Abstract
Background
Earlier attempts to deliver effective lung doses of surfactant by aerosolization were unsuccessful, mostly because of technical shortcomings. We aimed at quantifying the lung deposition of poractant alfa with a new supraglottic delivery system for surfactant atomization in an experimental neonatal model.
Methods
The method involved six sedated 1-day-old piglets lying in the lateral decubitus, spontaneously breathing on nasal-mask continuous positive airway pressure (nCPAP). A pharyngeal cannula housing a multi-channel air-blasting atomization catheter was placed through the mouth with its tip above the glottis entrance. In all, 200 mg kg−1 of a 99mTc-surfactant mixture was atomized through the catheter synchronously with inspiration. Six intubated control piglets received an equal amount of intratracheally instilled 99mTc-surfactant mixture. The percentage of the 99mTc-surfactant mixture deposited in the lungs was estimated by scintigraphy.
Results
Median (range) deposition in the lungs was 40% (24–68%) after atomization and 87% (55–95%) after instillation (p < 0.001). Overall, almost 80% of the deposited surfactant was in the dependent lung. Effective atomization time (atomizer on) was 28 (17–52) min, yielding an output rate of 0.1–0.2 mL min−1.
Conclusions
Without endotracheal intubation, in spontaneously breathing newborn piglets, this new supraglottic atomizer delivery system attained a median lung deposition of 40% of the nominal dose of surfactant.
Introduction
Respiratory distress syndrome is the most common disease affecting premature neonates.1 Endotracheal (ET) instillation of exogenous surfactant is still the standard recommended therapy2 with high-quality evidence demonstrating its clinical efficacy by dramatically improved survival rates since its introduction in the 1980s.3,4 Nevertheless, surfactant delivery via instillation is an invasive procedure that usually requires ET intubation and manipulation of the airways and is associated with several risks, such as transient hypoxia and bradycardia, changes in blood pressure, fluctuations in cerebral blood flow, pulmonary hemorrhage, and positive pressure ventilation.5–7
Because of these potential complications and the increasing use of noninvasive ventilation methods (NIV) in the treatment of mild-to-moderate degrees of neonatal respiratory distress syndrome, there has been a renewed interest in the implementation of less invasive techniques for surfactant administration to the premature surfactant-deficient infant. In the 1990s, the INSURE [INtubate, bolus SURfactant administration, prompt Extubation to nasal continuous positive airway pressure (nCPAP)] technique was introduced8,9 to preclude prolonged intubation and mechanical ventilation, and although it does reduce mechanical ventilation time and possibly the extent of barotrauma, it does not consistently decrease the incidence of bronchopulmonary dysplasia (BPD) or associated complications.10 In the past decade, new approaches to surfactant administration have been gathered under the denomination of minimally invasive surfactant therapy. Fifteen German centers showed decreased mortality and lower incidence of BPD when administering surfactant via a thin, flexible catheter inserted below the vocal cords in spontaneously breathing neonates while on nCPAP.11–13 However, the technique requires the use of a laryngoscope and often of a Magill forceps to properly place the catheter in the trachea.
Recent improvements in aerosol technology have encouraged new trials for the administration of nebulized or atomized surfactant obviating the need for intubation. Using a vibrating-mesh nebulizer to continuously deliver porcine surfactant (Curosurf®, Chiesi Farmaceutici SpA, Parma, Italy) to piglets on nCPAP, we reported a lung deposition of 14% of the total nebulized dosage.14 However, upper airway deposition (nose and pharynx) was 19%, supporting the observations of others that the placement of a nebulizer in the ventilator breathing circuit is often associated with a significant loss of surfactant in the upper airways, in the breathing circuit, and possibly to the ambient air.15 To optimize delivery, a new system that uses a special pharyngeal cannula and a multichannel flexible catheter for surfactant atomization was developed.16,17 The tip of the cannula holding the multichannel catheter is placed in the pharynx just above the glottic opening without requiring the use of a laryngoscope. The device allows surfactant atomization to take place directly above the glottis, therefore bypassing most of the upper airway, in synchrony with the inspiratory flow during nCPAP or other modalities of NIV.
This feasibility study aimed to quantify the lung deposition of the 99mTc-poractant alfa surfactant mixture in spontaneously breathing newborn piglets on nCPAP with this new delivery system for surfactant atomization.
Methods
The experiment was approved by the local committee on animal research ethics of Lund University, Sweden. Twelve-to-36 h-old full-term piglets were studied. The animals were randomly assigned to one of the two groups of six subjects each: one group received atomized surfactant, and the other received ET-instilled surfactant. In both, during surfactant administration, half of the animals laid on their right and half on their left side.
Anesthesia and monitoring
Animals were premedicated with intramuscular ketamine (6 mg), midazolam (0.4 mg), and atropine (0.2 mg). An ear vein was cannulated and continuous analgosedation with intravenous (i.v.) ketamine (1–3 mg kg−1 h−1) and dexmedetomidine (1–3 µg kg−1 h−1) was started. Intermittent dexmedetomidine (0.1 µg kg−1) or propofol (1–3 mg kg−1) were given i.v. as needed to ascertain animal comfort. A solution of 50 g glucose, 70 mmol sodium, 45 mmol chloride, and 25 mmol acetate per liter was infused i.v. at a rate of 10 mL kg−1 h−1.
A femoral artery was cannulated for arterial blood pressure monitoring and blood sampling. Electrocardiogram and pulse oximetry were continuously monitored. Data were sampled and recorded using a Philips IntelliVue M70 monitor (Philips Healthcare Sverige, Stockholm, Sweden). A self-adherent near-infrared spectroscopy probe (Pediatric SomaSensor probe, model SPFB-USA, Somanetics Corporation, Troy, MI, USA) was placed on a shaved spot on the center of the scalp and connected to a Somanetics INVOS 5100C Cerebral/Somatic Oximeter monitor for measurement of regional cerebral saturation (CrSO2); the monitor updated and stored the readings every 6 s.
A neurally adjusted ventilator assist (NAVA) catheter (Maquet Critical Care AB, Solna, Sweden) was used to monitor the integrated electrical activity of the diaphragm (Edi), which allowed assessment of the breathing pattern and aided in titrating the level of sedation.
Arterial blood gases
Arterial blood gases were obtained at baseline (10 min after preparation was completed), immediately before treatment, at 15 min of atomization or 15 min after instillation, and the end of treatment. The samples were analyzed on an ABL 700 blood gas analyzer (Radiometer, Copenhagen, Denmark).
Surfactant preparation
All animals received 200 mg kg−1 of porcine surfactant (Curosurf® 80 mg mL−1), thoroughly mixed with 300 MBq technetium-99m-nanocolloid (99mTc–nanocolloid, Nanocoll®, GE Healthcare AB, Stockholm, Sweden).18
The atomizer
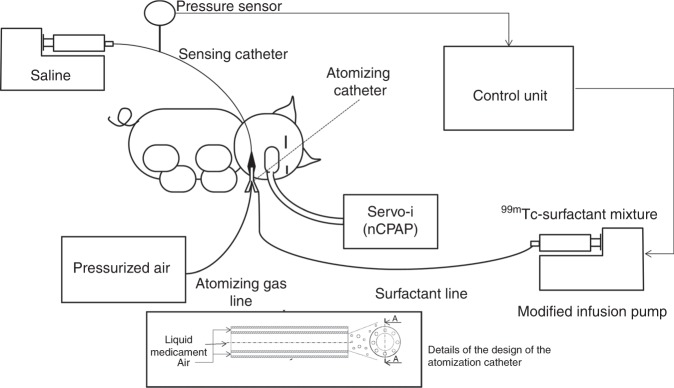
The investigational system consisted of an ad hoc designed pharyngeal cannula conveying a multi-lumen catheter connected to a modified infusion pump intermittently delivering the 99mTc- surfactant mixture into the catheter’s central lumen. The infusion pump was controlled by a dedicated electronic circuit activating the infusion of surfactant during inspiration only, which reduced the waste of surfactant. For atomization, pressurized medical air was applied to the catheter’s outer lumens at a pressure level able to produce an atomizing flow of 0.5 L min−1. With this airflow, the median particle size volume distribution (Dv50) ranged between 40 and 60 µm as determined by a laser diffraction method (Spraytech, Malvern Instruments, Malvern, UK) with the variability explained by the handmade manufacturing process of the catheter. For breath synchronization, an additional fluid-filled catheter (sensing catheter) was connected to a pressure sensor and secured along the pharyngeal cannula to measure the pressure oscillations in the pharyngeal cavity (Fig. 1). A second infusion pump injected a continuous flow of a saline solution at 1 mL h−1 preventing the unintentional occlusion of the sensing catheter.
Fig. 1.
Schematics of the delivery system.
Ventilation and surfactant administration in the atomization group
In the atomization group, 0.1 mL of nasal decongestant oxymetazoline hydrochloride (0.5 mg mL−1) was given in each nostril. To avoid undesirable reflexes, the animals inhaled 10 mg of lidocaine with 0.5 mg adrenaline for 3 min before airway manipulation. A snout mask manufactured using the thumb of a nitrile-rubber medical examination glove and a standard ET tube (ETT) connector was attached to the ventilator circuit Y-piece. Spontaneous breathing was maintained throughout surfactant atomization supported by the Servo-i ventilator (Maquet Critical Care AB, Solna, Sweden) in nCPAP mode. The mouth was kept closed to decrease leakage and to avoid interference with the delivery system (Fig. 1). CPAP was kept at 3 cm H2O and FiO2 at 0.4.
The surfactant delivery system was placed in the retro-pharynx with additional topical anesthesia with 1% lidocaine gel (Fig. 2). The correct placement, just above the epiglottis, i.e., not to interfere with the normal movement of the epiglottis during breathing, was confirmed using a fiberscope (Fig. 3). The surfactant was delivered in four aliquots interspaced with 5 min of pressure-support (PS) ventilation with inspiratory pressures 4–8 cm H2O above positive end expiratory pressure (PEEP) 4 cm H2O to avoid atelectasis and promote the delivery of the surfactant to the distal airways. During atomization, FiO2 was adjusted in increments of 5% as needed to keep SaO2 > 85%. After surfactant administration, the piglets were stabilized for 15 min and then intubated with a 2.5 cuffed ETT (Kimberley-Clark, Neenah, WI) to secure the airway before transport to the gamma camera in an adjacent building.
Fig. 2.
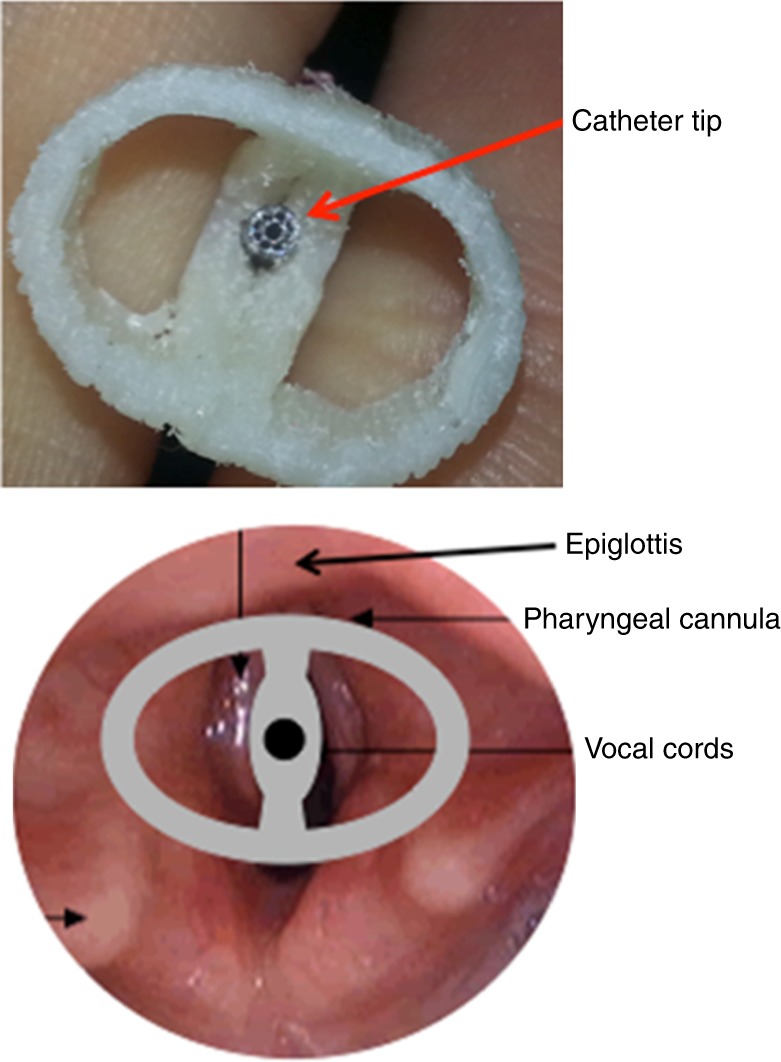
Close-up picture of the catheter tip in the center of the oropharyngeal cannula and its position at the laryngeal entrance.
Fig. 3.

Picture taken with the fiberscope confirming the correct position of the cannula in relation to the larynx entrance and a technical drawing of the cannula’s lateral view.
Ventilation and surfactant administration in the instillation group
In the instillation group, the trachea was intubated with a size 2.5 cuffed ETT after spraying the larynx with lidocaine. Correct tube placement was confirmed by careful thorax auscultation. The piglet was connected to the Servo-i ventilator, and spontaneous breathing maintained using the PS mode with PEEP 4 cm H2O and FiO2 0.5. Inspiratory pressure was adjusted to achieve a tidal volume of 6–10 mL kg−1, i.e., between 4 and 8 cm H2O above PEEP. Differently from the treatment group (alternating nCPAP and PS + PEEP), the animals were kept on PS mode throughout.
Surfactant bolus was instilled in the trachea using a catheter passed 0.5 cm beyond the tip of the ETT. After instillation, before the resumption of the previous ventilator settings, PEEP was increased to 10 cm H2O for 1 min to promote lung recruitment and surfactant distribution. The piglets were stabilized for 15 min before being transferred to the gamma camera.
Measurement of surfactant deposition
Piglets were placed prone in a gamma camera (Philips Skylight, Philips AB, Stockholm, Sweden) with dual heads, simultaneously acquiring one anterior and one posterior image. After a 3-min exposure, 25 MBq of 99mTc-macroaggregated albumin (Tc-MAA, TechneScan LyoMAA, Covidien Sverige AB, Solna, Sweden) was injected i.v., and a second exposure made. The 99mTc-MAA is trapped in the lung capillaries and is used to outline the lungs and to do internal calibration as earlier described.14 Deposition at various regions of interest was calculated from the mean of the anterior and posterior images and presented as a percentage of the total atomized or instilled surfactant dose.
At the end of the study, i.e., after the gamma scintigraphy examination, the piglets were killed using an excess dose of pentobarbital, fentanyl, and potassium.
Statistical analysis
Differences between groups were assessed with t test or alternatively Mann–Whitney test using Sigmaplot 14 (Systat Software Inc., San Jose, CA) after controlling for normality and variance. Differences within groups were tested with paired t test or alternatively Wilcoxon signed-rank test or repeated-measures analysis of variance on ranks. p values <0.05 were considered significant. Results are presented as median (range), if not otherwise stated.
Results
Body weight was significantly different between the groups, 0.9 kg (0.8–1.1 kg) in the instillation and 1.2 kg (1–1.7 kg) in the atomization group (p = 0.014). There were no statistically significant differences between the groups in gender distribution (four females and eight males). Hemoglobin concentration was 78 g L−1 (62–121 g L−1) in the instillation group and 80 g L−1 (55–109 g L−1) in the atomization group (differences not statistically significant). Blood gas parameters (Table 1), mean arterial pressure (MAP), heart rate (HR), and CrSO2 were similar at baseline.
Table 1.
Arterial blood gases.
| Group | Baseline | Start | 15 min | End |
|---|---|---|---|---|
| PaO2, kPa | ||||
| Atomizer | 19 (13–22) | 9.2 (6.1–19)a,b | 5.7 (3.7–13)b | 7.2 (6.8–12)a,b |
| Instillation | 16 (14–26) | 18 (14–24) | 9.6 (5.3–13)b | 14 (13–21) |
| PaCO2, kPa | ||||
| Atomizer | 5.8 (4.9–6.7) | 5.4 (4.8–6.4) | 6.8 (5.0–8.5) | 8.6 (5.7–11)a,b |
| Instillation | 7.5 (4.6–12) | 5.1 (4.5–5.8) | 6.8 (4.9–9.4) | 6.1 (5.6–7.2) |
| pH | ||||
| Atomizer | 7.49 (7.39–7.55) | 7.42 (7.33–7.49) | 7.41 (7.33–7.49) | 7.29 (7.24–7.44)a,b |
| Instillation | 7.44 (7.29–7.53) | 7.52 (7.47–7.65) | 7.42 (7.24–7.58) | 7.47 (7.41–7.50) |
Values are presented as median (range). Differences within groups were analyzed with RM ANOVA on ranks followed by Dunn’s post hoc test when indicated. Differences between groups at the different stages were analyzed with t test or Mann–Whitney test. p < 0.05 was considered significant
aDenotes significant changes between groups
bDenotes significant changes from Baseline
The percentage of the administered surfactant found in the lungs was 40% (24–68%) in the atomization group and 87% (55–95%) in the instillation group (p < 0.001) (Table 2). In the atomization group, 13% of the surfactant was observed in the nasopharynx and 2% in the gut. In the instillation group, one animal had only 55% of the surfactant deposited in the lungs, and this was the same animal that had 15.6% of the surfactant in the nasopharynx/ET tube (Table 2). Gamma camera images of a typical piglet in the atomization and the instillation group are presented in Fig. 4. In both groups, significantly more surfactant was detected in the dependent lung (p values < 0.001; Table 2) with no significant differences in the distribution ratios between the upper and lower lungs between the two groups.
Table 2.
Deposition of surfactant at various locations.
| Group | Upper lung, % | Dependent lung, % | Both lungs, % | Ratio of upper/both lungs | Nasopharynx–ET tube, % | Trachea, % | Gut, % |
|---|---|---|---|---|---|---|---|
| Atomizer | 4.7 (0.8–19) | 32 (23–50) | 40 (24–68) | 14 (3.3–35) | 13 (5.5–16) | 4 (3–6) | 1.9 (1–17) |
| Instillation | 9.3 (1.9–29) | 66 (53–92) | 87 (55–95) | 11 (3.5–31) | 5.4 (4.3–15.6) | 3 (1–8) | 2.1 (1.1–2.5) |
| p value | 0.41 | <0.001 | <0.001 | 0.80 | 0.057 | 0.965 | 0.937 |
Deposition median [range] as a percentage of total atomized dose or total instilled dose. n = 6 in both groups
p values are calculated using t test or Mann–Whitney test
Fig. 4.
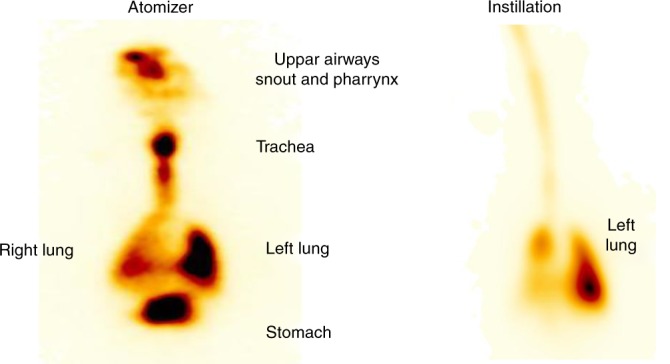
Example of a scintigram of a piglet in the atomizer group lying on the left side and one in the instillation group also lying on the left side.
The effective atomization time (the time the atomizer was on) for delivering 200 mg kg−1 of surfactant, i.e., 3.3 mL (2.7–4.4 mL), was 28 min (17–52 min), corresponding to an output rate of 0.1–0.2 mL min−1 in this setting.
There were significant differences in arterial blood gas measurements between groups due to inherent ventilatory dissimilarities related to study design (Table 1). At the start of the treatment, when the piglets had already been instrumented according to the protocol of the respective group, that is, either being deeper sedated and intubated in the instillation group or after placement of the oropharyngeal device in the atomization group, there was a significant difference between the two groups in PaO2, which was higher in the intubated animals. Likewise, differences in PaO2 (higher in instillation group, p = 0.002), PaCO2 (lower in instillation group, p = 0.04), and pH (lower in the atomization group, p = 0.005) were also observed at the end of the treatment.
There were no significant hemodynamic (HR, MAP) differences between groups at baseline, at the start of treatment, and 15 min after treatment. Compared to baseline, there were no within-group significant changes except that CrSO2 was lower at 1 min after the instillation of surfactant.
Discussion
We found that, with this new supraglottic atomization device, 40% of the surfactant dose atomized directly above the vocal cords of spontaneously breathing newborn piglets on nCPAP reached the lungs. It corresponds to almost half of the deposition observed in the control group that received the surfactant as direct instillation via ETT. This suggests that the new device can deliver considerable amounts of aerosolized surfactant to the lungs of spontaneously breathing subjects on nCPAP without the need for ET intubation.
Based on earlier animal studies, it has been suggested that the lung dose of surfactant needed to elicit a significant clinical effect is about 50 mg kg−1.19 Despite a few pilot trials of aerosolized surfactant delivered above the glottis entrance in neonates, there are only a few experimental studies assessing lung deposition of surfactant aerosolized above the glottis (Table 3). Rahmel et al.,20 using immature lambs breathing via nasal prongs, reported a lung deposition of only 1% of the administered recombinant surfactant protein-C surfactant aerosol, equivalent to 4 mg lipids per kg. The median lung deposition of 14% of the total administered poractant alfa surfactant dose (200 mg kg−1) reported by our group in non-intubated term newborn piglets in nCPAP with a mesh nebulizer placed in the respiratory circuit in non-synchronized mode corresponds to 28 mg kg−1 of lipids.14 Wagner et al. demonstrated that surfactant atomization could take place inside the trachea with an atomizer at the tip of a modified ETT and synchronized to the animals’ breath.21 Their average lung surfactant deposition using a radiolabeling technique was 86%. Nevertheless, placing the atomizer at the tip of the tube is technically challenging and does not avoid the disadvantages of ET intubation. With the presently tested breath-activated supraglottic delivery system, in premature spontaneously breathing lambs on nCPAP, using samarium (nonradioactive tracer)-labeled surfactant, Milesi et al. observed a median lung deposition of 32% of the administered dose (200 mg kg−1) of poractant alfa.22 In the present study with the same atomizing device, using a well-validated and accepted method for measuring lung deposition with radioactive labeling of the administered surfactant, we show for the first time, a lung surfactant deposition over 50 mg kg−1, i.e., median lipid delivery of 80 mg kg−1 for a surfactant dose of 200 mg kg−1. This device was conceived with the intent to avoid patient intubation, to reduce dead space, to avoid surfactant denaturalization,23 and to work synchronously with inspiration thereby reducing surfactant losses to the upper airways and environment.
Table 3.
Animal studies on nebulization and atomization of surfactant.
| Author | Year | Method | Deposition | Surfactant | Breathing | Ventilation mode | Animal |
|---|---|---|---|---|---|---|---|
| Lewis et al.26 | 1991 | Nebulization | 2.7% | Survanta | Intubated | IPPV | Lamb |
| Lewis et al.27 | 1991 | Nebulization | 3.6% | Survanta | Intubated | IPPV | Rabbit |
| Lewis et al.28 | 1993 | Nebulization | 6.1% | Survanta | Intubated | IPPV | Sheep |
| Dijk et al.18 | 1997 | Nebulization | 8.4% | Alveofact | Intubated | IPPV | Rabbit |
| Dijk et al.6 | 1998 | Nebulization | 9.8% | Alveofact | Intubated | HFV | Rabbit |
| Fok et al.29 | 1998 | Nebulization | 0.1–1% | Survanta/Exosurf | Intubated | IPPV | Rabbit |
| Wagner et al.21 | 2000 | Atomization | 86.5% | Poractant alfa | Intubated | IPPV | Rabbit |
| Rahmel et al.20 | 2012 | Aerosolized | 1% | rSP-C | Spontaneous | nCPAP | Lamb |
| Rey-Santano et al.30 | 2013 | Aerosolized | Not reported | Poractant alfa | Intubated | IPPV | Lamb |
| Linnér et al.14 | 2015 | Nebulization | 14% | Poractant alfa | Spontaneous | nCPAP | Pig |
| Milesi et al.16 | 2016 | Atomization | Not reported | Poractant alfa | Intubated | IPPV | Lamb |
| Hutten et al. 31 | 2015 | Nebulization | Not significant | Poractant alfa | Spontaneous | nCPAP | Lamb |
| Milesi et al.22 | 2017 | Atomization | 32% | Poractant alfa | Spontaneous | nCPAP | Lamb |
| Gregory et al. 32 | 2019 | Nebulization | 11.4% | Lucinactant | Spontaneous | nCPAP | Macaque |
Aerosol delivery to the lungs is influenced by many different factors related to the aerosol-generating system and the recipient. Particle size is one of the most important properties of an aerosol with an impact on its deposition and distribution in the airways. The optimal particle size for reaching the distal airways and alveoli is around 1–3 μm.24 Nonetheless, in the above-mentioned study by Wagner et al., intratracheal atomization of surfactant particles with a Sauter Mean Diameter >100 μm reached similar lung deposition and distribution as instillation.21 Their results are probably due to a more central deposition in the larger airways with distribution to non-expanded alveoli by diffusion gradient, by capillarity, or along the surface tension gradient, factors that help to avoid losses to exhalation. The mean particle size in our study was somewhat less, with a median diameter of 40–60 μm. Even so, the generated particles were probably big enough to increase the initial deposition in the large airways reducing the losses during expiration. Furthermore, the accurate positioning of the tip of the atomizer in the supraglottic region and the synchronicity of delivery with the inspiratory flow are two other factors contributing to improved lung deposition by eliminating unwarranted losses in the upper respiratory tract, as well as losses to the atmosphere and the delivery system.
The oropharyngeal cannula was designed with a shape allowing self-positioning during the blind introduction. We confirmed the correct placement with a fiberscope before starting atomization. However, the cannula did not perfectly match the geometry of the pig’s pharynges, with the lateral dimension of the actual pharynx being larger than the one of the cannulas. This allowed an excessive degree of freedom that might have led to dislodgement of the cannula during the surfactant delivery and could be one of the sources of the inter-subject variability in the deposition rates we observed.
The median time of atomization was 28 min, with a wide range of 17–52 min. Two main factors influenced the variability of atomization time: (1) The total amount of surfactant administered as the dose was defined per kg of body weight, and (2) As surfactant atomization was synchronized with the inspiratory phase, the actual atomization time during 1 min depended on the piglet’s spontaneous respiratory rate and inspiration:expiration ratio. Moreover, animals had sometimes periodic breathing, which further impacted on the atomization time. However, there was no correlation between total deposition and the time taken to atomize the entire amount of surfactant, suggesting that atomization time did not impact on the efficiency of the atomizer.
The changes in hemodynamics and oxygenation observed during our study are most certainly inherent to the model itself. That the instillation group had a slightly lower MAP and HR at 0 time (not statistically significant) is explained by the need for deeper sedation required for animal comfort, as MAP and HR decreased at the time of intubation and remained stable afterward. In contrast with reports showing drastic changes in hemodynamics and oxygenation during surfactant instillation,25 we only observed a transient fluctuation in CrSO2. PaO2 did not fall probably because we performed a recruitment maneuver with PEEP right after surfactant instillation. Blood gas parameters were better in the instillation group at the end of the experiment, but this is not surprising because these piglets were breathing in PS mode with an unobstructed secured airway (ETT). In the atomization group, shortly after the procedure, there was an increase in PaCO2 and a decrease in PaO2 corresponding to a SaO2 around 84%, probably reflecting ventilation–perfusion mismatch due to the extra fluid volume delivered to healthy lungs. Surfactant accumulation in the large airways was also a concern. Therefore, after a few pilot experiments, we chose to split the surfactant dose into four equal aliquots intercalated with 5 min of PS ventilation to promote redistribution of the accumulated fluid and lung recruitment.
We did not observe any major complications of the delivery system itself. During the pilot studies, we tested different gas flow rates to create the aerosol. With flow rates >0.75 L min−1, the piglets either decreased their breathing rate or stopped breathing (Heuring–Breuer reflex). It is difficult to speculate whether this would be of concern in human neonates with decreased lung compliance. With the sedation provided, the animals tolerated well the retro-pharyngeal airway. One of the animals developed a minor hematoma over the epiglottis.
The major limitation of our study is the use of term newborn piglets with normal lung function and normal endogenous surfactant pool under sedation/analgesia that precludes the evaluation of treatment efficacy. Owing to the presence of additional gas flow, we were not able to confidently measure tidal volume, which is an important variable in aerosol delivery. However, we indirectly monitored the work of breathing by the Edi signal obtained with the NAVA catheter and modified the level of CPAP and/or PS, to improve airway patency when needed.
In conclusion, in a model of sedated term newborn piglets on nCPAP, we showed that it was feasible to achieve a median lung deposition of 40% of the total administered dose of poractant alfa surfactant using a new device for supraglottic atomization. This is by far the highest observed lung deposition of surfactant administered above the vocal cords in the available medical literature. Delivery of atomized surfactant was completed without any major adverse events and the total amount of lipids deposited in the lungs, i.e., 80 mg kg−1 was well above the amount expected to induce a significant clinical effect what has been confirmed in Milesi et al. study in preterm lambs.22
Acknowledgements
This study was funded by Chiesi Farmaceutici SpA (Parma, Italy), which is the employer of F.B. and M.d.C. Chiesi Farmaceutici SpA also supplied the animal-derived surfactant Poractant alfa (Curosurf®, Chiesi Farmaceutici SpA, Parma, Italy) used in this study.
Author contributions
All named authors have made substantial contributions to the conception and design of the study, as well as to the acquisition, interpretation, and analysis of data. A.N., R.L., D.C.-G., and V.P.-d.-S. have drafted the article that has been critically revised for important intellectual content by F.B., M.d.C., I.M., E.Z., and R.L.D. All named authors have approved the final version of the manuscript and agreed to the article’s submission to Pediatric Research.
Competing interests
Chiesi Farmaceutici SpA, and Politecnico di Milano University, the home institution of I.M., E.Z., and R.L.D., own patents on the atomization of surfactant licensed to Chiesi Farmaceutici. The authors disclose off-label product use of surfactant where it is delivered by a different route of administration, an investigational device for the delivery of aerosol drugs, though indication (Respiratory Distress Syndrome, RDS) and dosage (200 mg kg−1) have not been changed. Not related to this study, Politecnico di Milano University, home institution of R.L.D., I.M., and E.Z., received research funding from Chiesi Farmaceutici SpA, ACUTRONIC Medical Systems AG, RESTECH Srl, and Philips AB. The other authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gupta S, Donn SM. Novel approaches to surfactant administration. Crit. Care Res. Pract. 2012;2012:278483. doi: 10.1155/2012/278483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakonidou S, Dhaliwal J. The management of neonatal respiratory distress syndrome in preterm infants (European Consensus Guidelines-2013 update) Arch. Dis. Child. Educ. Pract. Ed. 2015;100:257–259. doi: 10.1136/archdischild-2014-306642. [DOI] [PubMed] [Google Scholar]
- 3.Jobe A, Ikegami M. Surfactant for the treatment of respiratory distress syndrome. Am. Rev. Respir. Dis. 1987;136:1256–1275. doi: 10.1164/ajrccm/136.5.1256. [DOI] [PubMed] [Google Scholar]
- 4.Fujiwara T, et al. Artificial surfactant therapy in hyaline-membrane disease. Lancet. 1980;1:55–59. doi: 10.1016/S0140-6736(80)90489-4. [DOI] [PubMed] [Google Scholar]
- 5.Schipper JA, Mohammad GI, van Straaten HL, Koppe JG. The impact of surfactant replacement therapy on cerebral and systemic circulation and lung function. Eur. J. Pediatr. 1997;156:224–227. doi: 10.1007/s004310050588. [DOI] [PubMed] [Google Scholar]
- 6.Dijk PH, Heikamp A, Oetomo SB. Surfactant nebulization versus instillation during high frequency ventilation in surfactant-deficient rabbits. Pediatr. Res. 1998;44:699. doi: 10.1203/00006450-199811000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Katheria AC, Leone TA. Changes in hemodynamics after rescue surfactant administration. J. Perinatol. 2013;33:525–528. doi: 10.1038/jp.2012.166. [DOI] [PubMed] [Google Scholar]
- 8.Bohlin K, Jonsson B, Gustafsson AS, Blennow M. Continuous positive airway pressure and surfactant. Neonatology. 2008;93:309–315. doi: 10.1159/000121457. [DOI] [PubMed] [Google Scholar]
- 9.Bohlin K, Gudmundsdottir T, Katz-Salamon M, Jonsson B, Blennow M. Implementation of surfactant treatment during continuous positive airway pressure. J. Perinatol. 2007;27:422–427. doi: 10.1038/sj.jp.7211754. [DOI] [PubMed] [Google Scholar]
- 10.Göpel W, et al. Avoidance of mechanical ventilation by surfactant treatment of spontaneously breathing preterm infants (AMV): an open-label, randomised, controlled trial. Lancet. 2011;378:1627–1634. doi: 10.1016/S0140-6736(11)60986-0. [DOI] [PubMed] [Google Scholar]
- 11.Kanmaz, H. G. et al. Surfactant administration via thin catheter during spontaneous breathing: randomized controlled trial. Pediatrics131, e502–e509 (2013). [DOI] [PubMed]
- 12.Gopel W, et al. Less invasive surfactant administration is associated with improved pulmonary outcomes in spontaneously breathing preterm infants. Acta Paediatr. 2015;104:241–246. doi: 10.1111/apa.12883. [DOI] [PubMed] [Google Scholar]
- 13.Hartel C, et al. Less invasive surfactant administration and complications of preterm birth. Sci. Rep. 2018;8:8333. doi: 10.1038/s41598-018-26437-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linner R, Perez-de-Sa V, Cunha-Goncalves D. Lung deposition of nebulized surfactant in newborn piglets. Neonatology. 2015;107:277–282. doi: 10.1159/000369955. [DOI] [PubMed] [Google Scholar]
- 15.Mazela J, Polin RA. Aerosol delivery to ventilated newborn infants: historical challenges and new directions. Eur. J. Pediatr. 2011;170:433–444. doi: 10.1007/s00431-010-1292-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milesi I, et al. Intratracheal atomized surfactant provides similar outcomes as bolus surfactant in preterm lambs with respiratory distress syndrome. Pediatr. Res. 2016;80:92–100. doi: 10.1038/pr.2016.39. [DOI] [PubMed] [Google Scholar]
- 17.Dellacá, R. F. & Milesi, I. Method and system for the administration of a pulmonary surfactant by atomization. European patents 2012016523420120423 and WO2013160129 A1 (patent pending) (2013).
- 18.Dijk PH, Heikamp A, Bambang Oetomo S. Surfactant nebulisation: lung function, surfactant distribution and pulmonary blood flow distribution in lung lavaged rabbits. Intensive Care Med. 1997;23:1070–1076. doi: 10.1007/s001340050458. [DOI] [PubMed] [Google Scholar]
- 19.Robertson B, Halliday HL. Principles of surfactant replacement. Biochim. Biophys. Acta. 1998;1408:346–361. doi: 10.1016/S0925-4439(98)00080-5. [DOI] [PubMed] [Google Scholar]
- 20.Rahmel DK, et al. The non-intubated, spontaneously breathing, continuous positive airway pressure (CPAP) ventilated pre-term lamb: a unique animal model. Reprod. Toxicol. 2012;34:204–215. doi: 10.1016/j.reprotox.2012.05.089. [DOI] [PubMed] [Google Scholar]
- 21.Wagner MH, et al. Endotracheal surfactant atomization: an alternative to bolus instillation? Crit. Care Med. 2000;28:2540–2544. doi: 10.1097/00003246-200007000-00058. [DOI] [PubMed] [Google Scholar]
- 22.Milesi I, et al. Supraglottic atomization of surfactant in spontaneously breathing lambs receiving continuous positive airway pressure. Pediatr. Crit. Care Med. 2017;18:e428–e434. doi: 10.1097/PCC.0000000000001267. [DOI] [PubMed] [Google Scholar]
- 23.Dhand R. Aerosol delivery during mechanical ventilation: from basic techniques to new devices. J. Aerosol Med. Pulm. Drug Deliv. 2008;21:45–60. doi: 10.1089/jamp.2007.0663. [DOI] [PubMed] [Google Scholar]
- 24.Darquenne, C. in The ISAM Textbook of Aerosol Medicine (ed. Dhand, R.) Ch. 2.2 (Mary Ann Libert Publishing, Inc., Rochelle, NY., 2015).
- 25.Moen A, Yu XQ, Rootwelt T, Saugstad OD. Acute effects on systemic and pulmonary hemodynamics of intratracheal instillation of porcine surfactant or saline in surfactant-depleted newborn piglets. Pediatr. Res. 1997;41:486–492. doi: 10.1203/00006450-199704000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Lewis JF, Ikegami M, Jobe AH, Tabor B. Aerosolized surfactant treatment of preterm lambs. J. Appl. Physiol. 1985;1991:869–876. doi: 10.1152/jappl.1991.70.2.869. [DOI] [PubMed] [Google Scholar]
- 27.Lewis J, Ikegami M, Higuchi R, Jobe A, Absolom D. Nebulized vs. instilled exogenous surfactant in an adult lung injury model. J. Appl. Physiol. 1985;1991:1270–1276. doi: 10.1152/jappl.1991.71.4.1270. [DOI] [PubMed] [Google Scholar]
- 28.Lewis JF, et al. Lung function and surfactant distribution in saline-lavaged sheep given instilled vs. nebulized surfactant. J. Appl. Physiol. 1985;1993:1256–1264. doi: 10.1152/jappl.1993.74.3.1256. [DOI] [PubMed] [Google Scholar]
- 29.Fok TF, al-Essa M, Dolovich M, Rasid F, Kirpalani H. Nebulisation of surfactants in an animal model of neonatal respiratory distress. Arch. Dis. Child. Fetal Neonatal Ed. 1998;78:F3–F9. doi: 10.1136/fn.78.1.F3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rey-Santano C, et al. Acute and sustained effects of aerosolized vs. bolus surfactant therapy in premature lambs with respiratory distress syndrome. Pediatr. Res. 2013;73:639–646. doi: 10.1038/pr.2013.24. [DOI] [PubMed] [Google Scholar]
- 31.Hutten MC, et al. Nebulization of Poractant alfa via a vibrating membrane nebulizer in spontaneously breathing preterm lambs with binasal continuous positive pressure ventilation. Pediatr. Res. 2015;78:664–669. doi: 10.1038/pr.2015.165. [DOI] [PubMed] [Google Scholar]
- 32.Gregory, T. J., Irshad, H., Chand, R. & Kuehl, P. J. Deposition of aerosolized lucinactant in nonhuman primates. J. Aerosol Med. Pulm. Drug Deliv. 10.1089/jamp.2018.1505 (2019). [DOI] [PMC free article] [PubMed]