Abstract
Discovery of genes and molecular mechanism involved in cervical cancer development would promote the prevention and treatment. By comparing gene expression profiles of cervical carcinoma in situ (CCIS) and adjacent normal tissues, we identified a potential cancer-promoting gene, IMPA2. This study aimed to elucidate the role of IMPA2 and underlying molecular mechanisms in cervical cancer progression. To do this expression of IMPA2 was compared between human cervical cancer and corresponding adjacent normal cervical tissues firstly. CCK-8 assay, clone formation assay, wound healing assay, transwell assay, and tumor formation in nude mice were performed to demonstrate the effect of IMPA2 in cervical cancer proliferation and metastasis. Further proteomic profiling and western blotting explored the molecular pathway involved in the IMPA2-regulating process. The results showed that IMPA2 gene expression was upregulated in cervical cancer. Consistently, silencing of IMPA2 suppressed tumor formation in BALB/c nude mice. Short hairpin RNA (shRNA)-mediated IMPA2 silencing significantly inhibited proliferation and colony-forming abilities of cervical cancer cells, while IMPA2 overexpression had little impact. Also, IMPA2 silencing suppressed cellular migration, but overexpression promoted migration. Proteomics analysis revealed the involvement of mitogen-activated protein kinase (MAPK) pathway in tumor-promoting action of IMPA2. Significantly, the inhibition of IMPA2 activated ERK phosphorylation, and its inhibitory effects can be restored by using selective ERK inhibitor, FR180204. In conclusion, IMPA2 acts as an oncogene in the proliferation and migration of cervical cancer. IMPA2 downregulated ERK phosphorylation to promote cervical cancer. These findings identify a new mechanism underlying cervical cancer and suggest a regulating effect of IMPA2 in MAPK signaling pathway.
Subject terms: Cervical cancer, Tumour biomarkers
Introduction
Cervical cancer is the fourth leading cause of cancer-associated death regarding gynecological malignancies worldwide1, even though prevention and treatment have rapidly developed recently2,3. As we all know, the most significant cause of cervical cancer is persistent infection of human papillomavirus (HPV). HPV is detected in 99% of cervical cancer patients4, but most women infected with HPV failed to develop invasive cervical cancer5. Therefore, comprehensive understanding of molecular mechanisms underlying cervical cancer will promote the earlier diagnosis and effective treatment.
Genetic mutations were proved to play important roles in development of cervical cancer6. RNA sequencing provides an efficient and comprehensive method to identify the key genes and molecular pathways involved in cervical cancer pathogenesis7,8. Cervical carcinoma in situ (CCIS), without stromal invasion, and superficially invasive carcinomatosis, is the earliest stage in cancer progression. By comparing gene expression profiles of CCIS and adjacent normal tissues, it is possible to look for the most direct evidence of tumorigenesis and help early diagnosis. Here, we sought potential oncogenes of cervical cancer, by comparing mRNA expression profiles of tissue samples of CCIS with adjacent normal cervical tissue. IMPA2 gene was discovered to be significantly upregulated in CCIS tissues. IMPA2 located on chromosome 18p11.2, encodes myo-inositol monophosphatase 2 (IMPA2) with 288 amino acids9. IMPA2 has intrinsic IMPase activity that is completely dependent on magnesium10, and is involved in phosphatidylinositol signaling pathway, which is associated with cellular activities such as metabolism, secretion, cell growth, and differentiation11. Therefore, we speculated that IMPA2 may be a cancer-promoting gene in cervical cancer. However, most studies about IMPA2 focused on neuropsychiatric diseases and the pharmacological action of Lithium10,12,13. Recently, French et al.14 found that IMPA2 expression might affect accumulation of methotrexate polyglutamates (MTXPGs) in leukemia. In addition, Lin et al.15 indicated that IMPA2 downregulation leads to poor outcomes in clear cell renal cell carcinoma (ccRCC). This is contrary to our speculation on the role of IMPA2 in cervical cancers. As there are few published articles about the role of IMPA2 gene in cervical cancer, this study performed both in vitro and in vivo studies to discuss the relationship between cervical cancer and IMPA2.
Here, we discussed the role of IMPA2 and found that IMPA2 promoted the ability of proliferation, metastasis, and in vivo tumorigenesis of cervical cancer cells. Further proteomic analysis was performed to discuss the possible mechanisms regulated by IMPA2 in cervical cancer. The present study is proposed to identify the potential cancer-promoting action of IMPA2 in cervical cancer and explore possible pathways controlled by IMPA2 to further understanding the molecular mechanisms underlying cervical cancer.
Material and methods
Tissue sample selection
Cervical carcinoma in situ (CCIS) and adjacent normal tissues were obtained from three patients who underwent radical hysterectomy at the Second Xiangya Hospital, Central South University. All patients were diagnosed by multipoint biopsy. Table 1 showed the clinical characteristics of the patients. After tumor purity analysis performed by ESTIMATE algorithm, samples were collected for transcriptome analyses16. None of the three patients had received adjuvant therapy (chemotherapy or radiotherapy) prior to uterectomy. In addition, the other 58 patients with cervical cancer were enrolled from September 2015 to December 2017. Clinicopathologic features of the patients were shown in Table S1. Among them, 57.4% (35/61) were positive for HPV16, 19.7% (12/61) for HPV 58, 11.5% (7/61) for HPV 33, 11.5% (7/61) for HPV 52, 8.2% (5/61) for HPV51, and 8.2% (5/61) for HPV18. This study was approved by the Joint Ethics Committee of the Central South University Health Authority and performed following national guidelines. Written informed consent was obtained from all the patients. The clinical staging and clinicopathological classifications were determined according to the International Federation of Obstetrics and Gynecology (FIGO). Paired cervical cancer and adjacent normal tissues were collected at surgery, immediately frozen in liquid nitrogen and stored until total RNA or proteins were extracted.
Table 1.
Clinical characteristic of cervical cancer patients enrolled in this study.
| ID | Age at diagnosis (years) | Pathological type | FIGO stage | HPV types | Reproductive history (pregnancy-birth-abortion) |
|---|---|---|---|---|---|
| 1 | 47 | CIS | 0 | 51+/52+/44+ | 5-1-4 |
| 2 | 51 | CIS | 0 | 33+/53+ | 5-2-3 |
| 3 | 43 | CIS | 0 | 16+/53+ | 2-1-1 |
CIS carcinoma in situ, FIGO International Federation of Gynecology and Obstetrics, HPV Human Papilloma Virus.
Immunohistochemistry staining analysis
The immunohistochemical staining procedure was performed as previously described17. Cervical cancer samples for IMPA2 detecting were obtained from the Second Xiangya Hospital of Central South University. Samples for ERK and p-ERK detecting were xenografts from mice. The staining positivity was determined by the following formula: IRS = intensityscore × quantity score. The percentage of positive cells was divided into five score ranks: <10% (0), 10–25% (1), 25–50% (2),50–75% (3), and >75% (4). The intensity of staining was divided into four score ranks: no staining (0), light brown(1), brown (2), and dark brown (3). Two different pathologists evaluated all the specimens in a blinded manner. The antibodies used were as follows: anti-IMPA2 rabbit monoclonal antibody (1:100, GeneCopoeis, USA); anti-ERK (1:500, Abcam, UK), and anti-pERK (1:400, Cell Signaling, USA).
Cell culture
Cervical cancer cell line SiHa (#BNCC337881) and normal cervical epithelial cell line, HcerEpic (#BNCC340373) were purchased from the Cell Bank of BeNa culture collection (Beijing, China). Cervical cancer cell line HeLa (#GCC-UT0002CS) was purchased from the Cell Bank of Genechem (Shanghai, China). The cell line was cultured in Dulbecco’s modified eagle medium (DMEM) (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco, Grand Island, NY, USA) and 1% antibiotics at 37 °C in an atmosphere containing 5% CO2.
IMPA2 silencing
The short hairpin RNA (shRNA) targeting IMPA2 mRNA (shIMPA2) and the negative control were obtained from Ribobio (Guangzhou, China). Sequences of shIMPA2 were listed as follow: forward 5′‘-CCGGGCCTTACAGACGATTAACTATCTCGAGATAGTTAATCGTCTGAAGGCTTTTTG-3′; Reverse 5′-AATTCAAAAAGCCTTACAGACGATTAACTATCTCGAGATAGTTAATCGTCTGTAAGGC-3′. The lentivirus was packaged using GV115 vector, pHelper 1.0 vector and pHelper 2.0 vector, as well as Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA; Thermo Fisher Scientific) for the HEK293T cell and then collected after 48 h. The SiHa cell line was infected with lentivirus and polybrene (1:500; Shanghai Ji Kai Gene Chemical Technology Co., Ltd., Shanghai, China) according to the manufacturer’s instructions. The expression change of IMPA2 was determined by reverse-transcription polymerase chain reaction (RT-PCR) and Western blotting at 72 h after transfection.
In vivo tumor formation assay
A total of 14 health female BALB/c (nu/nu) nudes (4 weeks, 20–23 g) were purchased from Shanghai Lingchang Biotechnology limited company and fed in SPF Animal Laboratory of Central South University with sterile water and food. The mice were divided into 2 groups based on the randomized table, Normal Control (shCtrl) group and IMPA2 RNAi (shIMPA2) group. In all, 5 × 106 indicated stable cell lines were subcutaneously injected into right flank of BALB/c (nu/nu) mice in each group. Tumor sizes and weights were measured once a week. Mice were examined by in vivo fluorescence imaging system (Lumina LT, Perkin Elmer, USA) and killed for the analysis of tumor burden after 4 weeks. The tumors were stripped for follow-up experiments. All the experiments were strictly accordant with the care and use guidelines of experimental animal and approved by the Animal Protection Committee.
RNA isolation and quantitative real-time PCR
Total RNA was extracted using the Trizol reagent (Sangon Biotech, Shanghai, China). RNA (1 μg) was reverse transcribed into cDNA using Transcriptor First Strand cDNA Synthesis Kit (Roche Diagnostics, Germany) according to the supplier’s instructions. Quantitative real-time PCR analysis was performed with Stratagene Mx3000P qPCR system (Agilent Technologies, USA) using Thunderbird qPCR Mix (TOYOBO, Japan). CDNA samples were tested in triplicate and glyceraldehyde-3-phosphate dehydrogenase (GADPH) was used as a reference gene. The expression of IMPA2 was quantified by measuring Ct values and normalized using the 2−ΔΔCt method relative to GAPDH. The primer pairs used for qRT-PCR were designed using the primer3 program. Primers used were as follows: IMPA2 forward, 5′-GAAACCTCTCTCGCAACTCAG-3′, reverse, 5′-GGGCAGGACAGATCATCAGAA-3′. GADPH forward: 5′- GAACGGGAAGCT CACTGG-3′, reverse, 5′-GCCTGCTTCACCACCT TCT-3′.
Western blotting analysis
Details of Western blotting were previously described18. Cells at 80–90% confluence were lysed on ice in radioimmunoprecipitation assay buffer (RIPA; keygen biotech, China) containing PMFS complete protease inhibitor cocktail (keygen biotech, China). Protein concentration was determined by the BCA assay (keygen biotech, China). Equal protein samples (10 μg) were separated on 12% sodium dodecyl sulfate (SDS)/polyacrylamide gels, and transferred onto 0.45 µm polyvinylidene difluoride (PVDF) membranes (Immobilon-P; Millipore, Bedford, MA, USA). The antibodies used were as follow: anti-IMPA2 rabbit monoclonal antibody (1:1000; GeneCopoeis, USA); anti-ERK (1:10,000, Abcam, UK), anti-p38α (1:500, BBI China), anti-JNK1/2/3 (1:500, BBI China), p-ERK (1:500, Cell Signaling, USA), p-p38α (p-Thr180/Tyr182, 1:500, BBI China), and p-JNK1/2/3 (p-Th183/Ty185, 1:500, BBI China). Horseradish peroxidase (HRP)-conjugated goat anti‑rabbit immunoglobulin G (1:1000; BBI China) was used as second antibody and anti-GAPDH mouse monoclonal antibody (1:5000; BBI China) as a loading control. The final protein expression was detected by enhanced chemiluminescence (Bio-rad, Berkeley, CA, USA) according to the manufacturer’s suggested protocols. The band quantification was conducted using ImageJ (National Institutes of Health, Bethesda, MA, USA).
CCK-8 cell viability assays
Cells were seeded into a 96-well plate at 2 × 103 cells per well with 100 µl cultured medium and cultured for 24, 48, 72, and 96 h at 37 °C, 5% CO2. The cell viability was determined with CCK8 assay as previously described19. Each process was repeated three times.
Colony formation assay
Cells (1000/well) were plated in 6-well plates and cultured for 2 weeks. The colonies were washed with PBS three times and fixed with 4% formaldehyde for 10 min. Then, the colonies were stained with 1% crystal violet for 10 min. After washing, the colonies were counted. The experiment was carried out in triplicate for each cell line.
Wound healing and transwell migration assays
In the wound healing assay, cells (2×106/well) were seeded in 6-well plates. When the cells were 90% confluent, they were serum-starved for 24 h. A linear wound was created in the confluent monolayer using a 10-μl pipette tip. The wounds were observed and photographed immediately (time 0) and thereafter at 48 (magnification, ×200). Details of transwell migration assay was described previously20, 2 × 104 cells in 200 µl of serum-free medium were added to the top chamber of the transwell (8-μm pore size, BD Biosciences, New Jersey, USA). The bottom well contained growth medium with 20% FBS. After 24 h incubation at 37 °C, cells that had migrated to the lower face of the filters were fixed with 4% paraformaldehyde and stained with hematoxylin and finally counted under a magnification of ×200 (10 random fields/well). Each experiment was repeated at least 3 times.
Sample preparation for proteomic measurement
The cultured cells were scraped and collected in 1.5 mL Eppendorf tubes. Then, samples were sonicated three times on ice using a high intensity ultrasonic processor (Scientz) in lysis buffer (8 M urea, 1% Protease Inhibitor Cocktail). The remaining debris was removed by centrifugation at 12,000×g at 4 °C for 10 min. Finally, the supernatant was collected and the protein concentration was determined with BCA kit. The protein solution was reduced with 5 mM dithiothreitol for 30 min at 56 °C and alkylated with 11 mM iodoacetamide for 15 min at room temperature in darkness. The protein sample was then diluted by adding 100 mM NH4HCO3 to urea concentration <2 M. Finally, trypsin was added at 1:50 trypsin-to-protein mass ratio for the first digestion overnight and 1 : 100 trypsin-to-protein mass ratio for a second 4-h-digestion.
LC-MS/MS analysis
The tryptic peptides were separated by an EASY-nLC 1000 UPLC system and then subjected to NSI source followed by tandem mass spectrometry (MS/MS) in Q Exactive TM Plus (Thermo) coupled online to the UPLC. The resulting MS/MS data were processed using Maxquant search engine (v.1.5.2.8). Tandem mass spectra were searched against SwissProt Human database concatenated with reverse decoy database. Trypsin/P was specified as cleavage enzyme allowing up to two missing cleavages. The mass tolerance for precursor ions was set as 20 ppm in First search and 5 ppm in Main search, and the mass tolerance for fragment ions was set as 0.02 Da. Carbamidomethyl on Cys was specified as fixed modification, and oxidation on Met was specified as variable modifications. False positive rate (FDR) was adjusted to <1% and minimum score for peptides was set >40.
Bioinformatics analysis of differentially expressed proteins
Firstly, protein ID of the differentially expressed proteins (DEPs) were converted to UniProt ID according to the UniProt-GOA database (www. http://www.ebi.ac.uk/GOA/) and then mapped to Gene Ontology (GO) IDs. Proteins were classified by GO annotation based on three categories: biological process, cellular component, and molecular function. Then, Kyoto Encyclopedia of Genes and Genomes (KEGG) database was used to annotate protein pathway and to identify enriched pathways. KEGG online service tools KAAS and KEGG mapper help annotate protein’s KEGG database description and map the annotation result on the KEGG pathway database. Finally, a two-tailed Fisher’s exact test were used to test the enrichment of the DEP against all identified proteins. A corrected p-value <0.05 was considered significant.
Statistical analysis
The results were analyzed using SPSS 22.0 (Chicago, IL, USA) and GraphPad Prism 6 software (GraphPad Software, San Diego, CA, USA). The data were expressed as the mean ± SD. The t-test and one-way ANOVA Tukey’s post-hoc test were used to analyze the statistical significance of parametric data from two and more independent samples, respectively. Non-parametric Wilcoxon Signed-Ranks test, Friedman test, Mann–Whitney U test and Kruskal–Wallis test were used to analyze data from two related samples, three or more related samples, two independent samples and three or more independent samples, respectively.
Results
IMPA2 is overexpressed in cervical cancer
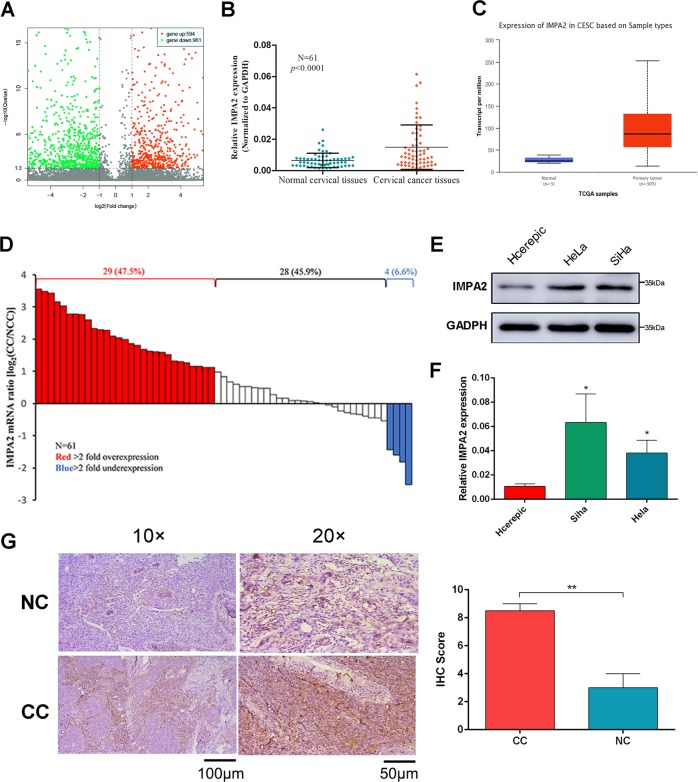
Comparing the gene expression profiles of CCIS and adjacent normal cervical samples resulted in 1555 differentially regulated genes at P < 0.05. Among the significant genes, 961 genes were up-regulated and 594 were down-regulated in CCIS (Fig. 1a). Of these, IMPA2 gene was upregulated 11 times. Further real-time PCR was used to determine the IMPA2 expression pattern in 61 pairs of cervical cancer tissues and their matched adjacent non-tumor cervical tissues. IMPA2 is significantly overexpressed in cervical cancer samples (P < 0.0001, Fig. 1b). Overexpression of more than 2-fold was displayed in 47.5% (29 of 61) of the cervical cancer samples compared with non-tumor samples (Fig. 1d). Analysis of a public CESC (Cervical squamous cell carcinoma) dataset from The Cancer Genome Atlas (TCGA) (https://tcga-data.nci.nih.gov/tcga/tcgaDownload.jsp) also showed significant IMPA2 overexpression (Fig. 1c)21. Expression of IMPA2 was also compared among cervical cancer cell lines and normal cervical epithelial cell line to prove the overexpression (Fig. 1e, f). Moreover, increased protein level of IMPA2 was also observed in paired cervical cancer samples by immunohistochemistry (IHC; Fig. 1g).
Fig. 1. IMPA2 was overexpressed in cervical cancer tissue and cell lines.
a Volcano plot of differentially expressed genes. b Relative expression of IMPA2 in cervical cancer tissues (n = 61) compared with that of adjacent normal tissues (n = 61) (P < 0.0001). c Expression of IMPA2 in normal and CESC tissues using public data from the TCGA-CESC dataset (Normal vs Primary tumor: P = 0.0004). d Waterfall plot analyses of IMPA2 mRNA levels in CC and matched non-tumor cervical specimens. Red and blue bars represent samples that show a relative IMPA2 fold change of ≥2 overexpression and underexpression, respectively (CC/NT). e IMPA2 expression was determined using western blotting and qRT-PCR (f) in SiHa and Hela cells. g Immunohistochemical (IHC) detection of IMPA2 in normal cervical samples (NC) and cervical cancer samples and the Immunoreactivity scores of IMPA2 were shown. Scale bar, 100 or 50 μm. Values were all presented as mean values ± SD. *P < 0.05.
Inhibition of IMPA2 suppresses tumorigenesis of SiHa cells in vivo
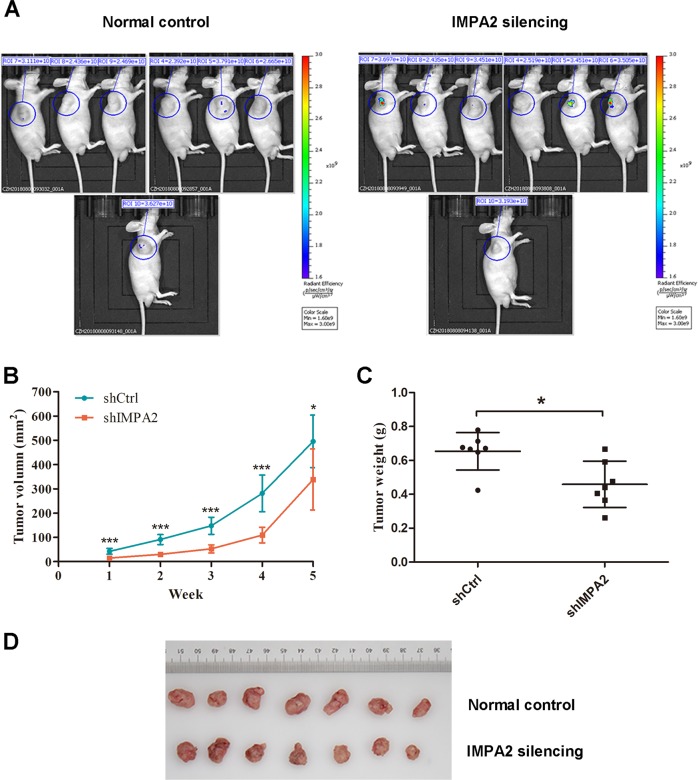
SiHa cells infected with IMPA2 shRNA or shRNA control were subcutaneously injected into each flank of nude mice. The tumor size of each nude mouse was measured and record weekly to draw the growth curve (Fig. 2b). After 5 weeks, all the mice were anesthetized to perform whole-body fluorescent imaging. As shown in Fig. 2a, no obvious metastasis was discovered in both groups. After that, all the mice were killed to harvest the xenografts. The tumor size and weight were measured and compared between groups. The IMPA2 silencing group showed significantly smaller tumor size (P = 0.0274) and lighter average weight (P = 0.0123) than the control group (Fig. 2c, d).
Fig. 2. Inhibition of IMPA2 suppresses tumor growth in vivo.
a Nude mice were transplanted subcutaneously with SiHa cells transfected with IMPA2 shRNA or the control shRNA. After 35 days, the mice were killed. Whole-body fluorescent imaging was performed for each nude mouse, and the representative pictures was recorded and presented. b Tumor volumes were measured weekly from week one to five post-injection. c Tumor weights were measured after the mice were killed. Bars indicate mean values ± SD. d A representative picture of the morphology of tumor xenografts after excision at the 35th day. *P < 0.05.
IMPA2 promotes the proliferation and clonogenicity of cervical cancer cells in vitro
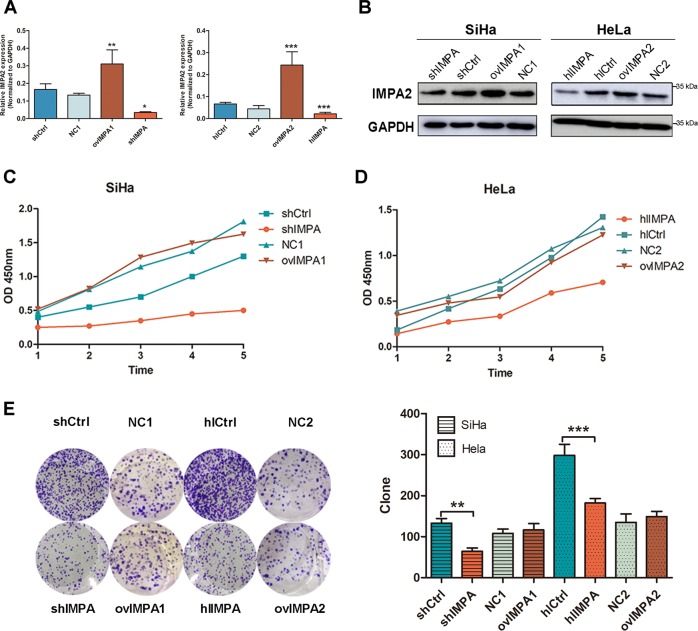
The role of IMPA2 in cervical cancer cells were detected after transfected with overexpressed plasmids and small interfering RNA (siRNA) lentivirus. The downregulation and overexpression efficiency were verified using qRT-PCR and western blotting (Fig. 3a, b). CCK8 cell viability assay (Fig. 3c, d) and colony formation assay (Fig. 3e) were performed to evaluate cell growth. As a result, silencing IMPA2 in SiHa and HeLa cells both significantly slowed the cell growth. Overexpression of IMPA2 failed to enhance the cell growth in both cervical cancer cells. Similarly, fewer and smaller colonies appeared in cells treated with IMPA2-siRNA than those in controls (P < 0.01). In contrast, no significant difference was found between the IMPA2 overexpression group and the control group.
Fig. 3. IMPA2 downregulation inhibits proliferation and clonogenicity of SiHa and Hela cells in vitro.
a, b ShRNA silence of IMPA2 dramatically downregulated IMPA2 expression at the RNA level and protein level when compared to the negative control shRNA (shCtrl) in SiHa cell line by qRT-PCR and western blotting. c, d CCK-8 assay was performed to measure proliferation in IMPA2 silencing and overexpressing SiHa or Hela cells and in the respective control cells. e The cell clonogenicity of IMPA2 silencing and overexpressing SiHa or HeLa cells and the respective control cells was analyzed using Celigo Image Cytometry. The data represent the mean ± SDs of three replicates, ***P < 0.001.
IMPA2 promotes the migration of cervical cancer cells in vitro
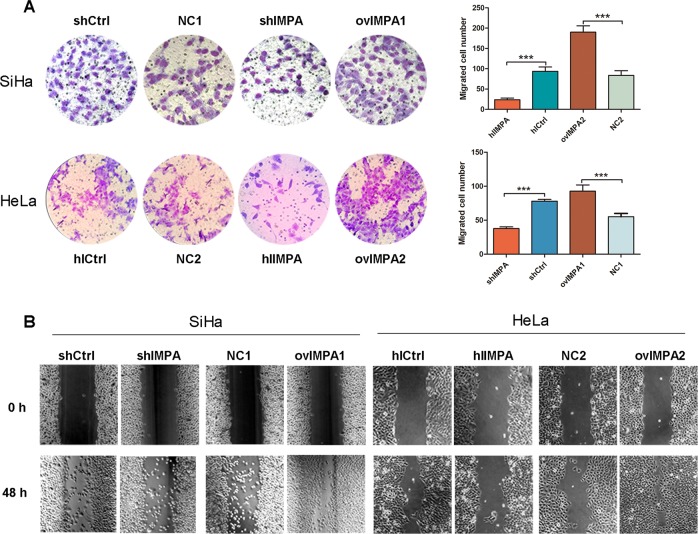
Wound-healing assay and transwell migration assay were conducted to examine the effect of IMPA2 on the migration and invasion of cervical cancer cells. IMPA2 silencing slowed the speed with which both cervical cancer cells filled the scratch, in comparison to the control in an obvious manner (Fig. 4b). The transwell assays yielded the similar results (P < 0.001, Fig. 4a). On the contrary, the cell migration capacity was improved (Fig. 4b) and the number of invaded cells were significantly higher (P < 0.001, Fig. 4a) in IMPA2 overexpression group.
Fig. 4. IMPA2 silencing blocks migration of SiHa and Hela cells in vitro.
a Cell migration of IMPA2 silencing and overexpressing SiHa or Hela cells and in the respective control cells was analyzed without Matrigel using transwell chamber. b Cell migration was analyzed by a wound healing assay. IMPA2 silencing and overexpressing SiHa or Hela cells were seeded and grown to full confluence. In total, 48 h after creating the wound, the wounding space was observed and photographed. The data represent the mean ± SDs of three replicates. **P < 0.01; ***P < 0.001.
Pathway analysis of differentially expressed protein between shCtrl and shIMPA2
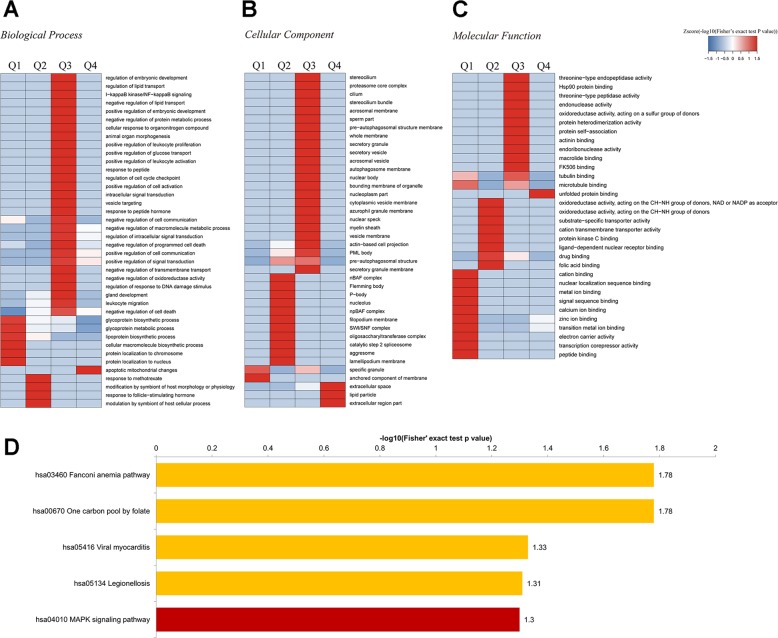
The proteomic profile of shCtrl and shIMPA2 (Fig. 5) was determined by LC-MS/MS. A total of 4364 proteins were identified. Using a cutoff value 1% FDR for statistical significance, 267 proteins were found to be differentially expressed: 112 significantly overexpressed (shIMPA2/shCtrl ratio ≥1.50) and 155 underexpressed (shIMPA2/shCtrl ratio ≤0.50). Further bioinformatics analysis was then performed to assess whether the DEPs were related to specific molecular pathways. In the Gene Ontology (GO) enrichment, 14 biological processes, 8 molecular functions, and 8 cellular components were enriched with statistical significance (Fig. 5a–c). Main biological processes include DNA repair, glycoprotein biosynthesis and metabolism, RNA biosynthesis, and cell death process. The analysis by cellular components showed that IMPA2 silencing produced a more profound impact on autophagosome-related structure and secretory granule membrane. Moreover, the analysis of molecular functions showed that proteins involved in the binding of transcript, protein kinase and drug, and folic acid, as well as many enzymatic activities like oxidoreductase and DNA polymerase. Cluster analysis based on shIMPA2/shCtrl ratio showed the involved biological processed in more detail (Fig. 5b). KEGG pathway enrichment analysis indicated that most of the DEPs were related to Fanconi anemia pathway, one carbon pool by folate, viral myocarditis, legionellosis, and MAPK signaling pathway (Fig. 5d). Eight DEPs involved in the MAPK pathway were significantly changed after inhibition of IMPA2, including four upregulated proteins (HSPA1L, RAP1A, NFKB1 and TAB1) and four downregulated proteins (MAP2K3, EPHA2, IL1RAP, and ECSIT). The fold change and primary information of these proteins were detailed in Table 2.
Fig. 5. Proteomic profile after inhibition of IMPA2 in SiHa cells.
a–c Functional enrichment of the differentially expressed proteins (DEPs) between shCtrl and shIMPA2 based on Gene ontology (GO) annotations. The results cover biologic processes (a), cellular component (b), and molecular function (c). d Enrichment of DEPs based on the KEGG pathway database. DEPs were divided into four parts according to the fold ratio of IMPA2 expression level between shCtrl and shIMPA2. Q1, 0 < ratio ≤ 1/2; Q2, 1/2 < ratio ≤ 1/1.5; Q3, 1.5 < ratio ≤ 2; Q4, ratio > 2.
Table 2.
Primary information and fold change of 8 DEPs involved in the MAPK pathway.
| Gene name | Protein accession | Protein description | SI/SN ratio |
|---|---|---|---|
| HSPA1L | P34931 | Heat shock 70-kDa protein 1-like | 2.016 |
| TAB1 | Q15750 | TGF-beta-activated kinase 1 and MAP3K7-binding protein 1 | 1.823 |
| RAP1A | P62834 | Ras-related protein Rap-1A | 1.573 |
| NFKB1 | P19838 | Nuclear factor NF-kappa-B p105 subunit | 1.551 |
| EPHA2 | P29317 | Ephrin type-A receptor 2 | 0.644 |
| MAP2K3 | P46734 | Dual specificity mitogen-activated protein kinase kinase 3 | 0.64 |
| IL1RAP | Q9NPH3 | Interleukin-1 receptor accessory protein | 0.587 |
| ECSIT | Q9BQ95 | “Evolutionarily conserved signaling intermediate in Toll pathway, mitochondrial” | 0.511 |
SI scilenced IMPA2 group, SN negative control group.
Inhibition of IMPA2 activates the ERK/MAPK pathway
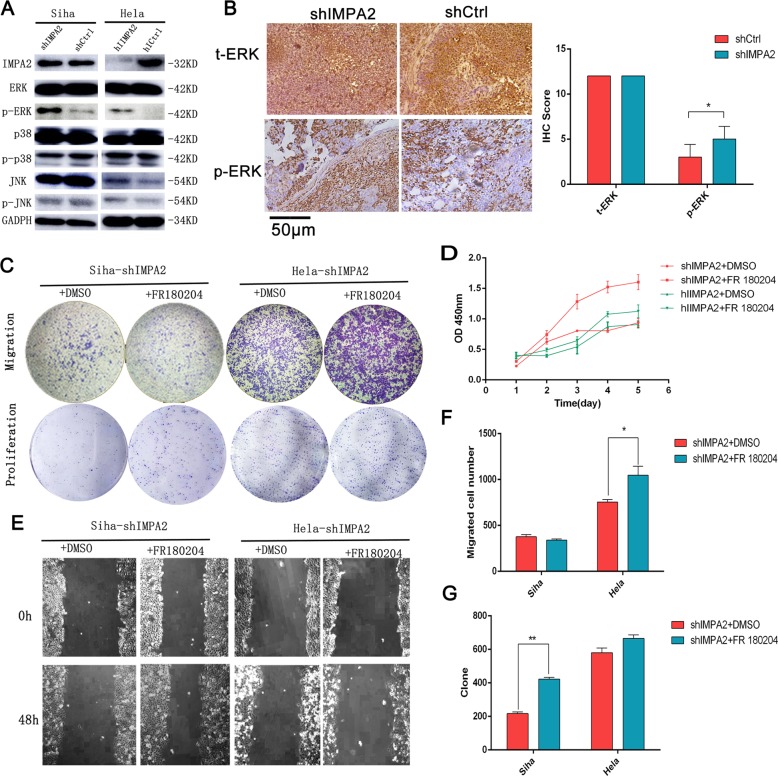
To understand further the mechanisms responsible for IMPA2-related cancer progression, MAPK signaling pathways were analyzed by Western blotting based on the proteomic profile results. We found that compared with the shCtrl group, IMPA2 silencing significantly increased ERK phosphorylation in SiHa and Hela cells. Total levels ERK showed no statistical differences. And IMPA2 had little effect on JNK and p38 phosphorylation (Fig. 6a). Furthermore, silenced IMPA2 activating ERK expression was also been verified in inxenografts by Immunohistochemical staining (Fig. 6b). Thereafter, ERK inhibitor FR180204(10 μmol) was added in IMPA2-silenced SiHa and Hela cells for 48 h. We found that FR180204 treatment significantly reversed the IMPA2-induced decrease in cell viability (Fig. 6d), cell migration and invasion in Hela cell (Fig. 6c, e), suggesting that activation of the ERK signaling pathway may result in inhibitory of proliferation and migration induced by IMPA2 in cervical cancer.
Fig. 6. Silenced IMPA2 activated ERK and the IMPA2-induced cell proliferation and migration were revised by ERK inhibitor.
a The expression levels of IMPA2 and MAPK signaling molecules ERK, p-ERK, p38, p-p38, JNK, p-JNK, and GAPDH protein in IMPA2 silencing SiHa or HeLa cells were detected by western blotting. b ERK and p-ERK expression of the inxenografts were detected by immunohistochemical staining and the immunoreactivity scores of t-ERK and p-ERK were shown. Scale bar, 50 μm. The migratory and proliferation potential of IMPA2 silencing SiHa or HeLa cells treated with FR 180204 or DMSO were analyzed by the transwell cell migration and colony formation assay (c). CCK-8 assay (d), wound healing assay (e). Number of migratory cells (f) and clone (g) was shown as mean ± SD from three independent experiments using triplicate measurements and statistically analyzed with Student’s t-test in each experiment. *p < 0.05, **p < 0.01.
Discussion
Cervical cancer progression is a continuous process from normal cervical to cervical intraepithelial neoplasia (CIN) and finally to cancer. Various molecules were involved22–25 besides the key role of human papillomavirus (HPV) infection. IMPA2 gene is a protein-coding gene for myo-inositol monophosphatase 29. This monophosphatase is one of the key enzymes in inositol phosphate metabolism and finally convert monophosphate into free inositol through dephosphorylation. IMPA2 is received in many studies to be associated with neuropsychiatric diseases, like schizophrenia26, epilepsy27, bipolar disorder11,28,29, and Huntington’s disease12,30. Also, IMPA2 inhibition is accepted as the therapeutic mechanism of lithium in bipolar disorders31. However, there is only one published article clearly demonstrated the relationship between IMPA2 expression and cancer progression. Lin et al. recently showed that IMPA2 downregulation constitutes a novel signature for cancer metastasis and poor outcomes in ccRCC15. On the contrary, our data of RNA sequencing (RNA-seq) showed an obvious upregulation of IMPA2 in cervical cancer. Therefore, our study investigated its ability to act as an oncogene and the possibility to be a potential diagnostic and prognostic biomarker as well as a therapeutic target in cervical cancer. In the present study, IMPA2 was upregulated in cervical cancer tissues when compared with pair matched normal tissues, which is consistent with the dataset of IMPA2 from TCGA. And about 47.5% (29/61) overexpressed more than 2-fold. However, the upregulation of IMPA2 failed to correlate with clinical characteristics, HPV types and overall survival.
Our results demonstrated that inhibition of IMPA2 could slower the proliferation and promote the migration of SiHa and Hela cells (Figs. 3, 4). And this effect of promoting tumorigenesis was also confirmed in vivo (Fig. 2). However, the in vivo result showed no migration or invasion in our shCtrl or shIMPA2 group. This can partly explain why IMPA2 was not associated with clinical and pathological characteristics well (Table S1). And as introduced previously, IMPA2 was identified upregulated in carcinoma in situ of cervix. We suspect that IMPA2 can be a promoting element in the early stage of cervical cancer, but the further migration or invasion still need other contributing factors. Collectively, the present study is the first report to demonstrate that IMPA2 may act as an oncogene in cervical cancer.
As the mechanism of promoting effect of IMPA2 has not been studied yet, we first traced back to the researches about mechanisms behind the role of IMPA2 in neuropsychiatric diseases. Most of the studies agreed that inhibition of IMPA2 might induce IP3 accumulation and /or inositol depletion, which can finally regulate autophagy to help treat neurodegenerative diseases or bipolar disorders12,30,31. However, the mechanism of IMPA2 in cancer is utterly unknown. We therefore performed proteomics analysis to look for clues of molecular regulation by IMPA2 in cervical cancer. In all, 267 proteins with different expression were detected, 149 of which have previously reported to be potentially related to biological processes of DNA damage repair, cell death, glycoprotein biosynthesis and metabolism, RNA biosynthesis, and embryonic development. Clustering analysis showed the involvement of cell death in more detail, as regulation of cell death, programmed cell death, and apoptotic mitochondrial changes were all significantly clustered.
The KEGG enrichment analysis for the identification of pathways unveils possible involvement of MAPK signaling pathway. MAPK plays an important role in regulating cell proliferation32, differentiation33,34, cell cycle arrest35, apoptosis24,36,37, immune function38, and autophagy36,39. The MAPK signaling pathway is shared by four distinct cascades40, including Jun amino-terminal kinases (JNK1/2/3), extracellular signal-related kinases (ERK1/2), p38-MAPK and ERK5. The expression of above key molecules of SiHa and Hela cells pre and post inhibition of IMPA2 were compared by western blotting. Phosphorylation of ERK was activated after IMPA2 silencing. JNK 1/2/3 and p38 remained unchanged (Fig. 6), suggesting that MAPK/ERK signaling pathway take part in IMPA2-induced tumorigenesis of cervical cancer.
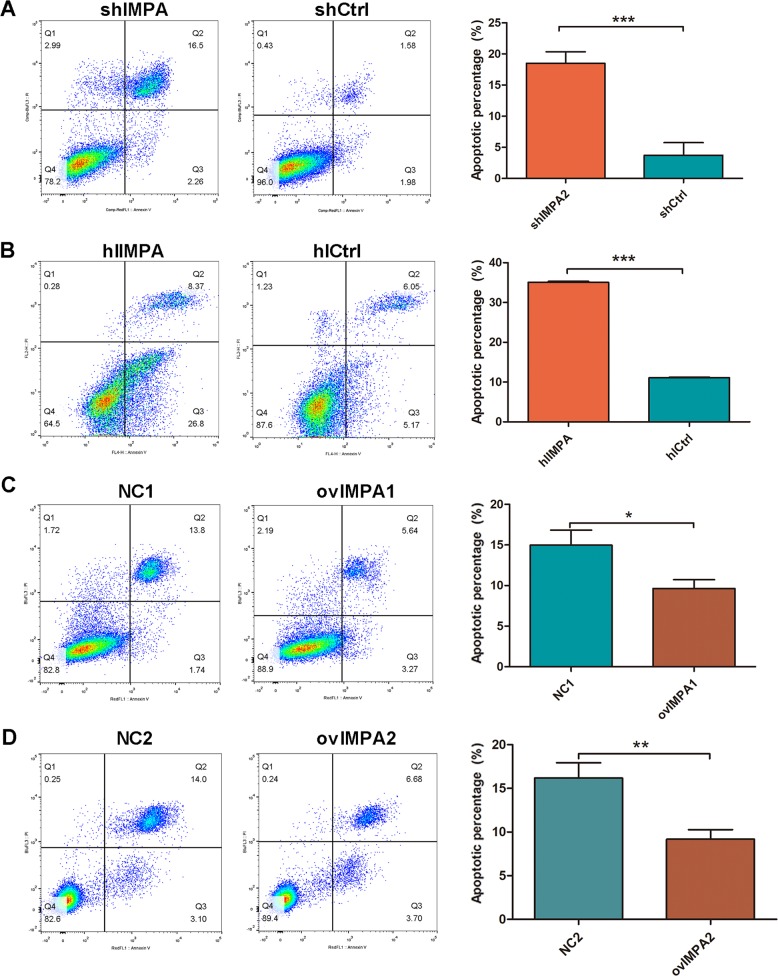
Many researches about chemotherapeutic drugs41,42, tumor-related gene43, microRNA38, and LncRNA44 have shown multiple effects on cervical cancer by regulation of MAPK signaling pathway. However, most of the studies agreed that phosphorylation of ERK38,45 was upregulated to promote the proliferation or (and) suppress the apoptosis33,46 of cervical cancer, while evidences showed contrary effect in this study (Fig. 6). When added ERK inhibitor in IMPA2-silenced cells, we could find that the proliferation capacity of Hela and migration of SiHa could not be revised completely (Fig. 6f, g). Therefore, we thought ERK cannot regulate tumorigenesis directly. Previous studies demonstrated that activation of ERK MAPK could also induce cytotoxicity or tumor inhibition in some cancers19,41,47. Some studies revealed that activation of ERK could promote apoptosis progress48,49. And as we all know, apoptosis is a common process of programmed cell death and plays a key role in maintaining cellular homeostasis in normal and cancer cells35,50. Therefore, we suspect that upregulated ERK decreased cervical cancer progression by activating apoptosis in IMPA2 silencing cells. And the primary apoptosis assay by using flow cytometry had initially verified our hypothesis (Fig. 7), but this hypothesis needs to be confirmed in a further study.
Fig. 7. IMPA2 silencing induced apoptosis of SiHa and Hela cells.
a Flow cytometry analysis of apoptosis showed that the knockdown of IMPA2 in SiHa cell elevated the percentage of apoptotic cells, and which was decreased when IMPA2 was overexpressed (b). The consistent results have been observed in IMPA2 silencing or overexpressing Hela cells (c, d).
In conclusion, IMPA2 is a novel tumor promotor that regulates ERK MAPK in cervical cancer. Thus, silencing IMPA2 could step down proliferation and metastasis of cervical cancer cells. It could also induce activation of ERK. These findings provided promising insights into developing novel cancer therapies by inhibiting the IMPA2 in cervical cancer.
Supplementary information
Correlation of the expression of IMPA2 with clinicopathologic features.
Results of tumor sample purity assessment
table of differentially expressed protein statistics of proteomics
Acknowledgements
This work was supported by grants from National Natural Science Foundation of China (81470133), Natural Science Foundation of Hunan Province (2019JJ40426), Fundamental Research Funds for the central Universities of Central South University (2018zzts928), and Hunan Provincial Department of Finance (201895).
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Edited by J.-E. Ricci
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Kan Zhang, Lei Liu
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41419-020-2507-z).
References
- 1.Bray F, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Petrosky E, et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb. Mortal. Wkly Rep. 2015;64:300–304. [PMC free article] [PubMed] [Google Scholar]
- 3.Duenas-Gonzalez A, Campbell S. Global strategies for the treatment of early-stage and advanced cervical cancer. Curr. Opin. Obstet. Gynecol. 2016;28:11–17. doi: 10.1097/GCO.0000000000000234. [DOI] [PubMed] [Google Scholar]
- 4.Marth C, et al. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017;28:iv72–iv83. doi: 10.1093/annonc/mdx220. [DOI] [PubMed] [Google Scholar]
- 5.Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N. Engl. J. Med. 1998;338:423–428. doi: 10.1056/NEJM199802123380703. [DOI] [PubMed] [Google Scholar]
- 6.Wu, Y. et al. Whole-exome and RNA sequencing reveals novel insights into the pathogenesis of HPV associated cervical cancer. Cancer Biomark10.3233/CBM-190055 (2019). [DOI] [PubMed]
- 7.Yang, R. et al. Combined transcriptome and proteome analysis of immortalized human keratinocytes expressing Human Papillomavirus 16 (HPV16) oncogenes reveals novel key factors and networks in HPV-induced carcinogenesis. mSphere10.1128/mSphere.00129-19 (2019). [DOI] [PMC free article] [PubMed]
- 8.Ojesina AI, et al. Landscape of genomic alterations in cervical carcinomas. Nature. 2014;506:371–375. doi: 10.1038/nature12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshikawa T, Turner G, Esterling LE, Sanders AR, Detera-Wadleigh SD. A novel human myo-inositol monophosphatase gene, IMP.18p, maps to a susceptibility region for bipolar disorder. Mol. Psychiatry. 1997;2:393–397. doi: 10.1038/sj.mp.4000325. [DOI] [PubMed] [Google Scholar]
- 10.Ohnishi T, et al. Spatial expression patterns and biochemical properties distinguish a second myo-inositol monophosphatase IMPA2 from IMPA1. J. Biol. Chem. 2007;282:637–646. doi: 10.1074/jbc.M604474200. [DOI] [PubMed] [Google Scholar]
- 11.Jimenez E, et al. Genetic variability at IMPA2, INPP1 and GSK3beta increases the risk of suicidal behavior in bipolar patients. Eur. Neuropsychopharmacol. 2013;23:1452–1462. doi: 10.1016/j.euroneuro.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Sarkar S, et al. Lithium induces autophagy by inhibiting inositol monophosphatase. J. Cell Biol. 2005;170:1101–1111. doi: 10.1083/jcb.200504035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohnishi T, et al. Human myo-inositol monophosphatase 2 rescues the nematode thermotaxis mutant ttx-7 more efficiently than IMPA1: functional and evolutionary considerations of the two mammalian myo-inositol monophosphatase genes. J. Neurochem. 2013;124:685–694. doi: 10.1111/jnc.12112. [DOI] [PubMed] [Google Scholar]
- 14.French D, et al. Acquired variation outweighs inherited variation in whole genome analysis of methotrexate polyglutamate accumulation in leukemia. Blood. 2009;113:4512–4520. doi: 10.1182/blood-2008-07-172106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin YF, et al. Dysregulation of the miR-25-IMPA2 axis promotes metastatic progression in clear cell renal cell carcinoma. EBioMedicine. 2019;45:220–230. doi: 10.1016/j.ebiom.2019.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mustafa DAM, et al. T lymphocytes facilitate brain metastasis of breast cancer by inducing Guanylate-Binding Protein 1 expression. Acta Neuropathol. 2018;135:581–599. doi: 10.1007/s00401-018-1806-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen TP, et al. Increased expression of SKP2 and phospho-MAPK/ERK1/2 and decreased expression of p27 during tumor progression of cervical neoplasms. Gynecol. Oncol. 2007;104:516–523. doi: 10.1016/j.ygyno.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 18.Yu Z, et al. SIX3, a tumor suppressor, inhibits astrocytoma tumorigenesis by transcriptional repression of AURKA/B. J. Hematol. Oncol. 2017;10:115. doi: 10.1186/s13045-017-0483-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Z, et al. The D Domain of LRRC4 anchors ERK1/2 in the cytoplasm and competitively inhibits MEK/ERK activation in glioma cells. J. Hematol. Oncol. 2016;9:130. doi: 10.1186/s13045-016-0355-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang ZY, et al. Upregulation of the long non-coding RNA AFAP1-AS1 affects the proliferation, invasion and survival of tongue squamous cell carcinoma via the Wnt/beta-catenin signaling pathway. Mol. Cancer. 2018;17:3. doi: 10.1186/s12943-017-0752-2. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Chandrashekar DS, et al. UALCAN: a portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia. 2017;19:649–658. doi: 10.1016/j.neo.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen SQ, et al. Analyzing simultaneous positive expression of EZH2 and P53 protein to improve predictive value in cervical squamous cell carcinoma. Int. J. Gynecol. Cancer. 2014;24:1653–1658. doi: 10.1097/IGC.0000000000000273. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y, et al. INPP4B restrains cell proliferation and metastasis via regulation of the PI3K/AKT/SGK pathway. J. Cell. Mol. Med. 2018;22:2935–2943. doi: 10.1111/jcmm.13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao S, et al. Fra-1 is downregulated in cervical cancer tissues and promotes cervical cancer cell apoptosis by p53 signaling pathway in vitro. Int. J. Oncol. 2015;46:1677–1684. doi: 10.3892/ijo.2015.2873. [DOI] [PubMed] [Google Scholar]
- 25.Yadav SS, et al. Epigenetic silencing of CXCR4 promotes loss of cell adhesion in cervical cancer. Biomed. Res. Int. 2014;2014:581403. doi: 10.1155/2014/581403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J, et al. A promoter polymorphism rs2075824 within IMPA2 gene affecting the transcription activity: possible relationship with schizophrenia. J. Cell. Mol. Med. 2017;21:658–664. doi: 10.1111/jcmm.13009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gurnett CA, Hedera P. New ideas in epilepsy genetics: novel epilepsy genes, copy number alterations, and gene regulation. Arch. Neurol. 2007;64:324–328. doi: 10.1001/archneur.64.3.324. [DOI] [PubMed] [Google Scholar]
- 28.Tomioka Y, et al. Association between genetic variation in the myo-inositol monophosphatase 2 (IMPA2) gene and age at onset of bipolar disorder. J. Affect. Disord. 2018;232:229–236. doi: 10.1016/j.jad.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 29.Yoon IS, et al. Altered IMPA2 gene expression and calcium homeostasis in bipolar disorder. Mol. Psychiatry. 2001;6:678–683. doi: 10.1038/sj.mp.4000901. [DOI] [PubMed] [Google Scholar]
- 30.Sarkar S, Rubinsztein DC. Inositol and IP3 levels regulate autophagy: biology and therapeutic speculations. Autophagy. 2006;2:132–134. doi: 10.4161/auto.2387. [DOI] [PubMed] [Google Scholar]
- 31.Sade Y, et al. IP3 accumulation and/or inositol depletion: two downstream lithium’s effects that may mediate its behavioral and cellular changes. Transl. Psychiatry. 2016;6:e968. doi: 10.1038/tp.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cuadrado A, Nebreda AR. Mechanisms and functions of p38 MAPK signalling. Biochem. J. 2010;429:403–417. doi: 10.1042/BJ20100323. [DOI] [PubMed] [Google Scholar]
- 33.Sun Y, et al. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduct. Res. 2015;35:600–604. doi: 10.3109/10799893.2015.1030412. [DOI] [PubMed] [Google Scholar]
- 34.Ventura JJ, et al. p38alpha MAP kinase is essential in lung stem and progenitor cell proliferation and differentiation. Nat. Genet. 2007;39:750–758. doi: 10.1038/ng2037. [DOI] [PubMed] [Google Scholar]
- 35.Hu X, et al. Polypeptide fraction from Arca subcrenata induces apoptosis and G2/M phase arrest in HeLa cells via ROS-mediated MAPKs pathways. Evid. Based Complement. Alternat. Med. 2015;2015:930249. doi: 10.1155/2015/930249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tan, G. X. et al. PP-22 promotes autophagy and apoptosis in the nasopharyngeal carcinoma cell line CNE-2 by inducing endoplasmic reticulum stress, downregulating STAT3 signaling, and modulating the MAPK pathway. J. Cell. Physiol.10.1002/jcp.27076 (2018). [DOI] [PubMed]
- 37.Chiu CC, et al. p38 MAPK and NF-kappaB pathways are involved in naphtho[1,2-b] furan-4,5-dione induced anti-proliferation and apoptosis of human hepatoma cells. Cancer Lett. 2010;295:92–99. doi: 10.1016/j.canlet.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 38.Li, Z. H., Li, L., Kang, L. P. & Wang, Y. MicroRNA-92a promotes tumor growth and suppresses immune function through activation of MAPK/ERK signaling pathway by inhibiting PTEN in mice bearing U14 cervical cancer. Cancer Med.10.1002/cam4.1329 (2018). [DOI] [PMC free article] [PubMed] [Retracted]
- 39.Sui X, et al. p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer Lett. 2014;344:174–179. doi: 10.1016/j.canlet.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 40.Hommes DW, Peppelenbosch MP, van Deventer SJ. Mitogen activated protein (MAP) kinase signal transduction pathways and novel anti-inflammatory targets. Gut. 2003;52:144–151. doi: 10.1136/gut.52.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Potocnjak I, Gobin I, Domitrovic R. Carvacrol induces cytotoxicity in human cervical cancer cells but causes cisplatin resistance: involvement of MEK-ERK activation. Phytother. Res. 2018;32:1090–1097. doi: 10.1002/ptr.6048. [DOI] [PubMed] [Google Scholar]
- 42.Vazhappilly, C. G. et al. Inhibition of SHP2 by new compounds induces differential effects on RAS/RAF/ERK and PI3K/AKT pathways in different cancer cell types. Investig. New Drugs10.1007/s10637-018-0626-5 (2018). [DOI] [PubMed]
- 43.Zheng HY, Shen FJ, Tong YQ, Li Y. PP2A inhibits cervical cancer cell migration by dephosphorylation of p-JNK, p-p38 and the p-ERK/MAPK signaling pathway. Curr. Med. Sci. 2018;38:115–123. doi: 10.1007/s11596-018-1854-9. [DOI] [PubMed] [Google Scholar]
- 44.Liu, X., Yang, Q., Yan, J., Zhang, X. & Zheng, M. LncRNA MNX1-AS1 promotes the progression of cervical cancer through activating MAPK pathway. J. Cell. Biochem.10.1002/jcb.27712 (2018). [DOI] [PubMed]
- 45.Sun Q, Liang Y, Zhang T, Wang K, Yang X. ER-alpha36 mediates estrogen-stimulated MAPK/ERK activation and regulates migration, invasion, proliferation in cervical cancer cells. Biochem. Biophys. Res. Commun. 2017;487:625–632. doi: 10.1016/j.bbrc.2017.04.105. [DOI] [PubMed] [Google Scholar]
- 46.Wang K, Zhu Y. Dexmedetomidine protects against oxygen-glucose deprivation/reoxygenation injury-induced apoptosis via the p38 MAPK/ERK signalling pathway. J. Int. Med. Res. 2018;46:675–686. doi: 10.1177/0300060517734460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen JC, Huang C, Lee IN, Wu YP, Tang CH. Amphiregulin enhances cell migration and resistance to doxorubicin in chondrosarcoma cells through the MAPK pathway. Mol. Carcinog. 2018;57:1816–1824. doi: 10.1002/mc.22899. [DOI] [PubMed] [Google Scholar]
- 48.Xiong W, et al. DAPK1-ERK signal mediates oxygen glucose deprivation reperfusion induced apoptosis in mouse N2a cells. J. Neurol. Sci. 2018;387:210–219. doi: 10.1016/j.jns.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 49.Zong D, et al. Notch1 regulates endothelial apoptosis via the ERK pathway in chronic obstructive pulmonary disease. Am. J. Physiol. Cell Physiol. 2018;315:C330–C340. doi: 10.1152/ajpcell.00182.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nass J, Efferth T. Insights into apoptotic proteins in chemotherapy: quantification techniques and informing therapy choice. Expert Rev. Proteomics. 2018;15:413–429. doi: 10.1080/14789450.2018.1468755. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Correlation of the expression of IMPA2 with clinicopathologic features.
Results of tumor sample purity assessment
table of differentially expressed protein statistics of proteomics