Abstract
The rs738409 G>C single nucleotide polymorphism occurring in the patatin-like phospholipase 3 gene has been identified as a novel genetic marker for hepatic steatosis. Recent studies also associated rs738409 with fibrosis in hepatitis C (HCV). Therefore, we sought to determine the impact of donor and recipient rs738409 genotype on the progression of fibrosis after liver transplantation for HCV. This cohort study included 101 patients infected with HCV who underwent liver transplantation between January 2008, and June 2011. Donor and recipient rs738409 genotypes were determined from donor wedge biopsies and recipient explants. The time to Ishak stage 3 fibrosis, or HCV-related mortality/graft loss was analyzed by the Cox model adjusting for HCV-Donor Risk Index, warm ischemic time, pretransplant Model for Endstage Liver Disease (MELD) and viral load. The rs738409 CC variant was present in 56% of donors and 57% of recipients. The median follow-up period was 620 days. A total of 39 patients developed the primary outcome of ≥stage 3 fibrosis or HCV-related mortality/graft loss, the time to which differed by donor (P = 0.019) but not recipient (P = 0.89) genotype. In the multivariate model, donor GC or GG variants had 2.53 times the risk (95% confidence interval [CI] 1.25–5.02, P = 0.008) compared to CC variants. In the alternative endpoint: stage 3 fibrosis or all-cause mortality/graft loss, the effect of donor genotype was attenuated but remained significant at 1.98 (95% CI 1.11–3.53).
Conclusions
The rs738409 genotype is an important predictor of posttransplant outcome in HCV. Liver, and not adipocytes, is the site at which this effect occurs. Our finding may be useful in donor selection for liver transplantation with HCV, and may guide decisions regarding early antiviral treatment.
Hepatitis C (HCV)-related endstage liver disease is the most common reason for liver transplantation in the United States. Recurrent HCV is nearly universal after transplantation.1 In addition, fibrosis progression is accelerated by transplantation. Cirrhosis develops in 20% to 40% of recipients within 5 years after transplantation,2 whereas only 3% to 20% of nontransplant patients develop cirrhosis within 20 years after HCV infection. Although a number of donor, recipient, and virological characteristics have been identified as prognostic indicators,3 fibrosis progression in individual patients is unpredictable and poorly understood. Hepatic lipid metabolism is modulated by HCV to enhance its replication.4 Steatosis occurs in most patients with HCV infection and directly correlates with the Histological Activity Index (HAI), more rapid progression of fibrosis, and poor response to antiviral treatment in the nontransplant setting.5 A single-nucleotide polymorphism (rs738409 C>G) has been associated with the prevalence and severity of both nonalcoholic fatty liver disease and alcoholic liver disease.6–8 This polymorphism is located in an exon of the patatin-like phospholipase 3 (PNPLA3) gene, which encodes the 481-amino acid protein adiponutrin. The sequence of adiponutrin is similar to that of adipose triglyceride lipase, and the protein appears to have both triglyceride lipase and acyl-coenzyme A-independent transacylase activity. The C-to-G transversion changes isoleucine to methionine at codon 148. The longer hydrophobic sidechain of the methionine blocks the substrate from binding to the active site, thereby abolishing triglyceride lipase activity and causing triglycerides to accumulate.9–11 Rs738409 has been associated with hepatic steatosis, fibrosis, response to treatment, and incidence of hepatocellular carcinoma (HCC) in the setting of HCV. However, the role of rs738409 in recurrent HCV after liver transplantation remains largely unknown.
Since PNPLA-3 is expressed in both adipocytes and hepatocytes, its effects on the outcome of the liver posttransplant is complicated by the need to examine both donor and recipient genotypes for possible effects. A recent preliminary report12 suggested that rs738409 in liver transplant recipients does not predict recurrent HCV fibrosis progression, but the effects of donor rs738409 were not considered. In the course of examining the factors that predict the outcome of HCV patients posttransplantation, we examined rs738409 genotypes of both donors and recipients. This work showed that donor, but not recipient, rs738409 genotype predicted recurrent HCV fibrosis progression after transplantation more strongly than any other examined factor.
Patients and Methods
Study Sample
Between January 1, 2008 and June 30, 2011, a total of 128 patients with HCV underwent orthotopic liver transplantation. We excluded patients who achieved sustained virologic response (SVR) before liver transplant or had undetectable viral load before transplant (n = 20). We excluded patients undergoing retransplantation (n = 6), and patients for whom donor genotyping data was not available (n = 1). A cohort of 101 patients was included in this analysis. The follow-up period ended April 1, 2013.
Human Subjects
Written informed consent for study participation including genetic studies was obtained from each patient. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the Human Subject Committee of the University of Kansas Medical Center. No donor organs were obtained from executed prisoners or other institutionalized persons.
Histological Assessment
Based on a standard protocol, patients underwent liver biopsies 6 months after liver transplantation for HCV and yearly thereafter, and at other times if liver enzymes were abnormal. All liver biopsies were collectively reviewed during regular liver transplant pathology conferences by hepatologists, transplant surgeons, and liver pathologists. Inflammation and fibrosis were graded and staged according to Ishak, except when inflammation and fibrosis were due to etiologies other than recurrent HCV (rejection or biliary obstruction). Steatosis was graded as none or present (≥5%) because only one donor reperfusion biopsy and only three follow-up biopsies had moderate or severe steatosis. Acute cellular rejection, if present, was graded according to the Banff rejection activity index. Chronic rejection was defined as >50% bile duct loss. Fibrosing cholestatic hepatitis was defined by histological features (cholestasis, ballooning, ductular reaction) and viral load >50 million IU/mL.
HCV-Related Mortality/Graft Loss
HCV-related mortality/graft loss was defined as death or retrains-plantation due to 1) fibrosing cholestatic hepatitis or 2) decompensated graft function related to progressive fibrosis in the absence of predominant ischemic biliopathy or chronic rejection.
Rs738409 Genotype
Donor and recipient rs738409 genotypes were determined from wedge biopsies (donors) and explants (recipients). When fresh frozen tissues were available, RNA was extracted and reverse transcribed, otherwise genomic DNA was extracted from formalin-fixed, paraffin-imbedded tissue with phenol:chloroform:isopropyl alcohol (25:24:1). Real-time polymerase chain reaction was performed using a C1000 Thermal Cycler (Bio-Rad, Hercules, CA) and TaqMan probe (Assay ID: C_7241_10, Product number: 4351379, Applied Biosystems, Foster City, CA), according to the manufacturers’ protocols. GC and GG genotypes were grouped together for analysis.
Data Analysis and Interpretation
The study endpoint (time to ≥stage 3 fibrosis, or HCV-related mortality/graft loss) was chosen to optimize statistical power based on the number of failures. HCV-related mortality/graft loss includes death or retransplantation from graft cirrhosis. Survival was analyzed using the Cox proportional hazard (PH) model. Multivariate analysis included donor and recipient rs738409 genotype, pretransplant log viral load, pretransplant MELD score, warm ischemic time, and HCV-Donor Risk Index (HCV-DRI),13 which is a composite score that takes into account donor age, diabetes, cause of death, height, aspartate transaminase (AST) level, and cold ischemic time. In the sensitivity analysis, the HCV-DRI was replaced with the DRI14 as well as individual components of HCV-DRI and DRI (donor age, gender, race, diabetes, height, AST, cause of death, and local versus regional). Rather than excluding 13 patients with missing data from statistical analysis, multiple imputation (SAS procedure IM) was used to replace each missing value with five imputed values in five complete datasets, based on the value of all other variables in the multivariate analysis. A multiple regression analysis was performed on each of the five datasets, and summary statistics were generated using the SAS procedure MIANALYSE. Kaplan-Meier survival curves were generated and compared using log-rank test. A general estimating equation with binomial distribution was used to analyze the repeated measurement of hepatic steatosis (<5% versus ≥5%), while a mixed effect model was used to compute the repeated measurement of HAI, 1–18. Categorical variables were compared by chi-square test. Continuous parametric and nonparametric variables were compared by t test and Wilcoxon two-sample test, respectively. Statistical analysis was carried out using SAS v. 9.2 (SAS Institute, Cary, NC).
Results
The rs738409 CC variant was detected in 56% of donors and 57% of recipients (P = 0.95). Table 1 shows donor and recipient characteristics stratified by donor rs738409 genotype. Donors with GC/GG genotype were more likely to be Hispanic. Recipients with donor GC/GG genotype were younger and more likely to have concurrent alcoholic cirrhosis. No other significant differences were found.
Table 1.
Donor and Recipient Characteristics
| Donor rs738409 CC | Donor rs738409 GC or GG | ||
|---|---|---|---|
| n = 57 | n = 44 | P | |
| Recipient characteristics | |||
| Recipient rs738409 | 0.95 | ||
| CC | 32 (56%) | 25 (57%) | |
| GC/GG | 25 (43%) | 19 (43%) | |
| Age, years | 56 (6) | 53 (6) | 0.01 |
| Gender | 0.68 | ||
| Male | 42/57 (74%) | 34/44 (77%) | |
| Female | 15/57 (26%) | 10/44 (23%) | |
| Ethnicity | 0.93 | ||
| White | 46/57 (81%) | 34/44 (77%) | |
| Black | 6/57 (11%) | 6/44 (14%) | |
| Hispanic | 3/57 (5%) | 3/44 (7%) | |
| Asian | 2/57 (3%) | 1/44 (2%) | |
| Diabetes (pre-OLT) | 15/57 (26.%) | 10/44 (23%) | 0.68 |
| MELD score (pre-OLT) | 21 (10) | 20 (10) | 0.86 |
| Alcoholic cirrhosis | 18/57 (32%) | 5/44 (12%) | 0.02 |
| Hepatocellular carcinoma | 15/57 (26%) | 16/44 (36%) | 0.28 |
| Simultaneous liver kidney | 3/57 (5%) | 6/44 (14%) | 0.14 |
| Hepatitis C genotype | 0.91 | ||
| 1 | 44/57 (77%) | 35/44 (80%) | |
| 2 | 6/57 (11%) | 3/44 (7%) | |
| 3 | 4/57 (7%) | 4/44 (9%) | |
| Indeterminant/missing | 3/57 (5%) | 2/44 (4%) | |
| Log viral load (Pre-OLT) | 5.6 (5.0–6.3) (n 5 56) | 5.9 (4.9–6.5) (n 5 40) | 0.35 |
| Donor characteristics | |||
| Age, years | 35 (13) | 32 (12) | 0.14 |
| Ethnicity | 0.11 | ||
| White | 46/57 (80%) | 30/44 (68%) | |
| Black | 8/57 (14%) | 6/44(14%) | |
| Hispanic | 1/57(2%) | 7/44(16%) | |
| Asian | 1/57 (2%) | 1 /44(2%) | |
| Other | 1/57(2%) | ||
| Diabetes | 0/57 (0%) | 1/44 (2%) | 0.25 |
| Cause of death | 0.65 | ||
| Anorexia | 15/57 (26%) | 7/44 (16%) | |
| CVA/stroke | 13/57 (23%) | 12/44 (27%) | |
| Head trauma | 27/57 (47%) | 23/44 (52%) | |
| Other | 2/57 (4%) | 2/44 (5%) | |
| Gender | 0.98 | ||
| Male | 39/57 (68%) | 30/44(68%) | |
| Female | 18/57 (32%) | 14/44 (32%) | |
| Height, cm | 173 (11) | 177 (11) | 0.13 |
| AST, IU/L | 42 (31-99) | 50 (29-108) | 0.69 |
| Cold ischemic time | 378 (114) | 379 (149) | 0.99 |
| Warm ischemic time | 52 (46-62) | 53 (47-64) | 0.38 |
| Early allograft dysfunction | |||
| Olthoff et al. | 11/57 (19%) | 8/44 (18%) | 0.89 |
| Modified Deschenes et al. | 5/57 (9%) | 1/43 (2%) | 0.17 |
| Wagener et al. | 7/57 (12%) | 12/44 (27%) | 0.056 |
| Immunosuppressant | |||
| Predominant | 0.27 | ||
| Tacrolimus | 47/57 (82%) | 32/44 (73%) | |
| Cyclosporin | 9/57 (16%) | 12/44 (27%) | |
| Rapamycin | 1/57 (2%) | ||
| Mycophenolate Mofetil >6 mo | 34/57 (60%) | 22/44 (50%) | 0.33 |
| Prednisone > 6mo | 17/57 (30%) | 11/44 (25%) | 0.59 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CVA, cerebrovascular accident; MELD, Model for End-Stage Liver Disease; OLT, orthotopic liver transplantation.
Olthoff et al.'s definition: either a total bilirubin level >10 mg/dL or an INR >1.6 on postoperative day 7 or an AST or ALT level >2000 IU/L within the first 7 days.
Modified Deschenes et al.'s definition: a peak total bilirubin level >10 mg/dL on postoperative days 2 to 7.
Wagener et al.'s definition: MELD score >18.9 on postoperative day 5.
Categorical variables are expressed as n (%) and compared by chi-square test. Continuous parametric variables are expressed as mean (standard deviation) and compared by t test. Continuous nonparametric variables are expressed as median (interquartile range) and compared by Wilcoxon two-sample test. N557 in the donor CC group and n = 44 in the donor GC/GG group unless otherwise specified.
Follow-up
The median follow-up period was 620 days (interquartile range [IQR] 317–975 days). A total of 39 patients developed the primary outcome of ≥stage 3 fibrosis, or HCV-related mortality/graft loss. Supporting Table 1 shows the condition when the censorship occurred. Among the 62 patients censored, 13 died or lost graft unrelated to HCV. Thirty-one patients had the last liver biopsy within 1 year of the follow-up end date. The most common reason for not having a liver biopsy within 1 year (n = 18) was SVR or actively undergoing HCV treatment (n = 10). The numbers at risk at 6, 12, 18, 24, and 30 months were 84, 63, 51, 40, and 29, respectively.
Primary Outcome
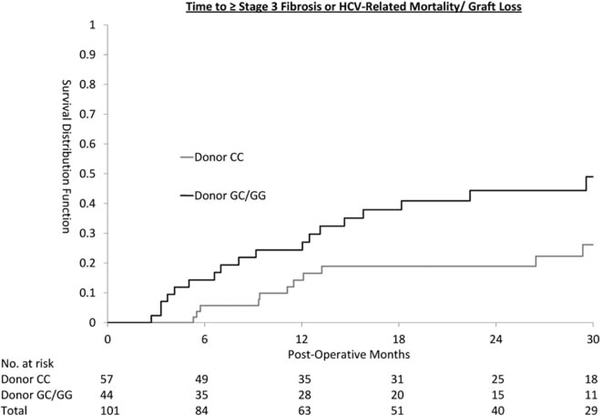
The time to primary outcome differed by donor genotype (Fig. 1; log-rank test P = 0.019) but not recipient genotype (P = 0.89). An endpoint occurred in 16/57 (28%) of patients who received transplants of donor CC genotype and 23/44 (52%) of those who received transplants of GC/GG donor genotype. Multivariate Cox PH regression analysis showed that the risk associated with donor GC/GG genotype was 2.53 times that of the CC genotype (95% confidence interval [CI] 1.28–5.02, P = 0.008).
Fig. 1.
Time to ≥ stage 3 fibrosis or HCV-related mortality/graft loss.
Donor and Recipient Genotype Interaction
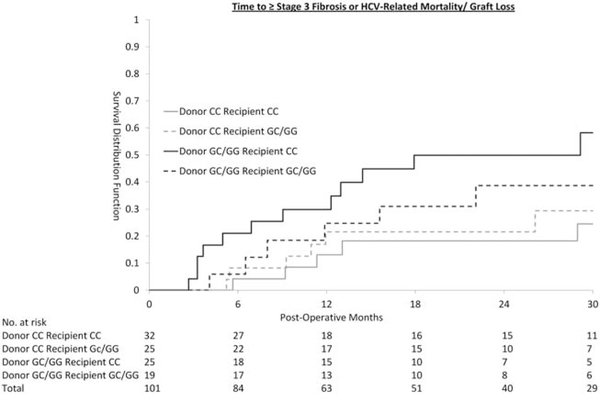
Figure 2 shows the survival curve for each of the donor and recipient genotype combinations. Although the survival curve (Fig. 2) suggested that CC recipients receiving a liver from GC/GG donors do worse than others, the interaction term for donor and recipient genotype is not statistically significant (P = 0.98), suggesting that it is the donor genotype, rather than recipient genotype or donor recipient combination, that affected the primary outcome.
Fig. 2.
Time to ≥ stage 3 fibrosis or HCV-related mortality/graft loss.
Alternative Multivariate Models
We generated several alternative multivariate models adjusting for donor confounders, and donor rs738409 genotype had a similar and significant effect in all models, regardless of which combination of other variables was used. In the primary analysis, the multivariate model adjusted for recipient rs738409, pretransplant log viral load, pretransplant MELD, warm ischemic time, and the HCV-DRI. In an alternative model that adjusted for the DRI14 rather than the HCV-DRI,13 donor GC/GG genotype had a hazard ratio (HR) of 2.56 (95% CI 1.26–5.21). In another multivariate model that adjusted for individual components of the HCV-DRI and DRI (donor age, gender, race, diabetes, height, AST, cause of death, and local versus regional), donor GC/GG genotype had an HR of 4.33 (95% CI 1.85–10.14). In the primary analysis missing data were handled by multiple imputation. If missing data were excluded instead (n = 92 after exclusion), the donor GC/GG genotype had an HR of 3.27 (95% CI 1.61–6.65).
Effect of Donor rs738409 Compared to Donor Age
To put the HR of donor rs738409 genotype into perspective, we compared it with donor age. In a multivariate model that included donor age instead of HCV-DRI, each 10-year increase in donor age had a parameter estimate of 0.30(95% CI 0.026–0.58) (HR = e30 = 1.30), while the donor rs738409 GC/GG genotype had a parameter estimate of 1.13 (95% CI 0.34–1.76) (HR = e1,13 = 3.09). Therefore, the effect of donor rs738409 genotype is equivalent to 38 (95%CI 6–68) years difference in donor age.
Retransplantation and Death
There were five retransplantations and six deaths among patients who received donors of CC genotype, and two retransplantations and eight deaths among those who received donors of GC/GG genotype. Table 2 shows that one case with donor CC genotype and four cases with donor GC/GG genotype could be attributed to HCV (fibrosing cholestatic hepatitis).
Table 2.
Causes of Death and Retransplantation
| Donor rs738409 | Event | n | Cause of Death/Retransplantation |
|---|---|---|---|
| CC | Death | 1 | Fibrosing cholestatic hepatitis |
| n = 57 | n = 6 | 3 | Recurrent hepatocellular carcinoma |
| 1 | Cardiovascular disease | ||
| 1 | Trauma | ||
| Retransplantation | 3 | Chronic rejection | |
| n = 5 | 1 | Ischemic cholangiopathy | |
| 1 | Submassive necrosis | ||
| GC/GG | Death | 2 | Fibrosing cholestatic hepatitis |
| n = 44 | n = 8 | 3 | Recurrent hepatocellular carcinoma |
| 2 | Cardiovascular disease | ||
| 1 | Hemophagocytic syndrome | ||
| Retransplantation | 2 | Fibrosing cholestatic hepatitis | |
| n = 2 | |||
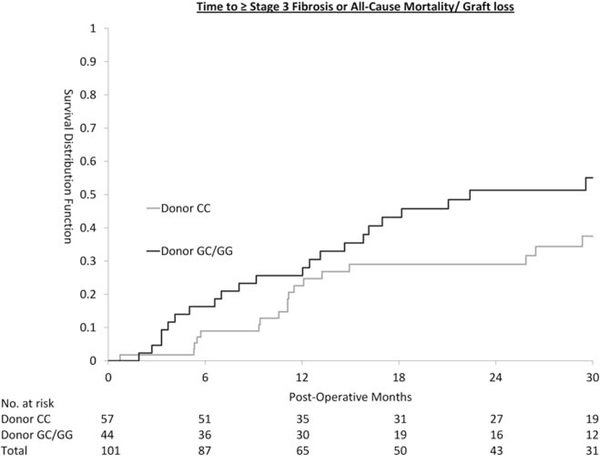
In the primary endpoint analysis, HCV-unrelated mortality/graft loss was censored. With the alternative endpoint of stage 3 fibrosis, all-cause mortality/graft loss (Fig. 3), the effect of donor GC/GG genotype was diluted by retransplantation and death unrelated to HCV. The multivariate HR remained significant at 1.98 (95% CI 1.11–3.53). The risk of death and retransplantation from all causes was comparable between patients who received transplant from CC versus GC/GG donor (log-rank P = 0.69).
Fig. 3.
Time to ≥ stage 3 fibrosis or all-cause mortality/graft loss.
Mode of Inheritance
Donor genotypes GC and GG were grouped together in the main analysis because of the relatively low frequency of the G alleles and because of previous reports of similar phenotype for these genotypes.15 When analyzed under the additive genetic model, each G allele was associated with an HR of 1.63 (1.006–2.63). When each donor genotype was analyzed separately, HR point estimates of GC genotype (HR 2.77, 95% CI 1.37–5.65) and GG genotype (HR 1.80, 95% CI 0.23–14.3) were similar. However, the difference between GG and CC genotypes was not significant because of the small number of donors with GG genotype.
Potential Mechanism
The posttransplant clinical course was evaluated in an attempt to identify the mechanism underlying the relationship between donor rs738409 genotype and fibrosis and survival. We evaluated the following factors, but found no significant differences between groups.
HCV Treatment Response
Before developing the primary outcome, 14 patients with CC donor and 8 with GC/GG donors underwent interferon/ribavirin treatment for HCV. The indication for treatment was usually ≥Ishak stage 2 fibrosis (n = 18). Selected patients with a modified HAI ≥7 and a very high transaminase were also treated (n = 4). SVR occurred in 62% of patients in each of the two groups. Adjusting for interferon/ribavirin treatment or for SVR as a time-dependent covariate did not change the HR of donor genotype towards the primary outcome (HR 2.71 95% CI 1.33–5.52 after adjusting for HCV treatment; HR 2.67 95% CI 1.32–5.42 after adjusting for SVR).
Steatosis
Steatosis at the time of transplantation was more common in grafts of donor GC/GG genotype than grafts of CC genotype (30% versus 25%), although this difference was not statistically significant (chi-square P = 0.66). Donor graft steatosis was not associated with the study endpoint (HR 1.26, 95% CI 0.59–2.56). Adjusting for donor graft steatosis did not change the HR associated with donor genotype (HR 2.77, 95% CI 1.37–5.57).
Thirty-three percent of patients with CC donor and 41% with GC/GG donors had at least ≥5% steatosis in the liver biopsy before or at the time of developing the primary endpoint (chi-square P = 0.28). Adjusting for any steatosis before or at the time of developing the primary endpoint did not change the HR between donor rs738409 genotype and the primary outcome (2.72 95% CI 1.35–5.48). Using general estimating equation with dichotomous outcome variable to account for the correlation of repeated measurements, donor rs738409 genotype was not associated with hepatic steatosis during follow-up biopsies (P = 0.33).
HAI
Using a mixed effect model to account for the correlation of repeated measurements, donor rs738409 genotype was not associated with HAI during follow-up biopsies (P = 0.56).
Diabetes
Diabetes was not present in 76 patients before transplantation. During follow-up, 26% of those with donor CC genotype and 35% of those with donor GC/GG genotype had developed new-onset diabetes after transplant before the primary outcome. The rate of developing diabetes was not significantly different (log-rank P = 0.18). Adjusting for diabetes as a time-dependent covariate did not change the HR of the donor rs738409 genotype towards the primary outcome (HR 2.44 95% CI 1.25–4.79).
Injury Early Allograft Dysfunction (EAD)16–18
Patients with GC/GG donor might be at increased risk of EAD (univariate odds ratio 2.7, 95% CI 0.95–7.5, P = 0.056, Table 1) based on Wagener et al.’s definition18 (MELD score >18.9 on postoperative day 5). However, excluding patients with EAD only accentuated the effect of donor rs738409 genotype on the primary outcome (HR 3.55, 95% CI 1.46–8.61).
Fibrosing Cholestatic Hepatitis
One patient with CC donor and four patients with GC/GG donors died or required retransplantation from fibrosing cholestatic hepatitis. The difference did not reach statistical significance (log-rank P = 0.09).
Discussion
We evaluated the relationship between rs738409 genotype (donor and recipient) and fibrosis progression and graft survival in a well-characterized sample of patients with HCV who underwent liver transplantation. We found that donor but not recipient GC/GG genotype was associated with increased risk of fibrosis progression, retransplantation, or death. The finding that recipient rs738409 genotype is not associated with these transplantation outcomes suggests that the liver, not adipose tissue, is the site at which this effect occurs. The causal pathway has not yet been identified; it does not appear to involve hepatic steatosis but may possibly involve processes that directly stimulate fibrogenesis.
The single-nucleotide polymorphism rs738409 in the PNPLA3 gene was first identified as a risk factor for steatosis in 2008 by the Dallas Heart Study19 and is associated with histological severity in nonalcoholic fatty liver disease (i.e., steatosis grade, lobular inflammation, Mallory-Denk bodies, and fibrosis stage).20 Rs738409 was found to be associated with alcoholic liver disease21 in 2010 and HCV in 2011. A Swiss cohort study22 reported that the rs738409 G allele was associated with hepatic steatosis in patients with HCV genotype non-3. In a large Italian cross-sectional study,23 it was further demonstrated that the G allele is associated with cirrhosis, HCC, and lower response to antiviral treatment. Another Italian cross-sectional study24 confirmed the association of the rs738409 genotype with HCC in HCV patients. In addition, a multicenter cross-sectional study in Belgium, Germany, and France25 reported that rs738409 was associated with fibrosis progression in patients with HCV. In this study, we evaluated the hypothesis that rs738409 polymorphisms in the donor PNPLA3 gene are the primary factor accounting for the differences in fibrosis development after liver transplantation in patients with HCV.
The use of a liver transplantation cohort in this study enabled us to accurately assess the relationship between rs738409 genotype and progression of fibrosis in HCV because it can separately examine both liver and host, identifying either liver or adipocyte as the relevant source. In addition, the duration of graft infection can be pinpointed to the time of transplantation. In addition, fibrosis progression is accelerated with immunosuppression, which allowed a sufficient number of endpoint events to occur over a relatively short follow-up period. Unlike a cross-sectional study, a transplantation cohort study can evaluate early death from fibrosing cholestatic hepatitis without survival bias. The protocol liver biopsy allows the tracking of fibrosis even in asymptomatic patients with normal laboratory test results.
There were a number of limitations in the current study. First, the posttransplant course can be complex (e.g., immunosuppressant change, rejection), which may not be adequately adjusted for in the multivariate analysis. However, receiving a graft with an rs738409 G allele is a random event. In this study, the donor characteristics were similar, and genotype was not available before transplantation. Therefore, the study design is analogous to a randomized trial and less susceptible to selection bias than typical observational studies. Second, fibrosis staging can be susceptible to misclassification bias. In addition to HCV, biliary obstruction and chronic rejection can also lead to fibrosis, but Ishak staging would not be appropriate. Therefore, the current study staged fibrosis in the setting of a pathology conference, where clinical data from the transplant surgeon, hepatologist, and pathologist were integrated. Although outcome based on fibrosis can be subjective, our study also included the endpoints retransplantation and death in the sensitivity analysis. Informative censoring could occur when patients died or lost the graft seemingly for reasons unrelated to HCV. With the alternative endpoint of stage 3 fibrosis, graft loss, or death from all causes, the effect of donor genotype was diluted but the HR remained significant. Informative censoring might also occur when patients did not undergo liver biopsy as a result of ongoing HCV treatment or SVR. This was addressed by adjusting SVR or HCV treatment as a time-dependent covariate in a secondary analysis. Again, the HR of the donor genotype was not affected and remained significant.
PNPLA3 is primarily expressed in the liver and, to a lesser extent, in adipose tissue.11 Results of in vitro assays showed that the wild-type PNPLA3 enzyme hydrolyzes emulsified triglyceride. The substitution of methionine for isoleucine at residue 148 restricts access of the substrate to the catalytic serine at residue 47, and the subsequent loss of lipase activity is thought to be the cause of hepatic steatosis.9 Although the loss of function in hepatic lipase can explain hepatic steatosis, it does not necessarily explain the link to fibrosis, HCC, and possible HCV treatment response. Furthermore, expression of PNPLA3-I148M results in increased triglyceride content, while knocking out the PNPLA3 gene does not affect triglyceride content.26 From this perspective I148M functions like a gain of function mutation. Liver transplantation provides a unique opportunity to test whether the effect of this gene is localized to the liver versus the adipose tissue because transplantation creates a chimeric individual. However, neither donor nor recipient rs738409 genotype was associated with hepatic steatosis in subsequent biopsies. In another liver transplant cohort of 237 that included HCV and non-HCV patients, Finkenstedt et al.27 similarly reported the lack of association between donor rs738409 and hepatic steatosis. Finkenstedt et al. reported that it was the recipient rs738409 GG genotype associated with hepatic steatosis, but our sample size was insufficient to validate this association. This brings us two puzzle pieces in the mechanism PNPLA3: the hepatic PNPLA3 is responsible for fibrosis progression in HCV but not for hepatic steatosis. Our finding, along with that of Finkenstedt et al., suggests that PNPLA3-mediated fibrosis progression and steatosis may be mediated through separate pathways. One possibility might be that PNPLA3 is present in stellate cells, where it alters their activation and/or response to external stimuli. If this were the case, there might be separate steatotic and fibrogenic effects of this protein.
While the recent literature27,28 has just started to touch on the relationship between donor rs738409 genotype and transplant outcome, many have reported the effect of donor graft steatosis. Severe graft steatosis is associated with lower patient and graft survival.29,30 Moderate graft steatosis may also be associated with lower survival, but few studies have included a sufficient number of donor grafts with moderate steatosis to demonstrate a significant difference. Doyle et al.32 reported that mild graft steatosis is not associated with lower patient or graft survival.23 However, mild steatosis was associated with increased coagulopathy, cholestasis, and reperfusion injury, although to a lesser degree than moderate steatosis.32 In liver transplantation for HCV, Subramanian et al.33 reported that moderate graft steatosis is associated with increased fibrosis at 1 year. In our study, only one patient received a graft with moderate steatosis. Donor graft with mild steatosis was not associated with fibrosis progression, retransplantation, or death and did not explain the relationship between rs738409 genotype and transplantation outcome.
Our findings demonstrate that donor rs738409 genotype is an important predictor of posttransplant outcome in HCV. This finding may be useful in donor selection for liver transplantation in patients with HCV and may guide decisions regarding early antiviral treatment. However, the relationship between donor rs738409 genotype and liver transplant patients without HCV has yet been evaluated. Furthermore, genotyping donors before organ allocation may present a logistical challenge.
Supplementary Material
Acknowledgments
Supported by the American Association for the Study of Liver Diseases Sheila Sherlock Clinical and Translational Research Award 2012–2014, and University of Kansas Liver Center Pilot Project Award to W.D.
Abbreviations
- ALT
alanine aminotransferase
- ST
aspartate transaminase
- CI
confidence interval
- DRI
Donor Risk Index
- HAI
Histology Activity Index
- HCV
hepatitis C
- HCV-DRI
hepatitis C, donor risk index
- HR
hazard ratio
- IQR
interquartile range
- MELD
Model for Endstage Liver Disease
- PH
proportional hazard
- PNPLA3
patatin-like phospholipase 3
- SVR
sustained virologic response
Footnotes
Potential conflict of interest: Nothing to report.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Shiffman ML, Stravitz RT, Contos MJ, Mills AS, Sterling RK, Luketic VA, et al. Histologic recurrence of chronic hepatitis C virus in patients after living donor and deceased donor liver transplantation. Liver Transpl 2004;10:1248–1255. [DOI] [PubMed] [Google Scholar]
- 2.Gane E The natural history and outcome of liver transplantation in hepatitis C virus-infected recipients. Liver Transpl 2003;9:S28–S34. [DOI] [PubMed] [Google Scholar]
- 3.Russo MW, Galanko J, Beavers K, Fried MW, Shrestha R. Patient and graft survival in hepatitis C recipients after adult living donor liver transplantation in the United States. Liver Transpl 2004;10: 340–346. [DOI] [PubMed] [Google Scholar]
- 4.McLauchlan J Lipid droplets and hepatitis C virus infection. Biochim Biophys Acta 2009;1791:552–559. [DOI] [PubMed] [Google Scholar]
- 5.Robinson JL, Doucette K. The natural history of hepatitis C virus infection acquired during childhood. Liver Int 2012;32:258–270. [DOI] [PubMed] [Google Scholar]
- 6.Valenti L, Al-Serri A, Daly AK, Galmozzi E, Rametta R, Dongiovanni P, et al. Homozygosity for the patatin-like phospholipase-3/adiponutrin I148M polymorphism influences liver fibrosis in patients with nonalcoholic fatty liver disease. Hepatology 2010;51:1209–1217. [DOI] [PubMed] [Google Scholar]
- 7.Sookoian S, Castano GO, Burgueno AL, Gianotti TF, Rosselli MS, Pirola CJ. A nonsynonymous gene variant in the adiponutrin gene is associated with nonalcoholic fatty liver disease severity. J Lipid Res 2009; 50:2111–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sookoian S, Castano GO, Burgueno AL, Fernandez Gianotti T, Rosselli MS, Pirola CJ. A nonsynonymous gene variant in adiponutrin gene is associated with nonalcoholic fatty liver disease severity. J Lipid Res 2009;50:2111–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He S, McPhaul C, Li JZ, Garuti R, Kinch L, Grishin NV, et al. A sequence variation (I148M) in PNPLA3 associated with nonalcoholic fatty liver disease disrupts triglyceride hydrolysis. J Biol Chem 2010; 285:6706–6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romeo S, Huang-Doran I, Baroni MG, Kotronen A. Unravelling the pathogenesis of fatty liver disease: patatin-like phospholipase domain-containing 3 protein. Curr Opin Lipidol 2010;21:247–252. [DOI] [PubMed] [Google Scholar]
- 11.Wilson PA, Gardner SD, Lambie NM, Commans SA, Crowther DJ. Characterization of the human patatin-like phospholipase family. J Lipid Res 2006;47:1940–1949. [DOI] [PubMed] [Google Scholar]
- 12.do ON, Eurich D, Trautwein C, Neuhaus P, Neumann UP, Wasmuth HE. The common I148 M variant of PNPLA3 does not predict fibrosis progression after liver transplantation for hepatitis C. Hepatology 2011;54:1483–1484. [DOI] [PubMed] [Google Scholar]
- 13.Maganty K, Levi D, Moon J, Bejarano PA, Arosemena L, Tzakis A, et al. Combined hepatocellular carcinoma and intrahepatic cholangiocarcinoma: outcome after liver transplantation. Dig Dis Sci 2010;55: 3597–3601. [DOI] [PubMed] [Google Scholar]
- 14.Feng S, Goodrich NP, Bragg-Gresham JL, Dykstra DM, Punch JD, DebRoy MA, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant 2006;6:783–790. [DOI] [PubMed] [Google Scholar]
- 15.Valenti L, Aghemo A, Stattermayer AF, Maggioni P, De Nicola S, Motta BM, et al. Implications of PNPLA3 polymorphism in chronic hepatitis C patients receiving peginterferon plus ribavirin. Aliment Pharmacol Ther 2012;35:1434–1442. [DOI] [PubMed] [Google Scholar]
- 16.Olthoff KM, Kulik L, Samstein B, Kaminski M, Abecassis M, Emond J, et al. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl 2010;16:943–949. [DOI] [PubMed] [Google Scholar]
- 17.Deschenes M, Belle SH, Krom RA, Zetterman RK, Lake JR. Early allograft dysfunction after liver transplantation: a definition and predictors of outcome. National Institute of Diabetes and Digestive and Kidney Diseases Liver Transplantation Database. Transplantation 1998;66:302–310. [DOI] [PubMed] [Google Scholar]
- 18.Wagener G, Raffel B, Young AT, Minhaz M, Emond J. Predicting early allograft failure and mortality after liver transplantation: the role of the postoperative model for end-stage liver disease score. Liver Transpl 2013;19:534–542. [DOI] [PubMed] [Google Scholar]
- 19.Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet 2008;40:1461–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rotman Y, Koh C, Zmuda JM, Kleiner DE, Liang TJ. The association of genetic variability in patatin-like phospholipase domain-containing protein 3 (PNPLA3) with histological severity of nonalcoholic fatty liver disease. Hepatology 2010;52:894–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tian C, Stokowski RP, Kershenobich D, Ballinger DG, Hinds DA. Variant in PNPLA3 is associated with alcoholic liver disease. Nat Genet 2010;42:21–23. [DOI] [PubMed] [Google Scholar]
- 22.Cai T, Dufour JF, Muellhaupt B, Gerlach T, Heim M, Moradpour D, et al. Viral genotype-specific role of PNPLA3, PPARG, MTTP, and IL28B in hepatitis C virus-associated steatosis. J Hepatol 2011;55:529–535. [DOI] [PubMed] [Google Scholar]
- 23.Valenti L, Rumi M, Galmozzi E, Aghemo A, Del Menico B, De Nicola S, et al. Patatin-like phospholipase domain-containing 3 I148M polymorphism, steatosis, and liver damage in chronic hepatitis C. Hepatology 2011;53:791–799. [DOI] [PubMed] [Google Scholar]
- 24.Corradini SG, Burza MA, Molinaro A, Romeo S. Patatin-like phospholipase domain containing 3 sequence variant and hepatocellular carcinoma. Hepatology 2011;53:1776; author reply 1777. [DOI] [PubMed] [Google Scholar]
- 25.Trepo E, Pradat P, Potthoff A, Momozawa Y, Quertinmont E, Gustot T, et al. Impact of patatin-like phospholipase-3 (rs738409 C>G) polymorphism on fibrosis progression and steatosis in chronic hepatitis C. Hepatology 2011;54:60–69. [DOI] [PubMed] [Google Scholar]
- 26.Chen W, Chang B, Li L, Chan L. Patatin-like phospholipase domain-containing 3/adiponutrin deficiency in mice is not associated with fatty liver disease. Hepatology 2010;52:1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finkenstedt A, Auer C, Glodny B, Posch U, Steitzer H, Lanzer G, et al. PNPLA3 rs738409-G in recipients of liver transplants is a risk factor for graft steatosis. Clin Gastroenterol Hepatol 2013. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 28.Watt KD, Dierkhising R, Fan C, Heimbach JK, Tillman H, Goldstein D, et al. Investigation of PNPLA3 and IL28B genotypes on diabetes and obesity after liver transplantation: insight into mechanisms of disease. Am J Transplant 2013;13:2450–2457. [DOI] [PubMed] [Google Scholar]
- 29.Busuttil RW, Tanaka K. The utility of marginal donors in liver transplantation. Liver Transpl 2003;9:651–663. [DOI] [PubMed] [Google Scholar]
- 30.Loinaz C, Gonzalez EM. Marginal donors in liver transplantation. Hepatogastroenterology 2000;47:256–263. [PubMed] [Google Scholar]
- 31.Marsman WA, Wiesner RH, Rodriguez L, Batts KP, Porayko MK, Hay JE, et al. Use of fatty donor liver is associated with diminished early patient and graft survival. Transplantation 1996;62:1246–1251. [DOI] [PubMed] [Google Scholar]
- 32.Doyle MB, Vachharajani N, Wellen JR, Anderson CD, Lowell JA, Shenoy S, et al. Short- and long-term outcomes after steatotic liver transplantation. Arch Surg 2010;145:653–660. [DOI] [PubMed] [Google Scholar]
- 33.Subramanian V, Seetharam AB, Vachharajani N, Tiriveedhi V, Angaswamy N, Ramachandran S, et al. Donor graft steatosis influences immunity to hepatitis C virus and allograft outcome after liver transplantation. Transplantation 2011;92:1259–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.