Abstract
Background
Male patients undergoing bariatric surgery have (historically) been considered higher risk than females. The aim of this study was to examine the disparity between genders undergoing laparoscopic sleeve gastrectomy (SG) and laparoscopic Roux-en-Y gastric bypass (RYGB) procedures and assess gender as an independent risk factor.
Methods
The MBSAQIP® Data Registry Participant User Files for 2015–2017 was reviewed for patients having primary SG and RYGB. Patients were divided into groups based on gender and procedure. Variables for major complications were grouped together, including but not limited to PE, stroke, and MI. Univariate and propensity matching analyses were performed.
Results
Of 429,664 cases, 20.58% were male. Univariate analysis demonstrated males were older (46.48 ± 11.96 vs. 43.71 ± 11.89 years, p < 0.0001), had higher BMI (46.58 ± 8.46 vs. 45.05 ± 7.75 kg/m2, p < 0.0001), and had higher incidence of comorbidities. Males had higher rates of major complications (1.72 vs. 1.05%; p < 0.0001) and 30-day mortality (0.18 vs. 0.07%, p < 0.0001). Significance was maintained after subgroup analysis of SG and RYGB. Propensity matched analysis demonstrated male gender was an independent risk factor for RYGB and SG, major complications [2.21 vs. 1.7%, p < 0.0001 (RYGB), 1.12 vs. 0.89%, p < 0.0001 (SG)], and mortality [0.23 vs. 0.12%, p < 0.0001 (RYGB), 0.10 vs. 0.05%; p < 0.0001 (SG)].
Conclusion
Males continue to represent a disproportionately small percentage of bariatric surgery patients despite having no difference in obesity rates compared to females. Male gender is an independent risk factor for major post-operative complications and 30-day mortality, even after controlling for comorbidities.
Keywords: Bariatric surgery, Sleeve gastrectomy, Roux-en-Y gastric bypass, Gender, MBSAQIP
The incidence and impact of obesity on public health is a significant global concern, and has reached epidemic proportions in the United States. According to the Center for Disease Control (CDC), obesity prevalence among adults increased from 30.5 to 39.8% between 2000 and 2016 [1]. Historically, the incidence of obesity among females has been higher than that of males. However, over the last two decades the obesity gender gap has continued to narrow in the United States [1], with the most recent CDC data (2015–2016) reporting no significant gender difference for obesity prevalence [2]. Despite these changes in obesity demographics, males continue to represent a significantly smaller proportion of obese patients undergoing bariatric surgery (analyses of the Bariatrics Outcomes Longitudinal Database and ACS-NSQIP databases report 79% female vs. 21% male patients underwent weight loss surgery (2005–2013) [3, 4]). Of further note, while an increase in male patients undergoing bariatric surgery was reported between 2002 (5.4%) and 2008 (21.0%), this trend appears to have now leveled off, with no significant year over year change since 2008 [5].
Historically, male patients have been considered higher risk for bariatric surgery compared to females. Studies utilizing the National Inpatient Sample (NIS) database report males present with significantly more comorbidities, at a more advanced age for bariatric surgery, and with higher risk adjusted morbidity and mortality rates. For example, a study by Carbonell et al. in 2000 reported males to have a mortality rate of 1.7%, a rate 4.7 times higher than females [6]. Similarly, while Young et al. report an overall decrease in mortality across both genders between 2002 and 2011, gender disparity remained with males having a mortality rate of 0.31%, a rate 3 times higher than females [5]. However, it should be noted in these studies that BMI data is not included in the NIS database, and therefore could not be adjusted for during multi-variant analysis.
The aim of this study was to examine the disparity between genders for patients undergoing bariatric surgery in the United States utilizing the MBSAQIP® database, and to assess the impact of gender as an independent risk factor for patients undergoing either a sleeve gastrectomy (SG) or Roux-en-Y gastric bypass (RYGB) procedure.
Methods
Institutional assurances
The Carolinas Medical Center Institutional Review Board certified that retrospective analyses of public, anonymized data sets (including the MBSAQIP® data registry) are deemed exempt from review.
Data source
Data was accessed from the MBSAQIP® Participant Use Files (PUF) gathered from more than 800 academic and community MBSAQIP® accredited centers between 2015 and 2016 (data consistency and reliability within the MBSAQIP® data registry is maintained by internal audit mechanisms [7]). The PUFs are available to all participating centers (upon request) and contain deidentified data that includes preoperative risk factors, intraoperative variables, and 30-day postoperative complications and mortality.
The MBSAQIP® data registry was queried for patients (18 years or older) undergoing laparoscopic RYGB or SG based on CPT codes 43644 (RYGB) and 43755 (SG). Exclusion criteria included patients with emergent, endo-therapy, gastric plication, revisional and balloon procedures, and cases with incomplete data. Major complications were defined as acute renal failure, cardiac arrest, coma > 24 h, cerebrovascular accident (CVA), myocardial infarction (MI), post-operative ventilator, progressive renal insufficiency, pulmonary embolism (PE), sepsis, septic shock, unplanned intubation, venous thrombo-embolism (VTE), organ space surgical site infection (SSI), and unplanned intensive care unit (ICU) admission. Minor complications were defined as post-operative SSI, urinary tract infection (UTI), wound disruption, and incisional SSI.
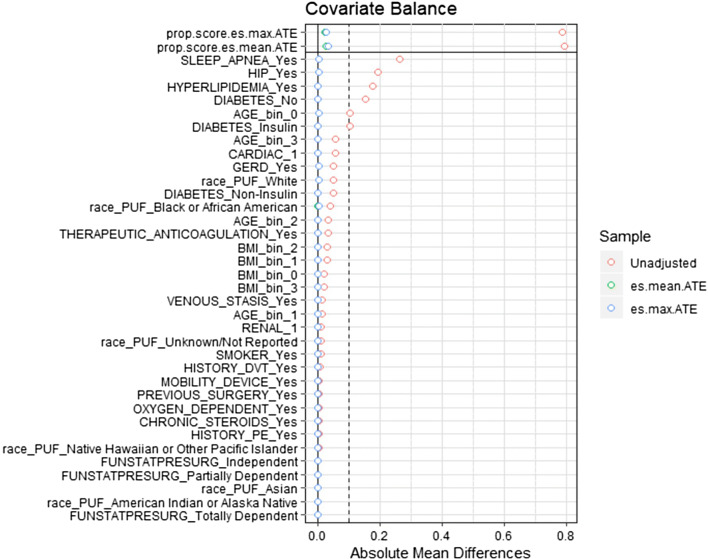
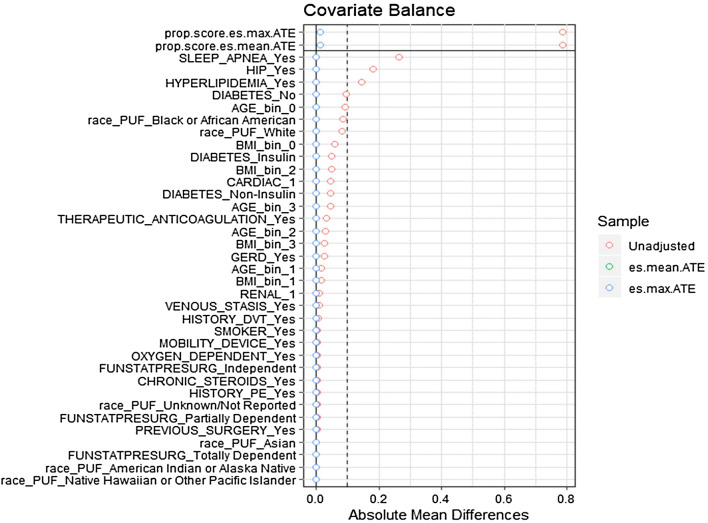
A univariate analysis was performed on patient demographics, comorbid risk factors, procedure details and outcomes for RYGB and SG. In a secondary set of analyses, propensity scores were calculated for patient demographics (age, BMI, race) and comorbid risk factors (gastroesophageal reflux disease (GERD), mobility status, hypertension requiring medication, cardiac conditions, hyperlipidemia, history of deep venous thromboembolism (VTE), venous stasis, anticoagulation therapy, renal conditions, previous foregut surgery, diabetes requiring insulin, smoking status, functional independence, oxygen usage, obstructive sleep apnea, and chronic steroid usage). Subjects were matched using a generalized boosted regression algorithm, an approach that provides effective weighting when utilizing a large number of matching covariates [8]. To assess the quality of the matching algorithm, distributions of covariates between male and female groups for RYGB and SG were assessed by absolute mean differences between groups (Figs. 1 and 2). Weights were applied to each subject and univariate analyses were repeated [9].
Fig. 1.
Propensity matching love plot Roux-en-Y gastric bypass. Covariates represented on the Y axis, mean difference between gender groups represented on the X axis. Post matching mean difference between males and females approaches zero for all covariates
Fig. 2.
Propensity matching love plot sleeve gastrectomy. Covariates represented on the Y axis, mean difference between gender groups represented on the X axis. Post matching mean difference between males and females approaches zero for all covariates
Statistical analysis
Statistical analysis was performed using R Software (V3.4.1). Propensity score weights were generated using the twang package. Chi square test for independence or paired t-tests were utilized for categorical and continuous data as appropriate. Due to the large number of patients in the MBSAQIP® database, a p value < 0.0001 was used to identify statistically significant differences of clinical relevance.
Results
Using the complete MBSAQIP® data set 555,239 cases were identified (2015–2017) from which 429,664 cases of primary RYGB and SG remained after exclusion criteria were applied. This patient population was then subdivided by gender resulting in 341,238 females (79.42%) and 88,426 males (20.58%), the patient demographics for which are detailed in Table 1. Performing a univariate analysis demonstrated males were slightly older that females (46.48 ± 11.96 vs. 43.71 ± 11.89 years, p < 0.0001), had clinically similar BMI (46.58 ± 8.46 vs. 45.05 ± 7.75 kg/m2, p < 0.0001), and had a significantly higher incidence of comorbidities compared to females (Table 1). Specifically, males had a higher incidence of cardiac history (MI or cardiac procedure) compared to females (7.14 vs. 2.17%, p < 0.0001), HTN (63.12% vs. 44.73%, p < 0.0001), renal insufficiency (1.72 vs. 0.55%, p < 0.0001), DM on insulin (13.66% vs. 7.32%, p < 0.0001), COPD (2.50% vs. 1.58%, p < 0.0001), and OSA (59.02 vs. 32.69%, p < 0.0001). These differences remained statistically different after performing a subgroup analysis of LRYGB and SG (Table 1).
Table 1.
Patient characteristics
| Gastric bypass | Sleeve gastrectomy | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Male | Female | p value | Male | Female | p value | Male | Female | p value | |
| N value | 23357 | 94242 | 65069 | 246996 | 88426 | 341238 | |||
| Frequency (%) | 19.86% | 80.14% | – | 20.85% | 79.15% | – | 20.85% | 79.15% | – |
| Mean age ± SD | 47.51 (11.72) | 44.36 (11.82) | < 0.0001** | 46.11 (12.03) | 43.46 (11.91) | < 0.0001** | 46.48 (11.96) | 43.71(11.89) | < 0.0001** |
| Mean BMI at surgery ± SD | 46.89 (8.46) | 45.94 (7.91) | < 0.0001** | 46.46 (8.45) | 44.72 (7.66) | < 0.0001** | 46.58 (8.46) | 45.05 (7.75) | < 0.0001** |
| White | 79.53 | 74.54 | < 0.0001** | 78.73 | 70.61 | < 0.0001** | 78.95 | 71.7 | < 0.0001** |
| Black | 10.62 | 14.79 | < 0.0001** | 12.13 | 20.69 | < 0.0001** | 11.73 | 19.06 | < 0.0001** |
| Race other | 9.85 | 10.68 | < 0.0001** | 9.14 | 8.7 | < 0.0001** | 9.33 | 9.25 | < 0.0001** |
| GERD | 34.28 | 39.37 | < 0.0001** | 26.07 | 28.66 | < 0.0001** | 28.24 | 31.62 | < 0.0001** |
| Limited ambulation | 2.3 | 1.81 | < 0.0001** | 1.87 | 1.45 | < 0.0001** | 1.99 | 1.55 | < 0.0001** |
| Cardiac history (MI or intervention) | 8.17 | 2.49 | < 0.0001** | 6.77 | 2.05 | < 0.0001** | 7.14 | 2.17 | < 0.0001** |
| HTN | 68.53 | 49.16 | < 0.0001** | 61.17 | 43.04 | < 0.0001** | 63.12 | 44.73 | < 0.0001** |
| Hyperlipidemia | 43.25 | 25.55 | < 0.0001** | 33.77 | 19.12 | < 0.0001** | 36.27 | 20.9 | < 0.0001** |
| History DVT | 2.41 | 1.7 | < 0.0001** | 2 | 1.33 | < 0.0001** | 2.11 | 1.44 | < 0.0001** |
| Venous stasis | 2.27 | 0.92 | < 0.0001** | 1.81 | 0.72 | < 0.0001** | 1.93 | 0.77 | < 0.0001** |
| Renal insufficiency or dialysis | 1.66 | 0.51 | < 0.0001** | 1.74 | 0.56 | < 0.0001** | 1.72 | 0.55 | < 0.0001** |
| Therapeutic anticoagulation | 5.34 | 2.06 | < 0.0001** | 5.02 | 1.84 | < 0.0001** | 5.1 | 1.9 | < 0.0001** |
| Previous foregut surgery | 1.4 | 1.8 | < 0.0001** | 1.38 | 1.6 | < 0.0001** | 1.38 | 1.65 | < 0.0001** |
| Diabetes insulin | 22.21 | 11.93 | < 0.0001** | 10.59 | 5.56 | < 0.0001** | 13.66 | 7.32 | < 0.0001** |
| Smoker | 7.54 | 8.45 | < 0.0001** | 9.06 | 8.63 | 0.0005* | 8.66 | 8.58 | 0.4555 |
| Total dependence | 0.32 | 0.31 | 0.0227* | 0.49 | 0.42 | < 0.0001** | 0.44 | 0.39 | < 0.0001** |
| COPD | 2.44 | 1.86 | < 0.0001** | 2.18 | 1.48 | < 0.0001** | 2.5 | 1.58 | < 0.0001** |
| Oxygen dependence | 1.12 | 0.84 | < 0.0001** | 0.95 | 0.57 | < 0.0001** | 1 | 0.64 | < 0.0001** |
| History of PE | 1.42 | 1.21 | 0.0093* | 1.34 | 1.04 | < 0.0001** | 1.36 | 1.09 | < 0.0001** |
| Sleep apnea | 64.73 | 38.3 | < 0.0001** | 56.97 | 30.55 | < 0.0001** | 59.02 | 32.69 | < 0.0001** |
| Chronic steroids | 1.32 | 1.57 | 0.006* | 1.54 | 1.83 | < 0.0001** | 1.48 | 1.76 | < 0.0001** |
*Significant (p value < 0.05)
**Highly significant (p value < 0.0001)
A similar univariate analysis of 30-day outcomes was then performed (Table 2). This analysis demonstrated males exhibited higher rates of major complications compared to females (1.72 vs. 1.05%, p < 0.0001), 30-day mortality (0.18 vs. 0.07%, p < 0.0001), and bleeding (0.15 vs. 0.08%, p < 0.0001). These differences remained statistically different after performing a subgroup analysis of RYGB and SG (Table 2), with the exception that males had higher leak rates than females overall (0.14 vs. 0.12%, p = 0.049), but this significance was lost during subgroup analysis of RYGB and SG (Table 2).
Table 2.
Univariate outcomes
| Gastric bypass | Sleeve gastrectomy | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Male | Female | p value | Male | Female | p value | Male | Female | p value | |
| OR time (min) ± SD | 124.53 (57.20) | 118.64 (53.56) | < 0.0001** | 77.80 (38.70) | 71.75 (36.12) | < 0.0001** | 90.14 (48.89) | 84.71 (46.65) | < 0.0001** |
| Approach converted | 0.36 | 0.27 | 0.0323* | 0.11 | 0.1 | 0.3031 | 0.18 | 0.14 | 0.0371* |
| Major complication | 2.62 | 1.61 | < 0.0001** | 1.4 | 0.83 | < 0.0001** | 1.72 | 1.05 | < 0.0001** |
| Minor complication | 1.49 | 1.9 | < 0.0001** | 0.54 | 0.72 | < 0.0001** | 0.79 | 1.04 | < 0.0001** |
| Acute renal failure | 0.24 | 0.09 | < 0.0001** | 0.11 | 0.04 | < 0.0001** | 0.14 | 0.05 | < 0.0001** |
| Cardiac arrest req CPR | 0.15 | 0.04 | < 0.0001** | 0.06 | 0.02 | < 0.0001** | 0.08 | 0.03 | < 0.0001** |
| Deep incisional SSI | 0.09 | 0.15 | 0.028* | 0.03 | 0.02 | 0.3732 | 0.05 | 0.06 | 0.1446 |
| Myocardial infarction | 0.11 | 0.03 | < 0.0001** | 0.05 | 0.02 | 0.0003* | 0.06 | 0.02 | < 0.0001** |
| Organ space SSI | 0.4 | 0.34 | 0.1822 | 0.16 | 0.14 | 0.3826 | 0.22 | 0.2 | 0.163 |
| Progressive renal failure | 0.24 | 0.08 | < 0.0001** | 0.12 | 0.03 | < 0.0001** | 0.15 | 0.04 | < 0.0001** |
| Pneumonia | 0.37 | 0.38 | 0.7097 | 0.12 | 0.13 | 0.5681 | 0.19 | 0.2 | 0.3999 |
| PE | 0.2 | 0.15 | 0.1035 | 0.09 | 0.08 | 0.8349 | 0.12 | 0.1 | 0.2619 |
| Sepsis | 0.19 | 0.18 | 0.6894 | 0.08 | 0.07 | 0.4611 | 0.11 | 0.1 | 0.4895 |
| Septic shock | 0.17 | 0.1 | 0.0026* | 0.05 | 0.03 | 0.0034* | 0.08 | 0.05 | < 0.0001** |
| Superficial SSI | 0.77 | 0.92 | 0.0336* | 0.22 | 0.24 | 0.4198 | 0.37 | 0.43 | 0.0144* |
| Discharge other than home | 0.87 | 0.52 | < 0.0001** | 0.51 | 0.4 | < 0.0001** | 0.6 | 0.43 | < 0.0001** |
| Unplanned intubation | 0.41 | 0.2 | < 0.0001** | 0.18 | 0.09 | < 0.0001** | 0.24 | 0.12 | < 0.0001** |
| UTI | 0.27 | 0.57 | < 0.0001** | 0.16 | 0.32 | < 0.0001** | 0.19 | 0.39 | < 0.0001** |
| VTE | 0.22 | 0.16 | 0.0434* | 0.22 | 0.16 | 0.0023* | 0.22 | 0.16 | 0.0003* |
| Unplanned ICU admission | 1.76 | 0.96 | < 0.0001** | 0.8 | 0.43 | < 0.0001** | 1.06 | 0.58 | < 0.0001** |
| 30 day mortality | 0.3 | 0.11 | < 0.0001** | 0.14 | 0.05 | < 0.0001** | 0.18 | 0.07 | < 0.0001** |
| Percent of deaths related to surgery | 56.52 | 52.83 | < 0.0001** | 49.45 | 50.42 | < 0.0001** | 52.5 | 51.56 | < 0.0001** |
| Reoperation 30 days | 2.25 | 2.2 | 0.6828 | 0.98 | 0.79 | < 0.0001** | 1.32 | 1.18 | 0.0011* |
| Readmission 30 days | 5.3 | 6 | < 0.0001** | 2.86 | 3.07 | < 0.0001** | 3.5 | 3.88 | < 0.0001** |
| Intervention 30 days | 2.14 | 2.48 | 0.0025* | 0.84 | 0.91 | 0.0772 | 1.18 | 1.34 | 0.0002* |
| Dehydration | 0.19 | 0.5 | < 0.0001** | 0.08 | 0.23 | < 0.0001** | 0.11 | 0.3 | < 0.0001** |
| VTE | 0.02 | 0.01 | 0.1368 | 0.02 | 0.02 | 0.1549 | 0.02 | 0.01 | 0.0512 |
| Bleeding | 0.37 | 0.22 | < 0.0001** | 0.07 | 0.03 | 0.0002* | 0.15 | 0.08 | < 0.0001** |
| Leak | 0.12 | 0.09 | 0.247 | 0.14 | 0.12 | 0.1131 | 0.14 | 0.12 | < 0.0490* |
*Significant (p value < 0.05)
**Highly significant (p value < 0.0001)
Propensity score matching was performed for the 341,238 females against the 88,426 males and patient demographics are presented in Table 3. Using this approach excellent matching was achieved as indicated by the lack of significant difference between age, BMI, and preoperative comorbidities (but not COPD) between were no longer significantly different between males and females (Table 3). The propensity matched outcomes within procedure subgroups (RYGB and SG) are presented in Table 4. This approach demonstrated that in both RYGB and SG, males maintained higher rates of 30-day major complications [2.21 vs. 1.7%; p < 0.0001 (RYGB), 1.12 vs. 0.89%; p < 0.0001 (SG)] and 30-day mortality [0.23 vs. 0.12%; p < 0.0001 (RYGB), 0.10 vs. 0.05%; p < 0.0001 (SG)] compared to females. Post-operative bleeding rates remained higher in males [0.32 vs. 0.23%, p = 0.0024 (RYGB), 0.06 vs. 0.03%, p = 0.0005 (SG)], but was no longer highly significant (p < 0.0001). Leak rates maintained no significant difference between males and females in both RYGB and SG. Of note though, female patients did have higher 30-day readmission rates compared to males in both RYGB and SG groups [6.03 vs. 4.97%; p < 0.0001 (RYGB) and 3.11 vs. 2.60%, p < 0.0001 (SG)].
Table 3.
Propensity score matched patient characteristics
| Gastric bypass | Sleeve gastrectomy | |||||
|---|---|---|---|---|---|---|
| Male | Female | p value | Male | Female | p value | |
| Mean age ± SD | 45.08 (11.88) | 44.98 (11.86) | 0.8123 | 43.94 (12.09) | 44.02 (11.96) | 0.9117 |
| Mean BMI at surgery ± SD | 46.26 (8.04) | 46.10 (8.02) | 0.9654 | 45.25 (7.83) | 45.05 (7.87) | 0.9983 |
| White | 75.87 | 75.56 | 0.6246 | 72.33 | 72.32 | 0.9995 |
| Black | 13.79 | 13.96 | 0.6246 | 18.88 | 18.9 | 0.9995 |
| Race other | 10.34 | 10.48 | 0.6246 | 8.79 | 8.9 | 0.9995 |
| GERD | 38.16 | 38.36 | 0.4727 | 28.08 | 28.11 | 0.8325 |
| Limited ambulation | 1.89 | 1.9 | 0.8504 | 1.52 | 1.53 | 0.7577 |
| Cardiac history (MI or intervention) | 3.58 | 3.6 | 0.8617 | 3.02 | 3.02 | 0.9934 |
| HTN | 53.23 | 52.99 | 0.8123 | 46.86 | 46.81 | 0.9117 |
| Hyperlipidemia | 29.08 | 29.02 | 0.8466 | 22.19 | 22.15 | 0.8006 |
| History DVT | 1.80 | 1.84 | 0.6319 | 1.44 | 1.47 | 0.5072 |
| Venous stasis | 1.13 | 1.15 | 0.6935 | 0.92 | 0.94 | 0.6093 |
| Renal insufficiency and dialysis | 0.71 | 0.72 | 0.8666 | 0.79 | 0.80 | 0.9405 |
|
therapeutic anticoagulation |
2.64 | 2.69 | 0.5923 | 2.48 | 2.49 | 0.8657 |
| Previous foregut surgery | 1.65 | 1.72 | 0.3669 | 1.55 | 1.54 | 0.8339 |
| Diabetes insulin | 14.01 | 13.97 | 0.9763 | 6.63 | 6.60 | 0.8188 |
| Smoker | 8.14 | 8.28 | 0.389 | 8.76 | 8.71 | 0.6715 |
| Dependency | 0.29 | 0.31 | 0.5291 | 0.42 | 0.43 | 0.7931 |
| COPD | 1.74 | 2.03 | 0.0003* | 1.57 | 1.65 | 0.0636 |
| Oxygen dependence | 0.83 | 0.89 | 0.3311 | 0.62 | 0.64 | 0.4063 |
| History of PE | 1.31 | 1.24 | 0.0704 | 1.05 | 1.10 | 0.222 |
| Sleep apnea | 43.83 | 43.52 | 0.286 | 36.18 | 36.04 | 0.4383 |
| Chronic steroids | 1.44 | 1.52 | 0.239 | 1.76 | 1.77 | 0.8191 |
*Significant (p value < 0.05)
**Highly significant (p value < 0.0001)
Table 4.
Propensity score matched outcomes
| Gastric bypass | Sleeve gastrectomy | |||||
|---|---|---|---|---|---|---|
| Male | Female | p value | Male | Female | p value | |
| OR time (min) ± SD | 122.48 (57.08) | 119.07 (53.73) | < 0.0001** | 76.42 (37.79) | 72.04 (36.27) | < 0.0001** |
| Approach converted | 0.27 | 0.28 | 0.7477 | 0.1 | 0.1 | 0.6644 |
| Major complication | 2.21 | 1.7 | < 0.0001** | 1.12 | 0.89 | < 0.0001** |
| Minor complication | 1.33 | 1.97 | < 0.0001** | 0.43 | 0.75 | < 0.0001** |
| Acute renal failure | 0.17 | 0.1 | 0.0019* | 0.08 | 0.04 | < 0.0001** |
| Cardiac arrest req CPR | 0.1 | 0.04 | < 0.0001** | 0.04 | 0.03 | 0.0496* |
| Deep incisional SSI | 0.08 | 0.16 | 0.0004* | 0.03 | 0.02 | 0.723 |
| Myocardial infarction | 0.08 | 0.04 | 0.0023* | 0.03 | 0.03 | 0.918 |
| Organ space SSI | 0.37 | 0.35 | 0.0423* | 0.15 | 0.15 | 0.9593 |
| Progressive renal failure | 0.17 | 0.08 | < 0.0001** | 0.08 | 0.04 | < 0.0001** |
| Pneumonia | 0.31 | 0.4 | 0.0068* | 0.1 | 0.14 | 0.0002* |
| PE | 0.18 | 0.15 | 0.242 | 0.09 | 0.08 | < 0.0001** |
| Sepsis | 0.17 | 0.18 | 0.6968 | 0.06 | 0.07 | 0.7341 |
| Septic shock | 0.14 | 0.1 | 0.052 | 0.04 | 0.03 | 0.6903 |
| Superficial SSI | 0.71 | 0.95 | < 0.0001** | 0.19 | 0.25 | 0.4944 |
| Discharge other than home | 0.72 | 0.54 | < 0.0001** | 0.43 | 0.41 | 0.0037* |
| Unplanned intubation | 0.33 | 0.21 | < 0.0001** | 0.12 | 0.1 | 0.025* |
| UTI | 0.21 | 0.59 | < 0.0001** | 0.12 | 0.34 | < 0.0001** |
| VTE | 0.2 | 0.16 | 0.1415 | 0.21 | 0.16 | 0.002* |
| Unplanned ICU admission | 1.46 | 1.02 | < 0.0001** | 0.61 | 0.48 | < 0.0001** |
| 30 day mortality | 0.23 | 0.12 | < 0.0001** | 0.1 | 0.05 | < 0.0001** |
| Percent of deaths related to surgery | 57.79 | 51.75 | < 0.0001** | 54.55 | 49.7 | < 0.0001** |
| Reoperation 30 days | 2.19 | 2.2 | 0.9436 | 0.91 | 0.81 | 0.001* |
| Readmission 30 days | 4.97 | 6.03 | < 0.0001** | 2.6 | 3.11 | < 0.0001** |
| Intervention 30 days | 2.14 | 2.48 | < 0.0001** | 0.84 | 0.91 | 0.0002* |
| Dehydration | 0.2 | 0.49 | < 0.0001** | 0.09 | 0.22 | < 0.0001** |
| VTE | 0.02 | 0.01 | 0.5307 | 0.02 | 0.02 | 0.1222 |
| Bleeding | 0.32 | 0.23 | 0.0024* | 0.06 | 0.03 | 0.0005* |
| Leak | 0.13 | 0.09 | 0.0663 | 0.13 | 0.12 | 0.3657 |
*Significant (p value < 0.05)
**Highly significant (p value < 0.0001)
Discussion
To our knowledge this is the first MBSAQIP database analysis evaluating morbidity and mortality in male and female patients after laparoscopic SG or laparoscopic RYGB. Based on approximate parity of the incidence of obesity in the United States [2], our analysis demonstrate females continue to represent a disproportionately high number of patients undergoing either SG or RYGB procedures. In addition, males tend to have a slightly higher BMI and are older when they undergo bariatric procedures compared to than females. Clinically, males also had a significantly higher incidence of comorbidities than females that was also reflected in higher 30-day morbidity and mortality rates following either SG or RYGB. This disparity between males and females remained true even after controlling for pre-operative comorbidities with propensity matching, leading us to the conclusion that male gender is an independent risk factor in patients undergoing SG or RYGB. Prior to propensity matching, cardiac disease had one of the largest gender gaps in the preoperative patient demographics with males having almost 4 times the incidence compared to females. In addition, males had higher post-operative MI and other cardiac complications. This increased cardiac risk was controlled for during propensity matching and resulting in lower and similar cardiac complication rates between genders and a significant reduction in mortality and major complication rates in males. We can translate this into clinical practice by trying to identify as much cardiac disease as possible and optimize these conditions as much as possible preoperatively. Since Males had more cardiac disease before surgery and more cardiac complications after, these patients would benefit the most from having a lower threshold to perform a thorough cardiac assessment.
The MBSAQIP data base does not allow us to determine the preoperative workups of each center, however its it likely that some patients made it to surgery with undiagnosed cardiac disease which may be a contributing factor as to why males maintained a higher 30-day mortality rate despite controlling for cardiac disease during propensity matching. To our knowledge there is not a study specifically assessing the validity of preoperative cardiac workups before bariatric surgery. This would make for an interesting and important future study to conduct.
The reasons behind gender disparity in bariatric surgery have been previously investigated and consistently report less males undergo bariatric surgery than females, despite having equal or better results. Several studies have focused on gender differences in weight loss following bariatric surgery with findings suggesting higher, equal, or less weight loss in males [10–12]. However, it should be noted that the difference in excess weight loss between males and females averaged only 5% in either direction in these reports, suggesting the clinical and overall health impact of such differences are likely to be small. Despite these similarities in weight loss between sexes, males are reported to have improved psychological benefits after bariatric surgery compared to women. For example, Kochkodan et al. evaluated surveys from more than 61,000 patients from the Michigan Bariatric Surgery Collaborative in 2018, and report males were found to have higher post-operative satisfaction, body image, and psychological wellbeing scores compared to females who underwent the same procedure concomitant with lower post-operative depression scores in males [13].
These finding pose several important clinical questions; if males and females have similar obesity rates and similar weight loss results after bariatric surgery, why does such a large gender gap remain between sexes for those undergoing bariatric surgery? From a broader perspective, the World Health Organization has investigated gender disparities in health care and report that (overall) men use health services less often than females, and are less likely to report symptoms when they do present to a health care provider. These findings correlate with the continued trend of male life expectancy growing at a slower rate than females, despite similar advances in healthcare [14]. This broad gender gap in healthcare also applies when considering obesity. Surveys report males to have lower body dissatisfaction scores, and to be more satisfied with their health and fitness overall, compared to females with similar BMIs [15] suggesting males may be less likely to seek medical treatment of their obesity because of their perceived “relative health”.
Similarly [since more women are likely to seek treatment for their obesity], when males do utilize health services, is there equivalence in efforts made by health care providers to counsel males on obesity and weight loss surgery? Chang and colleagues addressed this issue in 2017 by reviewing data from 66,263 clinic visits of patients with BMIs > 35 over a 15 year period (2000–2015) [16]. Data from this study report that despite males being counseled more frequently on bariatric surgery for weight loss (2.9% of males with BMI 42–45 kg/m2 vs. 2.0% of females; 6.2% of males with BMI > 45 kg/m2 vs. 3.7% of females) male patients were less likely to consider bariatric surgery than females of similar BMIs [16]. One potential explanation of these findings may be that males have higher obesity related quality of life scores and are more likely to think of bariatric surgery as being too risky [17]. These data suggest a more significant component of the bariatric surgery gender gap lies with disparities in perception males have for the impact of weight/weight loss on quality of life, as opposed to availability of counseling for weight loss surgery.
Previous studies investigating gender disparities and outcomes in patients undergoing bariatric surgery report males present to surgery with more comorbidities and with higher rates of morbidity and mortality following surgery [5, 6, 18, 19]. Similarly, our analysis identified males presented (on average) 3 years older and 1.55 BMI points heavier than females. In addition, males had significantly higher prevalence of comorbidities (including hypertension, MI or history of cardiac procedure, diabetes, COPD, OSA, and hyperlipidemia) indicating males were higher risk patients for bariatric surgery. Given the differences in preoperative health, it was of little surprise that males had higher post-operative 30-day major complications and mortality rates compared to females, even when sub-dividing patients into RYGB and SG procedures. Indeed, our findings were similar to previously published studies analyzing data from 2005 to 2011 in which males were reported as having higher morbidity and mortality rates compared to females after bariatric surgery [5, 6]. However, it should be noted the morbidity and mortality rates in our study were significantly lower for both genders than reported in these studies in line with the improved safety profile of bariatric surgery over the past decade. Our analysis did demonstrate a significant difference in leak rates between males and females. However, the difference was only 0.02% and we did not consider this to be clinically relevant.
In order to assess male gender as an independent risk factor, propensity matching was performed. After matching, the pre-operative demographics and comorbidity profiles were no longer different between males and females. However, 30-day major complications and mortality rates remained significantly higher in males than females (after both RYGB or SG), establishing male gender as an independent risk factor for patients undergoing primary RYGB or SG. These data reflect similar conclusions drawn from previous studies (albeit these studies having smaller patient cohorts than our analysis). For example, Carbon et al. used a multiple logistic regression approach to determine female gender to be protective against morbidity and mortality in patients undergoing gastric bypass (odds ratio of 0.65 and 0.34 respectively) [6]. while Lazzati et al. established male gender to be an independent risk factor in patients undergoing either, laparoscopic adjustable gastric band (LAGB), SG, or RYGB (male post-operative mortality odds ratio of 1.94) [18].
After accounting for differences in preoperative comorbidities, many of the post-operative complications that could be attributed to higher mortality rates in males lose significance, or are no longer highly significant (p < 0.0001) (e.g. post-operative MI and septic shock). However, complications which remained highly significant after accounting for differences in preoperative comorbidities included death related to surgery and operative time. Therefore, one possible reason males continue to have higher mortality, despite controlling for comorbidities, is the increased difficulty of the operation due to male gender. This operative difficulty could be because males tend to have more central obesity (and therefore thicker abdominal walls) and more intraabdominal adipose tissue compared to females. An exception to these findings was that females had higher 30-day readmission rates for both RYGB and SG procedures due to higher rates of post-operative dehydration that required re-admission. However, this difference may not be due to female gender being a risk factor for dehydration/re-admission per se, but because males are less likely (overall) to seek medical attention.
Limitations of this study include the observational nature of large registry database studies. Although the MBSAQIP® is collected in a prospective manner, there is still significant selection bias (as with any observational study). For instance, males tend to not present for bariatric surgery until they have significant comorbidities while females tend to have surgery before their health deteriorates. Additionally, propensity matching was difficult due to the number or unhealthy males and lack of sufficient unhealthy females to match with. Overall, these limitations should be balanced against strength of the study provided by the large sample size contained within the MBSAQIP® database that allows for detailed analysis of rare events (such as mortality) to be performed with sufficient statistical power.
Conclusions
Despite the similar prevalence of obesity in males and females the gender disparity among patients having bariatric surgery remains, with males representing only 20% of all bariatric surgery patients. While males do present to surgery with significantly more comorbidities than females, male gender remains as an independent risk factor for major post-operative complications and 30-day mortality, even when comorbidities are controlled for. Therefore, a more rigorous preoperative work up, including cardiac evaluation, should be considered in male patients prior to bariatric surgery.
Compliance with ethical standards
Disclosures
Keith S. Gersin, Timothy S. Kuwada are speakers for WL Gore and Abdelrahman Nimeri speaker for Medtronic. Nicholas Dugan, Selwan Barbat, Tanushree Prasad, Kyle J. Thompson, Iain H. Mckillop, Sean R. Maloney, Amanda Roberts have no conflicts of interest or financial ties to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fryar CD, Carroll MD, Ogden CL (2016) Prevalence of overweight, obesity, and extreme obesity among adults aged 20 and over: United States, 1960–1962 through 2013–2014. [Divisions of Health and Nutrition Examination Surveys]. https://www.cdc.gov/nchs/data/hestat/obesity_adult_13_14/obesity_adult_13_14.htm. Accessed 3 Mar 2019
- 2.Hales CM, Carroll MD, Fryar CD, Ogden CL (2017) Prevalence of obesity among adults and youth: United States, 2015–2016. [NCHS Data Brief No. 288]. https://www.cdc.gov/nchs/products/databriefs/db288.htm. Accessed 2 Mar 2019 [PubMed]
- 3.Benoit SC, et al. Use of bariatric outcomes longitudinal database (BOLD) to study variability in patient success after bariatric surgery. Obes Surg. 2014;24(6):936–943. doi: 10.1007/s11695-014-1197-y. [DOI] [PubMed] [Google Scholar]
- 4.Chen SY, et al. Assessment of postdischarge complications after bariatric surgery: a National Surgical Quality Improvement Program analysis. Surgery. 2015;158(3):777–786. doi: 10.1016/j.surg.2015.04.028. [DOI] [PubMed] [Google Scholar]
- 5.Young MT, Phelan MJ, Nguyen NT. A decade analysis of trends and outcomes of male vs female patients who underwent bariatric surgery. J Am Coll Surg. 2016;222(3):226–231. doi: 10.1016/j.jamcollsurg.2015.11.033. [DOI] [PubMed] [Google Scholar]
- 6.Carbonell AM, et al. National study of the effect of patient and hospital characteristics on bariatric surgery outcomes. Am Surg. 2005;71(4):308–314. [PubMed] [Google Scholar]
- 7.The Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program. User Guide for the MBSAQIP (2016) Participant Use Data File. 2018. https://www.facs.org/~/media/files/quality%20programs/bariatric/mbsaqip_2016_puf_user_guide.ashx. Accessed 4 Mar 2019
- 8.McCaffrey DF, Ridgeway G, Morral AR. Propensity score estimation with boosted regression for evaluating causal effects in observational studies. Psychol Methods. 2004;9(4):403–425. doi: 10.1037/1082-989X.9.4.403. [DOI] [PubMed] [Google Scholar]
- 9.McCaffrey DF, et al. A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Stat Med. 2013;32(19):3388–3414. doi: 10.1002/sim.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perrone F, et al. Gender influence on long-term weight loss and comorbidities after laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass: a prospective study with a 5-year follow-up. Obes Surg. 2016;26(2):276–281. doi: 10.1007/s11695-015-1746-z. [DOI] [PubMed] [Google Scholar]
- 11.Coleman KJ, et al. Three-year weight outcomes from a bariatric surgery registry in a large integrated healthcare system. Surg Obes Relat Dis. 2014;10(3):396–403. doi: 10.1016/j.soard.2014.02.044. [DOI] [PubMed] [Google Scholar]
- 12.Ortega E, et al. Predictive factors of excess body weight loss 1 year after laparoscopic bariatric surgery. Surg Endosc. 2012;26(6):1744–1750. doi: 10.1007/s00464-011-2104-4. [DOI] [PubMed] [Google Scholar]
- 13.Kochkodan J, Telem DA, Ghaferi AA. Physiologic and psychological gender differences in bariatric surgery. Surg Endosc. 2018;32(3):1382–1388. doi: 10.1007/s00464-017-5819-z. [DOI] [PubMed] [Google Scholar]
- 14.Baker P, et al. The men’s health gap: men must be included in the global health equity agenda. Bull World Health Org. 2014;92(8):618–620. doi: 10.2471/BLT.13.132795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fallon EA, Harris BS, Johnson P. Prevalence of body dissatisfaction among a United States adult sample. Eat Behav. 2014;15(1):151–158. doi: 10.1016/j.eatbeh.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Chang LS, Malmasi S, Hosomura N, Zhang H, Lei V, Rubin A, Ting C, Tong K, Turchin A. A5272—a weighty issue: gender disparities in bariatric surgery counseling. Surg Obes Relat Dis. 2017;13(10):S187–S188. doi: 10.1016/j.soard.2017.09.414. [DOI] [Google Scholar]
- 17.Wee CC, et al. Sex, race, and consideration of bariatric surgery among primary care patients with moderate to severe obesity. J Gen Intern Med. 2014;29(1):68–75. doi: 10.1007/s11606-013-2603-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lazzati A, et al. Reduction in early mortality outcomes after bariatric surgery in France between 2007 and 2012: a nationwide study of 133,000 obese patients. Surgery. 2016;159(2):467–474. doi: 10.1016/j.surg.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Stroh C, et al. Influences of gender on complication rate and outcome after Roux-en-Y gastric bypass: data analysis of more than 10,000 operations from the German Bariatric Surgery Registry. Obes Surg. 2014;24(10):1625–1633. doi: 10.1007/s11695-014-1252-8. [DOI] [PubMed] [Google Scholar]