Abstract
Genetic factors explain a major proportion of human height variation, but differences in mean stature have also been found between socio-economic categories suggesting a possible effect of environment. By utilizing a classical twin design which allows decomposing the variation of height into genetic and environmental components, we tested the hypothesis that environmental variation in height is greater in offspring of lower educated parents. Twin data from 29 cohorts including 65,978 complete twin pairs with information on height at ages 1 to 69 years and on parental education were pooled allowing the analyses at different ages and in three geographic-cultural regions (Europe, North America and Australia, and East Asia). Parental education mostly showed a positive association with offspring height, with significant associations in mid-childhood and from adolescence onwards. In variance decomposition modeling, the genetic and environmental variance components of height did not show a consistent relation to parental education. A random-effects meta-regression analysis of the aggregate-level data showed a trend towards greater shared environmental variation of height in low parental education families. In conclusion, in our very large dataset from twin cohorts around the globe, these results provide only weak evidence for the study hypothesis.
Subject terms: Heritable quantitative trait, Quantitative trait
Introduction
Since the late 19th and early 20th centuries1–3, family, twin and adoption studies have revealed that stature is among the most heritable quantitative traits in humans4. Genetic linkage studies have elucidated the location of genetic markers in the genome5 and genome-wide association (GWA) studies identified hundreds of loci related to height in different ancestry populations6–10. On the other hand, numerous environmental factors in childhood are known to affect growth; disadvantageous environmental conditions may decline the physical growth of children leading to shorter adult height11–13. Although nutrition and particularly the lack of dietary protein is the most relevant environmental factor affecting height, childhood diseases, particularly infections, also influence growth14. Such environmental exposures are generally shared by siblings to a large extent and would be expected to affect growth rather uniformly within families. These and other biological determinants are in turn related to socio-economic conditions manifesting as socio-economic height differences both between and within populations13. Accordingly, social and economic characteristics of childhood families, such as parental education and income, have generally been positively associated with the height of offspring15–17.
Twin studies have shown that environmental factors common to co-twins affect variation in height over the lifespan; the percentage of individual differences explained by the common environment was greatest in infancy (up to 50%), decreased over childhood and was generally absent or lower than 20% in adolescence and adulthood18,19. The classical twin design20 enables variance decomposition into common and unique environmental variance components and a genetic variance component. These components may all vary depending on particular exposures, e.g. exposure to a parental home with parents of lower or higher education. For example, heritability – i.e., the percentage of total variance explained by genetic variance – of height may not, be constant but dependent on the magnitude of environmental variation influencing the phenotype21. A poorer household environment may more often than a more affluent one, fail to provide basic necessities and can lead more frequent diseases stunting human growth13. This can be reflected in not only to shorter mean height but also higher environmental variation of height in poorer families with siblings being exposed to more similar household environments than non-siblings. On the other hand, in families with a higher socio-economic position, the environment is likely to be more uniformly good with fewer environmental factors restricting growth and thus leading to taller offspring and less environmental variation.
According to the bioecological model, at-risk environments will mask genetic differences between individuals, while enriched environments will amplify genetic differences22,23. This leads to the hypothesis, that the heritability of height should increase with higher parental socioeconomic position. To our knowledge, there are no previous studies testing this hypothesis and thus no direct evidence whether the heritability of height differs according to family social background and parental education. Further, such modifying effect of socio-economic characteristics might change over birth cohorts or could be different in males and females, if some cultures would encourage scare resources to be primarily shared with male offspring.
To examine the modification of genetic and environmental variance components by parental education, large datasets collected across a range of strata within society or across different countries are needed. The power to detect such effect was explored by Boomsma and Martin24 who concluded that heritability differences between groups of 0.3 or smaller requires large samples. Such information from large datasets was available from 29 twin cohorts participating in the CODATwins (COllaborative project of Development of Anthropometrical measures in Twins) project representing 15 countries from different parts of the world25. We utilized this database (i) to test whether parental education modifies the genetic and environmental variation of height in males and females from infancy through adulthood and (ii) to assess whether the possible modification effects vary between different geographic-cultural regions (Europe, North America and Australia, and East Asia).
Results
Descriptive statistics of height and parental education by age and sex for the pooled data (all cohorts together) are presented in Table 1 (the corresponding statistics by cultural-geographic region are presented in Supplementary table 1). Mean height showed the expected age pattern, and the difference between consecutive age groups was very similar in boys and girls during childhood. The exception was the slight decrease observed at 18 (males) and 20–69 (females) years, which reflects differences in the distribution of different cohorts within each age group. Mean height was generally tallest in Europe, somewhat shorter in North America and Australia and shortest in East Asia in both males and females. Paternal and maternal education generally decreased with age, which reflects the increasing education over birth cohorts since parents of younger twins were, on average, born later as compared to parents of older twins. Parental education was virtually identical for male and female twins during childhood and slightly greater in females from late adolescence. Parental education was generally lowest in Europe, reflecting that European twin cohorts were older than North American and Australian and East Asian cohorts (Supplementary table 1).
Table 1.
Number of measurements, means and standard deviations (SD) of height and parental education by age and sex.
| Age | Males | Females | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Height (cm) | Paternal education (years) | Maternal education (years) | Height (cm) | Paternal education (years) | Maternal education (years) | |||||||||
| N | Mean | SD | Mean | SD | Mean | SD | N | Mean | SD | Mean | SD | Mean | SD | |
| 1 | 13155 | 75.0 | 3.60 | 13.85 | 2.58 | 13.79 | 2.30 | 13631 | 73.5 | 3.67 | 13.86 | 2.61 | 13.82 | 2.31 |
| 2 | 10912 | 87.5 | 4.02 | 13.97 | 2.69 | 13.98 | 2.40 | 10930 | 86.2 | 4.15 | 13.91 | 2.75 | 13.99 | 2.43 |
| 3 | 10541 | 96.8 | 4.43 | 14.17 | 2.73 | 14.20 | 2.48 | 11087 | 95.8 | 4.50 | 14.13 | 2.75 | 14.20 | 2.50 |
| 4 | 3307 | 101.8 | 5.79 | 14.74 | 3.64 | 15.34 | 3.40 | 3327 | 100.6 | 5.72 | 14.74 | 3.68 | 15.45 | 3.38 |
| 5 | 6269 | 111.8 | 6.12 | 14.28 | 2.83 | 14.38 | 2.60 | 6341 | 111.0 | 6.28 | 14.27 | 2.84 | 14.31 | 2.52 |
| 6 | 1726 | 114.5 | 7.14 | 14.99 | 3.23 | 15.13 | 3.07 | 1796 | 113.8 | 6.64 | 15.04 | 3.29 | 15.22 | 3.09 |
| 7 | 6852 | 125.6 | 6.71 | 14.31 | 2.63 | 14.26 | 2.42 | 7228 | 124.9 | 6.55 | 14.31 | 2.68 | 14.22 | 2.45 |
| 8 | 4153 | 129.4 | 6.43 | 14.32 | 2.87 | 14.41 | 2.78 | 4261 | 128.4 | 6.57 | 14.32 | 2.93 | 14.35 | 2.77 |
| 9 | 3310 | 134.8 | 7.35 | 14.43 | 3.23 | 14.66 | 3.14 | 3266 | 133.9 | 7.47 | 14.55 | 3.28 | 14.77 | 3.13 |
| 10 | 6776 | 142.1 | 7.15 | 14.25 | 2.70 | 14.12 | 2.55 | 7136 | 141.5 | 7.35 | 14.21 | 2.62 | 14.00 | 2.42 |
| 11 | 3751 | 144.9 | 7.29 | 12.87 | 4.02 | 13.32 | 3.67 | 3779 | 145.3 | 7.73 | 12.92 | 4.06 | 13.36 | 3.67 |
| 12 | 6522 | 152.9 | 8.06 | 13.82 | 3.12 | 13.75 | 2.75 | 6750 | 154.0 | 8.10 | 13.83 | 3.17 | 13.74 | 2.77 |
| 13 | 2834 | 158.4 | 9.21 | 14.23 | 3.05 | 14.33 | 2.84 | 3102 | 157.8 | 7.67 | 14.23 | 2.96 | 14.13 | 2.78 |
| 14 | 4860 | 165.8 | 8.99 | 12.80 | 4.01 | 13.20 | 3.63 | 5402 | 162.2 | 6.93 | 12.84 | 3.96 | 13.29 | 3.52 |
| 15 | 2753 | 172.2 | 8.60 | 14.27 | 3.04 | 14.21 | 2.88 | 3027 | 164.4 | 7.40 | 14.25 | 2.97 | 14.18 | 2.70 |
| 16 | 3487 | 175.3 | 7.85 | 13.19 | 3.33 | 13.04 | 3.18 | 3979 | 164.7 | 6.82 | 13.10 | 3.27 | 13.04 | 3.11 |
| 17 | 4679 | 177.6 | 7.44 | 12.80 | 3.58 | 12.94 | 3.36 | 5187 | 165.7 | 6.88 | 12.97 | 3.50 | 13.06 | 3.20 |
| 18 | 3488 | 177.1 | 7.68 | 11.81 | 4.07 | 12.14 | 3.58 | 3230 | 165.8 | 7.21 | 12.44 | 3.83 | 12.61 | 3.45 |
| 19 | 2073 | 178.2 | 7.57 | 12.39 | 3.31 | 12.30 | 2.95 | 2547 | 165.7 | 7.19 | 13.13 | 3.08 | 12.90 | 2.84 |
| 20–69 | 25951 | 178.4 | 7.16 | 11.82 | 3.66 | 11.89 | 3.20 | 31205 | 164.5 | 6.76 | 12.25 | 3.54 | 12.15 | 3.18 |
Names list of the participating twin cohorts in this study: one cohort from Australia (Australian Twin Registry), five cohorts from East Asia (Korean Twin-Family Register, Ochanomizu University Twin Project, Qingdao Twin Registry of Children, South Korea Twin Registry, West Japan Twins and Higher Order Multiple Births Registry), 11 cohorts from Europe (Child and Adolescent Twin Study in Sweden, East Flanders Prospective Twin Survey, FinnTwin12, FinnTwin16, Gemini, Italian Twin Registry, Norwegian Twin Registry, Portugal Twin Cohort, TCHAD-study, Turkish Twin Study, Young Netherlands Twin Registry), one cohort from Middle East (Longitudinal Israeli Study of Twins) and 11 cohorts from North America (Boston University Twin Project, California Twin Program, Carolina African American Twin Study of Aging, Colorado Twin Registry, Michigan Twins Project, Mid Atlantic Twin Registry, Minnesota Twin Registry, NAS-NRC Study, University of Southern California Twin Study, Texas Twin Project, Vietnam Era Twin Registry).
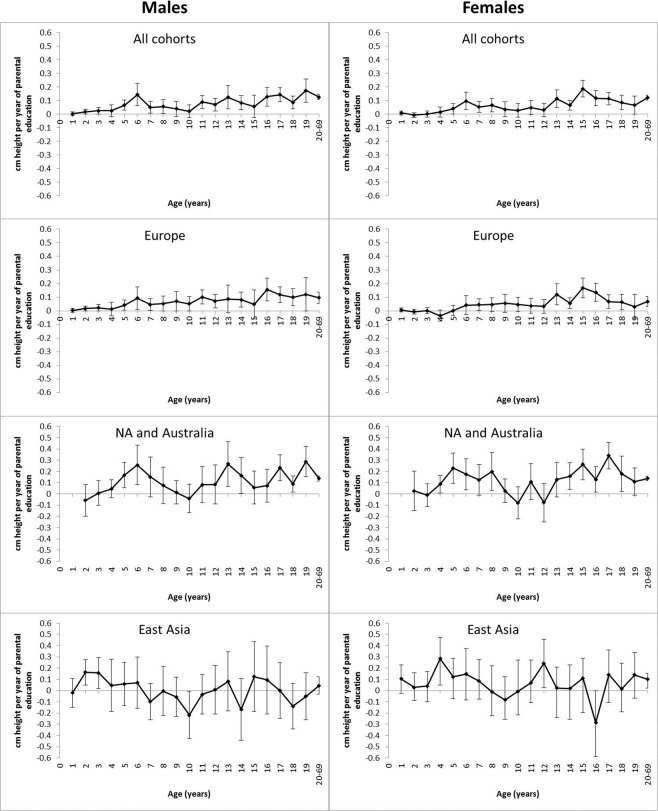
The associations between parental education (i.e., combined maternal and paternal) and offspring height, i.e. height difference in cm by one year difference of parental education, are presented in Fig. 1. From around age 5 years, parental education showed a generally positive association with offspring height; the pattern was similar in males and females, with significant associations in mid-childhood and from adolescence onwards. Regarding the geographic-cultural regions – which approximate ethnicity in the present study – the pattern in Europe was similar to that observed for the whole data set because it represents a large fraction of the total sample. In North America and Australia, the associations between parental education and offspring height were stronger than in Europe in some age groups, particularly in mid-childhood. In East Asia, the associations generally varied around zero and were not statistically significant. In North America and Australia and East Asia, the 95% confidence intervals (CIs) were, however, much broader than in Europe because of the smaller sample sizes.
Figure 1.
Mean height modification effects of parental education with 95% confidence intervals from 1 until 20–69 years of age by sex and geographic-cultural region.
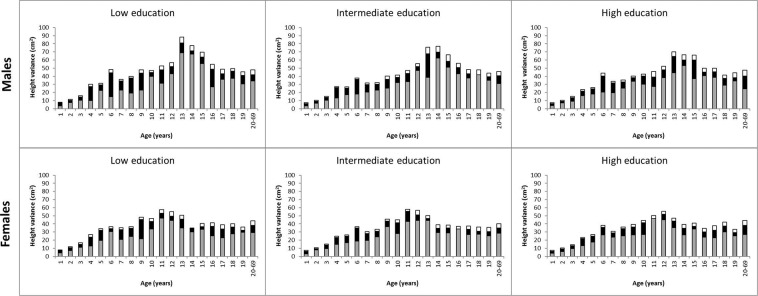
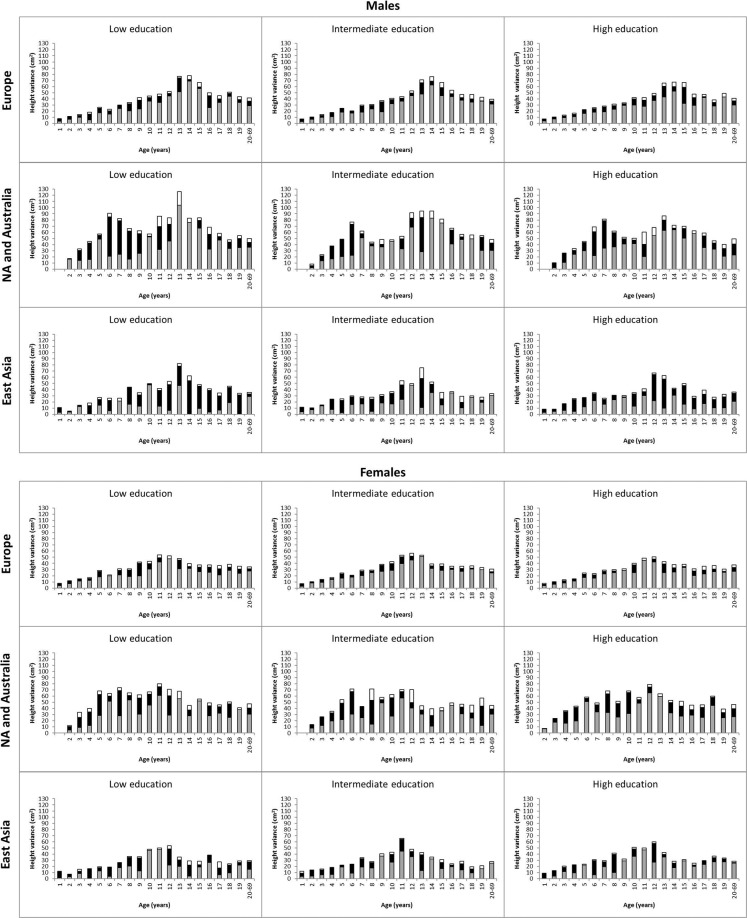
The total variance of height decomposed into additive genetic, shared environmental and unique environmental components in the three categories of parental education is shown in Fig. 2 (the estimates with 95% CIs are available in Supplementary Table 2). The total height variation was slightly greater in the lower than in the higher parental education level in some age-by-sex groups, but no consistent relation emerged by educational categories over ages. From age 13 years onwards, the total height variance was generally greater in males than in females. As indicated by overlapping CIs, genetic and environmental variances did not show any distinct relation across parental education categories from infancy through adulthood; the relative proportion of genetic and environmental variances did not show any relation either (Supplementary Table 3). Next, univariate variance decomposition modeling for height was carried out separately in the three geographic-cultural regions (Fig. 3 and Supplementary Tables 4 and 5). The total variance of height was greatest in North America and Australia and lowest in East Asia, but no distinct relation in the variance components (both total estimates and relative proportion) across the parental education levels emerged (seen as overlapping CIs). In East Asia, possibly due to the smaller sample sizes, the magnitude of the variance components between the educational categories varied more than in the other two geographic-cultural regions.
Figure 2.
Additive genetic (grey), shared environmental (black) and unique environmental (white) variances of height from 1 until 20–69 years of age by sex and parental education in all cohorts.
Figure 3.
Additive genetic (grey), shared environmental (black) and unique environmental (white) variances of height from 1 until 20–69 years of age by sex, parental education and geographic-cultural region.
Finally, we ran a random-effects meta-regression analysis of raw variance components of height (pooling all age groups and geographic-cultural regions together). The results showed some significant differences between the middle and low parental education categories (Table 2), when looking at the confidence intervals. In comparison with low parental education, for middle education shared environmental (c2) component of height was significantly smaller in males and in both sexes together. The point estimates for the other sex and variance components groups followed the same direction, but were not significant. Given the number of comparisons, we should be very careful in a substantive interpretation of these findings. Standardized variance components models gave very similar results (Supplementary Table 6).
Table 2.
Regression coefficients from meta-regression analyses of the aggregate-level data of raw variance components of height by parental education (reference category: low parental education).
| Intermediate parental education | High parental education | |
|---|---|---|
| Males | ||
| a2 | 2.13 (−1.48, 5.74) | −0.01 (−3.66, 3.63) |
| c2 | −3.27 (−6.31, −0.23) | −1.66 (−4.79, 1.46) |
| e2 | −0.15 (−0.68, 0.38) | −0.26 (−0.79, 0.27) |
| Females | ||
| a2 | 1.05 (−0.81, 2.91) | 0.26 (−1.65, 2.18) |
| c2 | −1.69 (−3.64, 0.26) | −1.57 (−3.55, 0.42) |
| e2 | −0.23 (−0.67, 0.21) | −0.36 (−0.80, 0.08) |
| Both sexes | ||
| a2 | 1.46 (−0.76, 3.69) | 0.67 (−1.58, 2.92) |
| c2 | −2.30 (−4.05, −0.55) | −1.58 (−3.37, 0.21) |
| e2 | −0.21 (−0.54, 0.13) | −0.31 (−0.65, 0.02) |
(): 95% Confidence Intervals.
Discussion
Questions about the modification of genetic and environmental variance components require very large and genetically informative data sets. Our large twin study pooling data for 65,978 complete twin pairs from 29 cohorts from 15 countries established that for human height there is a high and consistent heritability across parental education levels. The same result, i.e. similar genetic and environmental variances of height across parental education levels, was found in different geographic-cultural regions having different mean stature.
The meta-regression analysis also failed to provide substantial evidence for the study hypothesis that shared environmental variation of height tends to be greater in low parental education families; the evidence is weak considering the size of the dataset when pooling all data together. In a previous study from the CODATwins database, we found that there was no decrease in the environmental variance of adult height over the birth cohorts from the late 19th century to the late 20th century, nor any clear secular changes in the heritability18. Therefore, using two very different approaches –i.e., indirect information on the increasing standard of living over 100 years and the direct measures of socio-economic position of childhood family– we established that there is no or very little evidence of greater shared environmental variation in height in disadvantageous environments.
The offspring of better educated parents were generally taller, particularly in mid-childhood and from adolescence onwards, than those whose parents had lower education. Our findings for average height are in agreement with several population based studies showing a positive association between parents’ education and offspring height15,17,26,27. In a Chinese study, childhood height was also related to grandparents’ education, suggesting that socioeconomic conditions of current and previous generations may affect height28. In some societies, children from families with lower socioeconomic status (SES) may still have, on average, poorer diets and be more severely affected by infections than those from families with higher SES13–15. Comparison between geographic-cultural regions showed that parental education was more strongly related to height in North America and Australia than in Europe, which may reflect larger social inequalities in the former.
In the families of lower SES, environmental effects (e.g. malnutrition) on height may restrict individuals from reaching their genetic potential, leading to shorter stature. It is likely that there are differences in these environmental factors between low SES families; in high SES families, in contrast, the environment securing optimal growth is likely to be more homogeneous. These environmental influences would result in more between than within family variation in lower SES families, which according to the bioecological model is expected to increase shared environmental variation leading to lower heritability of height in lower as compared with higher SES families. It is thus interesting that even when we found the expected differences in mean height between families of high and low parental education, only very weak differences in genetic or environmental variances or in the heritability estimates of height were observed. It is theoretically possible that environmental factors affecting growth are so uniformly distributed in lower SES families that there is no variance of height explained by these environmental factors and thus the influence is not seen as shared environmental variation. However, we do not find this very likely since it would mean that families with high and low parental education form two distinctive but internally very homogenous groups. Further, this should be the case in all three cultural-geographic regions.
Finally, it is possible that the differences in height between the families of high and low parental education are not because of a causal effect of poorer living conditions on height but reflect genetic height differences. A study of children born in the 1990s found that higher education mothers had taller sons and daughters and that these differences in offspring height were fully explained by parental height26. This can be explained also by inheritance of socio-economic factors and not only genetic factors affecting height. However, there is also direct evidence on a modest genetic correlation (r = 0.13) between education and height based on linkage disequilibrium score regression analyses29. Thus, a not unreasonable hypothesis is that genetic variance of height can also differ by parental educational level. Such hypotheses will be testable in future studies, with the increasing availability of large genotyped cohorts (e.g.30).
The present study has several strengths. First of all, our large multinational database of twin cohorts, with data on parental education and height over childhood and adulthood, allows a comprehensive research of the genetic and environmental influences on individual height differences across parental education categories over lifespan in different cultural-geographic regions. We had sufficient statistical power to address these questions. The individual-based data, in comparison to literature based meta-analyses, provide important advantages such as better opportunities for statistical modeling and lack of publication bias. However, our study also has limitations. Ethnic-cultural groups are differently represented and the greatest proportion of the database is formed by Caucasian populations following Westernized lifestyles. In addition, most of the height measures were self-reported31, which increases measurement error and thus may bias our results toward greater estimates of unique environmental effects. However, this is not likely to explain the main result, i.e., relatively similar genetic and environmental variances of height across the categories of parental educational attainment. Also when pooling the estimates of variance components from different ages, we could not adjust the SEs by multiple observations at different ages, and thus, the 95% CIs are likely to be too narrow. Therefore, the main emphasis should be on the age-specific results, where only one observation from each individual is used.
In conclusion, there is no solid evidence that lower parental education is related to greater environmental variation in offspring height from infancy through adulthood. Thus, our findings indicate that the heritability estimates of height are quite uniform across parental education levels in spite of differences in mean height.
Materials and methods
Sample
This study is performed with data from the CODATwins project, which was planned to pool information on height and weight data from all twin projects in the world31. Additional information on paternal and maternal education was available for 29 twin cohorts from 15 countries. The participating twin cohorts are listed in Table 1 (footnote) and were described in detail elsewhere25,31.
In the original database, there were 137,867 twin individuals with a total of 311,087 height measurements at ages 1–69 years. Age was classified to single-year age groups from age 1 to 19 years (e.g. age 1 includes 0.5–1.5 years range) and one unique adult age group (20–69 years); height measures at ages ≥70 years were excluded because individuals in old age are more prone to develop osteoporosis leading to shorter height32. Outliers and implausible values were checked by visual inspection for each age and sex group and removed (0.1% of the measurements) to obtain an approximately normal distribution, resulting in 310,736 measurements. To confirm that all analyses are based on independent observations, we selected one height measure per individual in each age group by keeping the measurement at the youngest age (removing <10% of the measurements) resulting in 282,176 height measurements from 137,574 twin individuals. After excluding twins without data on their co-twins, we had 264,610 height measurements (132,305 paired height measurements; 38% monozygotic (MZ), 34% same- sex dizygotic (SSDZ) and 28% opposite-sex dizygotic (OSDZ) twin pairs) from 65,978 complete twin pairs (the number of observations by age and twin cohort is available on request). The different educational classifications used in the surveys were transformed as educational years by using the mean level of educational years in each category as described in detail elsewhere25.
In order to analyze possible differences in the genetic and environmental contribution on height across geographical-cultural regions, the cohorts were grouped in three regions: Europe (10 cohorts), North America and Australia (12 cohorts) and East Asia (5 cohorts) with 88,632, 34,087 and 8,873 paired height measurements, respectively. Two cohorts (Israel and Turkey) were not included in these sub-analyses by geographic-cultural region because the populations in these countries differ genetically from European populations33, and the data were too sparse to study these cohorts separately. The same classification was used also in our previous studies on the genetics of height in childhood19 and adulthood18 based on the CODATwins database.
All participants were volunteers and they or their parents/legal guardians gave informed consent when participating in their original study. Only a limited set of observational variables and anonymized data were delivered to the data management center at University of Helsinki. The pooled analysis was approved by the ethical committee of Department of Public Health, University of Helsinki, and the methods were carried out in accordance with the approved guidelines.
Statistical analyses
Statistical analyses were conducted using Stata statistical software (version 14.0; StataCorp, College Station, Texas, USA). First, all height measurements were adjusted for exact age and twin cohort within each age and sex group using linear regression model (height was used as the dependent variable and exact age and twin cohort as independent variables) and the resulting residuals were used as the outcome variable in the further statistical modeling. Twin cohorts were numbered as a nominal level variable in the regression analyses (i.e., a separate dummy variable was created for each twin cohort). Since paternal and maternal education (ranging from 0 to 30 years) may be differently associated with offspring birth year, we adjusted maternal and paternal education separately for twin cohort and birth year of their twin children (used as a proxy indicator for the birth years of parents) by fitting a regression model (maternal or paternal education was used as the dependent variable and twin cohort and birth year of their twin children as independent variables). Thus, the residuals indicate how much shorter or longer the parental education duration is as compared with that of the average person having a certain birth year in each twin cohort. These regression residuals were then summed up to get combined parental education and divided into three SD-based categories (<−0.5, −0.5 to +0.5, > +0.5), indicating low, intermediate and high parental education (31%, 40% and 29% of the observations, respectively).
We first studied the association between height and parental education separately for each age and sex group in all cohorts together as well as by the geographic-cultural regions. Linear regression models were used with parental education as the explanatory variable and height residuals as the outcome. The associations were adjusted for zygosity because of slight differences in height34 and parental education between MZ and DZ twins25. The non-independence within twin pairs was taken into account by using the cluster-option available in Stata35. This option takes into account that twin pairs rather than independent individuals are sampled and accordingly corrects the standard errors to be larger because of the less informative sample design.
To estimate genetic and environmental influences on the variation of height, we employed classic twin modeling based on linear structural equations36. MZ twins share the same genomic sequence, whereas DZ twins share, on average, 50% of their genes identical-by-descent. On this basis, it is possible to decompose the total variance of height into variance due to additive genetic effects (A: correlated 1.0 for MZ and 0.5 for DZ pairs), dominance genetic effects (D: 1.0 for MZ and 0.25 for DZ pairs), common (shared) environmental effects (C: by definition, correlated 1.0 for MZ and DZ pairs) and unique (non-shared) environmental effects (E: by definition, uncorrelated in MZ and DZ pairs). As in our previous studies in children18 and adults17, we found evidence of shared environmental variation but no evidence of dominance genetic variation in height. Thus, we used the additive genetic/shared environment/unique environment model in the analyses. Models were fitted separately for each parental education category by age and sex groups. A clear sex-specific genetic effect for height was found in childhood19 and adulthood18, and thus it was included in all models allowing the opposite-sex DZ genetic correlation to be lower than the 0.5. Because DZ twins were slightly taller than MZ twins from infancy to adulthood34, different means for MZ and DZ twins were allowed. All genetic models were fitted by the OpenMx package (version 2.0.1) in the R statistical platform31 using the maximum likelihood method.
In order to test whether variance components of height were significantly different between parental education categories, we ran a random-effects meta-regression analysis of the aggregate-level data of raw variance components. Adjustments were carried out for geographic-cultural regions and age categories, and models were run separately by sex and for both sexes together. However, it should be noted that in these analyses the SEs are not corrected for multiple observations and consequently the 95% CI are likely to be somewhat too narrow, possibly leading to a spurious support of the original hypothesis.
Supplementary information
Acknowledgements
This study was conducted within the CODATwins project (Academy of Finland #266592). The CATSS-Study is supported by the Swedish Research Council through the Swedish Initiative for Research on Microdata in the Social And Medical Sciences (SIMSAM) framework grant no 340-2013-5867, grants provided by the Stockholm County Council (ALF-projects), the Swedish Heart-Lung Foundation and the Swedish Asthma and Allergy Association’s Research Foundation. The Swedish Twin Registry is managed by Karolinska Institutet and receives funding through the Swedish Research Council under the grant no 2017-00641. Netherlands Twin Register acknowledges the Netherlands Organization for Scientific Research (NWO) and MagW/ZonMW grants 904-61-090, 985-10-002, 912-10-020, 904-61-193,480-04-004, 463-06-001, 451-04-034, 400-05-717, Addiction-31160008, Middelgroot-911-09-032, Spinozapremie 56-464-14192; Amsterdam Public Health (APH); the European Research Council (ERC - 230374), the Avera Institute, Sioux Falls, South Dakota (USA) and the KNAW Academy Professor Award (PAH/6635) to DIB. Data collection and analyses in Finnish twin cohorts have been supported by ENGAGE – European Network for Genetic and Genomic Epidemiology, FP7-HEALTH-F4-2007, grant agreement number 201413, National Institute of Alcohol Abuse and Alcoholism (grants AA-12502, AA-00145, and AA-09203 to R J Rose, the Academy of Finland Center of Excellence in Complex Disease Genetics (grant numbers: 213506, 129680), and the Academy of Finland (grants 100499, 205585, 118555, 141054, 265240, 263278, 308248, 312073 and 264146 to J Kaprio). Since its origin the East Flanders Prospective Survey has been partly supported by grants from the Fund of Scientific Research, Flanders and Twins, a non-profit Association for Scientific Research in Multiple Births (Belgium). Gemini was supported by a grant from Cancer Research UK (C1418/A7974). Data collection and research stemming from the Norwegian Twin Registry is supported, in part, from the European Union’s Seventh Framework Programmes ENGAGE Consortium (grant agreement HEALTH-F4-2007-201413, and BioSHaRE EU (grant agreement HEALTH-F4-2010-261433). Madeira data comes from the following project: Genetic and environmental influences on physical activity, fitness and health: the Madeira family study Project reference: POCI/DES/56834/2004 Founded by the Portuguese agency for research (The Foundation for Science and Technology [FCT]). S.Y. Öncel and F. Aliev are supported by Kırıkkale University Research Grant: KKU, 2009/43 and TUBITAK grant 114C117. K Silventoinen is supported by Osaka University’s International Joint Research Promotion Program. The Boston University Twin Project is funded by grants (#R01 HD068435 #R01 MH062375) from the National Institutes of Health to K. Saudino. California Twin Program was supported by The California Tobacco-Related Disease Research Program (7RT-0134H, 8RT-0107H, 6RT-0354H) and the National Institutes of Health (1R01ESO15150-01). The Carolina African American Twin Study of Aging (CAATSA) was funded by a grant from the National Institute on Aging (grant 1RO1-AG13662-01A2) to K. E. Whitfield. Colorado Twin Registry is funded byNIDA funded center grant DA011015, & Longitudinal Twin Study HD10333; Author Huibregtse is supported by 5T32DA017637 and 5T32AG052371. The Michigan State University Twin Registry has been supported by Michigan State University, as well as grants R01-MH081813, R01-MH0820-54, R01-MH092377-02, R21-MH070542-01, R03-MH63851-01 from the National Institute of Mental Health (NIMH), R01-HD066040 from the Eunice Kennedy Shriver National Institute for Child Health and Human Development (NICHD), and 11-SPG-2518 from the MSU Foundation. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIMH, the NICHD, or the National Institutes of Health. The University of Southern California Twin Study is funded by a grant from the National Institute of Mental Health (R01 MH58354). The Texas Twin Project is currently funded by grants AA023322 and HD081437 from the National Institutes of Health. Vietnam Era Twin Study of Aging was supported by National Institute of Health grants NIA R01 AG018384, R01 AG018386, R01 AG022381, and R01 AG022982, and, in part, with resources of the VA San Diego Center of Excellence for Stress and Mental Health. The Cooperative Studies Program of the Office of Research & Development of the United States Department of Veterans Affairs has provided financial support for the development and maintenance of the Vietnam Era Twin (VET) Registry. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA/NIH, or the VA. The NAS-NRC Twin Registry acknowledges financial support from the National Institutes of Health grant number R21 AG039572. Korean Twin-Family Register was supported by the Global Research Network Program of the National Research Foundation (NRF 2011-220-E00006). South Korea Twin Registry is supported by National Research Foundation of Korea (NRF-371-2011-1 B00047). The West Japan Twins and Higher Order Multiple Births Registry was supported by Grant-in-Aid for Scientific Research (B) (grant number 15H05105) from the Japan Society for the Promotion of Science. This research was facilitated through access to Twins Research Australia, a national resource supported by a Centre of Research Excellence Grant (ID: 1079102), from the National Health and Medical Research Council. Longitudinal Israeli Study of Twins was funded by the Starting Grant no. 240994 from the European Research Council (ERC) to Ariel Knafo.
Author contributions
A.J., in charge of data management, conducted the analyses, wrote the first draft of the manuscript and has primary responsibility of the final content; Y.Y., Y.-M.H., D.I.B., T.I.A.S., J.K., K.S., planned the study design of the CODATwins project, collected the data used in this study, commented the manuscript, read and approved the final version of the manuscript; M.S., M.T., S.M., D.L.F., J.A.M., A.K.-N., D.M., L.A., F.J., F.N., Z.P., K.J.S., T.L.C., J.L.H., V.U., C.A., P.K.E.M., W.C., A.E.H., T.M.M., T.L.N., K.E.W., J.S., J.K., J.L., S.L., C.H.L., A.F., E.M., L.N., V.T., L.A.B., C.T., R.P.C., B.M.H., C.A.D., R.F.V., R.F.J.L., A.B., K.L.K., J.L.S., H.H.M., R.F.K., M.M., S.P., M.G., D.A.B., J.R.H., I.B., T.S.N., K.P.H., E.M.T.-D.,C.E.F., W.S.K., M.J.L., P.L., M.B., C.E.Mv., G.W., S.Y.Ö., F.A., H.-U.J. collected the data used in this study, commented the manuscript, read and approved the final version of the manuscript, R.S., A.L., E.R., E.T. commented the manuscript, read and approved the final version of the manuscript.
Data availability
The data used in this study is owned by the third parties (the individual twin cohorts) and made available to us in condition that they will be used only in this meta-analysis.
For this reason, we do not have legal rights to re- deliver the data or to provide it to other third parties without permissions from the data owners. In order to replicate the results, each researcher need to apply the data set from each individual twin cohort owners and to harmonize the data as a metafile.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-64883-8.
References
- 1.Galton F. Regression towards mediocrity in heriditary stature. Journal of the Anthropological Institute. 1886;15:246–262. [Google Scholar]
- 2.Pearson K, Lee A. On the laws on inheritance in man. Biometrika. 1903;2:356–462. doi: 10.1093/biomet/2.3.356. [DOI] [Google Scholar]
- 3.Fisher RA. The correlation between relatives on the supposition of mendelian inheritance. Transactions of the Royal Society of Edinburgh. 1918;52:399–433. doi: 10.1017/S0080456800012163. [DOI] [Google Scholar]
- 4.Visscher PM, McEvoy B, Yang J. From galton to GWAS: Quantitative genetics of human height. Genet Res (Camb). 2010;92:371–379. doi: 10.1017/S0016672310000571. [DOI] [PubMed] [Google Scholar]
- 5.Perola M, et al. Combined genome scans for body stature in 6,602 european twins: Evidence for common caucasian loci. PLoS Genet. 2007;3:e97. doi: 10.1371/journal.pgen.0030097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho YS, et al. A large-scale genome-wide association study of asian populations uncovers genetic factors influencing eight quantitative traits. Nat Genet. 2009;41:527–534. doi: 10.1038/ng.357. [DOI] [PubMed] [Google Scholar]
- 7.Hao Y, et al. Genome-wide association study in han chinese identifies three novel loci for human height. Hum Genet. 2013;132:681–689. doi: 10.1007/s00439-013-1280-9. [DOI] [PubMed] [Google Scholar]
- 8.Allen HL, et al. Hundreds of variants clustered in genomic loci and biological pathways affect human height. Nature. 2010;467:832–838. doi: 10.1038/nature09410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.N’Diaye A, et al. Identification, replication, and fine-mapping of loci associated with adult height in individuals of african ancestry. PLoS Genet. 2011;7:e1002298. doi: 10.1371/journal.pgen.1002298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wood AR, et al. Defining the role of common variation in the genomic and biological architecture of adult human height. Nat Genet. 2014;46:1173–1186. doi: 10.1038/ng.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eveleth, P. B. & Tanner, J. M. “Worldwide variation in human growth” (Cambridge University Press, 1990)
- 12.Bogin, B. “The growth of humanity” (Wiley-Liss, 2001)
- 13.Steckel RH. Heights and human welfare: Recent developments and new directions. Explorations in Economic History. 2009;46:1–23. doi: 10.1016/j.eeh.2008.12.001. [DOI] [Google Scholar]
- 14.Bozzoli C, Deaton A, Quintana-Domeque C. Adult height and childhood disease. Demography. 2009;46:647–669. doi: 10.1353/dem.0.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silventoinen K. Determinants of variation in adult body height. J Biosoc Sci. 2003;35:263–285. doi: 10.1017/S0021932003002633. [DOI] [PubMed] [Google Scholar]
- 16.McCrory C, et al. Socioeconomic differences in children’s growth trajectories from infancy to early adulthood: Evidence from four european countries. J Epidemiol Community Health. 2017;71:981–989. doi: 10.1136/jech-2016-208556. [DOI] [PubMed] [Google Scholar]
- 17.Lakshman R, et al. Higher maternal education is associated with favourable growth of young children in different countries. J Epidemiol Community Health. 2013;67:595–602. doi: 10.1136/jech-2012-202021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jelenkovic, A. et al. Genetic and environmental influences on adult human height across birth cohorts from 1886 to 1994. Elife. 5, 10.7554/eLife.20320 (2016). [DOI] [PMC free article] [PubMed]
- 19.Jelenkovic A, et al. Genetic and environmental influences on height from infancy to early adulthood: An individual-based pooled analysis of 45 twin cohorts. Sci Rep. 2016;6:28496. doi: 10.1038/srep28496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boomsma D, Busjahn A, Peltonen L. Classical twin studies and beyond. Nat Rev Genet. 2002;3:872–882. doi: 10.1038/nrg932. [DOI] [PubMed] [Google Scholar]
- 21.Visscher PM, Hill WG, Wray NR. Heritability in the genomics era–concepts and misconceptions. Nat Rev Genet. 2008;9:255–266. doi: 10.1038/nrg2322. [DOI] [PubMed] [Google Scholar]
- 22.Bronfenbrenner U, Ceci SJ. Nature-nurture reconceptualized in developmental perspective: A bioecological model. Psychol Rev. 1994;101:568–586. doi: 10.1037/0033-295X.101.4.568. [DOI] [PubMed] [Google Scholar]
- 23.Scarr S, McCartney K. How people make their own environments: A theory of genotype greater than environment effects. Child Dev. 1983;54:424–435. doi: 10.1111/j.1467-8624.1983.tb03884.x. [DOI] [PubMed] [Google Scholar]
- 24.Boomsma, D. I. & Martin, N. G., Gene-environment interactions in Biological Psychiatry, D’Haenen, H, den Boer, JA & P, Wilner Eds. pp. 181–187 (John Wiley & Sons, 2002).
- 25.Silventoinen K, et al. Education in twins and their parents across birth cohorts over 100 years: An individual-level pooled analysis of 42-twin cohorts. Twin Res Hum Genet. 2017;20:395–405. doi: 10.1017/thg.2017.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galobardes B, et al. Social inequalities in height: Persisting differences today depend upon height of the parents. PLoS One. 2012;7:e29118. doi: 10.1371/journal.pone.0029118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matijasevich A, et al. Maternal education inequalities in height growth rates in early childhood: 2004 pelotas birth cohort study. Paediatr Perinat Epidemiol. 2012;26:236–249. doi: 10.1111/j.1365-3016.2011.01251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwok MK, Leung GM, Lam TH, Leung SS, Schooling CM. Grandparental education, parental education and child height: Evidence from hong kong’s “children of 1997” birth cohort. Ann Epidemiol. 2013;23:475–484. doi: 10.1016/j.annepidem.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 29.Zheng J, et al. LD hub: A centralized database and web interface to perform LD score regression that maximizes the potential of summary level GWAS data for SNP heritability and genetic correlation analysis. Bioinformatics. 2017;33:272–279. doi: 10.1093/bioinformatics/btw613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abdellaoui A, et al. Genetic correlates of social stratification in great britain. Nat Hum Behav. 2019;3:1332–1342. doi: 10.1038/s41562-019-0757-5. [DOI] [PubMed] [Google Scholar]
- 31.Silventoinen K, et al. The CODATwins project: The cohort description of COllaborative project of development of anthropometrical measures in twins to study macro-environmental variation in genetic and environmental effects on anthropometric traits. Twin Res Hum Genet. 2015;18:348–360. doi: 10.1017/thg.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Institute of Arthritis and Musculoskeletal and Skin Diseases, Handout on health: Osteoporosis. 2016, April,(2014).
- 33.Mallick S, et al. The simons genome diversity project: 300 genomes from 142 diverse populations. Nature. 2016;538:201–206. doi: 10.1038/nature18964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jelenkovic A, et al. Zygosity differences in height and body mass index of twins from infancy to old age: A study of the CODATwins project. Twin Res Hum Genet. 2015;18:557–570. doi: 10.1017/thg.2015.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000;56:645–646. doi: 10.1111/j.0006-341X.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- 36.Posthuma D, et al. Theory and practice in quantitative genetics. Twin Res. 2003;6:361–376. doi: 10.1375/136905203770326367. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this study is owned by the third parties (the individual twin cohorts) and made available to us in condition that they will be used only in this meta-analysis.
For this reason, we do not have legal rights to re- deliver the data or to provide it to other third parties without permissions from the data owners. In order to replicate the results, each researcher need to apply the data set from each individual twin cohort owners and to harmonize the data as a metafile.