Abstract
Critically ill patients often acquire neuropathy and/or myopathy labeled ICU-acquired weakness. The current insights into incidence, pathophysiology, diagnostic tools, risk factors, short- and long-term consequences and management of ICU-acquired weakness are narratively reviewed. PubMed was searched for combinations of “neuropathy”, “myopathy”, “neuromyopathy”, or “weakness” with “critical illness”, “critically ill”, “ICU”, “PICU”, “sepsis” or “burn”. ICU-acquired weakness affects limb and respiratory muscles with a widely varying prevalence depending on the study population. Pathophysiology remains incompletely understood but comprises complex structural/functional alterations within myofibers and neurons. Clinical and electrophysiological tools are used for diagnosis, each with advantages and limitations. Risk factors include age, weight, comorbidities, illness severity, organ failure, exposure to drugs negatively affecting myofibers and neurons, immobility and other intensive care-related factors. ICU-acquired weakness increases risk of in-ICU, in-hospital and long-term mortality, duration of mechanical ventilation and of hospitalization and augments healthcare-related costs, increases likelihood of prolonged care in rehabilitation centers and reduces physical function and quality of life in the long term. RCTs have shown preventive impact of avoiding hyperglycemia, of omitting early parenteral nutrition use and of minimizing sedation. Results of studies investigating the impact of early mobilization, neuromuscular electrical stimulation and of pharmacological interventions were inconsistent, with recent systematic reviews/meta-analyses revealing no or only low-quality evidence for benefit. ICU-acquired weakness predisposes to adverse short- and long-term outcomes. Only a few preventive, but no therapeutic, strategies exist. Further mechanistic research is needed to identify new targets for interventions to be tested in adequately powered RCTs.
Keywords: Critical illness, Muscle weakness, Diagnosis, Risk factors, Clinical outcome, Intervention
Take-home messages
| Critically ill patients frequently acquire muscle weakness while in the ICU, which adversely affects short- and long-term outcomes. No effective treatments are currently available whereas partial prevention of ICU-acquired weakness is possible by avoiding hyperglycemia, by postponing parenteral nutrition to beyond the first ICU week, and by minimizing sedation. Further mechanistic research is warranted in order to identify novel preventive and/or therapeutic strategies that can be tested in adequately powered RCTs. |
Introduction
Muscle weakness is a frequent problem in the intensive care unit (ICU). The weakness can be due to primary neuromuscular disorders that trigger the need for intensive care, such as Guillain–Barré Syndrome, myasthenia gravis, amyotrophic lateral sclerosis or multiple sclerosis, among others, but these conditions only account for < 0.5% of all ICU admissions [1]. More often, however, muscle weakness develops as a secondary disorder while patients are being treated for other life-threatening conditions. The latter has been labeled “ICU-acquired weakness” with the implication that this neuromuscular dysfunction has no plausible etiology other than the critical illness and its treatments [2]. ICU-acquired weakness is typically generalized, symmetrical, and affects limb (proximal more than distal) and respiratory muscles, whereas facial and ocular muscles are spared [3, 4]. Muscle tone is almost invariably reduced. Deep tendon reflexes can be reduced or normal. Weakness may originate from a neurogenic disturbance “critical illness polyneuropathy” (CIP), a myogenic disturbance “critical illness myopathy” (CIM), or a combination thereof labelled “critical illness neuromyopathy” [2, 5, 6]. Electrophysiological examination shows typical patterns of abnormalities. Also, the pronounced loss of muscle mass, that can exceed 10% over the 1st week in ICU, has been associated with functional impairment [7, 8]. Severe disuse muscle atrophy has been put forward as a separate entity of ICU-acquired weakness in the absence of electrophysiological abnormalities [4].
Prevalence of ICU-acquired weakness varies widely with the studied patient population and risk factors, the timing of assessment, the methods used for diagnosis, and inconsistent accounting for patients’ pre-hospital muscle function or overall functional status (often overlooking age-related frailty) [4, 6, 9–12]. A systematic review reported a median prevalence of 43% (interquartile range 25–75%) over 31 studies [13]. Diaphragm dysfunction may develop more often than limb muscle weakness [14].
We here review the current insights into the pathophysiology, diagnostic tools, risk factors, short- and long-term consequences and management of ICU-acquired weakness.
Pathophysiology
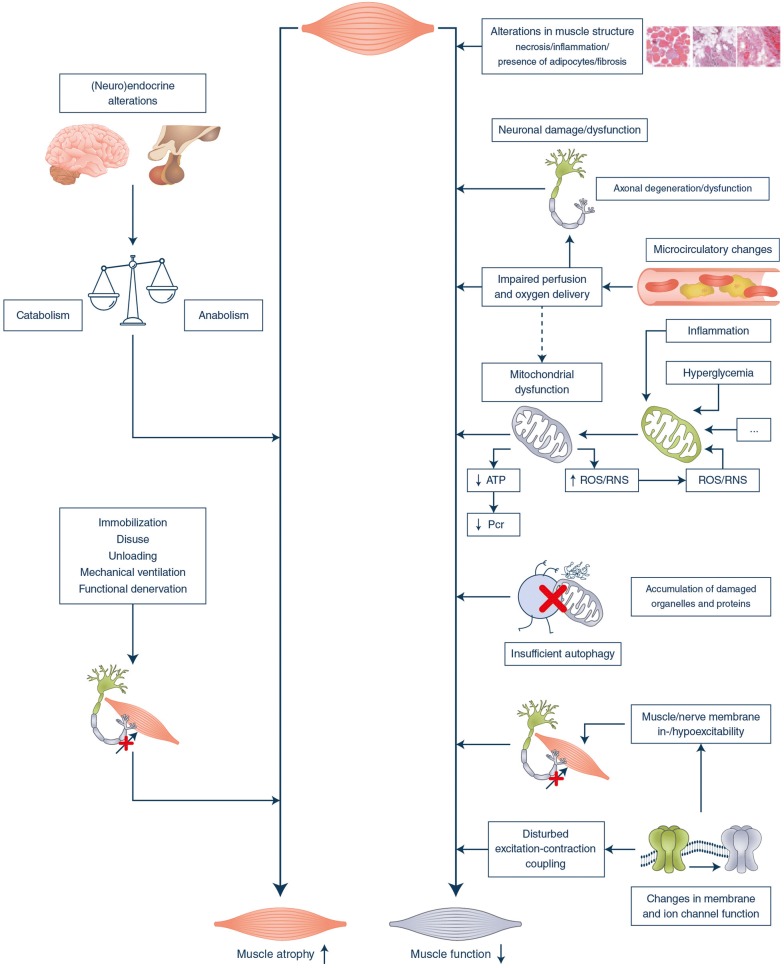
The pathophysiology of ICU-acquired weakness remains incompletely understood, in part explained by practical and ethical issues complicating the study of underlying mechanisms in human patients. Indeed, mechanistic studies require harvesting of muscle or nerve biopsies, which is an invasive procedure. Also, the possibility to interfere with biological processes in human patients, e.g., by administering activators or inhibitors of assumed central players, is inherently limited in view of potential risks for the patients. However, studies in animal models have added valuable insights and, together with available patient study results, allowed to attribute ICU-acquired weakness to complex structural/functional alterations within the central nervous system, the peripheral nerves and the myofibers. In Fig. 1, the major pathways assumed to be involved, comprising loss of muscle mass and loss of muscle function, are briefly summarized in a conceptual framework [5, 15–17].
Fig. 1.
Mechanisms implicated in the development of ICU-acquired weakness. A conceptual framework is shown of the major pathways that are assumed to be involved in the loss of muscle mass and loss of muscle function that contribute to the development of ICU-acquired weakness [5, 8, 15–17]. ATP adenosine triphosphate, PCr phosphocreatine, ROS/RNS reactive oxygen species/reactive nitrogen species. Mitochondria, proteins, neurons and ion channels indicated in green represent healthy organelles, molecules and cells, whereas grey symbols point to damaged/dysfunctional organelles, protein aggregates, cells and ion channels
Muscle atrophy
The catabolic state of critical illness, with reduced anabolic effector hormones and increased catabolic hormones [18], and the mechanical unloading due to immobilization/denervation, explain the pronounced muscle wasting that contributes to weakness of myogenic origin in ICU patients [7]. Such loss of muscle mass is due to imbalanced protein turnover, with a reduced protein synthesis relative to accelerated breakdown by activated proteolytic systems, such as the ubiquitin–proteasome system [7, 8, 16].
Muscle dysfunction
Several factors contribute to loss of muscle function during critical illness.
Structural muscle alterations
Muscle biopsies show signs of inflammation or necrosis, pronounced infiltration of muscle with (or myofiber conversion to) adipose tissue and fibrosis in a remarkably high proportion of critically ill patients [8].
Microcirculatory disturbances
Microcirculatory changes include vasodilation and increased permeability, which allow leukocyte extravasation and tissue infiltration, local cytokine production and edema formation with increased intercapillary distance [5, 6, 15]. These changes can compromise perfusion and oxygen delivery. Involvement of edema-induced compression damage to muscles and nerves remains debated. Nevertheless, hypoperfusion may contribute to neuronal injury, axonal degeneration, and to a chronic membrane depolarization of terminal motor axons.
Bioenergetic failure
Insufficient oxygen supply to mitochondria may compromise mitochondrial energy production. However, the mitochondrial dysfunction in critical illness appears explained by impaired oxygen utilization, due to direct mitochondrial damage further aggravated by inflammation, hyperglycemia and free radicals, rather than by impaired oxygen delivery [15]. Dysfunctional mitochondria not only compromise energy provision but also amplify the production of free radical and reactive oxygen species, eliciting a vicious cycle of macromolecular and organelle damage.
Inadequate autophagy activation
Initially, increased autophagy was assigned a detrimental role as a contributor to muscle atrophy [15]. However, it has become clear that this important cellular quality control mechanism is actually insufficiently activated during critical illness, allowing accumulation of damage to mitochondria and other cellular components [19]. Impaired clearance of such damage results in degenerative changes which compromise muscle function, thus contributing to ICU-acquired weakness [8, 19, 20].
Membrane and ion channel dysfunction
Sodium channel inactivation is thought to contribute to the rapid, reversible hypo-excitability or in-excitability of nerve and muscle membranes in patients with ICU-acquired weakness [15]. Altered intracellular calcium homeostasis further contributes to impaired muscle contractility by affecting the excitation–contraction coupling.
Central nervous system involvement
Recent evidence suggests that central nervous system involvement with failure of coordinated repetitive firing within the motor neurons can be a very early event, preceding electrical failure in axons and nerve–muscle coupling [17].
Diagnosis
Several techniques are used to diagnose ICU-acquired weakness. These methods assess peripheral and/or respiratory muscles. Tables 1 and 2 give an overview of currently available techniques, with their advantages and disadvantages.
Table 1.
Diagnosis of ICU-acquired weakness: assessment of peripheral muscles
| Technique | Measures | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Volitional functional testing | Functional measurement |
Patients need to be awake and cooperative and comprehend how to perform the measurements Does not differentiate CIPNM from deconditioning |
||
|
MRC sum score—6 categories 0: no contraction 1: contraction without movement 2: movement, gravity eliminated 3: movement against gravity 4: movement against resistance 5: normal muscle force |
Bilateral scoring of: Shoulder abduction Elbow flexion Wrist extension Hip flexion Knee extension Foot dorsiflexion Significant weakness: < 48/60 Severe weakness < 36/60 |
Gold standard Non-invasive, bedside testing Reliable and valid (at least for score 0–3) High inter-rater reliability (provided strict guidelines on adequacy and standardized test procedures and positions are followed) Overall estimation of motor function |
May be affected by positioning of the patient and availability of limbs for assessment (e.g., limitations by pain, dressings, immobilizing devices) Ordinal scale, lower sensitivity to more subtle changes in muscle function, difficulty in differentiation between score 4 and 5 Weak correlation with physical functioning |
[2, 3, 21, 22] |
|
MRC sum score—4 categories 0: paralysis 1: > 50% loss of strength 2: < 50% loss of strength 3: normal strength |
Same muscles as above weakness: < 24/36 to be validated |
Non-invasive, bedside testing Excellent inter-rater reliability Excellent accuracy in diagnosing weakness Requires less discrimination between grades than 6-grade score |
Concerns on potential subjectivity Further validation needed |
[23] |
| Hand-held dynamometry |
Handgrip strength weakness: < 11 kg for men, < 7 kg for women Quadriceps force |
Gold standard, quantitative measure Non-invasive, quick and easy bedside testing High inter-rater reliability High sensitivity and specificity |
Significant floor effect Uncertain whether representative of global muscle strength |
[3, 21, 23, 24] |
|
Scored Physical Function in Intensive Care Test (PFIT-s) Functional abilities scored 0–3 |
Shoulder flexion strength Knee extension strength Sit-to-stand assistance Step cadence |
Feasible and safe Inexpensive Evaluates patients’ functional abilities Validated, predictive of key outcomes |
Floor effect at admission Ceiling effect at discharge |
[25] |
|
Functional Status Score for the ICU Functional abilities scored 0–7 0: not able to perform 7: complete independence |
Rolling Transfer from spine to sit Sitting at the edge of bed Transfer from sit to stand Walking |
Feasible and safe Evaluates patients’ functional abilities |
Has not undergone additional psychometric testing for validation and scale analysis | [26] |
| Chelsea Critical Care Physical Assessment Tool |
Feasible and safe Evaluates patients’ functional abilities |
Has not undergone additional psychometric testing for validation and scale analysis | [27] | |
| Six-minute walk test | Distance walked in 6 min | Assesses functional capacity | Only in late phase | [28, 29] |
| Electrophysiology | ||||
| Full nerve conduction studies (NCS) and needle electromyography (EMG) |
CMAP amplitude and duration SNAP amplitude Nerve conduction velocity Fibrillation potentials Positive sharp waves Motor unit potentials |
Can delineate CIPNM from deconditioning |
Mildly invasive (EMG) Requires specialized training Partially requires patient cooperation (EMG) Anticoagulation therapy is a relative contra-indication |
[22, 30] |
| Single NCS |
Peroneal CMAP amplitude Sural SNAP amplitude |
Shorter testing duration than full four-limb NCS/EMG (5–10 min vs 60–90 min) Less painful than full NCS/EMG Non-invasive No need for volitional patient movement Good to excellent sensitivity Good specificity (peroneal nerve) |
Abnormal peroneal or sural NCS requires follow-up with full NCS/EMG (and ideally muscle strength testing) to confirm a CIPNM diagnosis | [31, 32] |
| Direct muscle stimulation | Muscle excitability |
Can distinguish between CIP and CIM Patient does not need to be awake and cooperative |
Requires specialized training Not widely available |
|
| Imaging | Patient does not need to be awake and cooperative | |||
| Ultrasonography |
Evaluation quantity and quality Muscle area and thickness Central tendon thickness Muscle angiogenic activity/vascularization Fasciculationsa Subcutaneous edema and intramuscular fluid Fat infiltration Intramuscular fibrous tissue Muscle necrosis and fasciitis (more advanced stages) |
Bedside Easy and relatively quick Non-invasive, painless test Allows repeated measurements, valid and practical for daily routine use Equipment available in most ICUs Relatively inexpensive Free of ionizing radiation Close correlation with MRI and CT data Abnormal muscle echogenicity is a good screening test and predictor of prognosis |
Does not measure muscle mass, muscle thickness underestimates muscle loss as compared with cross-sectional area Operator-dependent, precautions needed to obtain reproducible results Exactly same place for every evaluation Minimal amount of pressure Sufficient coverage of probe with gel Transducer perpendicular to imaged muscle Measure and control for SC tissue thickness Affected by obesity and edema Low accuracy to diagnose muscle weakness Does not discriminate between patients with or without weakness upon awakening |
[3, 4, 22, 30, 33–35] |
| Computed tomography (CT) |
Infiltration of muscle by adipose tissue Fat-free skeletal muscle |
Highly accurate, highly reliable Valid in patients with severe fluid retention Allows evaluation of the deepest muscles |
High cost, time-consuming Highly specialized staff and software needed Transport of patient outside ICU needed High level of radiation exposure (may be limited if only a single muscle group is assessed) Inappropriate for repeated monitoring |
[30, 33] |
| Magnetic resonance imaging (MRI) |
Infiltration of muscle by adipose tissue Fat-free skeletal muscle |
Highly accurate, highly reliable Valid in patients with severe fluid retention |
High cost, time-consuming Highly specialized staff and software needed Transport of patient outside ICU needed Inappropriate for repeated monitoring |
[33] |
| Dual-energy X-ray absorptiometry | Body composition |
Rapid Easily tolerated Allows whole-body scans |
Radiation exposure (minimal) Expensive Transport of patient outside ICU needed Specialized personnel needed Inaccurate with abnormal hydration status Inappropriate for routine, repeated monitoring |
[33] |
| Neutron activation analysis | Body composition |
Very accurate Valid in patients with severe fluid retention Most often preferred reference for evaluating/calibrating alternative techniques |
Time-consuming Radiation exposure Equipment available in only a few centers |
[33] |
| Bioelectrical impedance measurements | Body composition |
Non-invasive, highly acceptable to patients Rapid and inexpensive Portable, easily performed at the bedside No radiation exposure Possibility of repeated monitoring |
Distorted by hydration status/edema Affected by skin temperature Affected by body position Special device needed |
[33] |
| Biopsy analyses | ||||
| Nerve and muscle biopsies |
Degeneration and myelination status of nerve fibers Muscle fiber atrophy, necrosis, inflammation, fatty infiltration, fibrosis, vacuolation |
Have increased mechanistic understanding |
Invasive (nerve biopsy too invasive for routine clinical use) with risk of complications (bleeding, wound infection, pain) Specialized expertise needed for obtaining and interpreting samples Prognostic value poorly explored |
[8, 15, 30, 36] |
CMAP compound muscle action potential, CT computed tomography, CIPNP critical illness polyneuromyopathy, EMG electromyography, ICU intensive care unit, MRC Medical Research Council, MRI magnetic resonance imaging, NCS nerve conduction studies, SC subcutaneous, SNAP sensory nerve action potential
aSign of spontaneous activity in the muscle and increased excitability of impaired motor nerves
Table 2.
Diagnosis of ICU-acquired weakness: assessment of respiratory muscles
| Technique | Measures | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Volitional functional testing | ||||
| Functional measurements | Patients need to be awake and cooperative and comprehend how to perform the measurements | |||
| Maximal inspiratory and expiratory pressure |
Inspiratory muscle strength Expiratory muscle strength |
Measures global respiratory muscle strength High values exclude respiratory weakness Predictive of duration mechanical ventilation and mortality |
Low values may also represent poor technique | [24, 37, 38] |
| Transdiaphragmatic pressure |
Diaphragm strength weakness: Pdimax < 60 cm H2O |
Specific measure of diaphragm strength High values exclude respiratory weakness |
Invasive, requires esophageal + gastric balloons Difficult to obtain Low values may also represent poor technique |
[38–40] |
| Non-volitional functional testing | ||||
| Transdiaphragmatic pressure in response to bilateral twitch phrenic nerve stimulation |
Diaphragm strength weakness: Pdi,tw < 10 cm H2O |
Most objective Predictive of duration mechanical ventilation and mortality (better than maximum inspiratory pressure) |
Invasive Requires magnetic stimulation Technically difficult to perform |
[37–40] |
| Phrenic nerve conduction time | ||||
| Endotracheal tube pressure in response to bilateral phrenic nerve stimulation during airway occlusion | Weakness: Pet,tw < 11 cm H2O |
Invasive Requires magnetic stimulation Technically difficult to perform |
[4, 39] | |
| Imaging | ||||
| Chest X-rays | Diaphragm position | Readily available, bedside test | Low sensitivity and specificity | [38, 40] |
| Ultrasonography |
Diaphragmatic excursion weakness: < 11 mm Diaphragmatic thickening fraction weakness: < 20% |
Easy, bedside, non-invasive test Equipment available in most ICUs Relatively inexpensive Relatively good diagnostic performance to predict weaning outcome |
Limited value during assisted breathing | [4, 38–40] |
Pdimax maximal transdiaphragmatic pressure, Pdi,tw transdiaphragmatic pressure upon twitch phrenic nerve stimulation, Pet,tw endotracheal tube pressure upon phrenic nerve stimulation, SNAP sensory nerve action potential
Assessment of peripheral muscle strength
To diagnose ICU-acquired weakness, ideally, a clinical quantification of muscle strength should be performed. This inherently implies a volitional technique, which has the drawback that the patients must be awake and cooperative and must comprehend the assessor’s instructions. As patients are often unconscious or uncooperative, due to sedation or delirium, such clinical diagnosis is often not possible or is delayed. The most widely used volitional technique is the 6-grade Medical Research Council (MRC) sum score [2, 3, 21, 22]. This score yields a global estimation of motor function, pointing to clinically relevant muscle weakness when below 48 and severe muscle weakness when below 36 [11, 41]. However, differentiation in the higher range is difficult. A modified 4-grade score showed better inter-rater agreement for diagnosing ICU-acquired weakness than the classical 6-grade score [23], but requires further validation. The ordinal scale of the MRC sum score limits the sensitivity to detect more subtle changes in muscle function. In contrast, hand-held dynamometry for measuring handgrip and quadriceps strength provides a continuous quantitative measure, but representativeness for global muscle strength has been questioned [3, 21, 23, 24]. The “Scored Physical Function in Intensive Care Test”, “Functional Status Score for the ICU” and “Chelsea Critical Care Physical Assessment Tool” provide information about the patients’ functional abilities, but are less commonly used [25–27]. Finally, the 6-minute walking distance (6-MWD) assesses functional capacity, but is rather used to evaluate how patients perform at discharge and in post-ICU follow-up [27–29].
Electrophysiological assessments are also used to diagnose ICU-acquired weakness and can be applied to unconscious/uncooperative patients. Although CIP and CIM share many features on nerve conduction studies and electromyography, differentiation is possible in ideal circumstances, particularly when the patient is cooperative and voluntary muscle activation is possible (Table 3) [2, 5, 6, 22, 30, 42]. Single nerve conduction studies have shown promise as alternative for time-consuming full electrophysiological studies [22, 31, 32]. For differential diagnosis of CIP and CIM in uncooperative patients, direct muscle stimulation shows normal muscle excitability in CIP and reduced muscle excitability in CIM. However, expertise is not widely available and differential diagnosis is further complicated by the high co-occurrence of CIP and CIM.
Table 3.
Features of critical illness polyneuropathy and critical illness myopathy in electrophysiological and biopsy studies
| Critical illness polyneuropathy | Critical illness myopathy | |
|---|---|---|
| CMAP amplitude | Decreased | Decreased |
| CMAP duration | Normal | Increased |
| SNAP amplitude | Decreased | Normal |
| Nerve conduction velocity | Normal or near normal | Normal or near normal |
| EMG at rest | Fibrillation potentials/positive sharp waves | Fibrillation potentials/positive sharp waves |
| MUP voluntary muscle activation | Long duration, high amplitude, polyphasica | Short duration, low amplitudea |
| Repetitive nerve stimulation | Absence of decremental response | Absence of decremental response |
| Direct muscle stimulation | Normal muscle excitability | Reduced muscle excitability |
| Nerve biopsyb | Primary distal axonal degeneration of sensory nerve fibers, no demyelination | Normal |
| Muscle biopsy | Denervation atrophy of type 1 and 2 muscle fibers | Spectrum of abnormalities: myofiber atrophy, angulated fibers, necrosis, fatty degeneration, focal or diffuse loss of thick filaments |
Information obtained from [2, 5, 6, 15, 22, 30, 36]. Aggregate diagnostic criteria for CIP and CIM are reported in [5]
CMAP compound muscle action potential, EMG electromyography, MUP motor unit potential, SNAP sensory nerve action potential
aMUPs of long duration, high amplitude and polyphasic appearance can be detected in CIP as a sign of collateral reinnervation of denervated muscle fibers, whereas MUPs of short duration and low amplitude are observed in CIM as a sign of reduced functional muscle fibers within each motor unit
bSensory (sural) nerve and motor nerve (to gracilis muscle) biopsy are no longer advised except as a research procedure
A variety of imaging techniques have been evaluated to assess muscle mass, as a surrogate of muscle strength, some of which also can visualize muscle quality. Of these, ultrasonography is considered most promising [30, 33]. Although ultrasonography allows quick and repeated bedside evaluation of measures of muscle quantity and quality, it may underestimate muscle and protein loss [4, 30, 33, 34]. Furthermore, interpretation of the available studies is complicated by significant methodological defects, small sample sizes, and lack of standardization to control for operator dependency [30, 33]. The clinical relevance also remains to be determined [22, 35]. Computed tomography and magnetic resonance imaging can accurately and reliably detect infiltration of muscle by adipose tissue and quantify fat-free muscle mass, but are expensive, require specialized staff and software, and are logistically challenging [30, 33]. Furthermore, computed tomography exposes patients to a high level of radiation. Several of these limitations also apply to dual-energy X-ray absorptiometry and neutron activation analysis that assess body composition [33]. Bioelectrical impedance measurements also assess body composition, but results are affected by edema, skin temperature and positioning [33].
Finally, nerve and muscle biopsies can provide important information and have increased mechanistic understanding, but are invasive with potential for complications and require specialized expertise for obtaining the samples and interpreting findings [8, 15, 30, 36]. Biopsy analyses may allow differential diagnosis of CIP and CIM, but nerve biopsy is too invasive for routine clinical use and hence is no longer advised except in the context of scientific research (Table 3).
Clearly, many techniques have been developed to assess weakness. However, after exclusion of primary causes of neuromuscular diseases [1], diagnosis of ICU-acquired weakness from a practical point of view is currently limited to the use of clinical testing and electrophysiological studies.
Assessment of respiratory muscle strength
Volitional testing of global respiratory muscle strength via maximal inspiratory and expiratory pressure or of diaphragm strength via transdiaphragmatic pressure is again limited by the requirement of an awake and cooperative patient [24, 37–40]. Measurement of transdiaphragmatic or endotracheal tube pressure in response to phrenic nerve stimulation can circumvent this problem, but it is invasive, requires magnetic stimulation and is technically difficult [4, 37–40]. Also imaging techniques are being used. However, chest X-rays have low sensitivity and specificity and ultrasonography has limited value during assisted breathing [4, 38–40].
Risk factors
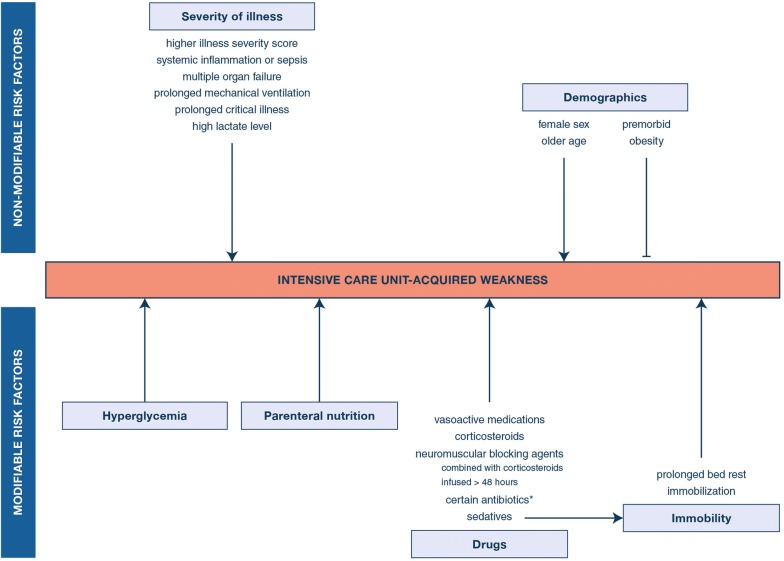
Several independent risk factors for developing ICU-acquired weakness have been identified, mostly from observational studies, although often not unequivocally (Fig. 2).
Fig. 2.
Overview of risk factors of ICU-acquired weakness. Observational and randomized controlled trials have identified a wide range of non-modifiable and modifiable risk factors associated with the risk of developing weakness in the ICU [11, 43–58]. *certain antibiotics, such as aminoglycosides and vancomycin, have been independently associated with ICU-acquired weakness, although not unequivocally [45, 57, 59]. Other antibiotics, such as clindamycin, erythromycin, quinolones, polymyxin, tetracycline and vancomycin may affect the neuromuscular junction, but have so far not been independently associated with ICU-acquired weakness [45, 60, 61]
A first group of risk factors are not modifiable The severity of critical illness is an important determinant. Thus, a higher severity of illness score, sepsis and inflammation, multiple organ failure, as well as a longer duration of mechanical ventilation and ICU stay, were found to be predictive [11, 43–45]. In fact, ICU-acquired weakness is most frequent in patients with persistent critical illness [46]. The relationship with mechanical ventilation could be reciprocal given that prolonged ventilation increases the risk of ICU-acquired weakness and diaphragmatic dysfunction which, vice versa, increase the risk of prolonged ventilation and failed weaning [13]. Another illness-related risk factor is a high lactate level [45]. Further, a higher risk of weakness may apply to women than to men, and to older as compared with younger patients [11, 43, 45]. Also, premorbid disability and frailty may predispose to the severity of weakness. Intriguingly, premorbid obesity was found to be an independent protective factor against development of ICU-acquired weakness and against muscle atrophy [47].
Some risk factors are modifiable These include the degree of hyperglycemia that develops in response to the severe stress of critical illness and the administration of parenteral nutrition (vide infra) [20, 45, 48–51], but also several drugs that are used to treat critically ill patients. For example, dose and duration of vasoactive medications, mostly β-agonists, are associated with a higher risk of ICU-acquired weakness [44]. In meta-analyses, the use of corticosteroids has been associated with risk of ICU-acquired weakness in heterogeneous patient populations [52] and when focusing exclusively on patients with sepsis [53]. However, one study suggested a protective effect of corticosteroids when hyperglycemia as a side effect was avoided [49]. The reported adverse relationship between the use of neuromuscular blocking agents (NMBAs) and muscle weakness remains uncertain [45, 54, 55]. An RCT comparing 48-h infusion of cisatracurium with placebo, both under deep sedation, in ARDS patients did not observe a significant impact [56]. However, a 48-h infusion of cisatracurium with concomitant deep sedation tended to increase the risk of ICU-acquired weakness as compared with absence of routine neuromuscular blockade and lighter sedation targets, with significance reached for weakness present on day 28, but not for weakness present on day 7 or at any time through day 28 [57]. Also, when co-administered with corticosteroids or infused for > 48 h, NMBAs may promote weakness [54]. Certain antibiotics, including aminoglycosides and vancomycin, have also been independently associated with ICU-acquired weakness [45, 58]. The association between sedatives and weakness may be indirect, as separating effects of sedatives from those of sedation-induced immobility and bed rest is difficult [62]. Continued sedation is thought to have a more pronounced effect on muscle atrophy and weakness than when a patient is conscious but immobile in the absence of sedation [9].
Acute and long-term consequences
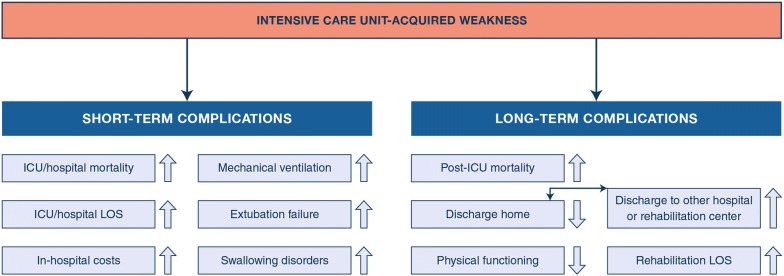
Developing ICU-acquired weakness elicits a range of acute and long-term adverse outcomes (Fig. 3).
Fig. 3.
Overview of short-term and long-term consequences of ICU-acquired weakness. The development of weakness in the ICU has been associated with a wide range of adverse consequences in the short term as well as the long term [14, 42, 63–76]. LOS length of stay
Short-term consequences
The development and severity of ICU-acquired weakness, as assessed clinically at awakening, have been independently associated with higher risk of in-ICU and in-hospital death [63, 64]. This association has mostly been documented for limb muscle weakness [14]. It has also been observed for diaphragm dysfunction, but not unequivocally [14, 65]. Limb and respiratory muscle weakness have further been identified as independent predictors of prolonged need of mechanical ventilation [66, 67]. Limb muscle weakness at extubation was independently associated with higher extubation failure rates in medical patients [68]. Among predominantly surgical patients with limb muscle weakness, 80% also showed diaphragmatic dysfunction [69]. Extubation failed in 50% of them, with need for reintubation within 72 h, among whom 50% died in ICU. Muscle weakness has also been associated with a longer duration of ICU and hospital stay and with increased in-hospital costs [14, 70, 71]. Neuromuscular weakness has further been suggested as a key mechanism contributing to ICU-acquired swallowing disorders, including post-extubation dysphagia [72]. Weakness of the abdominal muscles can impair effective cough [3].
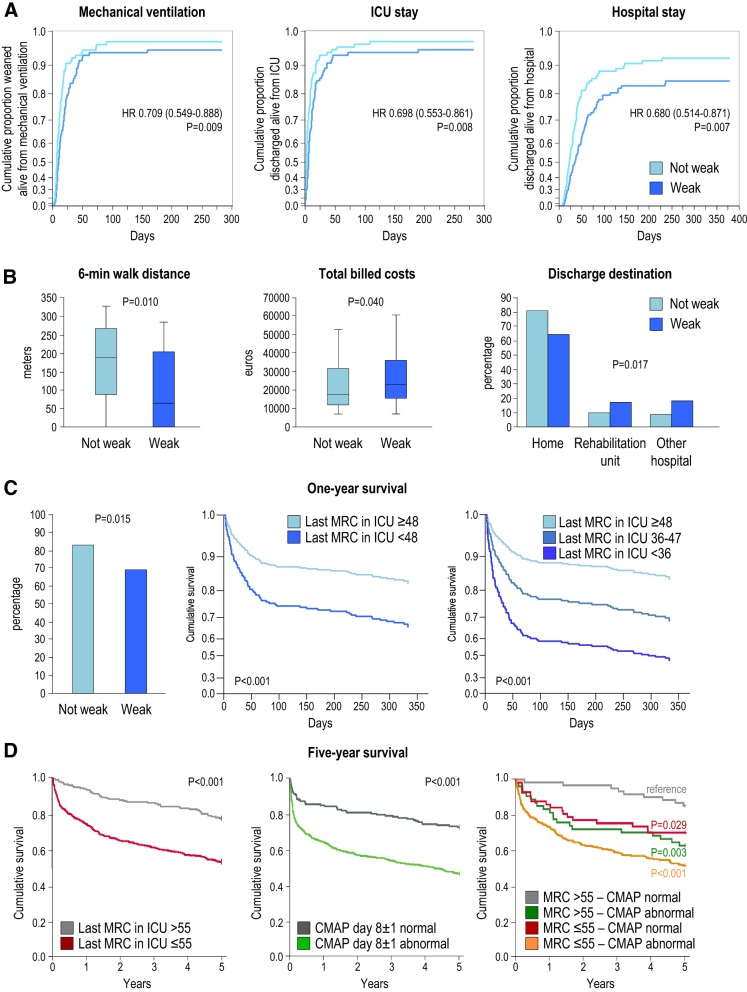
Associations do not necessarily reflect causality. However, a propensity score-matched analysis of patients with and without ICU-acquired weakness suggested that weakness may not merely be a marker but could also be a mediator of poor outcome [70]. Weak patients had a lower likelihood for earlier live weaning from mechanical ventilation, earlier live ICU discharge and earlier live hospital discharge than patients without weakness (Fig. 4a). In addition, in the group of weak patients, 6-MWD at hospital discharge was shorter, in-hospital costs were higher and more patients were discharged to rehabilitation centers or other hospitals (Fig. 4b).
Fig. 4.
Impact of ICU-acquired weakness on short-term outcome and one-year and five-year survival. a Kaplan–Meier plots show the cumulative proportion of well-matched long-stay patients (ICU stay > 7 days) with (MRC < 48 at first evaluation) and without ICU-acquired weakness (MRC ≥ 48 at first evaluation) over time who were alive and weaned from the ventilator, discharged alive from the ICU, and discharged alive from the hospital. Patients who died were censored after the last patient had been weaned alive, discharged alive from the ICU or discharged alive from the hospital, respectively. Plots were redrawn in JMP®Pro14.0.0 (SAS Institute, Cary, NC) from the data described in [70]. Hazard ratios and 95% confidence intervals below 1, for the effect of weakness versus no weakness, illustrate a lower chance of earlier live weaning, of earlier live ICU discharge and of earlier live hospital discharge for patients with as compared with patients without ICU-acquired weakness. b Medians, interquartile ranges and 10th and 90th percentiles of 6-min walking distance at hospital discharge and total billed costs, as well as the distribution of the discharge destination, are shown for well-matched long-stay patients (ICU stay > 7 days) with (MRC < 48 at first evaluation) and without ICU-acquired weakness (MRC ≥ 48 at first evaluation), illustrating worse acute morbidity and higher healthcare-related costs for weak patients, as reported in [70]. c One-year survival for matched long-stay patients (ICU stay > 7 days) with (MRC < 48 at first evaluation) and without ICU-acquired weakness (MRC ≥ 48 at first evaluation) are shown (left panel), together with Cox regression estimates for one-year survival for all long-stay patients with ICU-acquired weakness according to whether weakness persisted until final examination in the ICU or not (middle and right panel). The survival curves visually display the model predicted survival time for the “average” patient according to the Medical Research Council (MRC) sum score at final examination in the ICU as described in [70]. The middle panel compares patients who recovered from weakness (MRC ≥ 48 at last evaluation) with all patients who did not (MRC < 48 at last evaluation), whereas the right panel further distinguishes persistently weak patients into patients who remained moderately weak (MRC 36–47) or severely weak (MRC < 36). One-year survival was lower for weak patients as compared with not-weak patients. Survival was further lowered when weakness persisted and was more severe as compared with recovery of weakness at ICU discharge. d Five-year survival is shown for patients according to MRC sum score at final examination in the ICU > 55 versus ≤ 55 (left panel), according to normal or abnormal CMAP on day 8 ± 1 (middle panel), or according to the combined information of the MRC sum score at final examination in the ICU > 55 or ≤ 55 and normal or abnormal CMAP on day 8 ± 1 (right panel) (adapted from [77]). Five-year survival was lower for patients with an MRC sum score at final examination in the ICU ≤ 55 versus > 55 and for patients with an abnormal versus normal CMAP on day 8 ± 1. CMAP compound muscle action potential, HR hazard ratio, MRC Medical Research Council sum score
Clearly, ICU-acquired weakness has been associated with worse short-term outcome. However, weakness status or any minimal cutoff of the MRC score is not a sole determinant of whether a patient can be discharged from the ICU, as this decision is based on the patient no longer being dependent on vital organ support.
Long-term consequences
Survivors of critical illness face an increased risk of late death [70, 78], which is even higher when patients had experienced ICU-acquired weakness [70]. Indeed, in a propensity score-matched analysis, one-year mortality was higher in weak (MRC sum score < 48) than in not-weak patients (Fig. 4c) [70]. Strikingly, the likelihood of late death was further increased when weakness persisted until ICU discharge, and even more so for patients with a more severe degree of persistent weakness (MRC sum score < 36, Fig. 4c). An independent association with higher one-year mortality was also found for a reduced compound muscle action potential (CMAP) on ICU day 8, irrespective of clinical weakness diagnosed by the MRC sum score [42], and for respiratory muscle weakness evidenced by a low maximal inspiratory pressure [73]. Even a mild reduction in muscle strength not yet meeting the threshold of clinically relevant weakness (lower raw MRC sum score, but MRC sum score ≤ 55 being most predictive) and nerve/muscle dysfunction (abnormal CMAP) at ICU discharge have recently been independently associated with a worse five-year survival [77]. Being weak (MRC sum score < 48) at hospital discharge has also been associated with a worse five-year survival [74].
Following ICU discharge, ARDS patients showed a slow improvement in the severe wasting they suffered from in ICU and in 6-MWD [79–81]. However, even 5 years after ICU discharge, patients still experienced varying degrees of weakness and reduced walk and exercise ability [81]. New functional disabilities in activities of daily living also can persist for at least 8 years after sepsis [82]. A large heterogeneous cohort of critically ill patients showed lower handgrip force, shorter 6-MWD and reduced physical quality of life 5 years after ICU admission [78]. A small study observed reduced maximal voluntary contraction, rate of force development and endurance time 1 year after ICU discharge, but no signs of neural impairment [83]. Importantly, the acquisition of weakness in the ICU appeared to be a major independent determinant of long-term weakness, other morbidities and poor quality of life after ICU discharge [75, 76]. Weakness at ICU discharge, even if mild and not yet considered as clinically relevant, has recently been independently associated with lower handgrip force, lower respiratory muscle strength, lower 6-MWD and lower physical quality of life with lower physical independence 5 years later (MRC sum score ≤ 55 being most predictive), unlike an abnormal CMAP [77]. Interestingly, evaluation of patients 1 year after ICU discharge suggested that a myogenic origin of ICU-acquired weakness had a better prognosis with virtually full recovery as compared with a neuromyogenic origin that left 50–75% of the patients with persisting muscle weakness or even tetraparesis [84, 85]. This illustrates the importance of a differential diagnosis between CIP and CIM to predict long-term outcome of critically ill patients.
Whether the pathophysiology of the post-ICU weakness is different from that of in-ICU weakness is not clear, given that only few studies explored mechanisms underlying long-term weakness. A study of 11 prolonged critically ill patients assessed 6 months after ICU discharge showed persistent weakness in all tested patients owing to impaired voluntary contractile capacity, whereas quadriceps atrophy had resolved in 27% [86]. Muscle proteolysis, autophagy, inflammation and mitochondrial content had normalized, but patients with sustained atrophy showed less satellite cells, which may impair regenerative capacity. Co-expressed genes associated with the degree of muscle weakness and atrophy showed enrichment in genes involved in skeletal muscle regeneration and extracellular matrix deposition [87]. Interestingly, in mice, engraftment of mesenchymal stem cells improved muscle regeneration and strength after sepsis [88].
Preventive and therapeutic measures
Several interventions have been tested with the aim to prevent or treat ICU-acquired weakness, though with very limited success [15]. Unfortunately, there is currently still no effective treatment though prevention has been shown to work by targeting specific risk factors.
Avoiding hyperglycemia
Tight glycemic control, targeting normal fasting levels with insulin infusion, as compared with tolerating pronounced hyperglycemia, reduced morbidity and mortality of surgical, medical and pediatric ICU patients in the context of early use of parenteral nutrition [89–91]. However, the optimal glucose target remains controversial, as the largest multicenter RCT showed that intermediate blood glucose levels may be safer [92]. Nevertheless, strictly targeting fasting normoglycemia in surgical and medical ICU patients reduced the risk of developing electrophysiological signs of critical illness polyneuromyopathy [48, 49]. Achieving normoglycemia, rather than the administration of insulin, explained this lower risk. The polyneuromyopathy prevention in turn partially explained less need of prolonged mechanical ventilation, which suggested an impact of this intervention on clinically relevant ICU-acquired respiratory muscle weakness.
Avoiding early parenteral nutrition
The severe caloric deficit that arises from critical illness-associated anorexia and gastrointestinal dysfunction has been associated with muscle atrophy and weakness [93]. Muscle atrophy has long been regarded a major myogenic component of muscle weakness. Based on observational studies and expert opinion, to prevent muscle atrophy nutritional guidelines hence advocated early full nutrition, preferentially via the enteral route but otherwise supplemented via the parenteral route [94]. Major emphasis was put on giving enough protein as substrate for building muscle mass. However, large RCTs showed lack of benefit or even harm by early full nutrition and hence no longer support such aggressive feeding strategies. Indeed, supplementing insufficient enteral nutrition with early parenteral nutrition to caloric targets increased complications and delayed recovery of critically ill adults and children as compared with accepting the macronutrient deficit by withholding parenteral nutrition in the 1st week in ICU [28, 95]. Harm evoked by early parenteral nutrition comprised more ICU-acquired weakness and impaired recovery thereof, longer dependency on mechanical ventilation and a longer stay in the ICU [20, 28, 95]. Early parenteral nutrition to caloric targets also did not reduce muscle atrophy [20]. Macronutrient doses, and not the route of administration, explained the harm. More specifically, the early administration of amino acids, but not glucose or lipids, appeared to be the culprit [96, 97]. Rather than being used for synthesis of muscle proteins, the amino acids appeared to be largely broken down and shuttled to ureagenesis, possibly explaining the lack of benefit in RCTs investigating increased amino acid supplementation [97–100]. Amino acids also suppress autophagy in muscle, which has shown to be a mechanism contributing to ICU-acquired weakness [20]. Importantly, in these studies, micronutrients were infused in recommended doses in both groups to avoid deficiencies [28, 95] that have also been linked to muscle weakness, among other complications of critical illness [101]. In another RCT, also early full enteral feeding was unable to improve impaired physical function 1 year after ICU admission as compared with early hypocaloric feeding [102]. Further research is needed to establish the ideal timing of initiation beyond the 1st week, optimal dose and composition of artificial nutrition for critically ill patients [93].
Although clinical data are currently still lacking, a series of studies in mice, performed in search of mechanisms underlying protection against ICU-acquired weakness by obesity, revealed that increasing the availability of ketone bodies, either through stimulation of endogenous ketogenesis or through infusion of exogenous ketones, protected against sepsis-induced muscle weakness [103]. This interesting finding still needs to be further explored in patients.
Minimizing sedation and early mobilization
As immobility increases the risk of ICU-acquired weakness, (early) mobilization and physical rehabilitation were tested to reduce this risk. Such interventions evidently require a policy to minimize sedation [3, 9]. Interestingly, daily interruption of sedative infusions was shown to reduce the duration of mechanical ventilation and of ICU dependency [104]. Several modes of (early) mobilization and rehabilitation have been evaluated, but showed inconsistent results. Although several systematic reviews and meta-analyses concluded in favor of this intervention, a low quality of evidence was pointed out [105–109]. Tipping et al. concluded that active mobilization and rehabilitation improved muscle strength at ICU discharge, enhanced the probability of walking without assistance at hospital discharge and resulted in more days alive and out of hospital to day 180 [105]. High dose rehabilitation in the ICU was further suggested to improve some aspects of quality of life at 6 months. A more recent analysis concluded that early rehabilitation improved only short-term physical-related outcomes with low quality of evidence for a decreased risk of ICU-acquired weakness as compared with standard care or no early rehabilitation [106]. Other systematic reviews that compared early mobilization and/or active exercise with delayed exercise emphasized the low quality of evidence in favor of early intervention, given the small sample sizes of these studies, heterogeneity of studied interventions and outcomes, high risk of performance bias, high dropout rates, and inadequate descriptions of usual care [107, 108]. Methodological limitations also explained the low confidence in the observed associations of the improved inspiratory and expiratory muscle strength after inspiratory muscle training with shorter ventilator dependency [109]. However, several additional studies are ongoing which hopefully will shed light on the efficacy of early mobilization and rehabilitation.
Barriers to implementation in clinical practice comprise the patient’s capability to perform physical activity, safety concerns for patients, staff and caregivers, lack of expertise and lack of adequate staffing, equipment and funding to provide rehabilitation programs [110]. However, the incidence of potential safety events is low (2.6%) and consequences for patient management are even more rare (0.6%), provided that consensus guidelines are adequately considered [4, 111].
Neuromuscular electrical stimulation
Because poor alertness and cooperation precludes early active mobilization of a large proportion of patients, neuromuscular electrical stimulation (NMES) has been suggested as an alternative. Several mostly small RCTs have been performed in critically ill patients, with variable use of frequencies, intensities and duration of NMES and widely varying outcomes, limiting pooling and interpretation of data [112, 113]. Although some studies were promising [112], the most recent systematic review and meta-analysis did not reveal significant improvement in muscle strength or dependency on mechanical ventilation and intensive care with NMES as compared with usual care [113].
Drugs
A systematic review of initially promising pharmacological interventions such as the anabolic steroid oxandrolone, growth hormone, propranolol, immunoglobulin and glutamine therapy could not recommend any of these for adoption in routine practice [114].
Rehabilitation beyond ICU stay
Early interventions to prevent or attenuate the debilitating consequences of ICU-acquired weakness are obviously important. It remains unclear, however, whether later exercise-based rehabilitation on regular wards and after hospital discharge are beneficial, as studies have been small. Some studies showed a positive effect related to functional exercise capacity, unlike others, but a systematic review could not support benefits from post-ICU physical rehabilitation given the low quality of available evidence and heterogeneity [115]. Adequately powered RCTs are needed.
Conclusions and clinical implications
Muscle weakness is a frequent complication of critical illness, with devastating short- and long-term consequences and therefore requires prevention and/or treatment. Prevention seems at least in part possible, as avoiding hyperglycemia and postponing parenteral nutrition to beyond the 1st week in ICU, have been shown to reduce the burden of ICU-acquired weakness. Also early mobilization, which requires minimizing sedation, may be promising but several ongoing studies are awaited for to further clarify its impact on clinically relevant outcome measures. Further research is required to design new preventive and/or therapeutic strategies that can be tested in adequately powered RCTs. Based on animal studies [103], a first interesting candidate to explore could be the infusion of ketone bodies or strategies to activate ketogenesis.
Acknowledgements
This work was supported by the Methusalem program of the Flemish government (through the University of Leuven to GVdB and IV, METH14/06) and by European Research Council Advanced Grants (AdvG-2012–321670 and AdvG-2017–785809) to GVdB.
Author contributions
IV performed the literature search and wrote the first draft of the manuscript, which was critically reviewed by GVdB and NL.
Compliance with ethical standards
Conflicts of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Damian MS, Wijdicks EFM. The clinical management of neuromuscular disorders in intensive care. Neuromuscul Disord. 2019;29:85–96. doi: 10.1016/j.nmd.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Stevens RD, Marshall SA, Cornblath DR, Hoke A, Needham DM, de Jonghe B, Ali NA, Sharshar T. A framework for diagnosing and classifying intensive care unit-acquired weakness. Crit Care Med. 2009;37:S299–S308. doi: 10.1097/CCM.0b013e3181b6ef67. [DOI] [PubMed] [Google Scholar]
- 3.Latronico N, Herridge M, Hopkins RO, Angus D, Hart N, Hermans G, Iwashyna T, Arabi Y, Citerio G, Ely EW, Hall J, Mehta S, Puntillo K, Van den Hoeven J, Wunsch H, Cook D, Dos Santos C, Rubenfeld G, Vincent JL, Van den Berghe G, Azoulay E, Needham DM. The ICM research agenda on intensive care unit-acquired weakness. Intensive Care Med. 2017;43:1270–1281. doi: 10.1007/s00134-017-4757-5. [DOI] [PubMed] [Google Scholar]
- 4.Piva S, Fagoni N, Latronico N. Intensive care unit-acquired weakness: unanswered questions and targets for future research. F1000Res 8. 2019;8:508. doi: 10.12688/f1000research.17376.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Latronico N, Bolton CF. Critical illness polyneuropathy and myopathy: a major cause of muscle weakness and paralysis. Lancet Neurol. 2011;10:931–941. doi: 10.1016/S1474-4422(11)70178-8. [DOI] [PubMed] [Google Scholar]
- 6.Kress JP, Hall JB. ICU-acquired weakness and recovery from critical illness. N Engl J Med. 2014;370:1626–1635. doi: 10.1056/NEJMra1209390. [DOI] [PubMed] [Google Scholar]
- 7.Puthucheary ZA, Rawal J, McPhail M, Connolly B, Ratnayake G, Chan P, Hopkinson NS, Phadke R, Dew T, Sidhu PS, Velloso C, Seymour J, Agley CC, Selby A, Limb M, Edwards LM, Smith K, Rowlerson A, Rennie MJ, Moxham J, Harridge SD, Hart N, Montgomery HE. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310:1591–1600. doi: 10.1001/jama.2013.278481. [DOI] [PubMed] [Google Scholar]
- 8.Derde S, Hermans G, Derese I, Güiza F, Hedström Y, Wouters PJ, Bruyninckx F, D'Hoore A, Larsson L, Van den Berghe G, Vanhorebeek I. Muscle atrophy and preferential loss of myosin in prolonged critically ill patients. Crit Care Med. 2012;40:79–89. doi: 10.1097/CCM.0b013e31822d7c18. [DOI] [PubMed] [Google Scholar]
- 9.Parry SM, Puthucheary ZA. The impact of extended bed rest on the musculoskeletal system in the critical care environment. Extrem Physiol Med. 2015;4:16. doi: 10.1186/s13728-015-0036-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thabet Mahmoud A, Tawfik MAM, Abd El Naby SA, Abo El Fotoh WMM, Saleh NY, Abd El Hady NMS. Neurophysiological study of critical illness polyneuropathy and myopathy in mechanically ventilated children; additional aspects in paediatric critical illness comorbidities. Eur J Neurol. 2018;25:991–e76. doi: 10.1111/ene.13649. [DOI] [PubMed] [Google Scholar]
- 11.De Jonghe B, Sharshar T, Lefaucheur JP, Authier FJ, Durand-Zaleski I, Boussarsar M, Cerf C, Renaud E, Mesrati F, Carlet J, Raphaël JC, Outin H, Bastuji-Garin S; Groupe de Réflexion et d’Etude des Neuromyopathies en Réanimation Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA. 2002;288:2859–2867. doi: 10.1001/jama.288.22.2859. [DOI] [PubMed] [Google Scholar]
- 12.Hanna JS. Sarcopenia and critical illness: a deadly combination in the elderly. J Parenter Enteral Nutr. 2015;39:273–281. doi: 10.1177/0148607114567710. [DOI] [PubMed] [Google Scholar]
- 13.Fan E, Cheek F, Chlan L, Gosselink R, Hart N, Herridge MS, Hopkins RO, Hough CL, Kress JP, Latronico N, Moss M, Needham DM, Rich MM, Stevens RD, Wilson KC, Winkelman C, Zochodne DW, Ali NA; ATS Committee on ICU-acquired Weakness in Adults; American Thoracic Society An official American Thoracic Society Clinical Practice guideline: the diagnosis of intensive care unit-acquired weakness in adults. Am J Respir Crit Care Med. 2014;190:1437–46. doi: 10.1164/rccm.201411-2011ST. [DOI] [PubMed] [Google Scholar]
- 14.Dres M, Dubé BP, Mayaux J, Delemazure J, Reuter D, Brochard L, Similowski T, Demoule A. Coexistence and impact of limb muscle and diaphragm weakness at time of liberation from mechanical ventilation in medical intensive care unit patients. Am J Respir Crit Care Med. 2017;195:57–66. doi: 10.1164/rccm.201602-0367OC. [DOI] [PubMed] [Google Scholar]
- 15.Friedrich O, Reid MB, Van den Berghe G, Vanhorebeek I, Hermans G, Rich MM, Larsson L. The sick and the weak: neuropathies/myopathies in the critically ill. Physiol Rev. 2015;95:1025–1109. doi: 10.1152/physrev.00028.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Batt J, Herridge MS, Dos Santos CC. From skeletal muscle weakness to functional outcomes following critical illness: a translational biology perspective. Thorax. 2019;74:1091–1098. doi: 10.1136/thoraxjnl-2016-208312. [DOI] [PubMed] [Google Scholar]
- 17.Latronico N, Friedrich O. Electrophysiological investigations of peripheral nerves and muscles: a method for looking at cell dysfunction in the critically ill patients. Crit Care. 2019;23:33. doi: 10.1186/s13054-019-2331-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van den Berghe G. On the neuroendocrinopathy of critical illness. Perspectives for feeding and novel treatments. Am J Respir Crit Care Med. 2016;194:1337–1348. doi: 10.1164/rccm.201607-1516CI. [DOI] [PubMed] [Google Scholar]
- 19.Vanhorebeek I, Gunst J, Derde S, Derese I, Boussemaere M, Güiza F, Martinet W, Timmermans JP, D'Hoore A, Wouters PJ, Van den Berghe G. Insufficient activation of autophagy allows cellular damage to accumulate in critically ill patients. J Clin Endocrinol Metab. 2011;96:E633–E645. doi: 10.1210/jc.2010-2563. [DOI] [PubMed] [Google Scholar]
- 20.Hermans G, Casaer MP, Clerckx B, Güiza F, Vanhullebusch T, Derde S, Meersseman P, Derese I, Mesotten D, Wouters PJ, Van Cromphaut S, Debaveye Y, Gosselink R, Gunst J, Wilmer A, Van den Berghe G, Vanhorebeek I (2013) Effect of tolerating macronutrient deficit on the development of intensive-care unit acquired weakness: a subanalysis of the EPaNIC trial. Lancet Respir Med 1: 621–629 [DOI] [PubMed]
- 21.Vanpee G, Hermans G, Segers J, Gosselink R. Assessment of limb muscle strength in critically ill patients: a systematic review. Crit Care Med. 2014;42:701–711. doi: 10.1097/CCM.0000000000000030. [DOI] [PubMed] [Google Scholar]
- 22.Kelmenson DA, Quan D, Moss M. What is the diagnostic accuracy of single nerve conduction studies and muscle ultrasound to identify critical illness polyneuromyopathy: a prospective cohort study. Crit Care. 2018;22:342. doi: 10.1186/s13054-018-2281-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parry SM, Berney S, Granger CL, Dunlop DL, Murphy L, El-Ansary D, Koopman R, Denehy L. A new two-tier strength assessment approach to the diagnosis of weakness in intensive care: an observational study. Crit Care. 2015;19:52. doi: 10.1186/s13054-015-0780-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roberson AR, Starkweather A, Grossman C, Acevedo E, Salyer J. Influence of muscle strength on early mobility in critically ill adult patients: systematic literature review. Heart Lung. 2018;47:1–9. doi: 10.1016/j.hrtlng.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Denehy L, de Morton NA, Skinner EH, Edbrooke L, Haines K, Warrillow S, Berney S. A physical function test for use in the intensive care unit: validity, responsiveness, and predictive utility of the physical function ICU test (scored) Phys Ther. 2013;93:1636–1645. doi: 10.2522/ptj.20120310. [DOI] [PubMed] [Google Scholar]
- 26.Huang M, Chan KS, Zanni JM, Parry SM, Neto SG, Neto JA, da Silva VZ, Kho ME, Needham DM. Functional Status Score for the ICU: an international clinimetric analysis of validity, responsiveness, and minimal important difference. Crit Care Med. 2016;44:e1155–e1164. doi: 10.1097/CCM.0000000000001949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parry SM, Granger CL, Berney S, Jones J, Beach L, El-Ansary D, Koopman R, Denehy L. Assessment of impairment and activity limitations in the critically ill: a systematic review of measurement instruments and their clinimetric properties. Intensive Care Med. 2015;41:744–762. doi: 10.1007/s00134-015-3672-x. [DOI] [PubMed] [Google Scholar]
- 28.Casaer MP, Mesotten D, Hermans G, Wouters PJ, Schetz M, Meyfroidt G, Van Cromphaut S, Ingels C, Meersseman P, Muller J, Vlasselaers D, Debaveye Y, Desmet L, Dubois J, Van Assche A, Vanderheyden S, Wilmer A, Van den Berghe G. Early versus late parenteral nutrition in critically ill adults. N Engl J Med. 2011;365:506–517. doi: 10.1056/NEJMoa1102662. [DOI] [PubMed] [Google Scholar]
- 29.Chan KS, Pfoh ER, Denehy L, Elliott D, Holland AE, Dinglas VD, Needham DM. Construct validity and minimal important difference of 6-minute walk distance in survivors of acute respiratory failure. Chest. 2015;147:1316–1326. doi: 10.1378/chest.14-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Formenti P, Umbrello M, Coppola S, Froio S, Chiumello D. Clinical review: peripheral muscular ultrasound in the ICU. Ann Intensive Care. 2019;9:57. doi: 10.1186/s13613-019-0531-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Latronico N, Bertolini G, Guarneri B, Botteri M, Peli E, Andreoletti S, et al. Simplified electrophysiological evaluation of peripheral nerves in critically ill patients: the Italian multi-centre CRIMYNE study. Crit Care. 2007;11:R11. doi: 10.1186/cc5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Latronico N, Nattino G, Guarneri B, Fagoni N, Amantini A, Bertolini G, et al. Validation of the peroneal nerve test to diagnose critical illness polyneuropathy and myopathy in the intensive care unit: the multicentre Italian CRIMYNE-2 diagnostic accuracy study. F1000Research. 2014;3:127 [DOI] [PMC free article] [PubMed]
- 33.Joskova V, Patkova A, Havel E, Najpaverova S, Uramova D, Kovarik M, Zadak Z, Hronek M. Critical evaluation of muscle mass loss as a prognostic marker of morbidity in critically ill patients and methods for its determination. J Rehabil Med. 2018;50:696–704. doi: 10.2340/16501977-2368. [DOI] [PubMed] [Google Scholar]
- 34.Hernández-Socorro CR, Saavedra P, López-Fernández JC, Ruiz-Santana S. Assessment of muscle wasting in long-stay ICU patients using a new ultrasound protocol. Nutrients. 2018;10:E1849. doi: 10.3390/nu10121849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Witteveen E, Sommers J, Wieske L, Doorduin J, van Alfen N, Schultz MJ, van Schaik IN, Horn J, Verhamme C. Diagnostic accuracy of quantitative neuromuscular ultrasound for the diagnosis of intensive care unit-acquired weakness: a cross-sectional observational study. Ann Intensive Care. 2017;7:40. doi: 10.1186/s13613-017-0263-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Latronico N, Fenzi F, Recupero D, Guarneri B, Tomelleri G, Tonin P, De Maria G, Antonini L, Rizzuto N, Candiani A. Critical illness myopathy and neuropathy. Lancet. 1996;347:1579–1582. doi: 10.1016/s0140-6736(96)91074-0. [DOI] [PubMed] [Google Scholar]
- 37.Supinski GS, Westgate P, Callahan LA. Correlation of maximal inspiratory pressure to transdiaphragmatic twitch pressure in intensive care unit patients. Crit Care. 2016;20:77. doi: 10.1186/s13054-016-1247-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doorduin J, van Hees HW, van der Hoeven JG, Heunks LM. Monitoring of the respiratory muscles in the critically ill. Am J Respir Crit Care Med. 2013;187:20–27. doi: 10.1164/rccm.201206-1117CP. [DOI] [PubMed] [Google Scholar]
- 39.Dres M, Goligher EC, Heunks LMA, Brochard LJ. Critical illness-associated diaphragm weakness. Intensive Care Med. 2017;43:1441–1452. doi: 10.1007/s00134-017-4928-4. [DOI] [PubMed] [Google Scholar]
- 40.Qian Z, Yang M, Li L, Chen Y. Ultrasound assessment of diaphragmatic dysfunction as a predictor of weaning outcome from mechanical ventilation: a systematic review and meta-analysis. BMJ Open. 2018;8:e021189. doi: 10.1136/bmjopen-2017-021189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hermans G, Clerckx B, Vanhullebusch T, Segers J, Vanpee G, Robbeets C, Casaer MP, Wouters P, Gosselink R, Van den Berghe G. Interobserver agreement of Medical Research Council sum-score and handgrip strength in the intensive care unit. Muscle Nerve. 2012;45:18–25. doi: 10.1002/mus.22219. [DOI] [PubMed] [Google Scholar]
- 42.Hermans G, Van Mechelen H, Bruyninckx F, Vanhullebusch T, Clerckx B, Meersseman P, Debaveye Y, Casaer MP, Wilmer A, Wouters PJ, Vanhorebeek I, Gosselink R, Van den Berghe G. Predictive value for weakness and 1-year mortality of screening electrophysiology tests in the ICU. Intensive Care Med. 2015;41:2138–2148. doi: 10.1007/s00134-015-3979-7. [DOI] [PubMed] [Google Scholar]
- 43.Chlan LL, Tracy MF, Guttormson J, Savik K. Peripheral muscle strength and correlates of muscle weakness in patients receiving mechanical ventilation. Am J Crit Care. 2015;24:e91–98. doi: 10.4037/ajcc2015277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolfe KS, Patel BK, MacKenzie EL, Giovanni SP, Pohlman AS, Churpek MM, Hall JB, Kress JP. Impact of vasoactive medications on ICU-acquired weakness in mechanically ventilated patients. Chest. 2018;154:781–787. doi: 10.1016/j.chest.2018.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang T, Li Z, Jiang L, Wang Y, Xi X. Risk factors for intensive care unit-acquired weakness: a systematic review and meta-analysis. Acta Neurol Scand. 2018;138:104–114. doi: 10.1111/ane.12964. [DOI] [PubMed] [Google Scholar]
- 46.Latronico N. Critical illness polyneuropathy and myopathy 20 years later. No man's land? No, it is our land! Intensive Care Med. 2016;42:1790–1793. doi: 10.1007/s00134-016-4475-4. [DOI] [PubMed] [Google Scholar]
- 47.Goossens C, Marques MB, Derde S, Vander Perre S, Dufour T, Thiessen SE, Güiza F, Janssens T, Hermans G, Vanhorebeek I, De Bock K, Van den Berghe G, Langouche L. Premorbid obesity, but not nutrition, prevents critical illness-induced muscle wasting and weakness. J Cachexia Sarcopenia Muscle. 2017;8:89–101. doi: 10.1002/jcsm.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van den Berghe G, Schoonheydt K, Becx P, Bruyninckx F, Wouters PJ. Insulin therapy protects the central and peripheral nervous system of intensive care patients. Neurology. 2005;64:1348–1353. doi: 10.1212/01.WNL.0000158442.08857.FC. [DOI] [PubMed] [Google Scholar]
- 49.Hermans G, Wilmer A, Meersseman W, Milants I, Wouters PJ, Bobbaers H, Bruyninckx F, Van den Berghe G. Impact of intensive insulin therapy on neuromuscular complications and ventilator dependency in the medical intensive care unit. Am J Respir Crit Care Med. 2007;175:480–489. doi: 10.1164/rccm.200605-665OC. [DOI] [PubMed] [Google Scholar]
- 50.Hermans G, De Jonghe B, Bruyninckx F, Van den Berghe G (2014) Interventions for preventing critical illness polyneuropathy and critical illness myopathy. Cochrane Database Syst Rev 1: CD006832 [DOI] [PMC free article] [PubMed]
- 51.Garnacho-Montero J, Madrazo-Osuna J, Garcia-Garmendia JL, Ortiz-Leyba C, Jiménez-Jiménez FJ, Barrero-Almodóvar A, Garnacho-Montero MC, Moyano-Del-Estad MR. Critical illness polyneuropathy: risk factors and clinical consequences. A cohort study in septic patients. Intensive Care Med. 2001;27:1288–1296. doi: 10.1007/s001340101009. [DOI] [PubMed] [Google Scholar]
- 52.Yang T, Li Z, Jiang L, Xi X. Corticosteroid use and intensive care unit-acquired weakness: a systematic review and meta-analysis. Crit Care. 2018;22:187. doi: 10.1186/s13054-018-2111-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rochwerg B, Oczkowski SJ, Siemieniuk RAC, Agoritsas T, Belley-Cote E, D'Aragon F, Duan E, English S, Gossack-Keenan K, Alghuroba M, Szczeklik W, Menon K, Alhazzani W, Sevransky J, Vandvik PO, Annane D, Guyatt G. Corticosteroids in sepsis: an updated systematic review and meta-analysis. Crit Care Med. 2018;46:1411–1420. doi: 10.1097/CCM.0000000000003262. [DOI] [PubMed] [Google Scholar]
- 54.Bourenne J, Hraiech S, Roch A, Gainnier M, Papazian L, Forel JM. Sedation and neuromuscular blocking agents in acute respiratory distress syndrome. Ann Transl Med. 2017;5:291. doi: 10.21037/atm.2017.07.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Backer J, Hart N, Fan E. Neuromuscular blockade in the 21st century management of the critically ill patient. Chest. 2017;151:697–706. doi: 10.1016/j.chest.2016.10.040. [DOI] [PubMed] [Google Scholar]
- 56.Papazian L, Forel JM, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, Jaber S, Arnal JM, Perez D, Seghboyan JM, Constantin JM, Courant P, Lefrant JY, Guérin C, Prat G, Morange S, Roch A; ACURASYS Study Investigators Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363:1107–1116. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 57.Moss M, Huang DT, Brower RG, Ferguson ND, Ginde AA, Gong MN, Grissom CK, Gundel S, Hayden D, Hite RD, Hou PC, Hough CL, Iwashyna TJ, Khan A, Liu KD, Talmor D, Thompson BT, Ulysse CA, Yealy DM, Angus DC, Heart N, Lung, and Blood Institute PETAL Clinical Trials Network Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med. 2019;380:1997–2008. doi: 10.1056/NEJMoa1901686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wieske L, van Hest RM, Witteveen E, Verhamme C, Schultz MJ, van Schaik IN, Horn J. Is gentamycin affecting the neuromuscular system of critically ill patients? Intensive Care Med. 2015;41:727–728. doi: 10.1007/s00134-015-3731-3. [DOI] [PubMed] [Google Scholar]
- 59.Jolley SE, Bunnell AE, Hough CL. ICU-acquired weakness. Chest. 2016;150:1129–1140. doi: 10.1016/j.chest.2016.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anzueto A. Muscle dysfunction in the intensive care unit. Clin Chest Med. 1999;20:435–452. doi: 10.1016/s0272-5231(05)70151-9. [DOI] [PubMed] [Google Scholar]
- 61.Howard RS, Tan SV, Z’Graggen WJ. Weakness on the intensive care unit. Pract Neurol. 2008;8:280–295. doi: 10.1136/jnnp.2008.157263. [DOI] [PubMed] [Google Scholar]
- 62.Foster J. Complications of sedation in critical illness: an update. Crit Care Nurs Clin N Am. 2016;28:227–239. doi: 10.1016/j.cnc.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 63.Ali NA, O’Brien JM Jr, Hoffmann SP, Phillips G, Garland A, Finley JC, Almoosa K, Hejal R, Wolf KM, Lemeshow S, Connors AF Jr, Marsh CB; Midwest Critical Care Consortium Acquired weakness, handgrip strength, and mortality in critically ill patients. Am J Respir Crit Care Med. 2008;178:261–268. doi: 10.1164/rccm.200712-1829OC. [DOI] [PubMed] [Google Scholar]
- 64.Sharshar T, Bastuji-Garin S, Stevens RD, Durand MC, Malissin I, Rodriguez P, Cerf C, Outin H, De Jonghe B; Groupe de Réflexion et d'Etude des Neuromyopathies En Réanimation Presence and severity of intensive care unit-acquired paresis at time of awakening are associated with increased intensive care unit and hospital mortality. Crit Care Med. 2009;37:3047–3053. doi: 10.1097/CCM.0b013e3181b027e9. [DOI] [PubMed] [Google Scholar]
- 65.Medrinal C, Prieur G, Frenoy E, Combret Y, Gravier FE, Bonnevie T, Poncet A, Robledo Quesada A, Lamia B, Contal O. Is overlap of respiratory and limb muscle weakness at weaning from mechanical ventilation associated with poorer outcomes? Intensive Care Med. 2017;43:282–283. doi: 10.1007/s00134-016-4626-7. [DOI] [PubMed] [Google Scholar]
- 66.De Jonghe B, Bastuji-Garin S, Sharshar T, Outin H, Brochard L. Does ICU-acquired paresis lengthen weaning from mechanical ventilation? Intensive Care Med. 2004;30:1117–1121. doi: 10.1007/s00134-004-2174-z. [DOI] [PubMed] [Google Scholar]
- 67.De Jonghe B, Bastuji-Garin S, Durand MC, Malissin I, Rodrigues P, Cerf C, Outin H, Sharshar T; Groupe de Réflexion et d'Etude des Neuromyopathies en Réanimation Respiratory weakness is associated with limb weakness and delayed weaning in critical illness. Crit Care Med. 2007;35:2007–2015. doi: 10.1097/01.ccm.0000281450.01881.d8. [DOI] [PubMed] [Google Scholar]
- 68.Jeong BH, Nam J, Ko MG, Chung CR, Suh GY, Jeon K. Impact of limb weakness on extubation failure after planned extubation in medical patients. Respirology. 2019 doi: 10.1111/resp.13305. [DOI] [PubMed] [Google Scholar]
- 69.Jung B, Moury PH, Mahul M, de Jong A, Galia F, Prades A, Albaladejo P, Chanques G, Molinari N, Jaber S. Diaphragmatic dysfunction in patients with ICU-acquired weakness and its impact on extubation failure. Intensive Care Med. 2016;42:853–861. doi: 10.1007/s00134-015-4125-2. [DOI] [PubMed] [Google Scholar]
- 70.Hermans G, Van Mechelen H, Clerckx B, Vanhullebusch T, Mesotten D, Wilmer A, Casaer MP, Meersseman P, Debaveye Y, Van Cromphaut S, Wouters PJ, Gosselink R, Van den Berghe G. Acute outcomes and 1-year mortality of intensive care unit-acquired weakness. A cohort study and propensity-matched analysis. Am J Resp Crit Care Med. 2014;190:410–420. doi: 10.1164/rccm.201312-2257OC. [DOI] [PubMed] [Google Scholar]
- 71.Kelmenson DA, Held N, Allen RR, Quan D, Burnham EL, Clark BJ, Ho PM, Kiser TH, Vandivier RW, Moss M. Outcomes of ICU patients with a discharge diagnosis of critical illness polyneuromyopathy: a propensity-matched analysis. Crit Care Med. 2017;45:2055–2060. doi: 10.1097/CCM.0000000000002763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zuercher P, Moret CS, Dziewas R, Schefold JC. Dysphagia in the intensive care unit: epidemiology, mechanisms, and clinical management. Crit Care. 2019;23:103. doi: 10.1186/s13054-019-2400-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Medrinal C, Prieur G, Frenoy E, Robledo Quesada A, Poncet A, Bonnevie T, Gravier FE, Lamia B, Contal O. Respiratory weakness after mechanical ventilation is associated with one-year mortality - a prospective study. Crit Care. 2016;20:231. doi: 10.1186/s13054-016-1418-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dinglas VD, Aronson Friedman L, Colantuoni E, Mendez-Tellez PA, Shanholtz CB, Ciesla ND, Pronovost PJ, Needham DM. Muscle weakness and 5-year survival in acute respiratory distress syndrome survivors. Crit Care Med. 2017;45:446–453. doi: 10.1097/CCM.0000000000002208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wieske L, Dettling-Ihnenfeldt DS, Verhamme C, Nollet F, van Schaik IN, Schultz MJ, Horn J, van der Schaaf M. Impact of ICU-acquired weakness on post-ICU physical functioning: a follow-up study. Crit Care. 2015;19:196. doi: 10.1186/s13054-015-0937-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cunningham CJB, Finalyson HC, Henderson WR, O’Connor RJ, Travlos A. Impact of critical illness polyneuromyopathy in rehabilitation: a prospective observational study. PM R. 2018;10:494–500. doi: 10.1016/j.pmrj.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 77.Van Aerde N, Meersseman P, Debaveye Y, Wilmer A, Gunst J, Casaer MP, Bruyninckx F, Wouters PJ, Gosselink R, Van den Berghe G, Hermans G. Five-year impact of ICU-acquired neuromuscular complications: a prospective, observational study. Intensive Care Med. 2020 doi: 10.1007/s00134-020-05927-5. [DOI] [PubMed] [Google Scholar]
- 78.Hermans G, Van Aerde N, Meersseman P, Van Mechelen H, Debaveye Y, Wilmer A, Gunst J, Casaer MP, Dubois J, Wouters P, Gosselink R, Van den Berghe G. Five-year mortality and morbidity impact of prolonged versus brief ICU stay: a propensity score matched cohort study. Thorax. 2019;74:1037–1045. doi: 10.1136/thoraxjnl-2018-213020. [DOI] [PubMed] [Google Scholar]
- 79.Herridge MS, Moss M, Hough CL, Hopkins RO, Rice TW, Bienvenu OJ, Azoulay E. Recovery and outcomes after the acute respiratory distress syndrome (ARDS) in patients and their family caregivers. Intensive Care Med. 2016;42:725–738. doi: 10.1007/s00134-016-4321-8. [DOI] [PubMed] [Google Scholar]
- 80.Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, Cooper AB, Guest CB, Mazer CD, Mehta S, Stewart TE, Barr A, Cook D, Slutsky AS; Canadian Critical Care Trials Group One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348:683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 81.Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, Guest CB, Mazer CD, Mehta S, Stewart TE, Kudlow P, Cook D, Slutsky AS, Cheung AM; Canadian Critical Care Trials Group Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 82.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Poulsen JB, Rose MH, Jensen BR, Møller K, Perner A. Biomechanical and nonfunctional assessment of physical capacity in male ICU survivors. Crit Care Med. 2013;41:93–101. doi: 10.1097/CCM.0b013e31826a3f9e. [DOI] [PubMed] [Google Scholar]
- 84.Guarneri B, Bertolini G, Latronico N. Long-term outcome in patients with critical illness myopathy or neuropathy: the Italian multicentre CRIMYNE study. J Neurol Neurosurg Psychiatry. 2008;79:838–841. doi: 10.1136/jnnp.2007.142430. [DOI] [PubMed] [Google Scholar]
- 85.Koch S, Wollersheim T, Bierbrauer J, Haas K, Mörgeli R, Deja M, Spies CD, Spuler S, Krebs M, Weber-Carstens S. Long-term recovery in critical illness myopathy is complete, contrary to polyneuropathy. Muscle Nerve. 2014;50:431–436. doi: 10.1002/mus.24175. [DOI] [PubMed] [Google Scholar]
- 86.Dos Santos C, Hussain SN, Mathur S, Picard M, Herridge M, Correa J, Bain A, Guo Y, Advani A, Advani SL, Tomlinson G, Katzberg H, Streutker CJ, Cameron JI, Schols A, Gosker HR, Batt J; MEND ICU Group; RECOVER Program Investigators; Canadian Critical Care Translational Biology Group Mechanisms of chronic muscle wasting and dysfunction after an intensive care unit stay. A pilot study. Am J Respir Crit Care Med. 2016;194:821–830. doi: 10.1164/rccm.201512-2344OC. [DOI] [PubMed] [Google Scholar]
- 87.Walsh CJ, Batt J, Herridge MS, Mathur S, Bader GD, Hu P, Dos Santos CC. Transcriptomic analysis reveals abnormal muscle repair and remodeling in survivors of critical illness with sustained weakness. Sci Rep. 2016;6:29334. doi: 10.1038/srep29334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rocheteau P, Chatre L, Briand D, Mebarki M, Jouvion G, Bardon J, Crochemore C, Serrani P, Lecci PP, Latil M, Matot B, Carlier PG, Latronico N, Huchet C, Lafoux A, Sharshar T, Ricchetti M, Chrétien F. Sepsis induces long-term metabolic and mitochondrial muscle stem cell dysfunction amenable by mesenchymal stem cell therapy. Nat Commun. 2015;6:10145. doi: 10.1038/ncomms10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359–67. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 90.Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354:449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 91.Vlasselaers D, Milants I, Desmet L, Wouters PJ, Vanhorebeek I, van den Heuvel I, Mesotten D, Casaer MP, Meyfroidt G, Ingels C, Muller J, Van Cromphaut S, Schetz M, Van den Berghe G. Intensive insulin therapy for patients in paediatric intensive care: a prospective, randomised controlled study. Lancet. 2009;373:547–556. doi: 10.1016/S0140-6736(09)60044-1. [DOI] [PubMed] [Google Scholar]
- 92.NICE-SUGAR Study Investigators, Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, Hébert PC, Heritier S, Heyland DK, McArthur C, McDonald E, Mitchell I, Myburgh JA, Norton R, Potter J, Robinson BG, Ronco JJ Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 93.Gunst J, Van den Berghe G. Intensive care nutrition and post-intensive care recovery. Crit Care Clin. 2018;34:573–583. doi: 10.1016/j.ccc.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 94.Singer P, Berger MM, Van den Berghe G, Biolo G, Calder P, Forbes A, Griffiths R, Kreyman G, Leverve X, Pichard C, ESPEN ESPEN guidelines on parenteral nutrition: intensive care. Clin Nutr. 2009;28:387–400. doi: 10.1016/j.clnu.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 95.Fivez T, Kerklaan D, Mesotten D, Verbruggen S, Wouters PJ, Vanhorebeek I, Debaveye Y, Vlasselaers D, Desmet L, Casaer MP, Garcia Guerra G, Hanot J, Joffe A, Tibboel D, Joosten K, Van den Berghe G. Early versus late parenteral nutrition in critically ill children. N Engl J Med. 2016;374:1111–1122. doi: 10.1056/NEJMoa1514762. [DOI] [PubMed] [Google Scholar]
- 96.Casaer MP, Wilmer A, Hermans G, Wouters PJ, Mesotten D, Van den Berghe G. Role of disease and macronutrient dose in the randomized controlled EPaNIC trial: a post hoc analysis. Am J Respir Crit Care Med. 2013;187:247–255. doi: 10.1164/rccm.201206-0999OC. [DOI] [PubMed] [Google Scholar]
- 97.Vanhorebeek I, Verbruggen S, Casaer MP, Gunst J, Wouters PJ, Hanot J, Guerra GG, Vlasselaers D, Joosten K, Van den Berghe G. Effect of early supplemental parenteral nutrition in the paediatric ICU: a preplanned observational study of post-randomisation treatments in the PEPaNIC trial. Lancet Respir Med. 2017;5:475–483. doi: 10.1016/S2213-2600(17)30186-8. [DOI] [PubMed] [Google Scholar]
- 98.Gunst J, Vanhorebeek I, Casaer MP, Hermans G, Wouters PJ, Dubois J, Claes K, Schetz M, Van den Berghe G. Impact of early parenteral nutrition on metabolism and kidney injury. J Am Soc Nephrol. 2013;24:995–1005. doi: 10.1681/ASN.2012070732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Thiessen SE, Derde S, Derese I, Dufour T, Vega CA, Langouche L, Goossens C, Peersman N, Vermeersch P, Vander Perre S, Holst JJ, Wouters PJ, Vanhorebeek I, Van den Berghe G. Role of glucagon in catabolism and muscle wasting of critical illness and modulation by nutrition. Am J Respir Crit Care Med. 2017;196:1131–1143. doi: 10.1164/rccm.201702-0354OC. [DOI] [PubMed] [Google Scholar]
- 100.Gunst J, Vanhorebeek I, Thiessen SE, Van den Berghe G. Amino acid supplements in critically ill patients. Pharmacol Res. 2018;130:127–131. doi: 10.1016/j.phrs.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 101.Casaer MP, Bellomo R. Micronutrient deficiency in critical illness: an invisible foe? Intensive Care Med. 2019;45:1136–1139. doi: 10.1007/s00134-019-05678-y. [DOI] [PubMed] [Google Scholar]
- 102.Needham DM, Dinglas VD, Morris PE, Jackson JC, Hough CL, Mendez-Tellez PA, Wozniak AW, Colantuoni E, Ely EW, Rice TW, Hopkins RO, Network NIHNHLBIARDS. Physical and cognitive performance of patients with acute lung injury 1 year after initial trophic versus full enteral feeding. EDEN trial follow-up. Am J Respir Crit Care Med. 2013;188:567–576. doi: 10.1164/rccm.201304-0651OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Goossens C, Weckx R, Derde S, Dufour T, Vander Perre S, Pauwels L, Thiessen SE, Van Veldhoven PP, Van den Berghe G, Langouche L. Adipose tissue protects against sepsis-induced muscle weakness in mice: from lipolysis to ketones. Crit Care. 2019;23:236. doi: 10.1186/s13054-019-2506-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471–1477. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 105.Tipping CJ, Harrold M, Holland A, Romero L, Nisbet T, Hodgson CL. The effects of active mobilisation and rehabilitation in ICU on mortality and function: a systematic review. Intensive Care Med. 2017;43:171–183. doi: 10.1007/s00134-016-4612-0. [DOI] [PubMed] [Google Scholar]
- 106.Fuke R, Hifumi T, Kondo Y, Hatakeyama J, Takei T, Yamakawa K, Inoue S, Nishida O. Early rehabilitation to prevent postintensive care syndrome in patients with critical illness: a systematic review and meta-analysis. BMJ Open. 2018;8:e019998. doi: 10.1136/bmjopen-2017-019998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Doiron KA, Hoffmann TC, Beller EM. Eearly intervention (mobilization or active exercise) for critically ill adults in the intensive care unit. Cochrane Database Syst Rev. 2018;3:754. doi: 10.1002/14651858.CD010754.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang L, Hu W, Cai Z, Liu J, Wu J, Deng Y, Yu K, Chen X, Zhu L, Ma J, Qin Y. Early mobilization of critically ill patients in the intensive care unit: a systematic review and meta-analysis. PLoS ONE. 2019;14:e0223185. doi: 10.1371/journal.pone.0223185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vorona S, Sabatini U, Al-Maqbali S, Bertoni M, Dres M, Bissett B, Van Haren F, Martin AD, Urrea C, Brace D, Parotto M, Herridge MS, Adhikari NKJ, Fan E, Melo LT, Reid WD, Brochard LJ, Ferguson ND, Goligher EC. Inspiratory muscle rehabilitation in critically ill adults. A systematic review and meta-analysis. Ann Am Thorac Soc. 2018;15:735–744. doi: 10.1513/AnnalsATS.201712-961OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Parry SM, Knight LD, Connolly B, Baldwin C, Puthucheary Z, Morris P, Mortimore J, Hart N, Denehy L, Granger CL. Factors influencing physical activity and rehabilitation in survivors of critical illness: a systematic review of quantitative and qualitative studies. Intensive Care Med. 2017;43:531–542. doi: 10.1007/s00134-017-4685-4. [DOI] [PubMed] [Google Scholar]
- 111.Nydahl P, Sricharoenchai T, Chandra S, Kundt FS, Huang M, Fischill M, Needham DM. Safety of patient mobilization and rehabilitation in the intensive care unit. Systematic review with meta-analysis. Ann Am Thorac Soc. 2017;14:766–777. doi: 10.1513/AnnalsATS.201611-843SR. [DOI] [PubMed] [Google Scholar]
- 112.Burke D, Gorman E, Stokes D, Lennon O. An evaluation of neuromuscular electrical stimulation in critical care using the ICF framework: a systematic review and meta-analysis. Clin Respir J. 2016;10:407–420. doi: 10.1111/crj.12234. [DOI] [PubMed] [Google Scholar]
- 113.Zayed Y, Kheiri B, Barbarawi M, Chahine A, Rashdan L, Chintalapati S, Bachuwa G, Al-Sanouri I. Effects of neuromuscular electrical stimulation in critically ill patients: A systematic review and meta-analysis of randomised controlled trials. Aust Crit Care. 2019 doi: 10.1016/j.aucc.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 114.Shepherd SJ, Newman R, Brett SJ, Griffith DM; Enhancing Rehabilitation After Critical Illness Programme Study Investigators Pharmacological therapy for the prevention and treatment of weakness after critical illness: a systematic review. Crit Care Med. 2016;44:1198–1205. doi: 10.1097/CCM.0000000000001652. [DOI] [PubMed] [Google Scholar]
- 115.Connolly B, Salisbury L, O’Neill B, Geneen L, Douiri A, Grocott MP, Hart N, Walsh TS, Blackwood B. Exercise rehabilitation following intensive care unit discharge for recovery from critical illness: executive summary of a Cochrane Collaboration systematic review. J Cachexia Sarcopenia Muscle. 2016;7:520–526. doi: 10.1002/jcsm.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]