Summary
Adenosine is a key metabolic and immune checkpoint regulator implicated in the tumor escape from the host immune system. Major gaps in knowledge that impede the development of effective adenosine-based therapeutics include: (1) Lack of consideration of redundant pathways controlling ATP and adenosine levels; (2) lack of distinction between receptor-dependent and - independent effects of adenosine, and (3) focus on extracellular adenosine without consideration of intracellular metabolism and compartmentalization. In light of current clinical trials, we provide an overview of adenosine metabolism and point out the need for a more careful evaluation of the entire purinome in emerging cancer therapies.
Introduction
A central role of metabolism in cancer biology is well established. Otto Warburg proposed that the growth of cancer cells is linked to the generation of energy mainly through the anaerobic breakdown of glucose in contrast to the oxidative breakdown of pyruvate in healthy cells (Warburg, 1956). However, after the discovery of oncogenes and tumor suppressor genes, the significance of the Warburg effect was almost forgotten. Only recently, cancer has been re-evaluated as a mitochondrial metabolic disease (Martinez-Outschoorn et al., 2017; Seyfried et al., 2014). Currently, intense research efforts have focused on selective targeting of metabolic pathways of cellular ATP that may be altered during tumorigenesis, with particular emphasis on dysregulated energy homeostasis (Martinez-Outschoorn et al., 2017) and extracellular purinergic signaling (Di Virgilio et al., 2018; Vijayan et al., 2017). A role of extracellular adenosine (eADO) in cancer biology is also well established and has been covered in recent review articles (Bowser et al., 2017; de Leve et al., 2019; Vijayan et al., 2017). Thereby, the adenosine pathway is currently viewed as a significant barrier to the effectiveness of immune therapies and becomes an important therapeutic target in cancer. In view of diverse, tumor-promoting and -suppressing effects of extracellular ATP (eATP) and eADO, substantial attention has been given to the tumorigenic role of the key adenosine producing ectoenzymes, ecto-nucleoside triphosphate diphosphohydrolase-1 (NTPDase1/CD39) and ecto-5′-nucleotidase/CD73 (eN/CD73). However, it has now become clear that eADO levels and hence adenosine receptor activation depend on the complex interplay between several adenosine producing and inactivating as well as reverse ATP-generating pathways (Figure 1, Table 1). Furthermore, intracellular adenosine metabolism and compartmentalization of the adenosine system are expected to have an equally important, yet understudied role (Boison, 2013) (Figure 1). This review provides an overview of adenosine metabolism and its role in cancer, which determines not only the availability of eADO and the activation of adenosine receptors but also intracellular, adenosine receptor-independent biochemical and epigenetic functions.
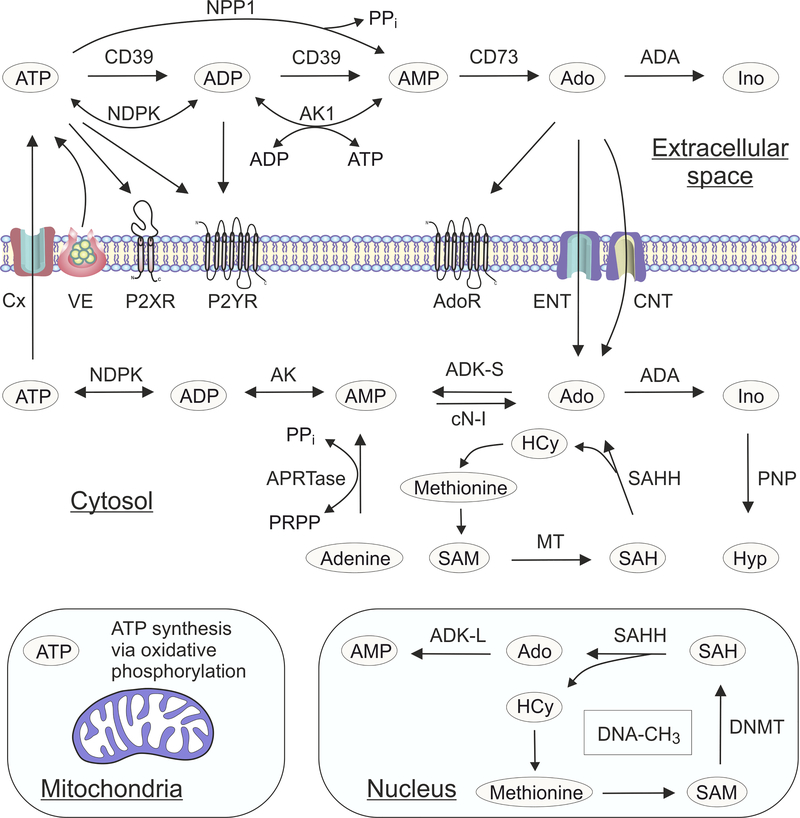
Figure 1. Compartmentalization of adenosine biochemistry.
Extracellular purine turnover is comprised of the release of endogenous ATP upon cell damage or via non-lytic mechanisms of vesicular exocytosis (VE), connexin hemichannels (Cx) and other ion channels and transporters; the triggering of signaling events via nucleotide (P2XR and P2YR) and adenosine (AdoR) selective receptors; the metabolism of nucleotides and nucleosides; and the uptake of nucleotide-derived adenosine via equilibrative (ENT) and concentrative (CNT) nucleoside transporters. General schemes of adenosine producing and removing pathways have included a role for the enzymes nucleoside triphosphate diphosphohydrolase-1 (NTPDase1/CD39), nucleotide pyrophosphatase/ phosphodiesterase-1 (NPP1), ecto-5′-nucleotidase/CD73 and adenosine deaminase (ADA). In addition, counteracting adenylate kinase-1 (AK1) and nucleotide diphosphokinase (NDPK) activities contribute to the regeneration of extracellular ATP via reversible phosphotransfer reactions. Intracellular adenosine metabolism depends on the cytoplasmic form of adenosine kinase (ADK-S), and the enzymes ADA, cytosolic nucleotidase (cN-I), purine nucleoside phosphorylase (PNP), and adenine phosphoribosyl transferase (APRTase). S-Adenosylhomocysteine (SAH) can also be hydrolyzed to adenosine (Ado) and homocysteine (HCy) by SAH hydrolase (SAHH), while S-adenosylmethionine (SAM) serves as the donor of a methyl group in the transmethylation reactions catalyzed by methyltransferases (MT). Because mitochondria are the main source of ATP production, mitochondrial bioenergetics is tightly linked to adenosine homeostasis. In the cell nucleus adenosine is part of the transmethylation pathway, which adds methyl groups to DNA (DNA-CH3) with DNA methyltransferase (DNMT). The nuclear form of adenosine kinase (ADK-L) drives the flux of methyl groups through the pathway leading to increased DNA and histone methylation. For the sake of clarity only the most important enzymes are mentioned.
Table 1 –
Major enzymes of adenosine metabolism
| Enzyme or enzyme family | EC number | Isozyme | Gene name | Alternative names and aliases | Catalytic reaction | Compartmentalization |
|---|---|---|---|---|---|---|
| Ecto-nucleoside triphosphate diphosphohydrolase (NTPDase) family | 3.6.1.5 | NTPDase1 NTPDase2 NTPDase3 NTPDase8 |
ENTPD1 ENTPD2 ENTPD3 ENTPD8 |
CD39, apyrase, ecto-ATPase, ATP-diphosphohydrolase | ATP + H20 → ADP + Pi ADP + H20 → AMP + Pi |
extracellular, microvesicles, soluble |
| Ecto-nucleotide pyrophosphatase/phosphodiesterase (NPP) family | 3.6.1.9 3.1.4.1 |
NPP1 NPP3 |
ENPP1 ENPP3 |
PC-1, CD203a Phosphodiesterase-1β, CD203c, gp130RB13–6, B10 | ATP + H20 → AMP + PPi ADPR → AMP + PPi Ap4A → ATP + AMP |
extracellular, soluble |
| Ecto-5′-nucleotidase (eN) | 3.1.3.5 | NT5E | CD73 | AMP + H20 → Adenosine + Pi | extracellular, microvesicles, soluble | |
| Alkaline phosphatase (ALP) family | 3.1.3.1 | TNAP | ALPL | Tissue-nonspecific alkaline phosphatase (TNAP) | ATP + 3H20 → Adenosine + 3Pi PPi → 2Pi |
extracellular, microvesicles, soluble |
| CD38 | 3.2.2.5 3.2.2.6 |
CD38 | NAD+ glycohydrolase, NAD nucleosidase, NADase, Cyclic ADP-ribose hydrolase, | NAD+ + H20 → ADPR + nicotinamide cyclic ADP-ribose + H2O → ADPR-D-ribose | extracellular, intracellular, soluble | |
| CD157 | 2.4.99.20 | BST1 | Cyclic ADP-ribosyl cyclase 2, ADP-ribosyltransferase | cyclic ADP-ribose + H2O → ADPR-D-ribose | extracellular | |
| Adenylate kinase (AK) | 2.7.4.10 | AK1 | AK1 | Myokinase | ATP + AMP ↔ 2ADP | extracellular, intracellular, soluble |
| Nucleoside diphosphate kinase (NDPK) | 2.7.4.6 | NDPK-A NDPK-B |
NME1 NME2 |
Nm23-H1, NME1, Nm23-H2, NME2 | ATP + NDP ↔ ADP + NTP | extracellular, intracellular, soluble |
| Adenosine deaminase (ADA) family | 3.1.3.1 | ADA1 ADA2 |
ADA1 ADA2 |
Adenosine + H20 → Inosine | extracellular, intracellular, soluble | |
| Purine nucleoside phosphorylase (PNP) | 2.4.2.2 | PNP | Inosine phosphorylase | Inosine + Pi ↔ hypoxanthine + alpha-D-ribose 1-phosphate | extracellular, intracellular | |
| Adenosine kinase (ADK) | 2.7.1.20 | ADK-S ADK-L |
ADK | Adenosine + ATP → AMP + ADP | intracellular intranuclear |
This table highlights the converting pathways for adenine nucleotides and adenosine. However, additional nucleotides and related compounds also serve as preferred substrates for these (ecto)enzymes.
An overview of cellular purine turnover
Clear signaling roles for extracellular nucleotides and adenosine have been established in several systems, including neurotransmission in the central nervous system, blood flow regulation, platelet activation, immunomodulation, vascular remodeling, and promotion of cell growth and proliferation (Di Virgilio et al., 2018; Ralevic and Burnstock, 1998) (Figure 2). Endogenous ATP can be released massively during necrosis, apoptosis and mechanical injury, but can also be released in a controlled manner in response to mechanical and chemical stimuli. Non-lytic mechanisms of ATP efflux include regulated vesicular exocytosis or non-vesicular nucleotide release via ion channels and nucleotide transporters, such as maxi anion channels, multidrug resistance proteins, and connexin/pannexin hemichannels (Lazarowski, 2012; Yegutkin, 2008). eATP generally functions as a “danger sensor” and “find-me” signal guiding phagocytic cells to the site of inflammation and alerting the immune system to the presence of pathogen-associated molecular patterns and tissue damage, and it is also crucial for the activation of the inflammasome and consecutive release of interleukin-1β (IL-1β) and other pro-inflammatory cytokines (Di Virgilio et al., 2018; Eltzschig et al., 2012). These effects are mediated via a series of ligand-gated (P2X) and metabotropic (P2Y) nucleotide-selective receptors (Figure 1) (Bours et al., 2006; Jacobson and Muller, 2016; Ralevic and Burnstock, 1998), and recent data provide evidence for the emerging roles of P2X7 receptors in immunomodulation and solid tumor progression (Borges da Silva et al., 2018; De Marchi et al., 2019; Di Virgilio et al., 2018). ATP-derived adenosine in turn has a non-redundant counteracting role in attenuating the inflammation and tissue damage (Bours et al., 2006; Chen et al., 2013; Linden et al., 2019) and, in addition, acts as a “guardian angel” in different human diseases through mediation of diverse neuroprotective, cardioprotective, anti-nociceptive, vasoactive and pro-angiogenic effects (Borea et al., 2016; Feoktistov et al., 2009; Ralevic and Burnstock, 1998) (Figure 2). Final termination of adenosinergic signaling is the result of a concerted effort between metabolism of eATP and eADO, nucleoside transport, as well as intracellular adenosine metabolism (Box 1, Figure 1). Thus, to understand the role of adenosine in cancer it is important to understand the status of the entire purinome.
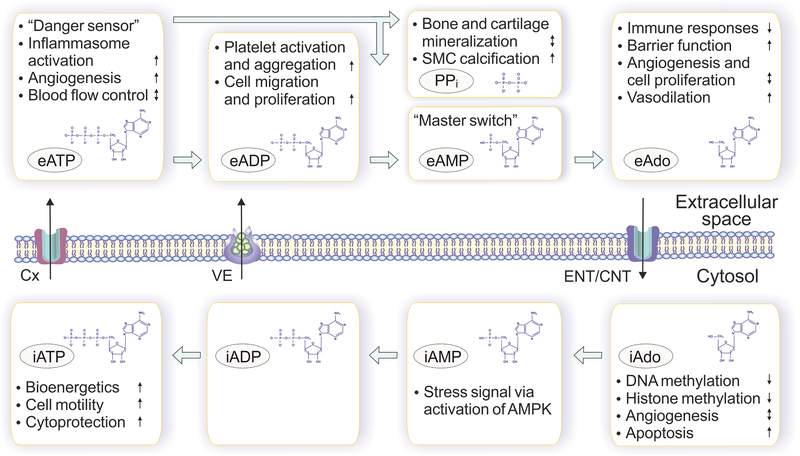
Figure 2. Extracellular (e) and intracellular (i) mechanisms of action of ATP, adenosine and other purines.
Intracellular ATP (iATP) is primarily utilized to drive energy-requiring processes such as active transport, cell motility and biosynthesis, whereas extracellular ATP (eATP) and its dephosphorylated metabolites are powerful signaling molecules, which trigger diverse cell-specific responses both in autocrine and paracrine fashions. Extracellular ATP and ADP (eADP) generally act as pro-inflammatory and prothrombotic molecules, while adenosine has a non-redundant counteracting role in attenuating inflammation and tissue damage. As for extracellular AMP (eAMP), it primarily serves as a “master switch” metabolite that determines the balance between anti-inflammatory adenosine-producing and pro-inflammatory ATP-regenerating pathways. Yet another important by-product of ecto-nucleotidase reaction, PPi, can also mediate diverse physiological effects, particularly ensuring bone matrix mineralization and smooth muscle cell (SMC) calcification. Along with commonly accepted adenosine receptor-mediated pathways, adenosine mediates crucial biological effects via receptor-independent mechanisms based on intracellular biochemical pathways, which affect methylation reactions and related signaling pathways.
Box 1. Cellular ATP turnover.
Most models of cellular ATP turnover depend on interactions between distinct processes including (i) release of ATP and other purines, (ii) binding to nucleotide- and adenosine-selective receptors, (iii) inactivation of nucleotides and nucleosides through ectoenzymes, and finally (iv) the re-uptake of nucleotide-derived adenosine for replenishing the intracellular ATP pool through complex phosphotransfer reactions (Figure 1). The released ATP triggers diverse signaling events via a series of ligand-gated (P2X) and metabotropic (P2Y) receptors, whereas adenosine primarily mediates its effects through the activation of G-protein coupled adenosine receptors (A1 A2A, A2B, A3). Several data also provide evidence that, along with commonly accepted adenosine receptor-mediated pathways, adenosine mediates crucial biological effects via receptor-independent mechanisms based on intracellular biochemical pathways, which affect methylation reactions and related signaling pathways. In particular, the adenosine-removing enzyme ADK, which exists in a cytoplasmic (ADK-S) and nuclear (ADK-L) isoform, plays a key role in the regulation of cell proliferation by controlling adenosine homeostasis and intracellular and intranuclear methylation reactions, which include DNA methylation and histone methylation (Figure 1). Those epigenetic functions of adenosine offer new therapeutic approaches for the treatment of cancer.
Extracellular purine metabolism
The major source of eADO is the sequential breakdown of eATP involving a number of different ectoenzymes (Yegutkin, 2008; Zimmermann et al., 2012), including the well-known anti-cancer targets NTPDase1/CD39 and eN/CD73 (Table 1, Figures 1 and 3). Along with the “classical routes” of ATP breakdown, eADO can also be generated from related compounds, including cAMP and nicotinamide adenine dinucleotide (NAD+), which is degraded by CD38 and another functionally related enzyme CD157 (Linden et al., 2019; Malavasi et al., 2008). In general, eATP serves as an immune stimulator, whereas eADO serves as an immune suppressor (Figure 2). Therefore, a major role of the ecto-nucleotidases is to implement a switch from an immune stimulating, inflammatory environment to an immunosuppressive, anti-inflammatory environment. Moreover, in the extracellular space ATP can be resynthesized via reverse adenylate kinase and nucleoside diphosphate kinase (NDPK) reactions (Yegutkin et al., 2001) (Box 2, Figure 1). Finally, eADO can be either directly inactivated on the cell surface via inosine to hypoxanthine through sequential activities of adenosine deaminase (ADA) and purine nucleoside phosphorylase (PNP) (Yegutkin, 2014), or by cellular uptake through specific equilibrative and concentrative nucleoside transporters (Boswell-Casteel and Hays, 2017) (Figures 1 and 3). In conclusion, eADO metabolism is not only determined by the specific make-up of different nucleotide-inactivating/adenosine-producing enzymes, but also by counteracting ATP-regenerating pathways, adenosine degrading pathways, and cellular adenosine uptake.
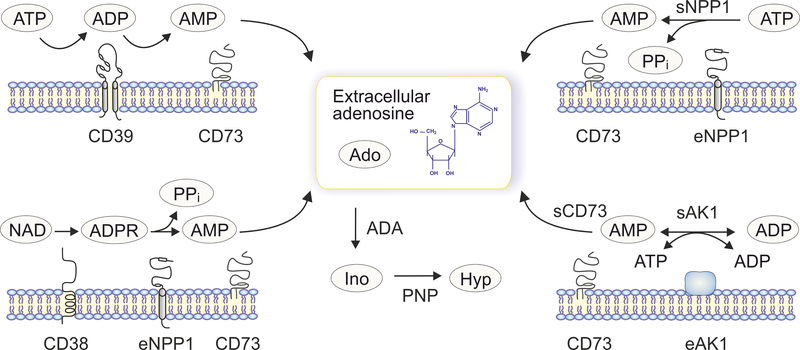
Figure 3. Redundancy of ectoenzymatic pathways controlling extracellular adenosine.
Along with the “classical” ATP-inactivating/adenosine-producing chain mediated via stepwise CD39 and CD73 reactions (upper left), additional ectoenzymatic pathways can contribute to the generation of eADO. Those include the direct breakdown of ATP via AMP into adenosine through the NPP1-CD73 axis (upper right), adenylate kinase-1 (AK1) mediated transphosphorylation of ADP and subsequent hydrolysis of the generated AMP by CD73 (lower right), as well as an alternative adenosine-producing route from extracellular NAD via the CD38-NPP1-CD73 axis (lower left). Importantly, both soluble (“s”) and extracellular membrane-bound (“e”) enzymes can contribute to these adenosine-producing pathways. The extracellularly generated adenosine can be taken up by the cells or further converted into extracellular inosine (Ino) and hypoxanthine (Hyp) via sequential ADA and PNP reactions.
Box 2. Interplay between ATP-consuming and ATP-regenerating pathways.
Under normal physiological conditions, the ecto-nucleotide kinase activities seem not to compete with ecto-nucleotidases for a limited pool of the released nucleotide substrate and therefore, the release of a particular nucleotide alone would cause its rapid dephosphorylation into adenosine and further to inosine/hypoxanthine. However, in the setting of inflammation, hypoxia and oxidative stress, acute changes in the specific ratios of nucleoside mono-, di- and triphosphates, in conjunction with feedforward inhibition of eN/CD73 activity by the precursor ATP/ADP, should determine the directional shift from the adenosine-generating route towards reverse ATP resynthesis through sequential AK and NDPK reactions (Figure 1). The presence of redundant pathways coordinately regulating ATP and adenosine levels implies the necessity of understanding the role of a particular ectoenzyme within the larger framework of other enzymes that are co-expressed to a variable extent among the mammalian cells and tissues and share similarities in substrate specificity.
Adenosine receptor dependent roles of adenosine
The role of adenosine receptors in cancer is well established and has been covered in recent review articles (Allard et al., 2017; Borea et al., 2016; Chen et al., 2013; Vijayan et al., 2017). Whereas all adenosine receptors (A1, A2A, A2B, and A3) have inherent pro- and anti-tumor effects and work in concert with each other, there is now general consensus that increased eADO in inflamed or cancerous tissue has an immunosuppressive effect largely mediated via excessive stimulation of Gs protein-coupled A2A receptors (A2AR) on immune cells, which in contrast to an abundance of immune receptors, do not have a reserve for the A2A receptors (Armstrong et al., 2001). The pioneering work of Sitkovsky and his team provided conclusive genetic evidence for the A2AR-immunoregulation link in cancer (Ohta et al., 2006; Ohta and Sitkovsky, 2001) and laid the foundation for the development of the first A2AR-based immunotherapies for cancer and the development of an entire new research field. Gs signaling triggered by eADO leads to activation of adenylate cyclase, the sustained accumulation of intracellular cAMP as a molecular basis for “T cell memory” (Koshiba et al., 1997), and finally the activation of cAMP-dependent protein kinase A, which negatively regulates the activation of T cell receptor (TCR) dependent transmembrane signaling providing an OFF signal to activated immune cells (Ohta and Sitkovsky, 2001). Using A2AR knockout mice Ohta et al demonstrated that A2AR deficiency in the host leads to complete tumor rejection (Ohta et al., 2006). The A2AR-dependent signaling cascade not only leads to the direct inhibition of TCR-activation, but also to the increased transcription of immunosuppressive genes in surrounding cells due to the phosphorylation by PKA of cAMP response element binding protein (CREB) (Lukashev et al., 2004; Sitkovsky, 2009). This mechanism plays a role under hypoxic conditions, where hypoxia inducible factor-1 alpha (HIF-1α) induces the accumulation of eADO and activation of the hypoxia response element (HRE) and cyclic AMP response element (CRE) (Sitkovsky et al., 2014). Those factors redirect gene transcription in CD4+ and CD8+ T cells, and in myeloid cells, directing the immune response towards an immunosuppressive repertoire of cytokines and molecules such as TGF-beta, IL-10, and CTLA-4. In addition, this mechanism promotes the development of Tregs and their inhibition of effector T-cells (Hatfield et al., 2019).
Receptor-independent, epigenetic roles of adenosine
Whereas the adenosine receptor-mediated effects of adenosine are well characterized, adenosine has additional, adenosine receptor-independent activities, which include a specific, emerging role of adenosine kinase (ADK), which exists in a cytoplasmic (ADK-S) and nuclear (ADK-L) isoform (Figure 1 and Table 1). Cytoplasmic ADK-S provides the major metabolic route of adenosine clearance under physiological conditions by phosphorylation of adenosine into AMP (Boison, 2013). Nuclear ADK-L links biochemically directly to the S-adenosylmethionine dependent transmethylation pathway, which drives DNA and histone methylation (Boison, 2013). By removing adenosine, a product of transmethylation, ADK-L controls the flux of methyl groups through the transmethylation pathway via mass action (Boison et al., 2002; Williams-Karnesky et al., 2013). High levels and activities of ADK-L are therefore associated with increased global DNA methylation (Williams-Karnesky et al., 2013). We propose that through this epigenetic role, ADK-L might contribute to the regulation of gene expression. Indeed, the epigenetics of cancer, which include changes in DNA and histone methylation signatures play a key role in driving the high proliferative rate of cancer cells (Du et al., 2015; Huang et al., 2015; Zahnow et al., 2016). Furthermore, AMP-activated protein kinase (AMPK), which is activated under conditions of metabolic stress and down-regulated in a high proportion of tumors (Hardie, 2015), is another likely candidate for adenosine receptor independent roles of adenosine in cancer (Virtanen et al., 2014). Overall, the complex interaction between receptor dependent and independent pathways of adenosine needs to be taken into account during evaluation of the efficacy of adenosine regulating treatments for cancer therapy.
Role of the CD39-CD73 axis in immunomodulation and tumor immune evasion
Over the past 10 years, a major focus in adenosine and cancer research has been the extracellular nucleotide phosphohydrolysis pathway and in particular the adenosine producing enzymes NTPDase1/CD39 and eN/CD73, which play a key role in turning an ATP-mediated immune-stimulating into an adenosine-mediated immunosuppressant tumor microenvironment (TME) involving the coordinated control of inflammatory responses and tumor-associated antigen-specific T-cell immunity (Figure 4). ATP released from stressed, apoptotic or necrotic cells binds to excitatory ATP-specific receptors and promotes rapid inflammation by amplifying TCR signaling in lymphocytes and promoting inflammasome activation in dendritic cells (DCs) and macrophages (Bours et al., 2006; Di Virgilio et al., 2018; Linden et al., 2019) (Box 3). Real-time measurements of pericellular ATP levels using a plasma membrane-targeted luciferase revealed the constitutive presence of high ATP in the TME and further correlated its changes to the expression levels of P2X7 receptors and ecto-nucleotidases (De Marchi et al., 2019; Di Virgilio et al., 2018) and also to anticancer immune responses induced by chemotherapeutic agents (Michaud et al., 2011). Recent data have also shown the important role of P2X7 receptor signaling in supporting the generation of long-lived memory CD8+ T-cells by sensing eATP and driving their metabolic reprogramming and mitochondrial maintenance (Borges da Silva et al., 2018). NTPDase1/CD39 is highly expressed in tumor endothelium and on the surface of most immune cells in the TME including macrophages, myeloid cells, and FOXP3+ regulatory T cells (Antonioli et al., 2013). CD39 stabilizes FOXP3+ Tregs and contributes to their suppressive function; in addition it boosts the differentiation and function of type 1 regulatory T (Tr1) cells, which produce IL-10, and limits the NLRP3 inflammasome activation in DCs (Takenaka et al., 2016). CD39+/CD8+ tumor-infiltrating lymphocytes from colon and lung tumors are highly enriched in tumor reactive T cells in the TME as well as the periphery but can also display hallmarks of highly exhausted cells in terms of both phenotypic and functional markers. In turn, the absence of CD39 defines a population of bystander CD8+ T cells whose phenotype is inconsistent with chronic antigen stimulation at the tumor site (Simoni et al., 2018).
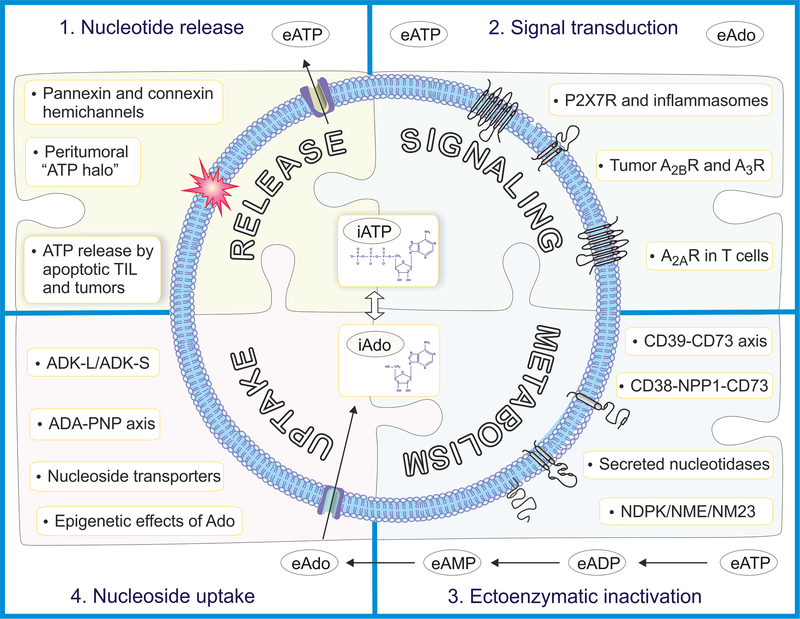
Figure 4. Key components of cellular purine turnover: promising targets in anti-cancer therapy.
Possible targets for anti-cancer therapy fall into four categories: (1) ATP release by apoptotic tumor-infiltrating lymphocytes (TIL) and tumors as well as the release of ATP through pannexin and connexin hemichannels creates a peritumoral “ATP halo”, which is also a source for the production of adenosine via ectoenzymes. Therefore, targeting the release of ATP would constitute a rational approach for anti-cancer therapy. (2) Both ATP and adenosine feed into signaling pathways, thought to promote cancer growth and malignancy. Possible therapeutic targets are P2X7 receptors and inflammasomes, A2A receptors on T cells, and A2B and A3 receptors on tumors. (3) The extracellular metabolism of ATP into adenosine via the CD39-CD73 axis, the CD38-NPP1-CD73 axis, or the NDPK/NME/NM23 axis in conjunction with secreted nucleotidases is a well-established target system in cancer therapy. (4) Finally, the intracellular uptake of adenosine and its intracellular and intranuclear metabolism constitutes a novel frontier for cancer therapy. In particular, the epigenetic effects of adenosine determined by the ADK-L/ADK-S ratio, may offer the therapeutic opportunity to modify the epigenetic signature of cancer cells through epigenetic reprogramming by adenosine. The intracellular equilibrium also depends on its uptake via nucleoside transporters and metabolism via the ADA-PNP axis.
Box 3. The hypoxia-adenosine link in cancer.
Tumors are hypoxic, primarily because of high oxygen demand by proliferating cells and insufficient supply of oxygen and nutrients due to restricted and unstable blood flow in the disorganized tumor vasculature. Tumor hypoxia, stabilization of the hypoxia-inducible factor (HIF) α-subunits (HIF1–3α), and lactate accumulation in turn have been associated with worsened disease outcome, thus making hypoxia a hallmark of cancer. The current view of the CD39-CD73 axis as an important anti-cancer target is based on the premises that the expression levels of NTPDase1/CD39 and eN/CD73 are up-regulated on surfaces of vascular endothelial, epithelial, lymphoid and tumor cells as a result of tissue hypoxia, inflammation and oxidative stress. Because hypoxia and inflammation are interrelated and coincidental processes, eADO metabolism and signaling might represent an essential HIF-driven pathway that dampens excessive inflammatory responses and induces barrier protection in conditions of low oxygen supply. Other constituents of purinergic signaling machinery can also be affected by hypoxia, including up-regulation of A2B receptors via selective activation of HIF1α and HIF2α pathways, diminished cellular adenosine uptake through ENTs, the inhibition of ADK and ADA activities, as well as activation of intracellular purine salvage pathways through complex phosphotransfer networks.
Because eN/CD73 mediates the subsequent breakdown of ATP/ADP-derived AMP into adenosine, this ectoenzyme represents another key component of the immunosuppressive functions of Treg cells (Antonioli et al., 2013). The expression of CD73 within the TME has therefore been considered as a prognostic biomarker for clinical outcomes in a wide spectrum of tumor types (Allard et al., 2017; Leone and Emens, 2018; Vijayan et al., 2017). The vast majority of these studies have demonstrated a statistically meaningful correlation between high CD73 expression and unfavorable clinical outcomes. Given that tumors often hijack normal cellular responses, it is possible that cancers with high eN/CD73 exploit the ability of eADO to regulate cell adhesion molecules and also F-actin re-arrangement for promoting tumor progression (Bowser and Broaddus, 2016). The activities of NTPDase1/CD39 and eN/CD73 contribute to the clearance of pro-inflammatory ATP in the hypoxic and metabolically abnormal TME, simultaneously leading to increased eADO levels and A2AR-mediated inhibition of effector immune cells, including cytotoxic CD8+ T lymphocytes (CTL), natural killer (NK) cells, macrophages and DCs (Vigano et al., 2019; Vijayan et al., 2017; Young et al., 2014). A recent study found that Treg cells undergo apoptosis due to oxidative stress within the TME, accompanied by the release of large amounts of ATP acting as a substrate for the NTPDase1/CD39 and eN/CD73 expressed on Treg cells. Generated eADO in turn triggered the immunosuppressive signaling pathway in tumor-infiltrating effector lymphocytes and APCs via activation of A2ARs and inhibited the spontaneous antitumor activity and treatment effects mediated by blockade of PD-1-PD-L1 and adoptive T cell transfer (Maj et al., 2017). These findings suggest the importance of combining strategies to control immunosuppression mediated by Treg cell—generated eADO.
CD39 and CD73: emerging targets in cancer immunotherapy
The above findings provide a solid background for the directional manipulation of the ATP–adenosine axis as a novel immunotherapeutic strategy to inhibit tumor cell–mediated immunosuppression (Box 4). Inhibition of CD39 with small-molecule inhibitors, polyoxotungstate-1 (POM-1) and ARL-67156, or CD39-blocking antibody OREG-103/BY40 markedly alleviated the CD39+ tumor cell–mediated suppression of CD4+ and CD8+ T cell responses and concurrently increased the cytotoxic activity of CTL and NK cells, leading to tumor cell killing (Bastid et al., 2015). NK cells were also increased in CD39-deficient mice and after the treatment of wild-type mice with POM-1. Excitingly, POM-1 suppressed metastases in four different tumor models and its anti-metastatic activity was abrogated in mice depleted of NK cells or after the neutralization of IFNg (Zhang et al., 2019). The combined treatment of mice transplanted with B16–10 melanoma cells with POM-1 and anti-PD-1 and CTLA4 antibodies reduced tumor growth and caused a strong synergistic boost in the survival of tumor-bearing mice (Sade-Feldman et al., 2018). Noteworthy, along with acting as a potent CD39 inhibitor, POM-1 and other polyoxometalates are capable of inhibiting the activities of other members of the NTPDase family and also NPP1 and alkaline phosphatase (Lee et al., 2015). Therefore, certain caution should be taken in the interpretation of data on the inhibitory effects of POM-1 on eATP metabolism and discriminating the role of CD39 within the larger network of other ectoenzymes co-expressed in the TME. The development of antibodies blocking NTPDase1/CD39 activity is underway, and several clinical and preclinical studies were initiated by Tizona Pharmaceutics (TTX-030; Table 2), Surface Oncology (SRF617; https://www.surfaceoncology.com/pipeline-programs/) and Innate Pharma [IPH52; (Perrot et al., 2019)] to develop novel first-in-class humanized or fully human anti-CD39 antibodies targeting the ATP-adenosine immune checkpoint pathway for cancer immunotherapy.
Box 4. Immune checkpoints.
Targeting immune checkpoints such as programmed cell death protein 1 (PD-1), programmed cell death-1 ligand-1 (PD-L1) and cytotoxic T lymphocyte antigen 4 (CTLA-4) has achieved noteworthy benefit in multiple cancers by blocking immuno-inhibitory signals and enabling patients to produce an effective antitumor response. Checkpoint therapies were developed to overcome the dysfunction or exhaustion of T cells resulting from chronic antigen exposure and suppression by the tumor or cells in its microenvironment. Whereas a subset of patients with advanced cancers respond to anti-CTLA4 or anti-PD1 mAb treatment (~20%), the majority of patients show modest or no clinical benefits to such single-agent therapies. It is becoming increasingly evident that targeting multiple immunosuppressive pathways has the potential to further improve the response rates without an increase in adverse events associated with excessive tissue damage, autoimmunity, and other immune-related off-side effects. The expression levels and activities of key adenosine-generating enzymes, NTPDase1/CD39 and eN/CD73, can be selectively up-regulated in the metabolically abnormal TME as a result of tissue hypoxia, inflammation, oxidative stress and epithelial-to-mesenchymal transition, thereby serving as a potential mechanism for checkpoint-blockage resistance.
Table 2 –
Ongoing clinical trials targeting adenosine metabolism in patients with malignancies
| Target | Drug +/− combination therapy |
Company | Cancer type | ClinicalTrials.gov identifier | Study phase |
|---|---|---|---|---|---|
| NTPDase1/CD39 | Human anti-CD39 Ab (TTX-030) +/− chemotherapy (docetaxel, gemcitabine, nab-paclitaxel), or anti-PD-1 mAb (pembrolizumab) |
Tizona Therapeutics | Solid tumors, lymphoma | NCT03884556 | I |
| eN/CD73 | anti-CD73 mAb (MEDI9447/oleclumab) +/− EGFR tyrosine kinase inhibitor (osimertinib), or A2R antagonist (AZD4635) |
MedImmune | NSCL carcinoma | NCT03381274 | I/II |
| eN/CD73 | anti-CD73 mAb (MEDI9447/oleclumab) +/− anti-PD-L1 mAbs (durvalumab, tremelilumab), or anti-OX40/CD134 mAb (MEDI0562/tavolimab) |
Nordic Society for Gynaecologic Oncology | Ovarian cancer | NCT03267589 | II |
| eN/CD73 | anti-CD73 mAb (MEDI9447/oleclumab) +/− anti-PD-L1 mAb (MEDI4736/durvalumab) |
MedImmune | Solid tumors | NCT02503774 | I |
| eN/CD73 | anti-CD73 mAb (MEDI9447/oleclumab) +/− chemotherapy (paclitaxel plus carboplatin), anti-PD-L1 mAbs (durvalumab) |
Jules Bordet Institute/AstraZeneca | TNBC | NCT03616886 (SYNERGY) | I/II |
| eN/CD73 | anti-CD73 mAb (BMS-986179) +/− anti-PD-1 mAb (nivolumab), or rHuPH20 |
Bristol-Myers Squibb | Advanced solid tumors | NCT02754141 | I/II |
| eN/CD73 | anti-CD73 mAb (NZV930/SRF373) +/− anti-PD-1 mAb (PDR001), or A2R antagonist (NIR178) |
Novartis Pharmaceuticals | Advanced solid tumors | NCT03549000 | I |
| eN/CD73 | anti-CD73 mAb (CPI-006) +/− anti-PD-1 mAb (pembrolizumab), or A2R antagonist (CPI-444) |
Corvus Pharmaceuticals | Advanced solid tumors, Non-Hodgkin lymphoma | NCT03454451 | I |
| eN/CD73 | anti-CD73 mAb (TJ004309/TJD5) +/− anti-PD-L1 mAb (atezolizumab) |
Tracon Pharmaceuticals | Solid tumors, metastatic cancer | NCT03835949 | I |
| eN/CD73 | small-molecule eN/CD73 inhibitor (AB680) | Arcus Biosciences | Healthy volunteers | NCT03677973 | I |
| eN/CD73 | small-molecule eN/CD73 inhibitor (AB680) +/− anti-PD-1 Ab (AB122) |
Arcus Biosciences | Advanced pancreatic cancer | NCT04104672 | I |
| ADA | ADA inhibitor pentostatin +/− chemotherapy (bendamustine), or anti-CD20 mAb (rituximab) |
National Cancer Institute | Hairy Cell Leukemia | NCT01059786 | II |
| CD38 | anti-CD38 mAb (Isatuximab/SAR650984) +/− anti-PD-1 mAb (cemiplimab) |
Sanofi | Lymphoma Prostate and NSCL cancers MM |
NCT03769181 NCT03367819 NCT03733717 |
I/II I/II I |
| CD38 | anti-CD38 mAb (Isatuximab) +/− standard immuno- and chemotherapy |
Sanofi | ALL, AML | NCT03769181 | II |
| CD38 | anti-CD38 mAb (Isatuximab) +/− immunotherapy (dexamethasone, lenalidomide), or proteasome inhibitor (bortezomib) |
University of Heidelberg Medical Center | MM | NCT03617731 | III |
| CD38 | anti-CD38 mAb (Isatuximab) monotherapy |
M.D. Anderson Cancer Center | Smoldering MM | NCT02960555 | II |
| CD38 | Human anti-CD38 Ab (MOR202/MOR03087) +/− immunotherapy (dexamethasone, pomalidomide, lenalidomide) |
MorphoSys AG | Relapsed or refractory MM | NCT01421186 | I/II |
| CD38 | anti-CD38 mAb (TAK079) +/− immunotherapy (dexamethasone, pomalidomide) |
Takeda - Millennium Pharmaceuticals | Relapsed or refractory MM | NCT03439280 | I/II |
| CD38 | Human anti-CD38 Ab (daratumumab) monotherapy |
M.D. Anderson Cancer Center | Bladder and kidney cancers Myeloid leukemia Plasma cell myeloma |
NCT03473730 NCT03067571 NCT03622775 |
I II II |
| CD38 | Human anti-CD38 Ab (daratumumab) monotherapy |
Dana-Farber Cancer Institute | Smoldering MM | NCT03236428 | II |
| CD38 | Human anti-CD38 Ab (daratumumab) +/−FMS tyrosine kinase inhibitor (JNJ-40346527) |
M.D. Anderson Cancer Center | Prostate cancer | NCT03177460 | I |
| CD38 | Human anti-CD38 Ab (daratumumab) +/− immunotherapy (ibrutinib) |
French Innovative Leukemia Organisation | Relapsed or refractory CLL | NCT03734198 | II |
| CD38 | CD38/CD3 bispecific antibody (GBR 1342) | Glenmark Pharmaceuticals | MM | NCT03309111 | I |
| CD38 | anti-CD38 mAb (SAR650984) +/− proteasome inhibitor (carfilzomib) |
University of California, San Francisco | MM | NCT02332850 | I |
| CD38 | Daratumumab labeled with zirconium-89 through deferoxamine (89Zr-DFO-daratumumab) | Memorial Sloan Kettering Cancer Center | PET imaging of MM | NCT03665155 | I/II |
| CD38 | Autologous CD38-A2 CAR2-expressing T lymphocytes | Sorrento Therapeutics | Relapsed or refractory MM | NCT03464916 | I |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CLL, chronic lymphocytic leukemia; EGFR, epidermal growth factor receptor; MM, multiple myeloma; NSCL cancer, non-small cell lung cancer; rHuPH20, novel drug delivery enzyme, fully human recombinant DNA-derived hyaluronidase; PET, positron emission tomography. Note, multiple clinical trials have been initiated recently for evaluating the therapeutic potential, effective dosage and possible side effects of daratumumab, isatuximab and other anti-CD38 antibodies in cancer patients, with over 50 recruiting trials being listed on ClinicalTrials.gov. Due to the lack of space, only a selection of these ongoing CD38-targeting trials have been listed here.
The directional manipulation of eN/CD73 activity and adenosinergic signaling in cancer cells has recently evolved into novel therapeutic approaches. Several of those approaches are focused on combined therapies with anti-CD73 antibodies and A2AR blockers. Surprisingly, those apparently redundant combination therapies have additional benefits based on non-enzymatic functions of CD73 and on CD73 independent sources of adenosine, as well as on the Fc binding domains of anti-CD73 antibodies, which enhanced the therapeutic response (Young et al., 2016). Based on those premises, several anti-CD73 antibodies and inhibitors have progressed to clinical trials (Table 2), with other CD73-inhibiting antibodies being tested in preclinical tumor models (Chen et al., 2019; Leone and Emens, 2018; Perrot et al., 2019; Vigano et al., 2019). In addition, the inhibition of the adenosinergic pathway could be potentially combined with cancer vaccine-induced tumor-reactive T cells, adoptively transferred tumor-reactive CD8+ T cell or NK cells, as well as T cells with chimeric activating receptors (CAR-T cells), or targeting the A2AR to enhance CAR-T cell efficacy (Beavis et al., 2017; Leone and Emens, 2018; Sitkovsky et al., 2014; Vijayan et al., 2017). Combinations of adenosinergic therapies with supplementation of oxygen via respiratory hyperoxia to reduce tumor hypoxia (Hatfield and Sitkovsky, 2015) or anti-angiogenic therapies (for example, anti-VEGF and anti-FGF) (Mahoney et al., 2015; Vijayan et al., 2017) may further improve immunotherapy approaches and enable tumor regression.
A potential limitation of those approaches is that anti-CD73 antibodies may not penetrate well into solid tumors. Alternative approaches targeting the CD73-adenosine axis include shRNA and siRNA gene silencing (Allard et al., 2014; Kordass et al., 2018), as well as development of nucleotide and nucleoside analogues acting as selective agonists or antagonists of adenosine receptors (Chen et al., 2013; Young et al., 2014), or site-specific CD73-activated prodrugs of A2A receptors (Flogel et al., 2012). Furthermore, novel small-molecule inhibitors with subnanomolar inhibitory potency towards human and rat eN/CD73 and high metabolic stability have been developed recently (Bhattarai et al., 2019; Bowman et al., 2019). Based on these results, the first clinical candidate (AB680) has recently been announced as a potential checkpoint inhibitor (Table 2).
Targeting CD38 in cancer
CD38 has been identified as an additional immune checkpoint, because the therapeutic blockade of programmed cell death protein 1 (PD-1) or its ligand PD-L1 can lead to upregulation of CD38 causing resistance to those checkpoint inhibitors (Chen et al., 2018). Increased CD38 suppresses CD8+ T cell function by increased adenosine receptor signaling; therefore, the combination of PD-L1 inhibitors with CD38 blockers improved antitumor immune responses (Chen et al., 2018). Consequently, CD38 has been identified as a cell-surface marker in hematological cancers, and cytotoxic anti-CD38 mAb daratumumab (Janssen Biotech) has been approved by the FDA, with several other CD38-binding antibodies being now in clinical trials, either alone or in combination with immunomodulatory agents or proteasome inhibitors (Table 2). These antibodies target different epitopes on the CD38 molecule and have pleiotropic mechanisms of action, including allosteric inhibition of NADase activity, Fc-dependent immune-effector mechanisms, direct apoptotic activity, and immunomodulatory effects by the elimination of CD38+ immune-suppressor cells (Chini et al., 2018; van de Donk et al., 2018). Furthermore, the activity of CD38 can be targeted using other pharmacological approaches, including CD38-specific CAR-T cells and nanobodies (Linden et al., 2019; Morandi et al., 2018), as well as flavonoids, NAD analogs, 4-amino-quinolines and other small-molecule enzyme inhibitors (Chini et al., 2018; Rajman et al., 2018). Importantly, NAD+ mainly regulates cellular functions through CD38, which possesses both NAD-glycohydrolase and ADP-ribose cyclase activities (Table 1), thereby giving rise to the generation of biologically active second messengers like cyclic ADP-ribose, nicotinic acid adenine dinucleotide phosphate (NAADP) and adenosine (Chini et al., 2018; Malavasi et al., 2008; van de Donk et al., 2018). Given the connection of CD38 with multiple intra- and extracellular metabolic and signaling pathways, further studies are required to elucidate whether these antitumor effects of anti-CD38 antibodies and small-molecule inhibitors are mediated by inhibition of ecto-NADase activity and concomitant attenuation of adenosinergic signaling pathways.
Secreted purinergic enzymes as circulating biomarkers and anti-metastatic targets
The constitutive presence of soluble and/or microparticle-associated forms of CD39, CD73 and other purinergic enzymes in the bloodstream and other biological fluids (Syn et al., 2016; Yegutkin, 2008; Zeiner et al., 2019; Zimmermann et al., 2012) might be an important auxiliary effector system for the local or systemic inactivation of acutely elevated nucleotides and the generation of eADO. Soluble NTPDase (apyrase) is considered a promising therapeutic drug, which inhibits platelet reactivity, thrombus formation and blood flow during acute vascular injury, ischemia-reperfusion and other prothrombotic conditions (Moeckel et al., 2014). High expression levels of CD39 and/or CD73 in extracellular vesicles derived from head and neck squamous cell carcinoma (Theodoraki et al., 2018), prostate cancer (Salimu et al., 2017), neuroblastoma (Morandi et al., 2019), and other neoplastic cell types and TIL (Syn et al., 2016) could further arbitrate the generation of an immunosuppressive environment by blunting the response of immune effector T cells and suppressing DC function. Therefore, current anti-metastatic therapeutic strategies aim to prevent the biogenesis, secretion and uptake of pathogenic tumor-derived exosomes via inhibition of heparanase/syndecan, vacuolar ATPases and several members of the Rab family (Salimu et al., 2017; Syn et al., 2016). Moreover, the ability of different tumors and hematopoietic cells to secrete other microvesicle-associated enzymes, adenylate kinase (Muller et al., 2017), NDPK (Yokdang et al., 2015), ADA (Theodoraki et al., 2018), NPP1 and CD38 (Morandi et al., 2019), might represent a currently underappreciated route regulating the pattern of nucleotide receptor stimulation/desensitization and facilitating the adaptation of disseminated tumor cells to the foreign soil. Identifying the mechanisms by which the extensive network of membrane-associated and secreted purinergic activities regulate intratumoral ATP and adenosine concentrations and the further translation of soluble and microparticle-associated enzymes as potential circulating biomarkers and anti-metastatic drugs will be an area of intense interest in the future.
ADK: novel therapeutic target for cancer
As outlined in Figure 1, ADK constitutes the major metabolic sink for adenosine. To understand ADK’s role as potential therapeutic target for cancer, it is important to realize the multifunctionality of the enzyme. Alternative promoter use and splicing yields two isoforms of mammalian ADK (Spychala et al., 1996). Human ADK mRNAs thereby encode proteins with sequence-derived molecular weights of 38.7 and 40.5 kD, differing only by 21 amino acids in their N-terminus. The long isoform ADK-L is located in the cell nucleus, where it acts as a regulator of DNA methylation (Williams-Karnesky et al., 2013). By removing adenosine, a metabolic product of DNA methylation, ADK-L drives the flux of methyl groups through the S-adenosylmethionine (SAM)-dependent transmethylation pathway (Williams-Karnesky et al., 2013). Thereby, high levels of ADK induce increased global DNA methylation, whereas high levels of adenosine (or low levels of ADK-L) prevent DNA methylation and lead to global DNA hypomethylation (Williams-Karnesky et al., 2013). Through this mechanism, ADK-L controls a novel, adenosine receptor-independent, epigenetic function of adenosine. In contrast, the short isoform ADK-S is located in the cytoplasm and thereby regulates intra- and extracellular levels of adenosine, with high levels of ADK-S promoting adenosine deficiency and reduced adenosine receptor activation, and low levels of ADK-S promoting high levels of extracellular adenosine and increased adenosine receptor activation(Pignataro et al., 2007). We propose that by reduced levels of ADK-S and increased adenosine release, a tumor evades immune surveillance and tumor suppression. Thus, ADK-L and ADK-S control the concentration of adenosine in nuclear and cytoplasmic/extracellular compartments, respectively, and thereby have distinct roles affecting cancer biology. A plethora of therapeutic opportunities exists to manipulate intracellular adenosine levels, ranging from metabolic and dietary interventions (Masino et al., 2011), to small molecule based pharmacological approaches (Kose et al., 2016; Toti et al., 2016), to gene therapy (Theofilas et al., 2011). However, in order to translate those approaches into the clinic, a more detailed understanding of the entire purinome in different types of cancers becomes mandatory.
Targeting adenosine metabolism in cancer – what are the challenges?
Much of the renewed interest in the ATP-adenosine axis over the last decade has been attributed to the ability of eATP and eADO to modulate diverse, often counteracting, pro- and anti-inflammatory effects via activation of nucleotide- and adenosine-specific receptors. The overarching conclusion is that cancer cells create an adenosine-rich immunosuppressive microenvironment through enhanced release of eATP (De Marchi et al., 2019; Di Virgilio et al., 2018), and persistent maintenance of high eADO levels in the tumor core (Blay et al., 1997; Ohta et al., 2006). However, intracellular adenosine metabolism remains understudied, but likely has an equally important role. Several open questions remain to be answered: Would the increased eADO production trigger enhanced intracellular adenosine clearance as a compensatory mechanism, or vice versa? Would a combination of increased eADO production combined with deficient intracellular adenosine clearance boost the adenosine signal in the microenvironment of a tumor? A further challenge is the fact that local adenosine concentrations rapidly fluctuate and are difficult to measure (Chen et al., 2013). Given the very short half-life of adenosine as an intermediate metabolite in the interstitial spaces between cells (~1–5 s) (Moser et al., 1989), more thorough research is required to assess intratumoral adenosine levels directly.
Another key issue in interpreting findings from the human cancer purinome is the question whether data obtained in rodent studies can be translated to humans. Whereas ADK is highly conserved in evolution and the genetic disruption of ADK expression in humans, mice, and plants causes highly comparable phenotypes related to transmethylation biochemistry (Bjursell et al., 2011; Boison et al., 2002; Moffatt et al., 2000), there seem to be major species-specific differences in other purinergic pathways, which need to be considered (Johnson et al., 2019; Joolharzadeh and St Hilaire, 2019). These observations suggest that both similarities and differences between biochemical pathways and metabolic rates have to be taken into account when using animal models to extrapolate biological and clinical phenomena into humans. Importantly, CD73 is not always upregulated in cancer cells and is not always associated with a poor prognosis (Bowser and Broaddus, 2016). A comprehensive analysis of the expression of key adenosine-producing and -removing enzymes in malignant versus surrounding benign tissues is also lacking. Therefore, eN/CD73 as a therapeutic target for cancer therapy needs to be understood within a broader context that also involves intracellular adenosine metabolizing pathways and additional enzymes such as NPP1 and TNAP, which are capable of contributing to the breakdown of eATP in a compensatory and synergistic fashion. Furthermore, the interplay between extracellular ATP-consuming/adenosine-generating and reverse ATP-regenerating pathways and their contribution to the maintenance of high intratumoral concentrations of pericellular ATP and concurrent inhibition of adenosinergic signaling in the metabolically abnormal TME has been rather neglected so far.
Translational value and feasibility of novel therapeutic targets
The realization that the equilibrium of adenosine homeostasis is more complex than previously recognized justifies an armamentarium of different regulating agents, which could significantly improve the efficacy of currently ongoing clinical trials primarily focused on selective targeting of the ATP-ADO axis. Novel therapeutic strategies discussed here fall into three different categories: (1) Small-molecule inhibitors or antibodies directed against NTPDase1/CD39, eN/CD73, CD38 and other adenosine producing ectoenzymes, with the desired outcome to prevent the formation of eADO and respective activation of A2AR. Importantly, enzyme blockers versus antibodies may have different effects on local nucleotide and nucleoside levels. (2) Directional manipulation of pro-inflammatory ATP levels in the TME via inhibition of secreted nucleotide-scavenging enzymes. (3) Finally, intracellular adenosine metabolism emerges as a novel and previously neglected strategy to manipulate adenosine levels in different compartments (extracellular, cytoplasmic, nuclear). New ADK inhibitors are in development (Kose et al., 2016; Toti et al., 2016), which may eventually permit the regulation of adenosine in a compartment-selective manner. A future therapeutic opportunity might be to engineer cancer tissue to overexpress ADK-S by gene therapy and thereby create a metabolic sink for adenosine, which would then alter the immunological characteristics of the TME. The development of new agents that enhance the activity of ADK-S (e.g. by allosteric interaction) is another feasible strategy to turn an adenosine-producing cancer cell into an adenosine-removing phenotype.
The future of adenosine-based therapeutic interventions for cancer patients will rely on genetic information with the goal to tailor treatments individually according to the purinergic genetic signature of each cancer. Such personalized therapies may become a reality as we more clearly understand the complex interactions of the purinome discussed here. For example blocking eN/CD73 appears to be a reasonable therapy in breast cancers characterized by pathological overexpression of eN/CD73 as suggested by a multiplex analysis of a phase III trial (Buisseret et al., 2018). However, those therapies might be futile, if adenosine receptor expression is downregulated, or if ADK is overexpressed at the same time. Future therapies therefore need to consider the genetic signatures of the entire purinome and strive to reconstitute purinergic network homeostasis. Techniques for the analysis of the purinome include genomic, proteomic, as well as metabolomic tools that should ideally be interpreted in conjunction as is now increasingly used in the emerging field of Integrative Personalized Medicine (Kidd et al., 2015). Adenosine regulating therapies in general appear to be relatively safe with a wide therapeutic margin. The reason for the wide safety margin might be the redundancy of adenosine metabolizing pathways, which enables the choice of therapeutics that selectively target pathways known to play a role in cancer biology. Overall, the identification of maladaptive changes in the cancer purinome of a patient would likely increase the safety profile of novel adenosine-regulating agents and offer the opportunity for the development of personalized purinomic interventions.
Conclusions and outlook
Adenosine metabolism has been explored and translated as a tractable therapeutic target for cancer treatment. The utility of CD39 or CD73-inhibiting antibodies and A2AR antagonists as checkpoint inhibitors to block adenosine production and signaling is well established, both conceptually as well as clinically. However, major gaps in knowledge that impede the development of more effective adenosine-based therapeutics include: (1) Lack of consideration of the redundancy of ectoenzymatic pathways controlling eATP and eADO levels in a coordinated manner. (2) Lack of distinction between adenosine receptor-mediated and receptor-independent effects of adenosine. (3) Focus on eADO without consideration of new pathways linked to intracellular adenosine metabolism and compartmentalization of the adenosine system. Furthermore, the realization that adenosine, via the regulation of DNA and histone methylation, exerts epigenetic activity opens up an entirely novel era of adenosine-based therapeutics that act independently of the adenosine receptors. Further studies are also required to elucidate the role of purinergic (ecto)enzymes in the coordinated control of adenosine metabolism and signaling, and to explore how these distinct, but apparently interrelated extra- and intracellular pathways interact in the course of tumor progression and metastasis (Figure 4). Therapeutic manipulation of the system offers unique opportunities to combine different adenosine receptor dependent and independent effects of adenosine into a more holistic treatment approach.
Acknowledgments
The authors are funded by the NIH (DB: NS103740, NS065957) and the Sigrid Juselius Foundation (GY).
Footnotes
Declaration of interests – The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Allard B, Longhi MS, Robson SC, and Stagg J (2017). The ectonucleotidases CD39 and CD73: Novel checkpoint inhibitor targets. Immunol Rev 276, 121–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard B, Turcotte M, Spring K, Pommey S, Royal I, and Stagg J (2014). Anti-CD73 therapy impairs tumor angiogenesis. Int J Cancer 134, 1466–1473. [DOI] [PubMed] [Google Scholar]
- Antonioli L, Blandizzi C, Pacher P, and Hasko G (2013). Immunity, inflammation and cancer: a leading role for adenosine. Nat Rev Cancer 13, 842–857. [DOI] [PubMed] [Google Scholar]
- Armstrong JM, Chen JF, Schwarzschild MA, Apasov S, Smith PT, Caldwell C, Chen P, Figler H, Sullivan G, Fink S, et al. (2001). Gene dose effect reveals no Gs-coupled A2A adenosine receptor reserve in murine T-lymphocytes: studies of cells from A2A-receptor-gene-deficient mice. Biochem J 354, 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastid J, Regairaz A, Bonnefoy N, Dejou C, Giustiniani J, Laheurte C, Cochaud S, Laprevotte E, Funck-Brentano E, Hemon P, et al. (2015). Inhibition of CD39 enzymatic function at the surface of tumor cells alleviates their immunosuppressive activity. Cancer Immunol Res 3, 254–265. [DOI] [PubMed] [Google Scholar]
- Beavis PA, Henderson MA, Giuffrida L, Mills JK, Sek K, Cross RS, Davenport AJ, John LB, Mardiana S, Slaney CY, et al. (2017). Targeting the adenosine 2A receptor enhances chimeric antigen receptor T cell efficacy. J Clin Invest 127, 929–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattarai S, Jan Pippel, Anne Meyer, Marianne Freundlieb, Constanze Schmies, Aliaa Abdelrahman, Amelie Fiene, Sang-Yong Lee, Herbert Zimmermann, El-Tayeb Ali, et al. (2019). X-Ray Co-Crystal Structure Guides the Way to Subnanomolar Competitive Ecto-5’-Nucleotidase (CD73) Inhibitors for Cancer Immunotherapy. Adv Ther 2, 1900075. [Google Scholar]
- Bjursell MK, Blom HJ, Cayuela JA, Engvall ML, Lesko N, Balasubramaniam S, Brandberg G, Halldin M, Falkenberg M, Jakobs C, et al. (2011). Adenosine kinase deficiency disrupts the methionine cycle and causes hypermethioninemia, encephalopathy, and abnormal liver function. Am J Hum Genet 89, 507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blay J, White TD, and Hoskin DW (1997). The extracellular fluid of solid carcinomas contains immunosuppressive concentrations of adenosine. Cancer Res 57, 2602–2605. [PubMed] [Google Scholar]
- Boison D (2013). Adenosine kinase: exploitation for therapeutic gain. Pharmacological Reviews 65, 906–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D, Scheurer L, Zumsteg V, Rulicke T, Litynski P, Fowler B, Brandner S, and Mohler H (2002). Neonatal hepatic steatosis by disruption of the adenosine kinase gene. Proc Natl Acad Sci USA 99, 6985–6990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borea PA, Gessi S, Merighi S, and Varani K (2016). Adenosine as a Multi-Signalling Guardian Angel in Human Diseases: When, Where and How Does it Exert its Protective Effects? Trends Pharmacol Sci 37, 419–434. [DOI] [PubMed] [Google Scholar]
- Borges da Silva H, Beura LK, Wang H, Hanse EA, Gore R, Scott MC, Walsh DA, Block KE, Fonseca R, Yan Y, et al. (2018). The purinergic receptor P2RX7 directs metabolic fitness of long-lived memory CD8(+) T cells. Nature 559, 264–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boswell-Casteel RC, and Hays FA (2017). Equilibrative nucleoside transporters-A review. Nucleosides Nucleotides Nucleic Acids 36, 7–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bours MJL, Swennen ELR, Di Virgilio F, Cronstein BN, and Dagnelie PC (2006). Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacology & Therapeutics 112, 358–404. [DOI] [PubMed] [Google Scholar]
- Bowman CE, da Silva RG, Pham A, and Young SW (2019). An Exceptionally Potent Inhibitor of Human CD73. Biochemistry 58, 3331–3334. [DOI] [PubMed] [Google Scholar]
- Bowser JL, and Broaddus RR (2016). CD73s protection of epithelial integrity: Thinking beyond the barrier. Tissue Barriers 4, e1224963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowser JL, Lee JW, Yuan X, and Eltzschig HK (2017). The hypoxia-adenosine link during inflammation. J Appl Physiol (1985) 123, 1303–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisseret L, Pommey S, Allard B, Garaud S, Bergeron M, Cousineau I, Ameye L, Bareche Y, Paesmans M, Crown JPA, et al. (2018). Clinical significance of CD73 in triple-negative breast cancer: multiplex analysis of a phase III clinical trial. Ann Oncol 29, 1056–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JF, Eltzschig HK, and Fredholm BB (2013). Adenosine receptors as drug targets--what are the challenges? Nat Rev Drug Discov 12, 265–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Diao L, Yang Y, Yi X, Rodriguez BL, Li Y, Villalobos PA, Cascone T, Liu X, Tan L, et al. (2018). CD38-Mediated Immunosuppression as a Mechanism of Tumor Cell Escape from PD-1/PD-L1 Blockade. Cancer Discov 8, 1156–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Fan J, Zhang M, Qin L, Dominguez D, Long A, Wang G, Ma R, Li H, Zhang Y, et al. (2019). CD73 expression on effector T cells sustained by TGF-beta facilitates tumor resistance to anti-4–1BB/CD137 therapy. Nat Commun 10, 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini EN, Chini CCS, Espindola Netto JM, de Oliveira GC, and van Schooten W (2018). The Pharmacology of CD38/NADase: An Emerging Target in Cancer and Diseases of Aging. Trends Pharmacol Sci 39, 424–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leve S, Wirsdorfer F, and Jendrossek V (2019). Targeting the Immunomodulatory CD73/Adenosine System to Improve the Therapeutic Gain of Radiotherapy. Front Immunol 10, 698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marchi E, Orioli E, Pegoraro A, Sangaletti S, Portararo P, Curti A, Colombo MP, Di Virgilio F, and Adinolfi E (2019). The P2X7 receptor modulates immune cells infiltration, ectonucleotidases expression and extracellular ATP levels in the tumor microenvironment. Oncogene 38, 3636–3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F, Sarti AC, Falzoni S, De Marchi E, and Adinolfi E (2018). Extracellular ATP and P2 purinergic signalling in the tumour microenvironment. Nat Rev Cancer 18, 601–618. [DOI] [PubMed] [Google Scholar]
- Du J, Johnson LM, Jacobsen SE, and Patel DJ (2015). DNA methylation pathways and their crosstalk with histone methylation. Nat Rev Mol Cell Biol 16, 519–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltzschig HK, Sitkovsky MV, and Robson SC (2012). Purinergic signaling during inflammation. N Engl J Med 367, 2322–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feoktistov I, Biaggioni I, and Cronstein BN (2009). Adenosine receptors in wound healing, fibrosis and angiogenesis. Handb Exp Pharmacol, 383–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flogel U, Burghoff S, van Lent PL, Temme S, Galbarz L, Ding Z, El-Tayeb A, Huels S, Bonner F, Borg N, et al. (2012). Selective activation of adenosine A2A receptors on immune cells by a CD73-dependent prodrug suppresses joint inflammation in experimental rheumatoid arthritis. Sci Transl Med 4, 146ra108. [DOI] [PubMed] [Google Scholar]
- Hardie DG (2015). Molecular Pathways: Is AMPK a Friend or a Foe in Cancer? Clin Cancer Res 21, 3836–3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield S, Veszeleiova K, Steingold J, Sethuraman J, and Sitkovsky M (2019). Mechanistic Justifications of Systemic Therapeutic Oxygenation of Tumors to Weaken the Hypoxia Inducible Factor 1alpha-Mediated Immunosuppression. Adv Exp Med Biol 1136, 113–121. [DOI] [PubMed] [Google Scholar]
- Hatfield SM, and Sitkovsky M (2015). Oxygenation to improve cancer vaccines, adoptive cell transfer and blockade of immunological negative regulators. Oncoimmunology 4, e1052934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang WY, Hsu SD, Huang HY, Sun YM, Chou CH, Weng SL, and Huang HD (2015). MethHC: a database of DNA methylation and gene expression in human cancer. Nucleic Acids Res 43, D856–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, and Muller CE (2016). Medicinal chemistry of adenosine, P2Y and P2X receptors. Neuropharmacology 104, 31–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TA, Jinnah HA, and Kamatani N (2019). Shortage of Cellular ATP as a Cause of Diseases and Strategies to Enhance ATP. Front Pharmacol 10, 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joolharzadeh P, and St Hilaire C (2019). CD73 (Cluster of Differentiation 73) and the Differences Between Mice and Humans. Arterioscler Thromb Vasc Biol 39, 339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd BA, Readhead BP, Eden C, Parekh S, and Dudley JT (2015). Integrative network modeling approaches to personalized cancer medicine. Per Med 12, 245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordass T, Osen W, and Eichmuller SB (2018). Controlling the Immune Suppressor: Transcription Factors and MicroRNAs Regulating CD73/NT5E. Front Immunol 9, 813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kose M, Schiedel AC, Bauer AA, Poschenrieder H, Burbiel JC, Akkinepally RR, Stachel HD, and Muller CE (2016). Focused screening to identify new adenosine kinase inhibitors. Bioorg Med Chem 24, 5127–5133. [DOI] [PubMed] [Google Scholar]
- Koshiba M, Kojima H, Huang S, Apasov S, and Sitkovsky MV (1997). Memory of extracellular adenosine A2A purinergic receptor-mediated signaling in murine T cells. J Biol Chem 272, 25881–25889. [DOI] [PubMed] [Google Scholar]
- Lazarowski ER (2012). Vesicular and conductive mechanisms of nucleotide release. Purinergic Signal 8, 359–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Fiene A, Li W, Hanck T, Brylev KA, Fedorov VE, Lecka J, Haider A, Pietzsch HJ, Zimmermann H, et al. (2015). Polyoxometalates--potent and selective ecto-nucleotidase inhibitors. Biochem Pharmacol 93, 171–181. [DOI] [PubMed] [Google Scholar]
- Leone RD, and Emens LA (2018). Targeting adenosine for cancer immunotherapy. J Immunother Cancer 6, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden J, Koch-Nolte F, and Dahl G (2019). Purine Release, Metabolism, and Signaling in the Inflammatory Response. Annu Rev Immunol. [DOI] [PubMed] [Google Scholar]
- Lukashev D, Ohta A, Apasov S, Chen JF, and Sitkovsky M (2004). Cutting edge: Physiologic attenuation of proinflammatory transcription by the Gs protein-coupled A2A adenosine receptor in vivo. J Immunol 173, 21–24. [DOI] [PubMed] [Google Scholar]
- Mahoney KM, Rennert PD, and Freeman GJ (2015). Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov 14, 561–584. [DOI] [PubMed] [Google Scholar]
- Maj T, Wang W, Crespo J, Zhang H, Wei S, Zhao L, Vatan L, Shao I, Szeliga W, Lyssiotis, Cv et al. (2017). Oxidative stress controls regulatory T cell apoptosis and suppressor activity and PD-L1-blockade resistance in tumor. Nat Immunol 18, 1332–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malavasi F, Deaglio S, Funaro A, Ferrero E, Horenstein AL, Ortolan E, Vaisitti T, and Aydin S (2008). Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology. Physiol Rev 88, 841–886. [DOI] [PubMed] [Google Scholar]
- Martinez-Outschoorn UE, Peiris-Pages M, Pestell RG, Sotgia F, and Lisanti MP (2017). Cancer metabolism: a therapeutic perspective. Nat Rev Clin Oncol 14, 11–31. [DOI] [PubMed] [Google Scholar]
- Masino SA, Li T, Theofilas P, Sandau US, Ruskin DN, Fredholm BB, Geiger JD, Aronica E, and Boison D (2011). A ketogenic diet suppresses seizures in mice through adenosine A1 receptors. J Clin Inv 121, 2679–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, Shen S, Kepp O, Scoazec M, Mignot G, et al. (2011). Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science 334, 1573–1577. [DOI] [PubMed] [Google Scholar]
- Moeckel D, Jeong SS, Sun X, Broekman MJ, Nguyen A, Drosopoulos JH, Marcus AJ, Robson SC, Chen R, and Abendschein D (2014). Optimizing human apyrase to treat arterial thrombosis and limit reperfusion injury without increasing bleeding risk. Sci Transl Med 6, 248ra105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffatt BA, Wang L, Allen MS, Stevens YY, Qin W, Snider J, and von Schwartzenberg K (2000). Adenosine kinase of Arabidopsis. Kinetic properties and gene expression. Plant Physiol 124, 1775–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morandi F, Horenstein AL, Costa F, Giuliani N, Pistoia V, and Malavasi F (2018). CD38: A Target for Immunotherapeutic Approaches in Multiple Myeloma. Front Immunol 9, 2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morandi F, Marimpietri D, Horenstein AL, Corrias MV, and Malavasi F (2019). Microvesicles expressing adenosinergic ectoenzymes and their potential role in modulating bone marrow infiltration by neuroblastoma cells. Oncoimmunology 8, e1574198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser GH, Schrader J, and Deussen A (1989). Turnover of adenosine in plasma of human and dog blood. Am J Physiol 256, C799–806. [DOI] [PubMed] [Google Scholar]
- Muller WEG, Wang S, Neufurth M, Kokkinopoulou M, Feng Q, Schroder HC, and Wang X (2017). Polyphosphate as a donor of high-energy phosphate for the synthesis of ADP and ATP. J Cell Sci 130, 2747–2756. [DOI] [PubMed] [Google Scholar]
- Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MK, Huang X, Caldwell S, Liu K, Smith P, et al. (2006). A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci U S A 103, 13132–13137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta A, and Sitkovsky M (2001). Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature 414, 916–920. [DOI] [PubMed] [Google Scholar]
- Perrot I, Michaud HA, Giraudon-Paoli M, Augier S, Docquier A, Gros L, Courtois R, Dejou C, Jecko D, Becquart Ov et al. (2019). Blocking Antibodies Targeting the CD39/CD73 Immunosuppressive Pathway Unleash Immune Responses in Combination Cancer Therapies. Cell Rep 27, 2411–2425 e2419. [DOI] [PubMed] [Google Scholar]
- Pignataro G, Simon RP, and Boison D (2007). Transgenic overexpression of adenosine kinase aggravates cell death in ischemia. J Cereb Blood Flow Metab 27, 1–5. [DOI] [PubMed] [Google Scholar]
- Rajman L, Chwalek K, and Sinclair DA (2018). Therapeutic Potential of NAD-Boosting Molecules: The In Vivo Evidence. Cell Metab 27, 529–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevic V, and Burnstock G (1998). Receptors for purines and pyrimidines. Pharmacol Rev 50, 413–492. [PubMed] [Google Scholar]
- Sade-Feldman M, Yizhak K, Bjorgaard SL, Ray JP, de Boer CG, Jenkins RW, Lieb DJ, Chen JH, Frederick DT, Barzily-Rokni Mv et al. (2018). Defining T Cell States Associated with Response to Checkpoint Immunotherapy in Melanoma. Cell 175, 998–1013 e1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimu J, Webber J, Gurney M, Al-Taei S, Clayton A, and Tabi Z (2017). Dominant immunosuppression of dendritic cell function by prostate-cancer-derived exosomes. J Extracell Vesicles 6, 1368823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyfried TN, Flores RE, Poff AM, and D’Agostino DP (2014). Cancer as a metabolic disease: implications for novel therapeutics. Carcinogenesis 35, 515–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoni Y, Becht E, Fehlings M, Loh CY, Koo SL, Teng KWW, Yeong JPS, Nahar R, Zhang T, Kared H, et al. (2018). Bystander CD8(+) T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature 557, 575–579. [DOI] [PubMed] [Google Scholar]
- Sitkovsky MV (2009). T regulatory cells: hypoxia-adenosinergic suppression and re-direction of the immune response. Trends Immunol 30, 102–108. [DOI] [PubMed] [Google Scholar]
- Sitkovsky MV, Hatfield S, Abbott R, Belikoff B, Lukashev D, and Ohta A (2014). Hostile, hypoxia-A2-adenosinergic tumor biology as the next barrier to overcome for tumor immunologists. Cancer Immunol Res 2, 598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spychala J, Datta NS, Takabayashi K, Datta M, Fox IH, Gribbin T, and Mitchell BS (1996). Cloning of human adenosine kinase cDNA: sequence similarity to microbial ribokinases and fructokinases. Proc Natl Acad Sci U S A 93, 1232–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syn N, Wang L, Sethi G, Thiery JP, and Goh BC (2016). Exosome-Mediated Metastasis: From Epithelial-Mesenchymal Transition to Escape from Immunosurveillance. Trends Pharmacol Sci 37, 606–617. [DOI] [PubMed] [Google Scholar]
- Takenaka MC, Robson S, and Quintana FJ (2016). Regulation of the T Cell Response by CD39. Trends Immunol 37, 427–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodoraki MN, Hoffmann TK, Jackson EK, and Whiteside TL (2018). Exosomes in HNSCC plasma as surrogate markers of tumour progression and immune competence. Clin Exp Immunol 194, 67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theofilas P, Brar S, Stewart K-A, Shen H-Y, Sandau US, Poulsen DJ, and Boison D (2011). Adenosine kinase as a target for therapeutic antisense strategies in epilepsy. Epilepsia 52, 589–601; PMCID: PMC3075862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toti KS, Osborne D, Ciancetta A, Boison D, and Jacobson KA (2016). South (S)- and North (N)-Methanocarba-7-Deazaadenosine Analogues as Inhibitors of Human Adenosine Kinase. Journal of Medicinal Chemistry 59, 6860–6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Donk N, Richardson PG, and Malavasi F (2018). CD38 antibodies in multiple myeloma: back to the future. Blood 131, 13–29. [DOI] [PubMed] [Google Scholar]
- Warburg O (1956). On respiratory impairment in cancer cells. Science 124, 269–270. [PubMed] [Google Scholar]
- Vigano S, Alatzoglou D, Irving M, Menetrier-Caux C, Caux C, Romero P, and Coukos G (2019). Targeting Adenosine in Cancer Immunotherapy to Enhance T-Cell Function. Front Immunol 10, 925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayan D, Young A, Teng MWL, and Smyth MJ (2017). Targeting immunosuppressive adenosine in cancer. Nat Rev Cancer 17, 709–724. [DOI] [PubMed] [Google Scholar]
- Williams-Karnesky RL, Sandau US, Lusardi TA, Lytle NK, Farrell JM, Pritchard EM, Kaplan DL, and Boison D (2013). Epigenetic changes induced by adenosine augmentation therapy prevent epileptogenesis. J Clin Inv 123, 3552–3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtanen SS, Kukkonen-Macchi A, Vainio M, Elima K, Harkonen PL, Jalkanen S, and Yegutkin GG (2014). Adenosine Inhibits Tumor Cell Invasion via Receptor-Independent Mechanisms. Mol Cancer Res 12, 1863–1874. [DOI] [PubMed] [Google Scholar]
- Yegutkin GG (2008). Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim Biophys Acta 1783, 673–694. [DOI] [PubMed] [Google Scholar]
- Yegutkin GG (2014). Enzymes involved in metabolism of extracellular nucleotides and nucleosides: Functional implications and measurement of activities. Crit Rev Biochem Mol Biol 49, 473–497. [DOI] [PubMed] [Google Scholar]
- Yegutkin GG, Henttinen T, and Jalkanen S (2001). Extracellular ATP formation on vascular endothelial cells is mediated by ecto-nucleotide kinase activities via phosphotransfer reactions. Faseb J 15, 251–260. [DOI] [PubMed] [Google Scholar]
- Yokdang N, Nordmeier S, Speirs K, Burkin HR, and Buxton IL (2015). Blockade of extracellular NM23 or its endothelial target slows breast cancer growth and metastasis. Integr Cancer Sci Ther 2, 192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young A, Mittal D, Stagg J, and Smyth MJ (2014). Targeting cancer-derived adenosine: new therapeutic approaches. Cancer Discov 4, 879–888. [DOI] [PubMed] [Google Scholar]
- Young A, Ngiow SF, Barkauskas DS, Sult E, Hay C, Blake SJ, Huang Q, Liu J, Takeda K, Teng MWL, et al. (2016). Co-inhibition of CD73 and A2AR Adenosine Signaling Improves Anti-tumor Immune Responses. Cancer Cell 30, 391–403. [DOI] [PubMed] [Google Scholar]
- Zahnow CA, Topper M, Stone M, Murray-Stewart T, Li H, Baylin SB, and Casero RA Jr. (2016). Inhibitors of DNA Methylation, Histone Deacetylation, and Histone Demethylation: A Perfect Combination for Cancer Therapy. Adv Cancer Res 130, 55–111. [DOI] [PubMed] [Google Scholar]
- Zeiner J, Loukovaara S, Losenkova K, Zuccarini M, Korhonen AM, Lehti K, Kauppinen A, Kaarniranta K, Muller CE, Jalkanen S, and Yegutkin GG (2019). Soluble and membrane-bound adenylate kinase and nucleotidases augment ATP-mediated inflammation in diabetic retinopathy eyes with vitreous hemorrhage. J Mol Med (Berl) 97, 341–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Vijayan D, Li XY, Robson SC, Geetha N, Teng MWL, and Smyth MJ (2019). The role of NK cells and CD39 in the immunological control of tumor metastases. Oncoimmunology 8, e1593809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann H, Zebisch M, and Strater N (2012). Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal 8, 437–502. [DOI] [PMC free article] [PubMed] [Google Scholar]