitoring, Risk factors
Introduction
Aflatoxins (AFs) are mycotoxins produced by the common fungi Aspergillus flavusand Aspergillus parasiticus1 and have been found in a wide range of crops such as maize, peanut, and walnut and their derived products2. There are four major aflatoxins (AFB1, AFB2, AFG1, and AFG2) produced by the two fungi that commonly found in contaminated crops3–5. AFB1 and AFB2 can be produced by A. flavus (both S and L strains) and A. parasiticus, whileAFG1 and AFG2 can be produced by A. flavus S strains and A. parasiticus5,6. AFB1 is considered the most toxic carcinogenwhich is classified as Group 1 human carcinogen by the International Agency for Research on Cancer (IARC)that induces mainly liver cancer7–9 and to a lesser degree rectal cancer10.
AFB1 is commonly found in cereals and nuts11, and it has attracted concern in lessdeveloped tropical regions12–15. Previous studies showed that AFs were found in 5%-30%of raw peanuts and peanut products in major peanut-producing regions in China16. Since some crops susceptible to AFs contamination, such as peanuts, are commonly consumed, it is hard to achieve zero exposure to AFs. Therefore, it is important to reduce the exposure to total AFsby establishing regulatory limits to AFs.The Codex Alimentarius Commission, the Joint Food and Agriculture Organization (FAO), and the World Health Organization (WHO) Food Standards Program jointly adopted a maximum level of 15 μg kg−1 for total AFsin unprocessed peanuts17. The European Commission regulation (EC) No. 1881/2006 set a maximum limit for AFB1 of 2 μg kg−1 for peanuts and cereals that are intended for direct consumption18. In China, the National Food Safety Standard set the limit of 20 µg kg−1 for AFB1 in peanut and its products and in maize and its products19. In addition to setting regulatory limits for AFB1,it is also necessary to conduct dietary exposure risk assessments in the population. A low-dose extrapolation approach introducedby the Joint FAO/WHO Expert Committee on Food Additives(JECFA)20 in 1997 and the margin of exposure (MOE) method proposed at the 64th JECFAmeeting in 200521 were both recommended and have been widely used worldwide14,22,23 to assess the risk of dietary exposure toAFB1.
JECFAperformed adietary exposure risk assessment for AFs as early as 1997. However, the data used were not considered representativebecause of the bias for the highest contamination level in food sampling24. In view of this scenario, national or regional AFhealth risk exposure assessments have beenundertakensince then,especially in tropical and subtropical regions12–15. In general, economically developed countries have a lower risk of health hazard assessment than developing or less developed countries. Dietary exposureto AFsestimated by the European Union ranged from 0.93 to 2.45 ng kg−1 bw day−1 for the lower bound to the upper bound25. In the United States, exposure was estimatedat 2.7 ngkg−1 bw day−1 26 In Asia, the population of Japan27, with anintake ranging from 0.003 to 0.004 ngkg−1 bw day−1, hasa lower riskthan those of Indonesia13 (from 0.02 to 427.8 ngkg−1 bw day−1) and Vietnam23 (from 35.0 to 43.7 ngkg−1 bw day−1).
Guangzhou, one of the major metropolitan areas in southern China, is located in Guangdong Province and has more than 14 million people. Due to the subtropical monsoon climate (with a relatively humid environment), Guangzhou,with coexisting urban and rural areas, has been facing the challenge of AFB1contamination in foodstuffs28–31. Warm and humid conditions are favourable for A.flavus growth in some types of food, such as peanut and maize. A survey of foodstuffs (rice, wheat flour, peanut and peanut oil, corn flour and corn oil, and soybean) that areprone to contamination by AFs found that the overall detection rate of AFB1 was 31.7%, with the highest concentration of 39.3 µg kg−1 found in peanut oil29. However, the health risk of AFB1 dietary exposure to local residents was unknown.
In this context, Guangzhou Center for Disease Control and Prevention conducted a surveillance programme for three consecutive years to monitor contamination of foodstuffsby AFB1. We present the probabilistic risk of dietary exposure to AFB1 among Guangzhou residents by using MOE andquantitative liver cancer risk approaches.
Materials and methods
Sampling
From January 2015 to December 2017, typical AFB1-contaminated foodstuffs, includingrice and rice products, wheat and wheat products, maize and maize products, vegetable oil(including home-made peanut oil), nuts, and tea,were bought from household supply retail shops covered in all eleven districts of Guangzhou. These foods were considered theprobable sources of AFB1exposure in Guangzhou29.
Individual streetswere set as the sampling units. Street information was obtained from local governmental authorities. Three streets (two central streets and one remote street) were randomly selectedand stratified by district and type of streets (central or remote) using computer-generatedrandom digits. A total of 33 streets (22 central streets and 11 remote streets) were selected as food sampling sites. Trained investigators bought foodstuffs fromsupermarkets, agricultural markets, retail shops, and family workshops. Finally, a total of 1854 single-species food samples were included in this study, and alist of sampling sites isshown in Supplementary Table 1.
Analytical procedure (high-performance liquid chromatography)
In accordance with a previouslyvalidated method, the procedure to determine AFB1in foods was applied with some slight modifications32–34. First,for solid samples, the sampling quantity should be more than 1 kg, and the sample should be crushed by a high-speed crusher and then sieved to make particles smaller than 2 mm. The test sieve should be mixed evenly, condensed to 100 g, and then stored in a sample bottle and sealed for storage until detection. The sampled amount of liquid samplesshould be greater than 1 L. For bagged, bottled and other packaged samples, at least 3 packages (the same batch or number) should be collected, all liquid samples should be mixed in a container with a homogenizer, and any 100 g (mL) of the liquid samples can be tested.The prepared samples were stored in a refrigerator at 0~4 °C for no more than 48 hours before analysis. Second,for solid samples, 5 g was weighed (accurate to 0.01 g) into a 50 mL centrifuge tube, 20.0 mL methanol-water solution (70 + 30) was added, and the sample was mixed by vortex, put into an ultrasonic oscillator for 20 min (or a homogenizer for 3 min), and centrifuged at 6000rmin−1 for 10 min (or applied to glass-fibre filter paper after homogenization).The supernatant was taken for later use.For vegetable oil, 5 g (accurate to 0.01 g) was placed into a 50 mL centrifuge tube, 20 mL methanol-water solution (70 + 30) was added, and the sample was mixed by vortex, put into ultrasonic oscillator for 20 min, and centrifuged at 6000rmin−1 for 10 min.The supernatant was taken for use.Whatman GF/A glass-fibre filter paper was used to filtrate 10 ml extract and to collect the filtrate in the clean container. In addition, 5 ml extract was diluted with 20 ml purified water and filtered before being tested. AFB1 content was determined by an HPLC-fluorescence detector (excitation, 360 nm; emission, 450 nm) (Waters Alliance e2695) by using post-column-photochemical reactor derivatization.
Quality control
The limit of detection (LOD)in our study was0.1 μg kg−1 and determined from a signal-to-noise ratio equal to 3:1,and the limit of quantification(LOQ) was determined as the point at which this ratio was more than 10:1. Recovery rates in each foodwere ascertained by spiking with AFB1, and the rates rangedfrom 95% to 105%.
Estimation of daily food consumption
Food consumption data were derived from a national food consumption survey of urban and rural residents in Guangzhou performed in 2011. Information on dietary intake was based on a three-day consecutive 24-h recall questionnaire in combination with the weighing method for edible cooking oil. Details of the methodology are available in our previously published manuscripts35,36. In sum, 2960 residents from 998 households were surveyed in the study. Among the subjects, 1416 were male and 1544 were female. Urban residents accounted for 63.8% (1888) of the total, and suburban residents accounted for 36.2% (1072). The age ranged from 3 to 86 years, and the mean age was 32 years. The age groupsof 3 to 6 years old, 7 to 17 years old, 18 to 59 years old, and 60 years old and above accounted for 6.7% (199), 21.5% (637), 58.6% (1734), and 13.2% (390) of the total people, respectively16,37,38.
In this study, vegetable oils collected in the survey included peanut oil, corn oil, tea seed oil, and soybean oil. According to the production conditions, vegetable oils were classified into commercial vegetable oil and bulk vegetable oil. Commercial vegetable oil was defined as the vegetable oil produced by licensed manufacturers and had underwent sampling inspection by the Chinese Food and Drug Administration. Bulk vegetable oil was produced by family workshops in the suburban areas, where this inspection usually did not in place. In this case, bulk vegetable oil referred to home-made peanut oil.
Estimation of daily intake of AFB1
The total dietary intake of AFB1 was calculated as an estimateddaily intake (EDI) by using Eq. (1)39.
| 1 |
EDI was the estimation of daily dietary AFB1 intake (ng kg−1 body weight day−1). Di was the daily consumption of each food in each age group (gperson−1 day−1). Mi was the mean level of AFB1 in each food category (ngkg−1). When AFB1 was not detected in certain types of food, Mi was then assumed to be LOD/240. The WHO recommended an alternative method to calculate the undetected value41–43. When the undetected samplevalueswere less than 60%, the non-detected value was replaced by the value of LOD / 2.When more than 80% of the sample values were not detected, the undetected valueswere replaced by 0 or LOD, respectively, as the lower bound and upper bound. However, in our study, the LODappears to be highcompared with those of other regions, such as Japan and Taiwan, China, where LODs ranged from0.001 to0.1 μg kg−1,but it was consistent withvalue in the Chinese National Food Contamination Monitoring Program.If the upper bound is used to estimate the mean value, the exposure risk might be overestimated. Our approach was to replace all the undetected values by LOD / 2, which ishighly conservative and doesn’t overestimatethe risk. W was the body weight of each respondent (kg). The average weight of respondents aged 3 to 6 years was determined to be 20 kg44, the average weight of respondents aged 7 to 17 years was determined to be 40 kg45, and members of the other two age groups were determined to average 60 kg46. Mean daily exposure to AFB1 was estimated by using the @RISK software.
Risk characterization
Margin of exposure (MOE)
TheMOE method estimates the risk of genotoxic carcinogens21. It calculates the risk by the ratio of carcinogenic dose (or population carcinogenic dose) to population intake. The higher the MOE value is, the lower the risk of genetic carcinogen exposure.
MOE is calculated as the ratio between the points of departure (PODs) on the dose-response curve (animal or population carcinogenic dose) for a critical effect and the exposure level of the population. The formula is as follows: MOEs = PODs/dietary exposure (EDI). The European Food Safety Authority (EFSA) Scientific Panel on Contaminants in the Food Chain proposed the use of a benchmark dose lower confidence limit for 10% extra risk (BMDL10) and a benchmark dose lower confidence limit for 1% extra risk (BMDL1) for characterizing the MOE as PODs25. The value of BMDL10 (the lower limit for the 95% confidence interval of the 10% incidence of liver cancer in the control group) was 340 ng kg−1 bw day−1 for AFB1as referenced by the EFSA25. In reference to the dose-response relationship based on the data of Peers47,48 and Carlborg49, the value of BMDL1 (the lower limit for the 95% statistical confidence interval of the 1% incidence of liver cancer in the control group) was estimated as 78 ng kg−1 bw day−1. The reference value for a chronic dose that causes 25% of test animals to develop liver cancer (T25) during their standard lifespan was varied. The most widely used values were390 ng kg−1bw day−1(according to Benford50)and 500 ng kg−1 bw day−1(recommended by Wogen51). For safety considerations, we referred to the conservative T25 value of 390 ng kg−1bw day−1as the POD.
Quantitative risk assessment of liver cancer
Quantitative liver cancer riskassessment is one of the popular low-dose extrapolationapproaches used for AFB1 dietary exposure risk assessment. The low-dose extrapolation approachassumes that there is a linear dose-response relationship between the carcinogenic dose and the incidence of cancer in a population within a low-dose response range15,52–54. This methodtakes advantage of the exposure andpotency of carcinogens, providing quantitative data on human carcinogenic risk.This method was consistent with the formula proposed by the JECFA20. Because hepatitis B could synergistically increase the risk of AFB1-induced liver cancer, we separately estimated the carcinogenic potency in people who had hepatitis B and in peoplewho were hepatitis B negative. Studies have shown that the carcinogenic efficacy of AF in hepatitis B virus carriers is 30 times higher than that in nonviral carriers27,53,55. For hepatitis B surface antigen-positive individuals (PHBsAg + ), the potency was 0.3 cancers per year ng−1 AFB1 kg−1bw day−1per 100,000 population. For hepatitis B surface antigen-negative individuals (PHBsAg-), the potency was 0.01 cancers per year ng−1AFB1 kg−1bw day−1per 100,000 population53,56,57.
The cancer riskwas estimated by using Eq. (2).
| 2 |
The prevalence of HBsAg+ was estimated to be 12.5% in Guangzhou, with 7.1% in urban areas and 16.1% in suburban areas58.
Statistical analysis
Descriptive analysis was performed to describe the concentration of AFB1 in foodstuffs by using the mean ± standard deviation. Probabilistic risk assessment model calculations for AFB1 dietary exposure, MOE values, and cancer risk were performed by @RISK software (Palisade Corporation, 7.6. Industrial, 2018) based on a Monte Carlo simulation with 10000 iterations. The results are displayed as the mean values (range from the 5th percentile to the 95th percentile).
Due to the difference in vegetable oil consumption habits between urban and suburban residents, we conducted a sub-analysis by stratifying suburban residents from urban residents to see the difference in dietary exposure to AFB1. We found that the dietary intake of home-made peanut oil was reported among only suburban residents because such oil was predominantly sold in suburban areas.
Results and discussion
AFB1 levels in foods
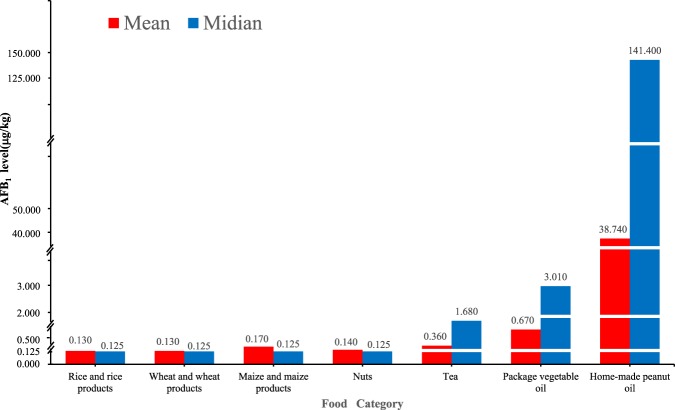
The levels of AFB1 in 1854 food samples are summarized in Table 1. The levels of AFB1 levels in food samples between 2015, 2016, and 2017 were comparable (see Supplementary Table 2).The mean level of AFB1 in all samples was 1.4 μg kg−1, and the 50th percentile (P50) and 95th percentile (P95) values were not detected (ND) and 2.2 μg kg−1, respectively. In total, 9.9% (184/1854) of the test samples had AFB1 levels above the LOD. Home-made peanut oil had the highest concentration of AFB1, with detected values ranging from 0.26 to 283.0 μg kg−1, a median value of 3.21 μg kg−1 and a mean value of 38.74 ±47.45 μg kg−1. In rice and rice products, wheat and wheat products, maize and maize products, and nuts, AFB1 concentration levels were very low, and most results were under the detection limit (Fig. 1).
Table 1.
AFB1 levels of foods in Guangzhou from2015 to 2017.
| Food Category | Number of samples | <LOD | AFB1 level(μg kg−1) | |||
|---|---|---|---|---|---|---|
| Mean ±standard deviation | P50 | P95 | Range | |||
| Rice and rice Products | 490 | 483 | 0.13 ± 0.001 | ND | ND | 0.28~1.00 |
| Wheat and wheat products | 436 | 430 | 0.13 ± 0.001 | ND | ND | 0.28~1.46 |
| Maize and maize Products | 339 | 336 | 0.17 ± 0.001 | ND | ND | 1.50~6.30 |
| Nuts | 96 | 93 | 0.14 ± 0.001 | ND | ND | 0.62~1.37 |
| Tea | 128 | 105 | 0.36 ± 0.62 | ND | 1.68 | 0.25~4.0 |
| Vegetable oil a | 365b | 223e | 6.32±25.99 | ND | 30.45 | 0.26~283.0 |
| 1. Commercial vegetable oil | 269c | 201 f | 0.67 ±1.81 | ND | 3.01 | 0.35~7.30 |
| 2 Home-made peanut oil | 96d | 22 g | 38.74 ±47.45 | 3.21 | 141.40 | 0.26~283.0 |
| Total | 1854 | 1670 | 1.40±11.94 | ND | 2.20 | 0.25~283.0 |
a = vegetable oil equalsthe sum of commercial vegetable oil and home-made peanut oil.
b = c+d.
e = f+g.
AFB1: Aflatoxin B1; LOD: Limit of detection; ND: Not detected.
Figure 1.
Aflatoxin B1 levels in seven kinds of foods in Guangzhou City.
Comparisonsof data from someSoutheast Asian countries show that the level of AFB1 contamination in some foods in Guangzhou, such as rice and maize, was relatively lower than that in some foods in Vietnam, where AFB1 contamination levels in maize, rice products and other cereals were 2.1~31.1 μg kg−1 59, 2.7 μg kg−1 and 3.2 μg kg−1 23, respectively. In addition, the contamination level of AFB1 in nuts (including peanut) was low in Guangzhou compared with other provinces of China16 and Malaysia (ranging from 0.40 μg kg−1 to 222 μg kg−1)12. However, the samples cited were raw peanut or maize samples, while in this study, all the samples were processed products. Generally, raw samples were relatively more contaminated with aflatoxins than were processed samples.For commercially processed samples, the levels of aflatoxin in peanut oil and maize were higher in Guangdong Province than in Fujian60 and Chongqing61,62. This situation promptedGuangzhouto pay attention to the contamination of AFB1 in peanut oil and find the source of the problem.
Our study is the first to include home-made peanut oil in the assessment of AFB1 in Guangzhou. The results showed that the alarmingly high AFB1 level in home-made peanut oil poses a potential public health threat among suburban residents in Guangzhou. Home-made peanut oil is widely consumed in many underdeveloped cities of China. People prefer home-made peanut oil because of traditional cooking styles and eating habits, particularly in rural areas28. Two factors might contribute to the contamination of home-made peanut oil by AFB1. One factor is that poor-qualityoil extraction machines and simple traditional procedures are unabletodegrade AFB1and that effective techniques to control AFB1 are difficult to apply in family workshops63. The other factor is that the peanuts used for oil extraction might becontaminated by AFB1 to different degrees.If the harvested peanuts that were not ready to be pressed soon for peanut oil were stored in a warm and humid environment,the AFB1 level could easily increase. If mouldy peanuts were not removed, the AFB1 level of peanut oil would hardly be reduced28.
Due to a lack of awareness of AFB1 contamination and the maximization of profits,oil mill ownerstend to use mouldy peanuts for oil extraction, which would not significantly affect the flavour of home-made peanut oil. This poor manufacturing practice is common because it is difficult for consumers to identify. Therefore, regulationand supervision of home-made peanut oil should be enhanced in Guangzhou.Findings from this study are also meaningful to regions where home-made peanut oilis widely available but whose production is unsupervised by food safety regulators.
Dietary AFB1 exposure
The EDI of AFB1 in each AFB1-detected food in all age groups is presented in Table 2. The EDI in each AFB1-detected food for urban and suburban areas is presented in Table 3.In all age groups, the intake was the highest for rice and rice products among the contributed foods, and rice and rice product intake was the main contributor to AFB1 exposure in Guangzhou.Despite the low dietary consumption of vegetable oil, it was the second contributor due to its high AFB1 concentration. Wheat and wheat products were the third contributor to therelatively high consumption. However, theAFB1 concentration in wheat and wheat products was low. In addition, maize and maize products, tea, and nuts had little effect on the EDI due to their low consumption and AFB1 concentrations.
Table 2.
Dietary consumption and AFB1 EDI for each AFB1-analysed food in different age groups in Guangzhou.
| Food category | 3~6years old | 7~17years old | 18~59years old | More than 60 years old | total | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dietary Consumption Reference Person± Standard Deviation (g day−1) | AFB1EDI (ngkg−1bwday−1)(90% confidence interval) | Dietary Consumption Reference Person± Standard Deviation (g day−1) | AFB1EDI (ng kg−1bwday−1)(90% confidence interval) | Dietary Consumption Reference Person±Standard Deviation (g day−1) | AFB1EDI (ng kg−1bwday−1)(90% confidence interval) | Dietary Consumption Reference Person± Standard Deviation (g day−1) | AFB1EDI (ng kg−1 bwday−1)(90% confidence interval) | Dietary Consumption Reference Person± Standard Deviation (g day−1) | AFB1 EDI (ng kg−1bwday−1)(90% confidence interval) | |
| Rice and rice products | 78.5 ± 45.5 | 0.50 (0.02~0.98) | 121.3 ± 73.8 | 0.38 (0.01~0.77) | 146.5 ± 90.5 | 0.31 (0.01~0.63) | 126.5 ± 90.7 | 0.27 (0.05~0.58) | 135.8 ± 86.9 | 0.29 (0.01~0.59) |
| Wheat and wheat products | 32.6 ± 22.3 | 0.21 (0.03~0.44) | 48.4 ± 38.2 | 0.16 (0.04~0.35) | 52.3 ± 38.7 | 0.11 (0.02~0.25) | 50.6 ± 33.1 | 0.12 (0.03~0.44) | 49.3 ± 36.3 | 0.11 (0.03~0.27) |
| Maize and maize products | 6.3 ± 4.8 | 0.04 (0.01~0.09) | 7.0 ± 4.5 | 0.02 (0.01~0.04) | 9.2 ± 8.7 | 0.02 (0.01~0.12) | 14.7 ± 3.1 | 0.03 (0.01~0.03) | 8.8 ± 5.3 | 0.02 (0.01~0.05) |
| Nuts | 2.5 ± 1.9 | 0.02 (0.01~0.03) | 2.2 ± 1.8 | 0.01 (0.01~0.02) | 2.3 ± 2.2 | 0.01 (0.01~0.02) | 1.14 ± 1.1 | 0.01 (0.01~0.02) | 2.0 ± 1.9 | 0.01 (0.01~0.02) |
| Tea | 0.1 ± 0.9 | 0.00 (0.00~0.02) | 2.2 ± 1.5 | 0.01 (0.01~0.02) | 4.5 ± 4.0 | 0.01 (0.01~0.05) | 3.8 ± 3.1 | 0.01 (0.01~0.03) | 3.6 ± 3.3 | 0.01 (0.01~0.03) |
| Vegetable oil | 11.3 ± 9.6 | 0.17 (0.01~3.37) | 22.3 ± 18.5 | 0.17 (0.01~3.31) | 26.4 ± 17.3 | 0.13 (0.01~2.43) | 9.6 ± 5.3 | 0.05 (0.01~0.88) | 26.6 ± 16.8 | 0.13 (0.01~2.50) |
| Total | 0.94 (0.29~4.24) | 0.75 (0.22~3.64) | 0.59 (0.20~3.11) | 0.48 (0.16~1.41) | 0.57 (0.21~3.16) | |||||
AFB1: Aflatoxin B1;EDI: Estimateddaily intake.
Table 3.
AFB1 exposure in each AFB1-analysed food between urban and suburban areas in Guangzhou.
| Food Category | Urban District | Suburban District | ||
|---|---|---|---|---|
| Dietary Consumption Reference Person (g day−1) | AFB1 EDI (ngkg−1bwday−1) | Dietary Consumption Reference Person (g day−1) | AFB1EDI (ngkg−1bwday−1) | |
| Rice and rice products | 118.5 ± 83.9 | 0.25 (0.04~0.54) | 159.3 ± 90.1 | 0.33 (0.02~0.65) |
| Wheat and wheat products | 51.6 ± 37.2 | 0.11 (0.02~0.24) | 47.4 ± 35.3 | 0.10 (0.02~0.23) |
| Maize and maize Products | 8.3 ± 5.8 | 0.02 (0.01~0.04) | 9.0 ± 4.9 | 0.02 (0.01~0.04) |
| Nuts | 2.5 ± 2.4 | 0.01 (0.01~0.03) | 1.8 ± 1.7 | 0.01 (0.01~0.02) |
| Tea | 3.5 ± 3.0 | 0.01 (0.01~0.03) | 3.7 ± 3.2 | 0.01 (0.01~0.03) |
| Vegetable oil a | 25.9 ± 14.8 | 0.14 (0.01~0.37) | 27.1 ± 18.1 | 1.78 (0.10~6.13) |
| 1. Commercial vegetable oil | 25.9 ± 14.8 | 0.14 (0.01~0.37) | 25.0 ± 17.8 | 0.13 (0.00~0.38) |
| 2. Home-made peanut oil | / | 2.1 ± 1.95 | 1.65 (0.05~5.72) | |
| Total | 0.29(0.08~0.56) | 2.26(0.35~6.59) | ||
A: Total vegetable oil intake was equal to commercial vegetable oil plus home-made peanut oil (a = 1 + 2).
AFB1: Aflatoxin B1;EDI: Estimateddaily intake.
The EDI of AFB1 in each age group was estimated to range from 0.48 ng kg−1bw day−1 to 0.94 ng kg−1bw day−1, and the average EDI was estimated to be 0.57 ng kg−1bw day−1(the 90% confidence interval extended from 0.21 to 3.16). Among all age groups, the 3–6 years of age group had the highest EDI, with a value of 0.94 ng kg−1bw day−1. The difference in EDI between urban and suburban residents was large, with 0.29 ng kg−1bw day−1 and 2.26 ng kg−1bw day−1 for urban and suburban residents, respectively. The main source of suburban resident exposure to AFB1 was home-made peanut oil.
Risk characterization using the MOE Approach
Table 4 presents the MOE values for AFB1 exposure. All MOE values were below the safe threshold of 10000. Probabilisticrisk analysis resultsshowed that most of the lower bound MOE values ranged from 10 to 100, indicating a concern for risk management.
Table 4.
Risk characterization of AFB1 exposure in different age groups and different regions in Guangzhou based on the MOE approach.
| Characteristic | POD(ng kg−1bwday−1) | Exposure (ng kg−1bwday−1) | MOE | |||||
|---|---|---|---|---|---|---|---|---|
| T25 | BMDL10 | BMDL1 | T25 | BMDL10 | BMDL1 | |||
| Age group | 390 | 340 | 78 | |||||
| 3~6 years old | 0.94 (0.29~4.24) | 417(65~1086) | 363 (62~931) | 83 (14~215) | ||||
| 7~17years old | 0.75 (0.22~3.64) | 519 (65~1382 | 453 (51~1234) | 104 (14~276) | ||||
| 18~59years old | 0.59 (0.20~3.11) | 654(96~1604) | 570 (98~1478) | 131 (20~347) | ||||
| More than 60 years old | 0.48 (0.16~1.41) | 812(231~2199) | 708 (204~1998) | 162 (48~455) | ||||
| Region | ||||||||
| Urban | 0.29 (0.08~0.56) | 1364 (657~4612) | 1189 (573~4020) | 273 (131~922) | ||||
| Suburban | 2.26(0.35~6.59) | 172 (57~1055) | 150 (50~920) | 34 (11~211) | ||||
| Total | 0.57 (0.21~3.16) | 681 (107~1719) | 594 (88~1509) | 136 (20~346) | ||||
AFB1: Aflatoxin B1; MOE: Margin of exposure; POD: Point of departure.
BMDL10: Benchmark dose lower confidence limit for 10%; BMDL1: Benchmark dose lower confidence limit for 1%; T25: The reference value of a chronic dose that causes 25% of test animals to develop liver cancer.
Age-group analysis suggestedthat we should pay close attention to the 3~6 years of age group, whose MOE value was the lowest. This result reflected that preschool children might have the highest risk of being exposed to AFB1.This agreed with the results from a studyfrom Taiwan in 2018 that found thatbabies and toddlers were at the highest risk of AFB1 exposure64.
Meanwhile, our results showed that the MOE value of suburban residents was lower than that of urban residents.AFB1 dietary exposure among urban residents in Guangzhou was similar to that of the urban residents in Shenzhen, an adjacent city to Guangzhou that is the most economically developed city in South China38. However, Guangzhou as a whole had a higher level of AFB1 risk than Shenzhen, probably because of the consumption of home-made peanut oil by suburban residents. In Guangxi Province, which neighbours Guangdong Province(where Guangzhouis located),grains and oil crops were also prone to mildew due to its subtropical climate with abundant year-round rainfall63. It should be noted that the residents of Guangxi Province have a similar habit of consuming home-made peanut oil. The mean AFB1 level of home-made peanut oil in the Guangxi study was 41.50 μg kg−1, slightly higher than the result in our study35. In a comparison of this study and studies from other low- and middle-income countries, dietary health risk exposure to AFB1 in Guangzhouappeared to be lower than that in other countries, and the risk of cancer was also lower than that in Indonesia13 and Vietnam23. The MOE values in our study were much greater than those in Japan27 and South Korea22,where socioeconomic statusisvery developed.
Risk characterization using quantitative risk assessment of liver cancer
The potential cancer risk of AFB1 in Guangzhou residents was estimated by age group and by region (Table 5). In general, the risk of liver cancer in the entire population was estimated at 0.0264 cancers(year100 000 people)−1on average, which was far less than the incidence of liver cancer in China of 24.6 cancers(year 100 000 people)−1 65. These results indicated that foods currently contaminated by AFB1 had low health risks for residents and that dietary exposure to AFB1may not account for the occurrence of liver cancer in Guangzhou.However, the EDI of suburban residents was nearly ten times higher than that of urban residents. The cancer risk among suburban residents was much higher than that among urban residents. These results were comparable to the results of a study conductedinGuangxi Province66, where dietary exposure to AFB1 was mainly caused by home-made peanut oil. Nonetheless, with the increasing vaccination rate for the hepatitis B vaccine in China, it is believed that the cancer risk will gradually decrease in the future.
Table 5.
Estimated cancer risk in different age groupsand different regions in Guangzhou residents.
| Characteristic | Exposure (ng kg−1bwday−1) | Fraction of population with hepatitis B | Annual hepatocellular carcinoma (HCC) incidence(cancers (year 100 000people)−1) |
|---|---|---|---|
| Age group | |||
| 3~6 years old | 0.94(0.29~4.24) | 12.45 | 0.0432(0.0001~1.4733) |
| 7~17years old | 0.75(0.22~3.64) | 0.0346(0.0001~1.3403) | |
| 18~59years old | 0.59(0.20~3.11) | 0.0275(0.0001~0.8368) | |
| More than 60 years old | 0.48(0.16~1.41) | 0.0221 (0.0001~0.4958) | |
| Region | |||
| Urban | 0.29(0.08~0.56) | 7.10 | 0.0088(0.0001~0.3507) |
| Suburban | 2.26(0.35~6.59) | 16.14 | 0.1284(0.0001~24.422) |
| Total | 0.57(0.21~3.16) | 12.45 | 0.0264(0.0058~1.3802) |
Uncertainty analysis and limitations
The entire process of food safety risk assessment has been accompanied by uncertainty. There are two main sources67,68. One source is extrapolation, wheredose levels inanimal studies exceed human exposure possibilities. Models used for extrapolation could cause results to differ by orders of magnitude, but uncertainty analysis can still improve transparency and assessment accuracy. The other source is data limitations, mainly including the inability to obtain theno observed adverse effect level(NOAEL), differences in exposure pathways, and differences in exposure time.Use of anuncertainty factor is a common method for dealing with these uncertainties68. Dividing the NOAEL obtained from animal experiments or other reference doses by theuncertainty factor can obtain a reference dose that is considered safe or without appreciable risk. The uncertaintyfactor is a coefficient that increases the level of protection of the health guidance value. The BMDL (with the uncertainty factor considered) used in the calculation of the exposure assessment in this study is a scientific method for dealing with data uncertainty.
Two factors need to be taken into consideration when these results are interpreted. First, AFs are jointly produced in nature, occurring as a mixture of AFB1, AFB2, AFG1, AFG2, etc.In our study, we assessed the risk of only AFB1, which wouldunderestimate the health risk of total AFs. However, AFB1 is the most toxic and frequent mycotoxinin AFs. Thus, the risk assessment forAFB1can reflect the overall risk of AFs. Second, although the consumption of home-made peanut oil among urban residents might be rare, the high concentration of AFB1 in home-made peanut oil requires the attention of the entire population. It would thus be necessary to expand the scale of home-made peanut oil consumption surveys to all residents instead of focusing on only suburban residents.
Conclusions
This study is one of the few studies on probabilisticrisk assessment of dietary exposure to AFB1in China. Instead of studying the limited category of AFB1-contaminatedfood that is found in most studies, our study covered a wide variety of foods that mightcontribute to contamination byAFB116,64,69. Thoughthe overall risk of dietary health risk exposure to AFB1 for liver cancer was low, there is a risk to health especially with continuous consumption. Furthermore, the health risk of suburban people was higher than that of urban people because of the common habit of consuming home-made peanut oil in the former group.In addition, 3~6-year-olds need special attention. Supervision of the production and sales of home-made peanut oil should be in place to reduce the risk of AFB1 exposure.
Supplementary information
Acknowledgements
This work was supported by the Project for KeyMedicine Discipline Construction of Guangzhou Municipality (grant number2017–2019-07) and a medicalscientificgrantfrom Guangdong Province, China (B2018154). Minling Ye from McGill University (CA) helped with English language editing.
Author contributions
Kuncai Chen is the corresponding author and was responsible for designing and organizing this study; Weiwei Zhang is the lead author and implemented the project, analysed the data and wrote the manuscript. Yufei Liu was responsible for project administration; Boheng Liang helped analyse the data; Yuhua Zhang and XianwuZhong contributed tothe foodsamples collected; XiaoyanLuo performed the experiments; Jie Huang and Yanyan Wang contributed to the food consumptionsurvey; Weibin Cheng provided important suggestions and revised the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-64295-8.
References
- 1.Saracci R, Wild CP. Fifty years of the International Agency for Research on Cancer (1965 to 2015) Int. J. Cancer. 2016;138:1309–1311. doi: 10.1002/ijc.29929. [DOI] [PubMed] [Google Scholar]
- 2.Wu F, Groopman JD, Pestka JJ. Public health impacts of foodborne mycotoxins. Annu. Rev. Food Sci. Technol. 2014;5:351–372. doi: 10.1146/annurev-food-030713-092431. [DOI] [PubMed] [Google Scholar]
- 3.Schroeder HW, Boller RA. Aflatoxin production of species and strains of the Aspergillus flavus group isolated from field crops. Appl. Microbiol. 1973;25:885–889. doi: 10.1128/AEM.25.6.885-889.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klich MA, Pitt JI. Differentiation of Aspergillus flavus from A. parasiticus and Other Closely Related Species. Trans. Br. Mycological Soc. 1988;91:99–108. doi: 10.1016/S0007-1536(88)80010-X. [DOI] [Google Scholar]
- 5.Frisvad JC, et al. Taxonomy of Aspergillus section Flavi and their production of aflatoxins, ochratoxins and other mycotoxins. Stud. Mycol. 2019;93:1–63. doi: 10.1016/j.simyco.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geiser DM, Dorner JW, Horn BW, Taylor JW. The phylogenetics of mycotoxin and sclerotium production in Aspergillus flavus and Aspergillus oryzae. Fungal Genet. Biol. 2000;31:169–179. doi: 10.1006/fgbi.2000.1215. [DOI] [PubMed] [Google Scholar]
- 7.Adamson RH, Correa P, Sieber SM, McIntire KR, Dalgard DW. Carcinogenicity of aflatoxin B1 in rhesus monkeys: two additional cases of primary liver cancer. J. Natl Cancer Inst. 1976;57:67–78. doi: 10.1093/jnci/57.1.67. [DOI] [PubMed] [Google Scholar]
- 8.Sengstag, C.The molecular mechanism of aflatoxin B1-induced liver cancer: is mitotic recombination involved?Mol Carcinog19, 147-152, doi:10.1002/(SICI)1098-2744(199707)19:3<147::AID-MC1>3.0.CO;2-B (1997). [PubMed]
- 9.Liu Y, Chang CC, Marsh GM, Wu F. Population attributable risk of aflatoxin-related liver cancer: systematic review and meta-analysis. Eur. J. Cancer. 2012;48:2125–2136. doi: 10.1016/j.ejca.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li QW, Lu CR, Ye M, Xiao WH, Liang J. Evaluation of DNA repair gene XRCC1 polymorphism in prediction and prognosis of hepatocellular carcinoma risk. Asian Pac. J. Cancer Prev. 2012;13:191–194. doi: 10.7314/APJCP.2012.13.1.191. [DOI] [PubMed] [Google Scholar]
- 11.Abrar M, et al. Aflatoxins: biosynthesis, occurrence, toxicity, and remedies. Crit. Rev. Food Sci. Nutr. 2013;53:862–874. doi: 10.1080/10408398.2011.563154. [DOI] [PubMed] [Google Scholar]
- 12.Leong YH, Rosma A, Latiff AA, Ahmad NI. Exposure assessment and risk characterization of aflatoxin B1 in Malaysia. Mycotoxin Res. 2011;27:207–214. doi: 10.1007/s12550-011-0097-4. [DOI] [PubMed] [Google Scholar]
- 13.Nugraha A, Khotimah K, Rietjens I. Risk assessment of aflatoxin B1 exposure from maize and peanut consumption in Indonesia using the margin of exposure and liver cancer risk estimation approaches. Food Chem. Toxicol. 2018;113:134–144. doi: 10.1016/j.fct.2018.01.036. [DOI] [PubMed] [Google Scholar]
- 14.Sun G, et al. Co-contamination of aflatoxin B1 and fumonisin B1 in food and human dietary exposure in three areas of China. Food Addit. Contam. Part. A Chem. Anal. Control. Expo. Risk Assess. 2011;28:461–470. doi: 10.1080/19440049.2010.544678. [DOI] [PubMed] [Google Scholar]
- 15.Asim M, Sarma MP, Thayumanavan L, Kar P. Role of aflatoxin B1 as a risk for primary liver cancer in north Indian population. Clin. Biochem. 2011;44:1235–1240. doi: 10.1016/j.clinbiochem.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 16.Ding X, et al. Risk Assessment on Dietary Exposure to Aflatoxin B(1) in Post-Harvest Peanuts in the Yangtze River Ecological Region. Toxins. 2015;7:4157–4174. doi: 10.3390/toxins7104157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.CODEX. in General Standard for Contaminants and Toxins in Food and Feed(Codex Alimentarius Comission (2015).
- 18.European Commission (EC). in Setting maximum levels for certain contaminants in foodstuffs Vol. No. 1881/2006 (2006).
- 19.National Health and Family Planning Commission of thePeople’s Republic of China. (2017).
- 20.Evaluation of certain food additives and contaminants. Forty-ninth report of the Joint FAO/WHO Expert Committee on Food Additives. World Health Organ. Tech. Rep. Ser. 1999;884(i-viii):1–96. [PubMed] [Google Scholar]
- 21.European Food Safety Authority. Opinion of the Scientific Committee on a request from EFSA related to Exposure Assessments. EFSA J. 2005;3:249. doi: 10.2903/j.efsa.2005.249. [DOI] [Google Scholar]
- 22.Ok HE, et al. Natural occurrence of aflatoxin B1 in marketed foods and risk estimates of dietary exposure in Koreans. J. Food Prot. 2007;70:2824–2828. doi: 10.4315/0362-028x-70.12.2824. [DOI] [PubMed] [Google Scholar]
- 23.Huong BTM, Tuyen LD, Tuan DH, Brimer L, Dalsgaard A. Dietary exposure to aflatoxin B1, ochratoxin A and fuminisins of adults in Lao Cai province, Viet Nam: A total dietary study approach. Food Chem. Toxicol. 2016;98:127–133. doi: 10.1016/j.fct.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 24.IARC. Monographs on the Evaluation of Carcinogenic Risks to Humans. (INTERNATIONAL AGENCY FOR RESEARCH ON CANCER (2002).
- 25.EFSAPanelon Contaminants in the Food Chain. Opinion of the Scientific Panel on contaminants in the food chain [CONTAM] related heptachlor as an undesirable substance in animal feed. EFSA J. 2007;5:478. doi: 10.2903/j.efsa.2007.478. [DOI] [Google Scholar]
- 26.Summary of Evaluations Performed by the Joint FAOWHO Expert Committee on Food Additives (JECFA). Nutrition Reviews58, 90, 10.1111/j.1753-4887.2000.tb01846.x (2000).
- 27.Sugita-Konishi Y, et al. Exposure to aflatoxins in Japan: risk assessment for aflatoxinB1. Food Addit. Contam. Part. A Chem. Anal. Control. Expo. Risk Assess. 2010;27:365–372. doi: 10.1080/19440040903317497. [DOI] [PubMed] [Google Scholar]
- 28.Qi N, et al. Aflatoxin B1 in peanut oil from Western Guangdong, China, during 2016-2017. Food Addit. Contam. Part. B Surveill. 2019;12:45–51. doi: 10.1080/19393210.2018.1544173. [DOI] [PubMed] [Google Scholar]
- 29.Zhang W, et al. [Analysis on contamination of aflatoxinB1 in food and oil in Guangzhou from 2009 to 2013] Chin. J. Food Hyg. 2015;27:4. doi: 10.13590/j.cjfh.2015.03.015. [DOI] [Google Scholar]
- 30.Gao X, et al. Aflatoxin contamination of corn samples collected from six regions of China. J. Hyg. Res. 2011;40:46–49. doi: 10.1631/jzus.B1000265. [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Liu XM. Contamination of aflatoxins in different kinds of foods in China. Biomed. Env. Sci. 2007;20:483–487. [PubMed] [Google Scholar]
- 32.Bircan C. The determination of aflatoxins in spices by immunoaffinity column extraction using HPLC. Int. J. Food Sci. Technol. 2010;40:929–934. doi: 10.1111/j.1365-2621.2005.01025.x. [DOI] [Google Scholar]
- 33.Roch OG, Blunden G, Haig DJ, Coker RD, Gay C. Determination of aflatoxins in groundnut meal by high-performance liquid chromatography: a comparison of two methods of derivatisation of aflatoxinB1. Br. J. Biomed. Sci. 1995;52:312–316. [PubMed] [Google Scholar]
- 34.Chen, L., Molla, A. E., Getu, K. M., Ma, A.&Wan, C.Determination of Aflatoxins in Edible Oils from China and Ethiopia Using Immunoaffinity Column and HPLC-MS/MS.Journal of Aoac International, 10.5740/jaoacint.18-0106 (2019). [DOI] [PubMed]
- 35.Zhang Y, et al. [Food consumption and nutrients intake among residents in Guangzhou city] Chin. J. Public. Health. 2017;33:4. doi: 10.11847/zgggws2017-33-06-26. [DOI] [Google Scholar]
- 36.Yuexin, Y.China Food Composition. (Peking University Medical Press (2009).
- 37.Wang J, Liu XM, Zhang ZQ. [Exposure assessment of liver cancer attributed to dietary aflatoxins exposure in Chinese residents] Zhonghua Yu Fang. Yi Xue Za Zhi. 2009;43:478–481. doi: 10.1016/j.chb.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 38.Li K, Qiu F, Jiang L, Yang M. [Dietary exposure assessment of aflatoxin of foodstuff and edible oil from Shenzhen residents] J. Hyg. Res. 2014;43:6. [PubMed] [Google Scholar]
- 39.Evaluation of certain food additives. Seventy-first report of the Joint FAO/WHO Expert Committee on Food Additives. ReportNo. 0512-3054, 1-80 (2010). [PubMed]
- 40.European Food Safety Authority Food and Agriculture Organization of the United Nations, World Health Organization; Towards a harmonised Total Diet Study approach: a guidance document. EFSA J. 2011;9:2450. doi: 10.2903/j.efsa.2011.2450. [DOI] [Google Scholar]
- 41.Vlachonikolis IG, Marriott FH. Evaluation of censored contamination data. Food Addit. Contam. 1995;12:637–644. doi: 10.1080/02652039509374352. [DOI] [PubMed] [Google Scholar]
- 42.Hecht H, Honikel KO. Assessment of data sets containing a considerable number of values below the detection limits. Z. Lebensm. Unters. Forsch. 1995;201:592–597. doi: 10.1007/bf01201592. [DOI] [PubMed] [Google Scholar]
- 43.Kulmbach Germany: WHORegional Office for Europe. Second workshop on reliable evaluation of low-level contamination of food. (1995).
- 44.Lin Z, Chen D. [A sampling survey of growth and nutritional status in preschool children in Yuexiu District of Guangzhou] Chin. Prim. Health Care. 2010;24:44–45. doi: 10.3969/j.issn.1001-568X.2010.08.019. [DOI] [Google Scholar]
- 45.Gao D, Dong Y, Yang Y, Zou Z, Ma J. [Secular trends of height and weight in Chinese children from 2005 to 2014] Chin. J. Sch. Health. 2018;39:252–255. doi: 10.16835/j.cnki.1000-9817.2018.02.027. [DOI] [Google Scholar]
- 46.China Institute of Nutrition and Health. China Nutrition Data Yearbook. (2012).
- 47.Peers FG, Gilman GA, Linsell CA. Dietary aflatoxins and human liver cancer. A study in Swaziland. Int. J. Cancer. 1976;17:167–176. doi: 10.1002/ijc.2910170204. [DOI] [PubMed] [Google Scholar]
- 48.Peers F, Bosch X, Kaldor J, Linsell A, Pluijmen M. Aflatoxin exposure, hepatitis B virus infection and liver cancer in Swaziland. Int. J. Cancer. 1987;39:545–553. doi: 10.1002/ijc.2910390502. [DOI] [PubMed] [Google Scholar]
- 49.Carlborg FW. Cancer, mathematical models and aflatoxin. Food Cosmet. Toxicol. 1979;17:159–166. doi: 10.1016/0015-6264(79)90216-5. [DOI] [PubMed] [Google Scholar]
- 50.Benford D, Leblanc JC, Setzer RW. Application of the margin of exposure (MoE) approach to substances in food that are genotoxic and carcinogenic: example: aflatoxin B1 (AFB1) Food Chem. Toxicol. 2010;48(Suppl 1):S34–41. doi: 10.1016/j.fct.2009.10.037. [DOI] [PubMed] [Google Scholar]
- 51.Newberne PM. Carcinogenic effects of low dietary levels of aflatoxin B1 in rats. Food Cosmetics Toxicol. 1974;12:681–685. doi: 10.1016/0015-6264(74)90239-9. [DOI] [PubMed] [Google Scholar]
- 52.Barraud L, et al. The role of duck hepatitis B virus and aflatoxin B1 in the induction of oxidative stress in the liver. Cancer Detect. Prev. 2001;25:192–201. doi: 10.1007/s002800000224. [DOI] [PubMed] [Google Scholar]
- 53.Kew MC. Synergistic interaction between aflatoxin B1 and hepatitis B virus in hepatocarcinogenesis. Liver Int. 2003;23:405–409. doi: 10.1111/j.1478-3231.2003.00869.x. [DOI] [PubMed] [Google Scholar]
- 54.Campbell TC. Correspondence re: G-S. Qian, et al., A follow-up study of urinary markers of aflatoxin exposure and liver cancer risk in Shanghai, People’s Republic of China. Cancer Epidemiol., Biomarkers & Prev., 3:3-10, 1994, and C.C. Harris, Solving the viral-chemical puzzle of human liver carcinogenesis. Cancer Epidemiol., Biomarkers & Prev., 3:1-2, 1994. Cancer Epidemiol. Biomarkers Prev. 1994;3:519–521. [PubMed] [Google Scholar]
- 55.Li Y, et al. Synergistic effect of hepatitis B virus and aflatoxin B1 in hepatocarcinogenesis in tree shrews. Ann. Acad. Med. Singap. 1999;28:67–71. [PubMed] [Google Scholar]
- 56.Wu HC, et al. Urinary 15-F2t-isoprostane, aflatoxin B1 exposure and hepatitis B virus infection and hepatocellular carcinoma in Taiwan. Carcinogenesis. 2008;29:971–976. doi: 10.1093/carcin/bgn057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang JS, et al. Temporal patterns of aflatoxin-albumin adducts in hepatitis B surface antigen-positive and antigen-negative residents of Daxin, Qidong County, People’s Republic of China. Cancer Epidemiol. Biomarkers Prev. 1996;5:253–261. [PubMed] [Google Scholar]
- 58.Liu J, Cai Y, Wang M. [Epidemiological survey of hepatitis B virus surface antigen positive in Guangzhou in 2008] Zhonghua Yu Fang. Yi Xue Za Zhi. 2010;44:3. doi: 10.3760/cma.j.issn.0253-9624.2010.03.029. [DOI] [Google Scholar]
- 59.Trung T, et al. Fungal mycoflora and contamination of maize from Vietnam with aflatoxin B1 and fumonisinB1. World Mycotoxin J. 2008;1:8. doi: 10.3920/WMJ2008.x010. [DOI] [Google Scholar]
- 60.Qiu W, Fu W. [Contamination of aflatoxins in peanuts and peanut products from Fujian] Chin. J. Health Laboratory Technol. 2012;22:2446–2448. [Google Scholar]
- 61.Zhang X, Ding J, Li S, Cheng Y. Survey of aflatoxin contamination in foods sold in Wanzhou District, Chongqing, 2013-2014. J. Practical Preventive Med. 2016;23:48–50. doi: 10.3969/j.issn.1006-3110.2016.04.013. [DOI] [Google Scholar]
- 62.Xu W, Liu D, Han X, Lu D, Li F. [Survey of aflatoxin contamination in edible vegetable oils sold in parts of China in 2015] Chin. J. Food Hyg. 2018;14:776–779. doi: 10.13590/j.cjfh.2018.01.014. [DOI] [Google Scholar]
- 63.Cheng H, et al. Exposure risk assessment of aflatoxin B1 in edible vegetable oil by using the margin of exposure in Guangxi. Chin. J. Food Hyg. 2017;29:4. doi: 10.13590/j.cjfh.2017.04.022. [DOI] [Google Scholar]
- 64.Wang, X., Lien, K. W.&Ling, M. P.Probabilistic Health Risk Assessment for Dietary Exposure to Aflatoxin in Peanut and Peanut Products in Taiwan. Food Control, S0956713518301828, 10.1016/j.foodcont.2018.04.021 (2018).
- 65.Zuo, T., Zheng, R., Zeng, H., Zhang, S.&Chen, W.[Analysis of liver cancer incidence and trend in China]. Zhonghua zhong liu za zhi37, 10.3760/cma.j.issn.0253-3766.2015.09.013 (2015). [PubMed]
- 66.Ding X, Li P, Bai Y, Zhou H. Aflatoxin B 1 in post-harvest peanuts and dietary risk in China. Food Control. 2012;23:143–148. doi: 10.1016/j.foodcont.2011.06.026. [DOI] [Google Scholar]
- 67.Edler L, et al. Selection of appropriate tumour data sets for Benchmark Dose Modelling (BMD) and derivation of a Margin of Exposure (MoE) for substances that are genotoxic and carcinogenic: considerations of biological relevance of tumour type, data quality and uncertainty assessment. Food Chem. Toxicol. 2014;70:264–289. doi: 10.1016/j.fct.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 68.International Programme on Chemical Safety (IPCS). Uncertainty and dataquality in exposure assessment. (2008).
- 69.Guo YD, Chen L, Yuan YH, Yue TL. Dietary Exposure and Risk Assessment of Aflatoxin B_1 in Corn-based Foods in China Using Probabilistic Approach. Food Sci. 2013;34:24–27. doi: 10.7506/spkx1002-6630-201311006. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.