Abstract
Purpose of review
SGLT2 inhibitors are a new class of antihyperglycemic drugs that protect kidneys and hearts of type 2 diabetic (T2DM) patients with preserved kidney function from failing. Here we discuss new insights on renal protection.
Recent findings
Also in T2DM patients with CKD, SGLT2 inhibition causes an immediate functional reduction in glomerular filtration rate (GFR) and reduces blood pressure and preserves kidney and heart function in the long-term, despite a lesser antihyperglycemic effect. According to modeling studies, the GFR reduction reduces the tubular transport work and metabolic demand, thereby improving renal cortical oxygenation. In humans, the latter is linked to protection from CKD. Urine metabolomics in T2DM patients suggested improved renal mitochondrial function in response to SGLT2 inhibition, and experimental studies indicated improved tubular autophagy. Modeling studies predicted that also in diabetic CKD, SGLT2 inhibition is natriuretic and potentially stimulates erythropoiesis by mimicking systemic hypoxia in the kidney. Meta-analyses indicated that SGLT2 inhibition also reduces risk and severity of acute kidney injury in T2DM patients. Studies in nondiabetic mice implied inhibition of the renal urate transporter URAT1 in the uricosuric effect of SGLT2 inhibition.
Summary
Renoprotection of SGLT2 inhibition involves blood glucose-dependent and independent effects and extends to CKD.
Keywords: acute kidney injury, chronic kidney disease, diabetic nephropathy, glucose transport, SGLT2 inhibitors
INTRODUCTION
Type 2 diabetes mellitus (T2DM) is a growing public health concern worldwide with increasing prevalence and high mortality rate. Normalizing blood glucose levels early on is important to lower the risk of macrovascular and microvascular complications, including diabetic nephropathy and cardiovascular disease and mortality [1]. Inhibitors of the sodium-glucose cotransporter 2 (SGLT2) are a new class of antihyperglycemic drugs recently approved in T2DM, which inhibit renal glucose reabsorption in the early proximal tubule, thereby enhancing urinary glucose excretion and lowering blood glucose levels. SGLT2 inhibitors act on their target from the extracellular surface of the brush border cell membrane [2], which they reach by glomerular filtration. and as recently indicated for empagliflozin, also by tubular secretion [3▪]. The use of SGLT2 inhibitors is associated with pleiotropic beneficial effects on the renal and cardiovascular system [4,5], and these drugs reduce the risk of renal and heart failure as demonstrated in multiple large clinical outcome trials in patients with T2DM and preserved kidney function [6]. This review aims to provide a brief update on the most recent clinical and experimental studies that shed new light on the therapeutic potential of SGLT2 inhibitors and the involved renoprotective mechanisms.
SGLT2 INHIBITION IMPROVES CARDIOVASCULAR AND RENAL OUTCOMES IN TYPE 2 DIABETES MELLITUS PATIENTS WITH PRESERVED KIDNEY FUNCTION AND ESTABLISHED CHRONIC KIDNEY DISEASE
SGLT2 inhibition improved renal and cardiovascular outcomes in T2DM patient with high cardiovascular risk [7]. The initial trials were conducted in patients with an overall relatively well preserved kidney function, although subanalyses indicated that the beneficial effects may extend to patients with CKD [8]. SGLT2 inhibitors block renal tubular glucose reabsorption and thus their glucoselowering efficacy depends on the amount of filtered glucose, and thus glomerular filtration rate (GFR). Consequently, SGLT2 inhibitors have not been indicated in T2DM patients with more advanced CKD. Recently, the Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial assessed the effects of canagliflozin treatment vs. placebo on renal outcomes and secondary cardiovascular outcomes in T2DM patients with albuminuric CKD [9▪▪]. The 4401 patients enrolled had an eGFR of 30 to less than 90ml/min/1.73m2 and albuminuria (ratio albumin-to-creatinine >300 to 5000 mg/g) and were followed over a median period of 2.62 years. Patients were receiving renin-angiotensin system (RAS) blockers as standard treatment. Canagliflozin reduced the risk of the primary outcome composite of ESRD (dialysis, transplantation, or a sustained estimated GFR of <15ml/min/1.73m2) by 30%, reduced the risk of the renal-specific composite of ESRD, a doubling of the creatinine level or death from renal causes, by 34% and reduced the risk of ESRD by 32% vs. placebo. Canagliflozin also lowered the risk of cardiovascular death, myocardial infarction, stroke, and lowered the rate of hospitalization for heart failure [9▪▪]. These findings are consistent with a previous systematic review and meta-analysis of multiple randomized controlled trials that included patients with T2DM and CKD treated with an SGLT2 inhibitor [10]. Reduction in HbA1c was modest in T2DM patients with CKD compared with the reductions observed in the general T2DM population [11]; however, the beneficial effects of SGLT2 inhibitors on cardiovascular and renal outcomes was preserved without raising additional safety concerns [10]. Overall, these studies suggest that SGLT2 inhibition prevents cardiovascular and renal outcomes in T2DM patients with CKD through mechanisms that are at least in part independent of blood glucose reduction. On the basis of the findings of the CREDENCE trial, the US Food and Drugs Administration (FDA) approved canagliflozin (Invokana) to treat diabetic kidney disease (DKD) and reduce the risk of hospitalization for heart failure in patients with T2DM and DKD [12]. Mathematical modeling indicated that the natriuretic and diuretic effect of SGLT2 inhibition is preserved in CKD because of a high load of glucose on the single nephron level. This high glucose load was the consequence of higher levels of blood glucose and single nephron hyperfiltration in remaining nephrons, and induced paracellular sodium secretion in the proximal tubule thereby preserving a natriuretic, kaliuretic and diuretic effect despite reduced nephron number [13▪▪] (Fig. 1).
FIGURE 1.
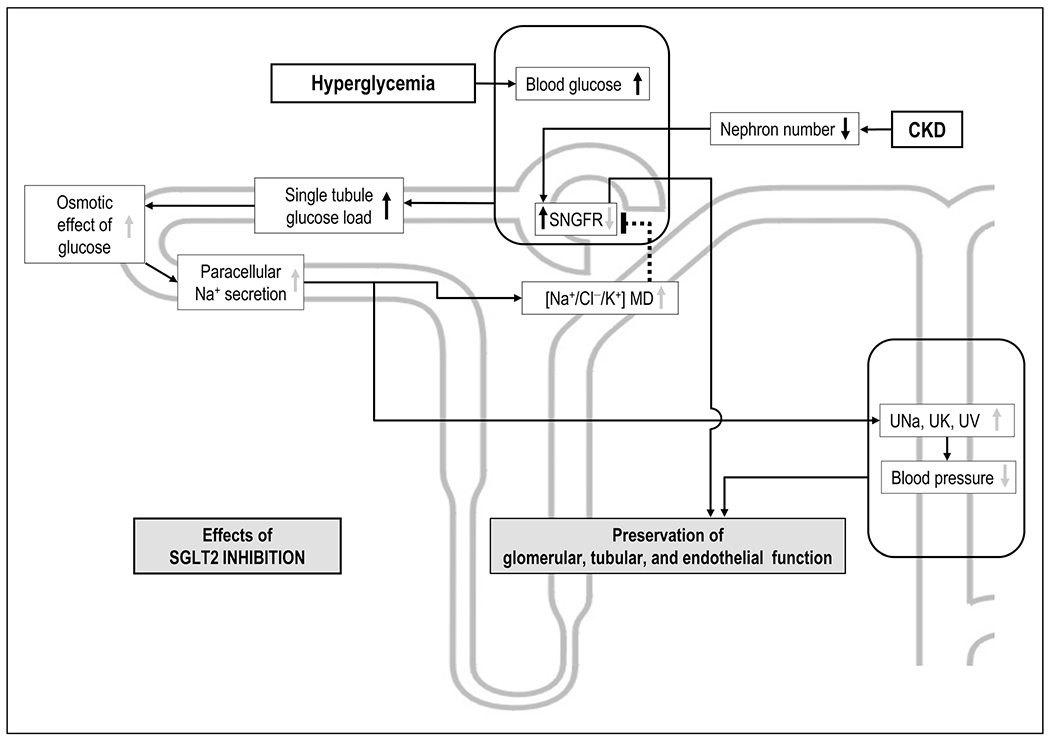
Proposed natriuretic effect of SGLT2 inhibition in diabetic chronic kidney disease. Depicted are effects of hyperglycemia or CKD (black arrows) and the effect of SGLT2 inhibition (grey arrows). CKD lowers nephron number and increases single nephron GFR in remaining nephrons. Together with hyperglycemia, this results in high single tubule glucose load. Mathematical modeling predicts that SGLT2 inhibition under these conditions increases the osmotic effect of luminal glucose to an extent that paracellular sodium secretion is induced in the proximal tubule, thereby preserving a robust natriuretic, diuretic and kaliuretic effect. This may contribute to the preserved blood pressure-lowering effect of SGLT2 in diabetic CKD. CKD, chronic kidney disease; GFR, glomerular filtration rate; MD, macula densa; SNGFR, single nephron glomerular filtration rate; UK, urinary excretion of potassium; UNa, urinary excretion of sodium; UV, urinary flow rate.
Recent meta-analyses also reported that SGLT2 inhibition is associated with a robust and consistent reduction in acute kidney injury (AKI) [14▪,15▪]. In accordance, dapagliflozin treatment decreased urinary levels of markers of glomerular and tubular injury in T2DM patients [16,17], an effect also shown in diabetic rats [18,19] (Fig. 2). Luseogliflozin prevented renal capillary rarefaction and reduced hypoxia and fibrosis in a murine model of renal ischemia–reperfusion [20▪]. SGLT2 gene-knockout in mice, however, did not affect ischemia–reperfusion injury and subsequent GFR recovery in preliminary studies in the bilateral renal artery clamping model of ischemia–reperfusion injury [21]. In comparison, Nespoux et al. [22] showed that gene-knockout of SGLT1 did not affect the initial kidney injury but improved kidney recovery in this model. Renal SGLT1 expression appeared to recover before SGLT2 in this model, which may have enhanced the transport burden in the outer medulla during recovery from ischemia–reperfusion injury.
FIGURE 2.
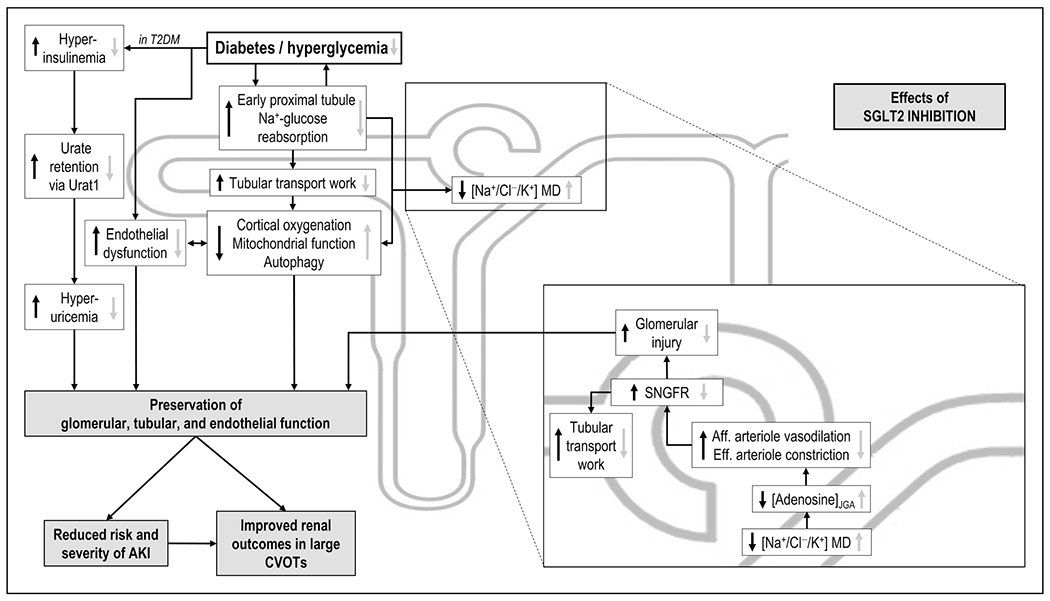
Proposed kidney protective effects of SGLT2 inhibition. Depicted are effects of diabetes/hyperglycemia (black arrows) and the effect of SGLT2 inhibition (grey arrows). Included is the tubular hypothesis of SGLT2 inhibition-mediated renoprotection in diabetes, including decreased glomerular hyperfiltration via adenosine-dependent hemodynamic changes, which reduce the tubular transport burden and preserve GFR in the long-term. Depicted are also the various influences of SGLT2 inhibition on renal metabolism and mitochondrial and endothelial integrity; as well as the uricosuric effect of SGLT2 inhibition. AKI, acute kidney injury; CVOTs, cardiovascular outcome trials; GFR, glomerular filtration rate; JGA juxtaglomerular apparatus; MD, macula densa; SNGFR, single nephron glomerular filtration rate; T2DM, type 2 diabetes mellitus; URAT1, urate transporter 1.
Currently, the randomized placebo-controlled CKD outcome trials Dapa-CKD and Empa-Kidney (ClinicalTrials.gov Identifiers: NCT03036150 and NCT03594110, respectively) are conducted in patients with established kidney disease, with or without diabetes, and treatment with the SGLT2 inhibitor dapagliflozin or empagliflozin, respectively. These trials are expected to provide further insights into the beneficial effects of SGLT2 inhibitors in CKD populations beyond glycemic control [23,24].
SGLT2 AND SGLT1 CAN EXPLAIN ALL RENAL GLUCOSE REABSORPTION IN NONDIABETIC AND DIABETIC MICE
In normoglycemia and with normal GFR, the two kidneys of a healthy individual filter up to 180 g of glucose daily. Glucose is a valuable energy substrate and, as a consequence, filtered glucose is basically completely reabsorbed (99.9%) by the tubular system, primarily through SGLT2 and SGLT1 in the proximal tubule. Studies in nondiabetic mice showed that SGLT2 reabsorbs all filtered glucose in the early proximal tubule (S1/S2 segments) and the majority of glucose (~97%) on the whole kidney level. SGLT1 reabsorbs the remaining glucose delivered to the late proximal tubule (S2/S3 segments) (for review see [4,5]). Recent studies confirmed that SGLT2 and SGLT1 can explain all renal glucose reabsorption in nondiabetic mice and extended the finding to genetic models of T2DM (db/db) and T1DM (Akita) [25▪▪].
Hyperglycemia enhances glomerular filtration of glucose. To prevent the renal loss of glucose, human kidneys increase their glucose reabsorption maximum capacity by ~20% to up to 600 g/day. This is associated with tubular growth [4,5]. Yakovleva et al. [26] used quantitative drug–disease systems modelling to propose that higher renal glucose reabsorption in T2DM patients vs. healthy individuals is associated with 54 and 28% greater transport maximum (Vmax) for SGLT1 and SGLT2, respectively. Studies in humans with T2DM [27,28] and genetic rodent models of T2DM and T1DM support an upregulation of renal SGLT2 protein expression [27,29,30], whereas the response in SGLT1 is less clear [4,5].
The early proximal tubule not only reabsorbs glucose (via SGLT2) but also generates glucose by gluconeogenesis. The latter not only occurs particularly in the postprandial phase but also in response to metabolic acidosis, as tubular gluconeogenesis is linked to bicarbonate formation. In this regard, Onishi et al. [31] linked enhanced renal gluconeogenesis to an inhibition of SGLT2 expression inasmuch as upregulation of renal gluconeogenesis to compensate for impaired bicarbonate reabsorption in mice lacking tubular NHE3 was associated with marked suppression of SGLT2 protein expression, probably to prevent glucose overload in these cells. In other words, if diabetes is associated with metabolic acidosis, then the resulting increase in renal gluconeogenesis can lower renal SGLT2 expression. The studies by Onishi et al. [31] further indicated that in this setting of suppressed SGLT2 expression, the downstream shift of glucose was associated with upregulation of SGLT1-mediated glucose reabsorption suggesting a coordinated response.
The uricosuric and blood urate-lowering effect of SGLT2 inhibitors is a class effect [4]. Using knockout mouse models for SGLT2, SGLT1, URAT1 and GLUT9, studies by Novikov et al. [32▪] indicated a role of luminal glucose delivery and inhibition of the proximal tubule urate transporter URAT1 in the uricosuric effect of the SGLT2 inhibitor canagliflozin. Insulin enhances URAT1 activity [33]. Therefore, SGLT2 inhibition may reduce URAT1 activity by enhancing luminal glucose delivery or lowering insulin levels (Fig. 2), or SGLT2 and URAT1 may functionally interact in the proximal tubule, such that inhibition of SGLT2 partially inhibits URAT1.
SGLT2 INHIBITORS LOWER GLOMERULAR HYPERFILTRATION AND PRESERVE GLOMERULAR FILTRATION RATE IN THE LONG-TERM
SGLT-mediated glucose transport is coupled with sodium reabsorption. Thus, tubular growth and enhanced glucose reabsorption in the proximal tubule of the diabetic kidney is associated with enhanced sodium and fluid reabsorption (Figs. 2 and 3). The latter decreases fluid and NaCl delivery to the downstream juxtaglomerular apparatus (JGA). In the JGA, a group of specialized cells, the macula densa cells, release ATP into the interstitium of the JGA in proportion to the luminal NaCl concentration. The released ATP is broken down to adenosine. Adenosine is the mediator of the tubuloglomerular feedback (TGF). Adenosine lowers hydrostatic glomerular capillary pressure and GFR primarily via activation of vasoconstrictor adenosine A1 receptors on the afferent arteriole, with a smaller contribution of vasodilator adenosine A2 receptors on the efferent arteriole under some conditions [34,35]. By establishing an inverse relationship between NaCl delivery to the macula and GFR, the TGF helps to stabilize distal tubule NaCl delivery. In diabetes, the reduced delivery of NaCl to the macula densa causes an increase in GFR through the physiology of TGF (Fig. 2). The increase in proximal tubule reabsorption also reduces tubular back pressure, which further increases GFR. The increase in GFR restores fluid and electrolyte load to the distal tubule and helps to restore sodium and fluid balance. This forms the basis for the tubular hypothesis of diabetic glomerular hyperfiltration and the immediate and desirable GFR-lowering effect of SGLT2 inhibition [4,5] (Fig. 2).
FIGURE 3.
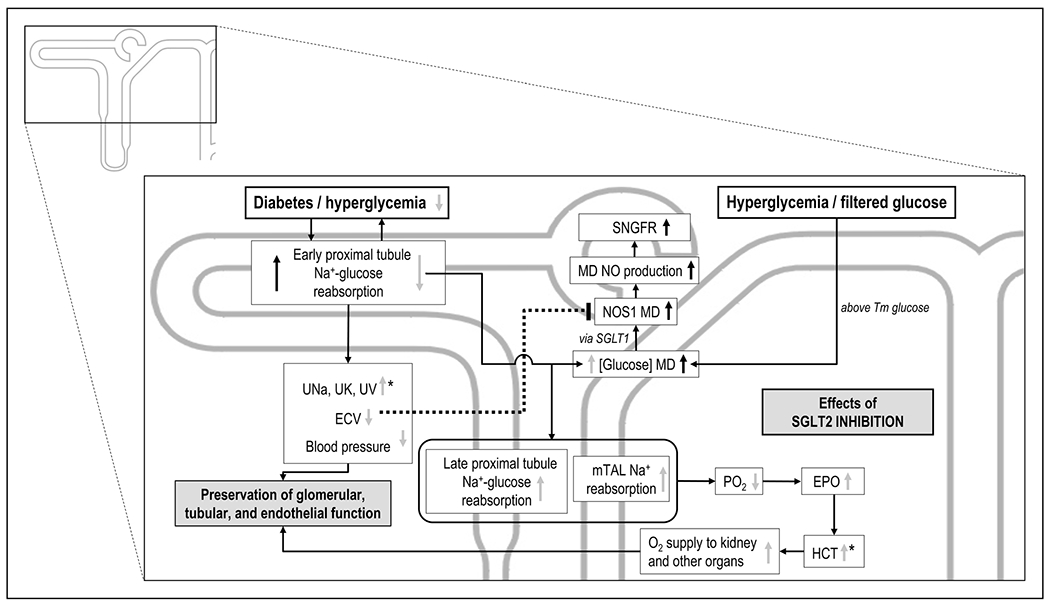
Proposed role for SGLT1 and NOS1 in macula densa in the modulation of glomerular filtration rate and potential interactions with SGLT2 inhibition. Depicted are effects of diabetes/hyperglycemia (black arrows) and the effect of SGLT2 inhibition (grey arrows). Depicted is the sensing of luminal glucose at the macula densa by SGLT1 and the link to GFR via NOS1. Shown are also the complex influences of SGLT2 inhibition on this pathway, including effects on macula densa glucose delivery and effective circulating volume (ECV). Depicted is also the shift in Na transport activity to the outer medulla upon SGLT2 blockade and the consequences on renal medullary oxygenation. Thus, SGLT2 inhibition may mimic systemic hypoxia to the oxygen-sensing structures in the deep cortex and outer medulla, and through an increase in erythropoietin release enhance hematocrit and oxygen delivery to the kidney and other organs. The asterisks indicate that increased UV also contributes to the increase in HCT, that is, the plasma concentration effect. EPO, erythropoietin; GFR, glomerular filtration rate; HCT, hematocrit; MD, macula densa; mTAL, medullary thick ascending limb; NO, nitric oxide; NOS1, nitric oxide synthase 1; PO2, oxygen tension; SGLT1, sodium-glucose cotransporter 1; SNGFR, single nephron glomerular filtration rate; Tm, tubular maximum transport capacity; T2DM, type 2 diabetes mellitus; UK, urinary excretion of potassium; UNa, urinary excretion of sodium; UV, urinary flow rate.
Diabetic hyperfiltration contributes to the development and progression of DKD as it increases the tubular transport burden. Lowering GFR at the onset of treatment forms the therapeutic basis for therapies targeting the renin–angiotensin system and SGLT2 inhibition, thereby alleviating tubular workload. The large SGLT2 inhibitor cardiovascular outcomes trials in T2DM patients demonstrated that empagliflozin, canagliflozin, and dapagliflozin reduce hyperfiltration at the onset of therapy and slow the decline in eGFR in the long-term [36–38]. Most importantly, the GFR reduction in response to SGLT2 inhibition is reversible after drug discontinuation, consistent with the functional mechanisms described above. Similar responses were observed in subgroups of patients with lower kidney function, with a rapid and reversible decrease in eGFR followed by better preservation of GFR in the long-term [36,37]. Similarly, new data in CREDENCE showed that during the first 3 weeks of treatment, eGFR reduction was greater in the canagliflozin group vs. placebo (−3.72 ± 0.25 vs. −0.55 ± 0.25 ml/min/1.73 m2). Thereafter, eGFR decline was slower in the canagliflozin group vs. placebo (−1.85±0.13 vs. −4.59±0.14 ml/min/1.73m2/year) [9▪▪]. Additional analyses of the CANVAS-Program trial showed that canagliflozin slowed eGFR reduction across T2DM patients with different levels of albuminuria, and canagliflozin absolute effect was greater in patients with the highest albuminuria level vs. placebo [39].
In accordance with the discussed role of the TGF mechanism in diabetic hyperfiltration, the increase in GFR in response to streptozotocin (STZ)-diabetes was blunted in adenosine A1 receptor knockout mice, which are TGF-less in response to increasing or decreasing (in diabetes) the macula densa NaCl signal [40]. Moreover, SGLT2 inhibition with empagliflozin increased urinary adenosine excretion in T1DM patients [41]. Using direct in-vivo visualization technique, Kidokoro et al. demonstrated that glomerular afferent arteriole diameter is 50–60% greater and associated with increased SNGFR in Akita T1DM mice compared with nondiabetic mice. Empagliflozin acutely lowered glomerular hyperfiltration within 30 min and reduced afferent arteriole diameter by ~15% compared with vehicle. This was associated with greater urinary adenosine levels. Empagliflozin effects were abolished by pharmacological blockade of the A1 receptor but not by COX-2/NOS1 inhibition, consistent with the notion that SGLT2 inhibition reduces diabetic hyperfiltration predominantly via adenosine-mediated vasoconstriction of the afferent arteriole [42] (Fig. 2). Preliminary studies in patients with T2DM suggested that SGLT2 inhibition may also lower GFR by reducing filtration fraction [43], which may reflect the vasodilator effect of adenosine on the efferent arteriole (Fig. 2).
By reducing glomerular hyperfiltration and inhibiting proximal tubular hyperreabsorption, SGLT2 inhibition reduces active tubular transport work and, thereby reduces the energy demand and oxygen consumption, primarily in the kidney cortex, as predicted by mathematical modeling of the rat nephron with normal and reduced nephron number [13▪▪,44], and consistent with experimental data using the SGLT2/SGLT1 inhibitor phlorizin [45] (Fig. 2). In this regard, recent studies by Prujim et al. indicated that reduced cortical oxygenation predicts progressive decline of renal function in CKD [46▪]. By reducing glomerular hyperfiltration and hyperglycemia, SGLT2 inhibition also reduces albuminuria, tubular growth, and tubulointerstitial inflammation [4,5]. Studies by Cassis et al. [47▪] proposed that podocytes may express SGLT2 in protein overload conditions.
SGLT2 INHIBITION MAY IMPROVE KIDNEY METABOLISM AND AUTOPHAGY
The greatest transport burden in the diabetic kidney is on the early proximal tubule [13▪▪,44]. By inhibiting SGLT2, the transport burden is more equally distributed among the tubular segments. Moreover, by lowering GFR, the total tubular transport burden is reduced. These effects of SGLT2 inhibition may also help to preserve mitochondrial function and tubular cell metabolism, which is expected to preserve tubular function and GFR in the long-term. Preliminary studies in T1DM Akita mice and patients with T2DM, showed that diabetes increased the urinary ratio of lactate to pyruvate, which may reflect a metabolic shift from mitochondrial oxidation to more glycolysis, and that this was reversed by an SGLT2 inhibitor [48]. A study in patients with T2DM and albuminuria found that dapagliflozin treatment increased urinary metabolites previously linked to mitochondrial metabolism compared with placebo, thus suggesting that dapagliflozin improves mitochondrial function in diabetes [49▪]. Studies in Akita mice showed that empagliflozin reduced the renal accumulation of p62, providing first evidence that SGLT2 inhibition may improve autophagy in the diabetic kidney [50]. Lee et al. showed that empagliflozin treatment improved mitochondrial fragmentation and enhanced renal proximal tubule cell autophagic activity under high glucose conditions and in STZ-treated mice, potentially involving the AMPK and mTOR pathway, thereby leading to lesser apoptosis and tubulointerstitial fibrosis [51▪]. Similarly, the SGLT2 inhibitor ipragliflozin reversed the tubular and mitochondrial damage caused by high-fat diet in mice, independent of blood glucose levels [52]. Thus, SGLT2 inhibitors may exert renoprotection and preserve kidney integrity by improving tubular energetics, mitochondria integrity and function, as well as autophagy (Fig. 2).
SGLT2 INHIBITION MAY MIMIC SYSTEMIC HYPOXIA TO THE OXYGEN SENSOR IN THE KIDNEY THEREBY STIMULATING ERYTHROPOIESIS AND IMPROVING OXYGEN DELIVERY TO KIDNEY AND HEART
SGLT2 blockade shifts Na and glucose reabsorption to downstream tubular segments in the outer medulla, a kidney region with physiologically low PO2 (Fig. 3). The resulting increase in oxygen consumption may further reduce medullary PO2. This can trigger hypoxia-inducible factor (HIF)-1 and HIF-2 dependent pathways, upregulate downstream effectors, such as erythropoietin (EPO), and be protective in the long-term by improving cortical and medullary oxygen delivery [5,13▪▪] (Fig. 3). Mathematical modeling studies proposed that these effects of SGLT2 inhibition are preserved in CKD [13▪▪].
EXTENDING THE TUBULAR HYPOTHESIS OF DIABETIC HYPERFILTRATION TO A NEW ROLE OF SGLT1 IN THE MACULA DENSA: IMPLICATIONS FOR SGLT2 INHIBITION
Recent studies identified the SGLT1-nitric oxide synthase 1 (NOS1) axis in macula densa cells as a new contributor to glomerular hyperfiltration in diabetes. Zhang et al. demonstrated SGLT1 expression at the luminal membrane of macula densa cells in humans and found that acute hyperglycemia in mice increases GFR by a mechanisms that involve NOS1 in the macula densa [53▪▪]. Moreover, studies in isolated perfused juxtamedullary nephrons and micropuncture studies revealed that increased tubular glucose blunts the TGF response, and that this effect is mediated by SGLT1, which senses luminal glucose and enhances MD NOS1 protein expression and phosphorylation, resulting in increased NO production thereby attenuating the vasoconstrictor effect of TGF [53▪▪] (Fig. 3). Moreover, Song et al. [25▪▪] demonstrated that the concept is relevant in T1DM Akita and STZ-diabetic mice, inasmuch as glomerular hyperfiltration is attenuated in mice lacking SGLT1 with minimal or no effects on blood glucose levels. Moreover, absence of SGLT1 prevents the increase in MD NOS1 expression observed in T1DM Akita mice or in response to an SGLT2 inhibitor in nondiabetic mice. Notably, SGLT2 inhibition in T1DM Akita mice lowers MD NOS1 expression. This is consistent with the notion that multiple factors regulate MD NOS1 (Fig. 3). The authors speculated that the natriuretic and diuretic effect of the SGLT2 inhibitor reduces effective circulating volume [54], a stimulator of MD NOS1, thereby opposing any macula densa glucose effect and causing a net reduction (Fig. 3). This net response was likely facilitated by the fact that in more severe conditions of hyperglycemia, chronic SGLT2 inhibition is not expected to change MD glucose because of the associated reduction in GFR and blood glucose [25▪▪]. Nevertheless, this new MD SGLT1 NOS1 mechanism may play a role in patients who show a blunted GFR-lowering effect in response to initiating SGLT2 inhibition.
CONCLUSION
SGLT2 inhibitors target renal glucose reabsorption in the proximal tubule and induce pleiotropic beneficial effects on the renal and cardiovascular systems. The CREDENCE trial indicated that SGLT2 inhibitors also improve renal and cardiovascular outcomes in T2DM patients with CKD despite a lesser blood glucose effect. Clinical and experimental studies and mathematical modeling provided further mechanistic insights on blood glucose-dependent and independent mechanisms underlying the renoprotective effects of SGLT2 inhibition.
KEY POINTS.
SGLT2 inhibition improves renal and cardiovascular outcomes in patients with CKD despite lesser blood glucose-lowering indicating a role for effects independent of glycemia control, and as a consequence, studies to define the effects of SGLT2 inhibitors on renal and cardiovascular outcomes in nondiabetic patients are on the way.
By lowering hyperfiltration at the onset of therapy, SGLT2 inhibition reduces tubular metabolic demand and helps preserving GFR in the long-term, which possibly involves improving cortical oxygenation and preserving tubular mitochondrial function and autophagy.
Mathematical modeling provided new insights that may explain why renal and cardiovascular effects in response to SGLT2 inhibition are preserved in CKD despite reduced blood glucose effects.
SGLT2 inhibition also decreases the risk and severity of AKI, a known contributor to CKD development.
SGLT1 in the macula densa senses luminal glucose and via stimulation of NOS1 contributes to diabetic glomerular hyperfiltration, a pathway that is expected to modulate the GFR response to SGLT2 inhibition.
Acknowledgements
Financial support and sponsorship
The authors were supported by NIH grants R01DK112042, R01DK106102, R01HL139836, RF1AG061296, the UAB/UCSD O’Brien Center of Acute Kidney Injury NIH-P30DK079337, the Department of Veterans Affairs (all to V.V.), and by The University of Edinburgh College of Medicine & Veterinary Medicine PhD Studentship (to J.N.).
Footnotes
Conflicts of interest
Over the past 36 months, V.V. has served as a consultant and received honoraria from Astra-Zeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Janssen Pharmaceutical and Merck, and received grant support for investigator-initiated research from Astra-Zeneca, Bayer, Boehringer Ingelheim, Fresenius, and Janssen Pharmaceutical. J.N. has no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
- 1.Laiteerapong N, Ham SA, Gao Y, et al. The legacy effect in type 2 diabetes: impact of early glycemic control on future complications (The Diabetes & Aging Study). Diabetes Care 2019; 42:416–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghezzi C, Hirayama BA, Gorraitz E, et al. SGLT2 inhibitors act from the extracellular surface of the cell membrane. Physiol Rep 2014; 2:e12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.▪.Fu Y, Breljak D, Onishi A, et al. Organic anion transporter OAT3 enhances the glucosuric effect of the SGLT2 inhibitor empagliflozin. Am J Physiol Renal Physiol 2018; 315:F386–F394. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study indicates that the SGLT2 inhibitor empagliflozin reaches its target in the brush border of the proximal tubule in part by tubular secretion.
- 4.Vallon V, Thomson SC. Targeting renal glucose reabsorption to treat hyper-glycaemia: the pleiotropic effects of SGLT2 inhibition. Diabetologia 2017; 60:215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nespoux J, Vallon V. SGLT2 inhibition and kidney protection. Clin Sci (Lond) 2018; 132:1329–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zelniker TA, Wiviott SD, Raz I, et al. Comparison ofthe effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus: systematic review and meta-analysis of cardiovascular outcomes trials. Circulation 2019; 139:2022–2031. [DOI] [PubMed] [Google Scholar]
- 7.Cardiorenal outcomes in the CANVAS, DECLARE-TIMI 58, and EMPA-REG OUTCOME trials: a systematic review. Rev Cardiovasc Med 2018; 19:41–49; Available at: https://rcm.imrpress.org/EN/10.31083/j.rcm.2018.02.907. [Accessed 15 August 2019] [DOI] [PubMed] [Google Scholar]
- 8.Mosenzon O, Wiviott SD, Cahn A, et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE-TIMI 58 randomised trial. Lancet Diabetes Endocrinol 2019; 7:606–617. [DOI] [PubMed] [Google Scholar]
- 9.▪▪.Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019; 380:2295–2306. [DOI] [PubMed] [Google Scholar]; This study shows that the SGLT2 inhibitor canagliflozin reduced the risk of kidney failure and cardiovascular events in T2DM patients with kidney disease. As previously shown in T2DM patients with preserved kidney function, eGFR decreased at onset of canagliflozin treatment, followed by better preservation of GFR in the long-term.
- 10.Toyama T, Neuen BL, Jun M, et al. Effect of SGLT2 inhibitors on cardiovascular, renal and safety outcomes in patients with type 2 diabetes mellitus and chronic kidney disease: a systematic review and meta-analysis. Diabetes Obes Metab 2019; 21:1237–1250. [DOI] [PubMed] [Google Scholar]
- 11.Petrykiv S, Sjostrom CD, Greasley PJ, et al. Differential effects ofdapagliflozin on cardiovascular risk factors at varying degrees of renal function. Clin J Am Soc Nephrol 2017; 12:751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raritan NJ. U.S. FDA approves INVOKANA® (canagliflozin) to treat diabetic kidney disease (DKD) and reduce the risk of hospitalization for heart failure in patients with type 2 diabetes (T2D) and DKD. PRNewswire. 30 September 2019; Available at: https://www.prnewswire.com/news-releases/us-fda-approves-invokana-canagliflozin-to-treat-diabetic-kidney-disease-dkd-and-reduce-the-risk-of-hospitalization-for-heart-failure-in-patients-with-type-2-diabetes-t2d-and-dkd-300927348.html. [Accessed 23 October 2019] [Google Scholar]
- 13.▪▪.Layton AT, Vallon V. SGLT2 inhibition in a kidney with reduced nephron number: modeling and analysis of solute transport and metabolism. Am J Physiol Renal Physiol 2018; 314:F969–F984. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using mathematical modeling of the rat nephron, this study predicts that the natriuretic and diuretic effect of SGLT2 inhibition is preserved in CKD despite reduced nephron number, and that SGLT2 inhibition improves kidney cortex oxygenation by lowering GFR and proximal tubule transport activity, and potentially stimulates erythropoiesis by mimicking systemic hypoxia in the kidney.
- 14.▪.Gilbert RE, Thorpe KE. Acute kidney injury with sodium-glucose co-transporter-2 inhibitors: a meta-analysis of cardiovascular outcome trials. Diabetes Obesity Metab 2019; 21:1996–2000. [DOI] [PubMed] [Google Scholar]; This meta-analysis of the three major CVOTs of SGLT2 inhibitors suggests that SGLT2 inhibition may protect T2DM patients from AKI.
- 15.▪.Neuen BL, Young T, Heerspink HJL, et al. SGLT2 inhibitorsforthe prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 2019; 7:845–854. [DOI] [PubMed] [Google Scholar]; This meta-analysis of the four major CVOTs of SGLT2 inhibitors, including CREDENCE, shows that SGLT2 inhibition reduces the risk of AKI in T2DM patients.
- 16.Dekkers CCJ, Petrykiv S, Laverman GD, et al. Effects of the SGLT-2 inhibitor dapagliflozin on glomerular and tubular injury markers. Diabetes Obes Metab 2018; 20:1988–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Satirapoj B, Korkiatpitak P, Supasyndh O. Effect of sodium-glucose cotransporter 2 inhibitor on proximal tubular function and injury in patients with type 2 diabetes: a randomized controlled trial. Clin Kidney J 2019; 12:326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oraby MA, El-Yamany MF, Safar MM, et al. Dapagliflozin attenuates early markers of diabetic nephropathy in fructose-streptozotocin-induced diabetes in rats. Biomed Pharmacother 2019; 109:910–920. [DOI] [PubMed] [Google Scholar]
- 19.Jaikumkao K, Pongchaidecha A, Chueakula N, et al. Dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, slows the progression of renal complications through the suppression of renal inflammation, endoplasmic reticulum stress and apoptosis in prediabetic rats. Diabetes Obes Metab 2018; 20:2617–2626. [DOI] [PubMed] [Google Scholar]
- 20.▪.Zhang Y, Nakano D, Guan Y, et al. Asodium-glucose cotransporter 2 inhibitor attenuates renal capillary injury and fibrosis by a vascular endothelial growth factor–dependent pathway after renal injury in mice. Kidney Int 2018; 94:524–535. [DOI] [PubMed] [Google Scholar]; These experimental studies indicate that pharmacological SGLT2 inhibition can attenuate acute kidney injury in a mouse model of ischemia and reperfusion.
- 21.Nespoux J, Patel R, Huang W, et al. Gene deletion of the Na-glucose cotransporter SGLT2 does not affect kidney injury or recovery in a murine model of severe AKI [abstract]. J Am Soc Nephrol 2018; 29:444. [Google Scholar]
- 22.Nespoux J, Patel R, Hudkins KL, et al. Gene deletion of the Na + -glucose cotransporter SGLT1 ameliorates kidney recovery in a murine model ofacute kidney injury induced by ischemia-reperfusion. Am J Physiol Renal Physiol 2019; 316:F1201 –F1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pecoits-Filho R, Perkovic V. Are SGLT2 inhibitors ready for prime time for CKD? Clin J Am Soc Nephrol 2018; 13:318–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herrington WG, Preiss D, Haynes R, et al. The potential for improving cardiorenal outcomes by sodium-glucose co-transporter-2 inhibition in people with chronic kidney disease: a rationale forthe EMPA-KIDNEY study. Clin KidneyJ 2018; 11:749–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.▪▪.Song P, Huang W, Onishi A, et al. Knockout of Na + -glucose cotransporter SGLT1 mitigates diabetes-induced upregulation of nitric oxide synthase NOS1 in the macula densa and glomerular hyperfiltration. Am J Physiol Renal Physiol 2019; 317:F207–F217. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study in T1DM Akita mice indicates that SGLT1 is a determinant of glucose-driven upregulation of NOS1 expression in the macula densa and diabetic hyperfiltration. The study also shows additive effects of SGLT2 and SGLT1 inhibition on blood glucose, glomerular hyperfiltration, kidney weight, and glomerular size.
- 26.Yakovleva T, Sokolov V, Chu L, et al. Urinaryglucose excretion contributions of SGLT2 vs. SGLT1 transporters: a quantitative systems pharmacology analysis in healthy and T2DM subjects administered SGLT2 inhibitors. Diabetes Obes Metab 2019; 21:2684–2693. [DOI] [PubMed] [Google Scholar]
- 27.Umino H, Hasegawa K, Minakuchi H, et al. High basolateral glucose increases sodium-glucose cotransporter 2 and reduces sirtuin-1 in renal tubules through glucose transporter-2 detection. Sci Rep 2018; 8:6791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang XX, LeviJ, LuoY, et al. SGLT2 protein expression is increased in human diabetic nephropathy: SGLT2 protein inhibition decreases renal lipid accumulation, inflammation, and the development of nephropathy in diabetic mice. J Biol Chem 2017; 292:5335–5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vallon V, Rose M, Gerasimova M, et al. Knockout of Na-glucose transporter SGLT2 attenuates hyperglycemia and glomerular hyperfiltration but not kidney growth or injury in diabetes mellitus. Am J Physiol Renal Physiol 2013; 304:F156–F167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wen L, Zhang Z, Peng R, et al. Whole transcriptome analysis of diabetic nephropathy in the db/db mouse model of type 2 diabetes. J Cell Biochem 2019; 120:17520–17533. [DOI] [PubMed] [Google Scholar]
- 31.Onishi A, Fu Y, Darshi M, et al. Effectof renaltubule-specific knockdown ofthe Na +/H + exchanger NHE3 in Akita diabetic mice. Am J Physiol Renal Physiol 2019; 317:F419–F434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.▪.Novikov A, Fu Y, Huang W, et al. SGLT2 inhibition and renal urate excretion: role of luminal glucose, GLUT9, and URAT1. Am J Physiol Renal Physiol 2019; 316:F173–F185. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study indicates that the uricosuric effect of the SGLT2 inhibitor canagliflozin involves inhibition of the tubular urate transporter URAT1.
- 33.Toyoki D, Shibata S, Kuribayashi-Okuma E, et al. Insulin stimulates uric acid reabsorption via regulating urate transporter 1 and ATP-binding cassette subfamily G member 2. Am J Physiol Renal Physiol 2017; 313:F826–F834. [DOI] [PubMed] [Google Scholar]
- 34.Vallon V, Miihlbauer B, Osswald H. Adenosine and kidney function. Physiol Rev 2006; 86:901–940. [DOI] [PubMed] [Google Scholar]
- 35.Vallon V, Unwin RJ, Inscho EW, et al. Extracellular nucleotides and P2 receptors in renal function. Physiol Rev 2020; 100:211–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perkovic V, Jardine M, Vijapurkar U, Meininger G. Renal effects of canagliflozin in type 2 diabetes mellitus. Curr Med Res Opin 2015; 31:2219–2231. [DOI] [PubMed] [Google Scholar]
- 37.Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. New Engl J Med 2016; 375: 323–334. [DOI] [PubMed] [Google Scholar]
- 38.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019; 380:347–357. [DOI] [PubMed] [Google Scholar]
- 39.Neuen BL, Ohkuma T, Neal B, et al. Effect of canagliflozin on renal and cardiovascular outcomes across different levels of albuminuria: data from the CANVAS program. J Am Soc Nephrol 2019; 30:2229–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vallon V, Schroth J, Satriano J, et al. Adenosine A1 receptors determine glomerular hyperfiltration and the salt paradox in early streptozotocin diabetes mellitus. Nephron Physiol 2009; 111:30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rajasekeran H, Lytvyn Y, Bozovic A, et al. Urinary adenosine excretion in type 1 diabetes. Am J Physiol Renal Physiol 2017; 313:F184–F191. [DOI] [PubMed] [Google Scholar]
- 42.Kidokoro K, Cherney DZI, Bozovic A, et al. Evaluation of glomerular hemodynamic function by empagliflozin in diabetic mice using in vivo imaging. Circulation 2019; 140:303–315. [DOI] [PubMed] [Google Scholar]
- 43.Van Bommel EJ, Muskiet MA, Van Baar MJ, et al. 243-OR: ADA Presidents’ select abstract: dapagliflozin reduces measured GFR by reducing renal efferent arteriolar resistance in type 2 diabetes. Diabetes 2019; 68(Suppl 1); 243–OR. [Google Scholar]
- 44.Layton AT, Vallon V, Edwards A. Predicted consequences of diabetes and SGLT inhibition on transport and oxygen consumption along a rat nephron. Am J Physiol Renal Physiol 2016; 310:F1269–F1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Neill J, Fasching A, Pihl L, et al. Acute SGLT inhibition normalizes O 2 tension in the renal cortex but causes hypoxia in the renal medulla in anaesthetized control and diabetic rats. Am J Physiol Renal Physiol 2015; 309:F227–F234. [DOI] [PubMed] [Google Scholar]
- 46.▪.Pruijm M, Milani B, Pivin E, et al. Reduced cortical oxygenation predicts a progressive decline of renal function in patients with chronic kidney disease. Kidney Int 2018; 93:932–940. [DOI] [PubMed] [Google Scholar]; This study found that low renal cortical oxygenation is an independent predictor of renal function decline in patients with CKD.
- 47.▪.Cassis P, Locatelli M, Cerullo D, et al. SGLT2 inhibitor dapagliflozin limits podocyte damage in proteinuric nondiabetic nephropathy. JCI Insight 2018; 3:; pii: 98720. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study proposes that proteinuric nondiabetic nephropathy can induce SGLT2 expression in podocytes where it contributes to podocyte damage.
- 48.Darshi M, Onishi A, Kim JJ, et al. Metabolic reprogramming in diabetic kidney disease can be restored via SGLT2 inhibition [abstract]. J Am Soc Nephrol 2017; 28:439.27297947 [Google Scholar]
- 49.▪.Mulder S, Heerspink HJL, Darshi M, et al. Effects of dapagliflozin on urinary metabolites in people with type 2 diabetes. Diabetes Obes Metab 2019; 21:2422–2428. [DOI] [PubMed] [Google Scholar]; This study found that dapagliflozin increased urinary metabolites linked to mitochondria in T2DM patients, suggesting that SGLT2 inhibition may improve mitochondrial function in diabetes.
- 50.Vallon V, Gerasimova M, Rose MA, et al. SGLT2 inhibitor empagliflozin reduces renal growth and albuminuria in proportion to hyperglycemia and prevents glomerular hyperfiltration in diabetic Akita mice. Am J Physiol Renal Physiol 2014; 306:F194–F204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.▪.LeeYH Kim SH, Kang JM, et al. Empagliflozin attenuates diabetictubulopathy by improving mitochondrial fragmentation and autophagy. Am J Physiol Renal Physiol 2019; 317:F767–F780. [DOI] [PubMed] [Google Scholar]; This study found that empagliflozin improved tubular mitochondrial dynamics and increased autophagic activity in in-vitro and in-vivo models of diabetes.
- 52.Takagi S, Li J, Takagaki Y, et al. Ipragliflozin improves mitochondrial abnormalities in renal tubules induced by a high-fat diet. J Diabetes Investig 2018; 9:1025–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.▪▪.Zhang J, Wei J, Jiang S, et al. Macula densa SGLT1-NOS1-tubuloglomerular feedback pathway, a new mechanism for glomerular hyperfiltration during hyperglycemia. J Am Soc Nephrol 2019; 30:578–593. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates that acute increases in luminal glucose at the macula densa are sensed by SGLT1 in the maculadensato upregulatethe expression and activity of NOS1. Enhanced NO formation then blunts the vasoconstrictor influence of TGF and increases in GFR.
- 54.Masuda T, Watanabe Y, Fukuda K, et al. Unmasking a sustained negative effect of SGLT2 inhibition on body fluid volume in the rat. Am J Physiol Renal Physiol 2018; 315:F653–F664. [DOI] [PMC free article] [PubMed] [Google Scholar]


