Highlights
-
•
MRI is suited for tracking volumetric changes/accumulating doses in the esophagus.
-
•
Introduced medial axis of esophagus to calculate inter-fraction positional uncertainty.
-
•
Planned and accumulated esophagus dose-volume parameter differences are significant.
-
•
Longitudinal expansion of esophagus may link to acute esophagitis.
Keywords: MRI, Esophagus, Adaptive radiotherapy, Lung cancer, Dose accumulation
Abstract
Background and purpose
Minimizing acute esophagitis (AE) in locally advanced non-small cell lung cancer (LA-NSCLC) is critical given the proximity between the esophagus and the tumor. In this pilot study, we developed a clinical platform for quantification of accumulated doses and volumetric changes of esophagus via weekly Magnetic Resonance Imaging (MRI) for adaptive radiotherapy (RT).
Material and methods
Eleven patients treated via intensity-modulated RT to 60–70 Gy in 2–3 Gy-fractions with concurrent chemotherapy underwent weekly MRIs. Eight patients developed AE grade 2 (AE2), 3–6 weeks after RT started. First, weekly MRI esophagus contours were rigidly propagated to planning CT and the distances between the medial esophageal axes were calculated as positional uncertainties. Then, the weekly MRI were deformably registered to the planning CT and the total dose delivered to esophagus was accumulated. Weekly Maximum Esophagus Expansion (MEex) was calculated using the Jacobian map. Eventually, esophageal dose parameters (Mean Esophagus Dose (MED), V90% and D5cc) between the planned and accumulated dose were compared.
Results
Positional esophagus uncertainties were 6.8 ± 1.8 mm across patients. For the entire cohort at the end of RT: the median accumulated MED was significantly higher than the planned dose (24 Gy vs. 21 Gy p = 0.006). The median V90% and D5cc were 12.5 cm3 vs. 11.5 cm3 (p = 0.05) and 61 Gy vs. 60 Gy (p = 0.01), for accumulated and planned dose, respectively. The median MEex was 24% and was significantly associated with AE2 (p = 0.008).
Conclusions
MRI is well suited for tracking esophagus volumetric changes and accumulating doses. Longitudinal esophagus expansion could reflect radiation-induced inflammation that may link to AE.
1. Introduction
Minimizing radiation-induced acute esophagitis (AE) in locally advanced non-small cell lung cancer (LA-NSCLC) is critical given the high rate of AE due to the proximity between the esophagus and the large tumors [1]. The esophagus is a mobile structure and the planned esophagus dose may not accurately represent the actual accumulated esophagus dose due to anatomical changes and setup uncertainties arising from e.g. physiological variations and respiratory motion over the course of radiotherapy (RT) [2]. In some situations, these physiological/anatomical variations may have clinical consequences such that the planned dose and the accumulated dose are notably different. Discrepancies between the planned and on-treatment esophagus structures are challenging to detect using Computed Tomography (CT) or Cone-Beam CT (CBCT) as a sole imaging modality due to low soft tissue contrast [3], [4]. Instead, these changes are better quantified using Magnetic Resonance Imaging (MRI) [3], [5], [6]. With the introduction of the MR-Linac, MRI becomes a well-suited imaging technique to track anatomical changes for response assessment and dose accumulation, both of which are crucial for adaptive re-planning. For instance, high-resolution T2-weighted MRI has been shown to enable detailed imaging of the anatomical layers of the esophageal wall and surrounding tissues [3], thus, motivating the use of MRI to quantify local changes for dose accumulation.
Among methods for dose accumulation, Deformable Image Registration (DIR) is a widely used technique to find the local structural change between the planning and weekly images [5]. Subsequently, the accumulated dose can be calculated by deforming either the structure of interest or the dose map [2], [7], [8]. Although CT has been the primary imaging modality for this purpose, a few groups have shown that CBCT, which is typically used for patient setup correction and treatment response evaluation [9], could be an alternative modality [7], [10], [11], [12].
In this pilot study, we examined weekly accumulated Mean Esophagus Dose (MED), and weekly Maximum Esophagus Expansion (MEex) using MRIs and compared them against the planning scan and the planned dose to investigate their relationship with AE. Radiation-induced esophageal injuries and abnormalities cause expanded esophageal wall (>5 mm) [13], [14], therefore MEex could reflect inflammation and/or edema in the esophagus that may link to AE [15], [16], [17]. In addition, we introduced a novel method to calculate inter-fraction positional uncertainty between the planned and weekly esophagus using the medial axis of the esophagus. The ultimate goal was to develop a clinical platform for robust evaluation of delivered esophageal dose via weekly MRI (wMRI) acquired during the RT course, and provide timely guidance to the decision making of adaptive radiotherapy.
2. Material and methods
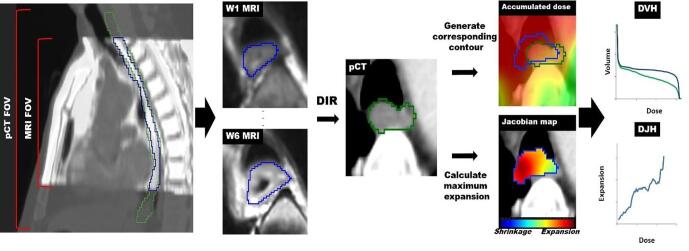
The main workflow of our method is summarized in Fig. 1. Weekly MRIs are deformably registered to pCT and the corresponding esophagus contours are generated representing the weekly esophagus changes. Then accumulated dose to the generated esophagus contour is calculated on planning coordinate and compared against the planned contour. Subsequently, weekly esophagus maximum expansion is obtained from the Jacobian map, and the correlations with the accumulated dose are estimated.
Fig. 1.
Main workflow. pCT = planning CT. DVH = Dose-volume histogram. DJH = Dose-Jacobian histogram. Blue and green contours are weekly and planning esophagus contours, respectively.
2.1. Dataset and clinical parameters
Eleven LA-NSCLC patients were enrolled in an IRB approved study to undergo weekly MRI during RT (Protocol# 15–073). Patients were treated via intensity-modulated RT in 2–3 Gy daily fractions in a five days/week fractionation (total dose 50–73 Gy), 9/11 with concurrent chemotherapy. At simulation, the patients were immobilized to have a free-breathing CT scan used for treatment planning and a 4DCT to evaluate tumor motion. Planning CT images (pCT) were used to define the Gross Tumor Volume (GTV) and to contour esophagus. All patients had daily kV orthogonal radiographs for daily setup (matched on bone) and a weekly CBCT to monitor positional uncertainties/tumor changes. A respiratory triggered (at exhalation) T2-weighted weekly MRI scan (TR/TE = 3000–6000/120 ms, 43 slices, NSA = 2, FOV = 300 × 222 × 150 mm, resolution = 0.85 × 0.85 × 3.5 mm3) was acquired post-treatment on a Philips Ingenia 3 Tesla scanner with vendor geometric distortion corrections. The purpose of the MRI scans was to assess the feasibility of weekly MRI to visualize pathologically involved lymph nodes (LN) in NSCLC during chemo-radiation for early therapy response assessment. The patient enrollment criteria in the protocol were: i) pathologic involvement of mediastinal LNs. ii) One or more LN that measured >1 cm. iii) Planned conventionally fractionated RT. iv) had >10 RT fractions. All patients underwent the same weekly MRI protocol. To estimate the potential inter-fraction positional discrepancy between the post-treatment wMRIs and the actual treatment setup where dose is calculated, first, a visual comparison was performed to evaluate differences between esophagus on end-exhalation of 4DCT and the free-breathing planning CT and no noticeable difference was seen. In addition, we took advantage of weekly CBCTs (used for treatment positional setup) as a ground-truth to calculate the inter-fraction esophagus positional uncertainties using medial axis technique introduced in section 2.5 and compared it with the uncertainties computed between pCT and wMRIs (section 3.2 for the results).
A total of 76 image sets (11 planning CT, 65 MRI) were acquired. The resolution of pCT was 1.17 × 1.17 × 3 mm3. The esophagus and GTV on the pCT and wMRIs were contoured by an experienced radiation oncologist and represented the ground-truth contour.
Acute esophagitis was assessed according to the Common Terminology Criteria for Adverse Events v.4.0 [18] and eight patients developed Grade 2 (AE2), 3–6 weeks after RT started (4 weeks median). We focused on AE2, because an oral supplement medication is indicated only after AE2 is diagnosed according to CTCAE.
The volumetric change of esophagus at the end of the treatment relative to the start ranged from −18% shrinkage to 82% expansion. Patient characteristics are summarized in Table 1.
Table 1.
Clinical and demographic characteristics of the cohort.
| Clinical Characteristic | Number of patients |
|---|---|
| Age (years) | |
| Mean ± SD, Range | 63 ± 7, 51–74 |
| Sex | |
| male/female | 7/4 |
| Histology | |
| Adenocarcinoma/Squamous/Other | 8/1/2 |
| Prescription dose (Gy) | |
| 50/60/66/73 | 1/7/2/1 |
| Acute Esophagitis (AE2) | |
| AE2/Non-AE | 8/3 |
| Tumor Location | |
| LUL/RUL/RML/LLL | 3/6/1/1 |
| Tumor Staging | |
| T2/T3/T4/Unspecified | 1/8/1/1 |
| Lymph Node Invasion | |
| Yes/No | 9/2 |
(LUL = Left-Upper-Lobe, LLL = Left-Lower-Lobe, RUL = Right-Upper-Lobe, RML = Right-Middle-Lobe).
2.2. Image registration
A multiresolution DIR was performed for two purposes:
-
1)
MRI-pCT registration to calculate the weekly accumulated dose in esophagus (MED): wMRIs (target) were deformably registered to pCT (reference) (Section 2.3).
-
2)
MRI-MRI registration to quantify weekly maximum expansion in esophagus (MEex): wMRI were registered from week to week. Each MRI at current week (target) was deformably registered to its previous week MRI (reference) (Section 2.4).
Prior to these DIR, first wMRIs were rigidly registered to their pCT images to roughly align the global structures by matching the center of the tumor (GTV). Before the DIR for dose accumulation and expansion quantification, tumor motion due to inter-fractional anatomy variation and respiratory motion was corrected using a rigidity penalty term [19] embedded in the DIR framework. This method enforces local rigidity on both the tumor and esophagus and preserves their structures while allowing only their surrounding tissues to register to its reference images [20]. This would minimize the inter-fractional differences in the surroundings of esophagus between the two images.
Then, a second B-spline regularized diffeomorphic registration [20], [21] was performed between the two images that could more accurately capture the local changes in esophagus for dose accumulation and maximum expansion quantification. The transformation in a diffeomorphic registration is obtained using a Symmetrized Large Deformation Diffeomorphic Metric Mapping (LDDMM) algorithm [21] that maps the corresponding points between two images by finding a geodesic solution. The integrated B-spline regularization fits the Deformation Vector Field (DVF) to a B-spline object to capture large differences. This gives free-form elasticity to the converging/diverging vectors that represents a morphological shrinkage/expansion. Three levels of multi-resolution registration with B-spline mesh size of 64 mm at the coarsest level was used with Mutual Information as similarity energy. The mesh size was reduced by a factor of two at each sequential level. The optimization step size was set to 0.1 and the number of iterations (100, 70, 30) at each level.
Histogram matching [22] was used for intensity standardization between the weekly MRIs. To account for different Field-Of-View (FOV) between pCT and wMRI, the pCT was cropped to the FOV of the wMRI images for image registration.
2.3. Dose accumulation via DIR
After the rigid registrations, wMRIs are transferred to planning coordinate system and the corresponding points between the esophagus on pCT and esophagus on the wMRIs were linked based on voxel-by-voxel correspondence built via second MRI-pCT DIR (section 2.2). The MRI-pCT DIR deformed/shrunk the expanded esophagus on wMRI and aligned it to the original esophagus on pCT (wMRI → pCT). However, to calculate the accumulated dose to the expanded weekly esophagus, the inverse of the MRI-pCT DIR transformation was computed [22] on the planning coordinate (pCT-MRI transformation) that reversed the direction of changes to pCT → wMRI. Consequently, by applying pCT-MRI transformation (the inverse) to the planning esophagus contour, a new esophagus volume/contour can be generated which represents the weekly esophagus structural changes (e.g. expansion, shift etc.) on the planning scan. The generated contours had the FOV of the weekly images which was smaller than the FOV of pCT (Fig. 1) and did not include the upper (proximal) or the lower (distal) parts of the esophagus. Therefore, we reconstructed these two regions by smoothly joining them to the original planning esophagus contours and built the complete corresponding contours. Dose calculation with the generated contours reflects the esophagus dose that is consistent with the initial plan generated using the pCT. The prescribed dose map on the planning coordinate was scaled to 6 weeks and for each week, the dose from the previous week was accumulated on the current week. Then dose-volume parameters i.e. MED, absolute V90% (the absolute volume receiving at least 90% of the prescription dose) and D5cc were calculated on the esophagus contour generated by DIR and compared against the planned esophagus contour. The accumulated dose was evaluated in both the entire esophagus and the portion of the esophagus corresponding to the MRI FOV. All the significance tests and the associations were calculated using Wilcoxon rank sum test in R [23].
2.4. Quantification of the maximum esophagus expansion
Weekly local volumetric esophagus expansion was quantified using Jacobian maps (J) calculated from DIR between the wMRI images. Each MRI at current week was registered to its previous week MRI: J was calculated at each voxel as the determinant of the gradient of the DVF that measured the ratio of local volume change where J > 1 indicates local volume expansion, J < 1 shrinkage and J = 1 no change [24]. The Jacobian integral defined as [(Mean J – 1) × baseline volume] measured the net local volume change [20], [24]. To quantify the maximum esophagus expansion (MEex), the average Jacobian value of a 3 × 3 × 3 voxel of cubic region (0.1 cm3) that encompassed the point with the maximum Jacobian value (the maximum anatomical change) was calculated. MEex was calculated on wMRIs coordinates. To calculate the voxel-wise correlation between the accumulated esophagus dose on pCT and its corresponding weekly expansion, the weekly Jacobian maps were rigidly transferred to pCT using the same transformations that were used to rigidly register wMRIs to pCT for dose accumulation.
2.5. Positional uncertainty using medial axis
To account for inter-fraction positional variations between the planning and the weekly esophagus, the medial axis was calculated on the planning and each weekly esophagus after rigidly aligning each MRI to its pCT. The medial axis is computed using the first non-trivial eigenfunction of the Laplace-Beltrami Operator [25] on the esophagus contour where its minimum and maximum represent the two points with the greatest geodesic distance on the contour’s surface. Connecting the centers of mass along this eigenfunction, gives the medial axis for the given 3D esophagus contour. The average Euclidean distance between the medial axes on the pCT and wMRIs represented the positional error for each week.
2.6. Registration evaluation
For geometric evaluation of MRI-pCT registrations, Dice Similarity Coefficient (DSC) and Hausdorff distance between the registered wMRI esophagus contours on pCT and the ground-truth planning esophagus contours were calculated. For MRI-MRI registrations, these metrics were calculated between the registered esophagus contour of the current week and the ground-truth esophagus contour of the previous week [26], [28].
Dosimetric evaluation was also performed for MRI-pCT registration by comparing the dose-volume parameters i.e. MED, V90% and D5cc between the corresponding esophagus contours generated by DIR on pCT (section 2.3) and the ground-truth wMRI esophagus contours rigidly propagated on pCT.
In addition, we investigated the spatial uncertainties of the registrations. We introduced known errors by 3D shifting the dose accumulated contours generated using DIR to 6 directions i.e. Left, Posterior, Inferior, Right, Anterior, and Superior with an amplitude of 1 mm. Then the average MED and maximum esophageal dose of these errors were compared to the ground-truth MED to obtain the dosimetric uncertainty with respect to the introduced errors. We repeated the experiments with 2 mm shifts.
Eventually, to validate MEex and the Jacobian map, we calculated the differences between the local volume change of esophagus obtained using Jacobian integral versus the volume change obtained from the ground-truth contours by the physician [20], [27].
3. Results
3.1. Registration results
The average DSC for MRI-pCT registrations were 0.80 ± 0.04 (95% confidence-interval: 0.79–0.81) and maximum, 95th percentile and average Hausdorff distances were 6.3 mm, 3.0 mm and 1.2 mm across the patients, respectively. For MRI-MRI registrations DSC was 0.81 ± 0.04 (95% confidence-interval: 0.80–0.82) and Hausdorff distances were 6.0 mm, 2.9 mm and 0.95 mm. Average dosimetric differences between MRI-pCT registration vs. the ground-truth were: ΔMED = 0.3 ± 0.4 Gy, ΔV90%=0.6 ± 1.3 cm3, ΔD5cc = 0.4 ± 0.9 Gy (p > 0.2).
Mean absolute dose difference between the ground-truth MED and 1 mm shifted MED was 0.4 ± 0.4 Gy and for maximum esophageal dose was 0.2 ± 0.5 Gy, for all the patients which yields the percent difference of 1.8% with respect to the population mean MED (24 Gy). For 2 mm shift, MED and maximum dose differences were 0.5 ± 0.5 Gy (2% difference to 24 Gy) and 0.3 ± 0.6 Gy, respectively.
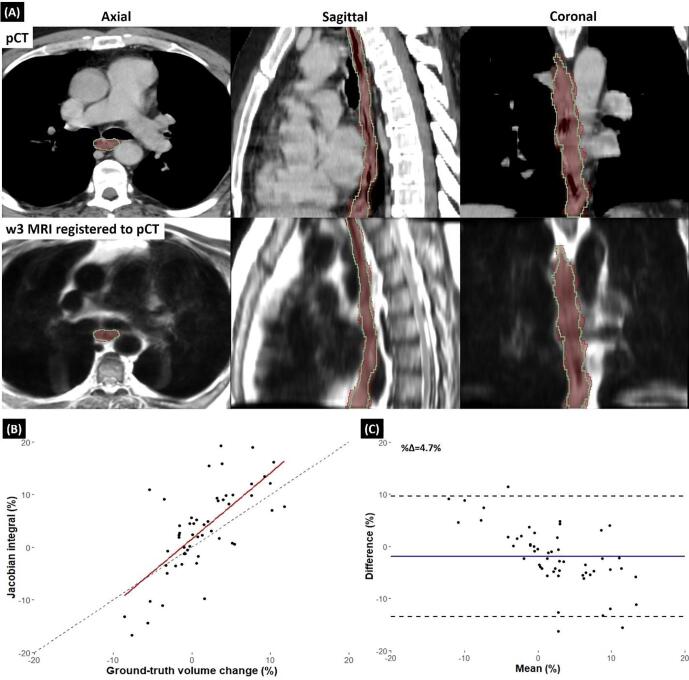
Deformable registration results of a case illustrated in Fig. 2-A showed a good alignment between week 3 MRI and the corresponding pCT image. There was a reasonable correlation between the weekly Jacobian integral and ground-truth volume change in the cohort (Fig. 2-B) and also, the agreement between the two methods showed 4.7% mean absolute percentage difference (Fig. 2-C).
Fig. 2.
(A-Top): pCT of a patient in axial, sagittal and coronal view. Esophagus contour propagated from week 3 MRI (green) to the pCT aligned well to the planning ground-truth contour (red color wash) and the esophagus boundary was clear after registration. (A-Bottom): Registered week 3 MRI to the pCT image using the same transformation that propagated the contour in the top row. (B) Scatter plot showing correlation between local net volume change of esophagus calculated using Jacobian integral and ground-truth volume change of esophagus. Dashed line is identity line. (C) Bland-Altman plot illustrating the agreement between the Jacobian integral and ground-truth volume change of esophagus. Solid blue line shows the mean difference. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2. Positional uncertainty of esophagus
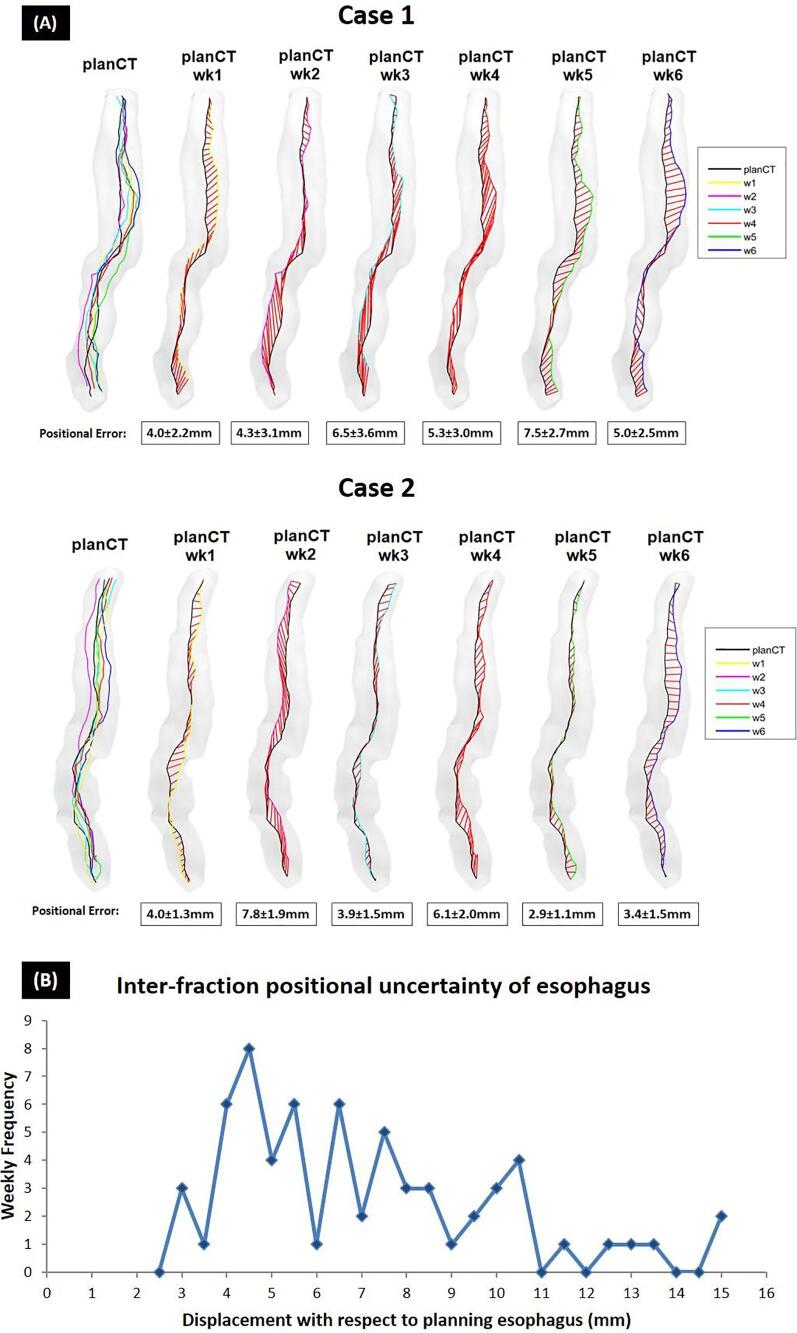
The inter-fraction positional uncertainties of esophagus were 6.8 ± 1.8 mm (3D) across patients with a maximum of 14.7 mm. This error was larger than 1cm in 22% of the weeks with >4–5 mm error for most cases (Fig. 3-B). Medial axes distances between the esophagus on pCT and the wMRIs of two typical patients are illustrated in Fig. 3-A where the average inter-fraction positional uncertainty differences calculated on wCBCTs versus wMRIs for the first and the second patients were 2.3 mm and 1.8 mm, respectively.
Fig. 3.
(A) Inter-fraction positional uncertainty between pCT and wMRIs esophagus of two typical cases calculated using medial axis. Grade shading structures demonstrate the planning esophagus contour. The black lines in the middle show medial axis of the planning esophagus and the colored lines (yellow for w1 to blue for w6) are the corresponding weekly esophagus medial axes. The red lines in between represent the distance between the corresponding points on the black and the colored medial axes which mean ± SD were calculated. (B) Histogram of displacement of weekly esophagus with respect to the planning esophagus for all the weeks.
3.3. Esophagus dose accumulation and maximum expansion quantification
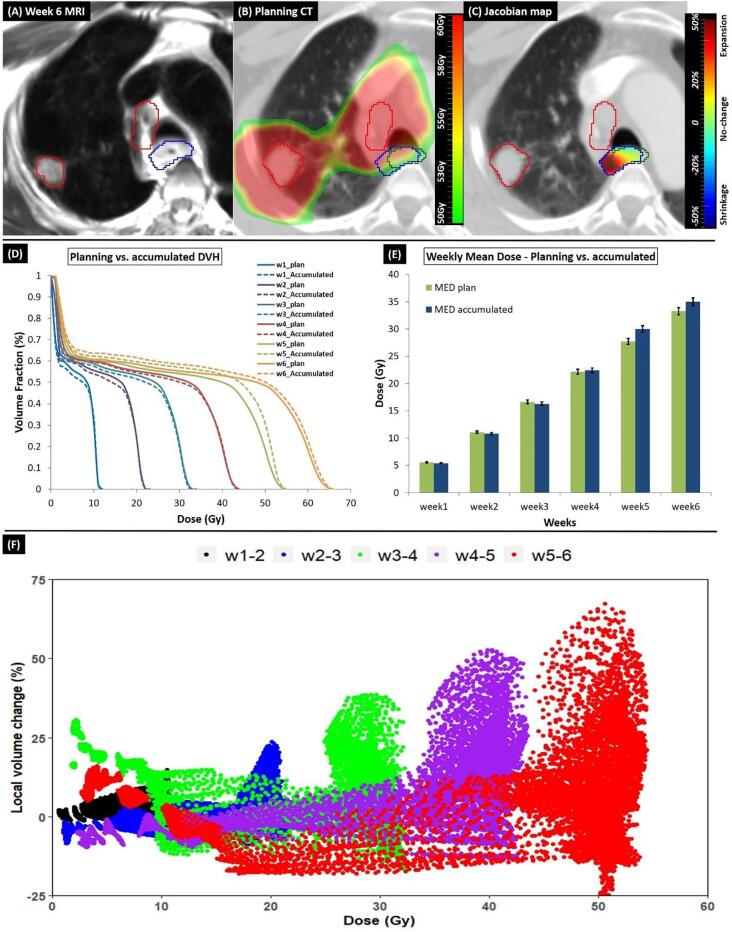
Fig. 4 shows the process of dose accumulation. This patient developed AE2 at week 5 where accumulated dose was drastically higher than the planned dose at that week (Fig. 4-D, E). Moreover, accumulated versus the planned dose V90% and D5cc at the final week were 24.0 cm3 vs. 20.6 cm3 and 62.0 Gy vs. 61.0 Gy, respectively. The Jacobian map showed large expansion in the region close to the tumor (Fig. 4-C) and when the accumulated dose was higher, the corresponding volumetric change was much larger (R = 0.68, p < 0.01) (Fig. 4-F).
Fig. 4.
(A) Week 6 MRI of a case that developed AE2 at week 5. Blue and red contours are the week 6 esophagus and GTV, respectively. (B) Accumulated week 6 dose map overlaid on pCT. The esophagus contour generated on pCT via DIR (blue) showed local expansion compared to the planning esophagus (green) due to proximity to the GTV (red contour). (C) Jacobian map showed large expansion (D) Planned vs. weekly DVH and (E) Comparing planned and the accumulated MED. Error bars indicates 2% errors for each value. (F) Scatter plot shows voxel-wise correlation between accumulated dose and the local volumetric change in esophagus calculated using wMRIs. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
For this case, MEex was as high as 66% and net volume change at the week AE2 was developed was the highest (10%).
3.4. Dose-volume parameter calculation
The population median accumulated MED at the end of RT was 24 Gy (9.6 Gy-35 Gy), which was higher than the planned dose of 21 Gy (p = 0.006); individual ΔMED (wMRI MED − planned MED) of up to 4.9 Gy were seen. The population median V90% and D5cc were 12.5 cm3 vs. 11.5 cm3 (p = 0.05) and 61 Gy vs. 60 Gy (p = 0.01), for accumulated and planned dose, respectively. Dose-volume parameters calculated for the accumulated dose using wMRIs were significantly higher than the planned dose at the final week and at the week patients developed AE2 (Table 2). Four patients (36%) had ΔMED larger than the population mean. Note that ΔMED within the MRI FOV was much larger than that of the entire esophagus on the planning scan.
Table 2.
Median differences of dose-volume parameters between accumulated weekly MRI vs. pCT (Δ = wMRI − planned) for the entire population.
| Final week (planned vs. accumulated) |
AE2 week (planned vs. accumulated) |
|||
|---|---|---|---|---|
| Parameters | Median (range) | p-value | Median (range) | p-value |
| ΔMED (Gy) | 1.0 (0.2–4.9) | 0.006 | 0.8 (0.1–2.3) | 0.07 |
| ΔMEDMRI FOV (Gy) | 5.2 (1.2–6.1) | 0.001 | 2.9 (0.5–6.8) | 0.008 |
| ΔV90% (cm3) | 1.2 (0.1–9.1) | 0.05 | 0.6 (0.1–5.6) | 0.7 |
| ΔD5cc (Gy) | 1.2 (0.2–5.7) | 0.01 | 1.1 (0.2–5.7) | 0.03 |
The median MEex at the final week for the entire population was 24% (11%–66%; Pearson correlation with MED was R = 0.50, p < 0.001). MEex was significantly associated with AE2 (p = 0.008) with a moderate correlation of R = 0.40.
4. Discussion
To the best of our knowledge, this is the first study investigating the feasibility of accumulating esophagus dose via DIR using MRI in LA-NSCLC patients. Furthermore, none of the studies so far explored the relationship between dose and local expansion for esophagus as an OAR. T2 MRI in lung, provides decent lesion-to-background contrast that helps detection of tumor infiltration into the chest wall/mediastinum and also to assess secondary changes due to the therapeutic effect of chemotherapy and radiation-induced inflammation.
We introduced a novel method to calculate inter-fraction positional uncertainty using medial axis of esophagus. Using this, systematic and random positional errors for the esophagus were generated, which were 6.8 ± 1.8 mm. Gao et al. [29] also reported mid-esophagus shift of 5.5 ± 2.0 mm in lung cancer patients. Ultimately, the distribution of these errors can be retrospectively modelled as a Probability Density Function and incorporated into an optimization framework to further reduce the dose to the esophagus. Such a development would pave a road for an adaptive robust optimization approach for treatment planning to reduce AE in lung cancer patients [30].
Moreover, in an experiment, we used medial axis to estimate the potential positional discrepancy between wMRIs and the treatment setup for the two patients in Fig. 3-A, by using wCBCTs as the ground-truth. We found the average difference was 2 mm and considering different acquisition process between MRI and CBCT and daily variations in patient’s anatomy, ~2 mm inter-fraction error may be reasonable and falls within the average registration error i.e. 1 mm-3 mm.
In addition to the entire esophagus volume used in the clinical settings, the MRI FOV was chosen to include part of the body that encompassed the Planning Target Volume (PTV i.e high dose region) where radiation-induced esophageal changes would be expected. ΔMED in that region was much larger than for the entire esophagus and may have larger impact in development of AE.
In this study, the rigid registrations were matched to the center of the tumor (GTV). However, alternatively the alignment could be performed with respect to the bony structures or other salient structures e.g. carina. To test this, we compared our rigid transformations with the transformations that are frequently used in the clinic (aligned to spine) and the differences were small with Δx = 0.2 mm, Δy = 0.8 mm, Δz = 2 mm. The largest difference was seen in the SI direction due to the slice thickness (~3 mm) relative to in-plane resolutions (~1 mm).
The challenge in MRI-pCT registration was mostly due to esophagus volume/structures appearing considerably different on MRI than on pCT for some cases. The esophagus volumes on week1 MRIs were 7%-73% (median 47%) smaller compared to their esophagus volume on pCT, in the entire cohort. This is mostly because higher soft tissue contrast in MRI images provided finer visualization of esophagus boundaries and soft tissue textures compared to the pCT which led to more accurate detection of esophagus boundaries. Another reason was that some patients had oral contrast in the esophagus on their pCT, which resulted in larger esophagus volumes compared to the wMRIs.
In the study by Niedzielski et al. [17], the association between volumetric esophagus expansion using Jacobian map and AE on weekly CTs in NSCLC was investigated. Similar to our study, they also reported MEex as a significant predictor of AE that suggests MEex could reflect inflammation and/or edema in the esophagus that may link to AE [14], [15], [16]. They did not perform a dosimetric analysis and the reported median MEex was ~40% (AE2).
The framework introduced in this study could benefit the online and real-time adaptive radiotherapy of lung cancer. On the MR-Linac platform, the accumulated dose along with the well-visualized esophagus of the day can be utilized to guide the optimization of the treatment plan to spare the esophagus in the “adapt to position/shape” approach. Such a re-planning can also focus on reducing dose to where esophagus shows significant expansion revealed by the Jacobian calculation, therefore mitigating the most severe esophagus complications.
AE may limit the delivery of the prescribed tumor doses in LA-NSCLC. Esophageal accumulated dose and maximum expansion information can be used to build a model for early prediction of AE and to guide the plan early in treatment for adaptive re-planning. Such a model would have the potential to minimize the risk of AE while respecting the prescribed dose or even open for dose escalation.
Finally, the main limitation of this study was that it was a retrospective analysis of a small patient cohort (n = 11), which was also reflected by the relatively low correlations between MEex, MED and AE. Moreover, the observed associations don’t imply causation but are considered hypothesis-generating, hence the accuracy and stability of the framework should be validated in a larger and independent patient cohort. Note that one could also establish a more meaningful association with AE by limiting the evaluation to the expansion area only within the high-dose region to exclude the nonessential distant deformations.
In summary, we showed that the differences between the planned and accumulated esophagus dose-volume parameters at the end of RT were significant for the cohort. This may convey the current challenge of image-guided RT to spare the mobile esophagus structure during lung cancer treatment. In this sense, MRI was well suited for accurately tracking accumulating doses and volumetric change of esophagus that on a larger cohort would be highly feasible on MR-guided systems such as MR-Linac.
Disclosure
Supported by master research agreement between Memorial Sloan Kettering Cancer Center and Varian Medical Systems, United States. Supported by NCI/NIH P30 CA 008748 and R01 CA 198121.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Thor M., Deasy J., Iyer A., Bendau E., Fontanella A., Apte A. Towards personalized dose-prescription in locally advanced nonsmall cell lung cancer: Validation of published normal tissue complication robability models. Radiother Oncol. 2019 doi: 10.1016/j.radonc.2019.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Velec M., Moseley J.L., Eccles C.L., Craig T., Sharpe M.B., Dawson L.A. Effect of breathing motion on radiotherapy dose accumulation in the abdomen using deformable registration. Int J Radiat Oncol Biol Phys. 2011;80:265–272. doi: 10.1016/j.ijrobp.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Rossum P.S.N., van Lier A., Lips I.M., Meijer G.J., Reerink O., van Vulpen M. Imaging of oesophageal cancer with FDG-PET/CT and MRI. Clin Radiol. 2015;70:81–95. doi: 10.1016/j.crad.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 4.Botros M.E., Gore E.M., Johnstone C., Knechtges P.M., Paulson E.S. MR simulation for esophageal cancer: imaging protocol and gross tumor volume comparison between MRI, CT, and PET/CT. Int J Radiat Oncol Biol Phys. 2015;93:S191-S. doi: 10.1016/j.ijrobp.2015.07.458. [DOI] [Google Scholar]
- 5.Sonke J.J., Aznar M., Rasch C. Adaptive radiotherapy for anatomical changes. Semin Radiat Oncol. 2019;29:245–257. doi: 10.1016/j.semradonc.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Bainbridge H., Salem A., Tijssen R.H.N., Dubec M., Wetscherek A., Van Es C. Magnetic resonance imaging in precision radiation therapy for lung cancer. Transl Lung Cancer Res. 2017;6(689–707) doi: 10.21037/tlcr.2017.09.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qin A., Gersten D., Liang J., Liu Q., Grill I., Guerrero T. A clinical 3D/4D CBCT-based treatment dose monitoring system. J Appl Clin Med Phys. 2018;19:166–176. doi: 10.1002/acm2.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chetty I.J., Rosu-Bubulac M. Deformable registration for dose accumulation. Semin Radiat Oncol. 2019;29:198–208. doi: 10.1016/j.semradonc.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 9.van Nunen A., van der Sangen M.J.C., van Boxtel M., van Haaren P.M.A. Cone-Beam CT-based position verification for oesophageal cancer: Evaluation of registration methods and anatomical changes during radiotherapy. Techn Innov Patient Supp Radiat Oncol. 2017;3–4:30–36. doi: 10.1016/j.tipsro.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kong V.C., Marshall A., Chan H.B. Cone beam computed tomography: the challenges and strategies in its application for dose accumulation. J Med Imaging Radiat Sci. 2016;47:92–97. doi: 10.1016/j.jmir.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Orlandini L.C., Coppola M., Fulcheri C., Cernusco L., Wang P., Cionini L. Dose tracking assessment for image-guided radiotherapy of the prostate bed and the impact on clinical workflow. Radiat Oncol. 2017;12:78. doi: 10.1186/s13014-017-0815-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moteabbed M., Sharp G.C., Wang Y., Trofimov A., Efstathiou J.A., Lu H.M. Validation of a deformable image registration technique for cone beam CT-based dose verification. Med Phys. 2015;42:196–205. doi: 10.1118/1.4903292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mesurolle B., Qanadli S.D., Merad M., Mignon F., Baldeyrou P., Tardivon A. Unusual radiologic findings in the thorax after radiation therapy. Radiographics. 2000;20:67–81. doi: 10.1148/radiographics.20.1.g00ja1167. [DOI] [PubMed] [Google Scholar]
- 14.Berkovich G.Y., Levine M.S., Miller W.T., Jr. CT findings in patients with esophagitis. AJR Am J Roentgenol. 2000;175:1431–1434. doi: 10.2214/ajr.175.5.1751431. [DOI] [PubMed] [Google Scholar]
- 15.Niedzielski J., Yang J., Stingo F., Liao Z., Gomez D., Mohan R. A novel methodology using CT imaging biomarkers to quantify radiation sensitivity in the esophagus with application to clinical trials. Sci Rep. 2017;7:6034. doi: 10.1038/s41598-017-05003-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Court L.E., Tucker S.L., Gomez D., Liao Z., Zhang J., Kry S. A technique to use CT images for in vivo detection and quantification of the spatial distribution of radiation-induced esophagitis. J Appl Clin Med Phys. 2013;14:4195. doi: 10.1120/jacmp.v14i3.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niedzielski J.S., Yang J., Stingo F., Martel M.K., Mohan R., Gomez D.R. Objectively quantifying radiation esophagitis with novel computed tomography-based metrics. Int J Radiat Oncol Biol Phys. 2016;94:385–393. doi: 10.1016/j.ijrobp.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Institutes of Health NCI. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. US DEPARTMENT OF HEALTH AND HUMAN SERVICES. 4 ed2009.
- 19.Staring M., Klein S., Pluim J.P. A rigidity penalty term for nonrigid registration. Med Phys. 2007;34:4098–4108. doi: 10.1118/1.2776236. [DOI] [PubMed] [Google Scholar]
- 20.Riyahi S., Choi W., Liu C., Nadeem S., Tan S., Zhong H. Quantification of local metabolic tumor volume changes by registering blended PET-CT images for prediction of pathologic tumor response. Lect Notes Comput Sci. 2018;11076:31–41. doi: 10.1007/978-3-030-00807-9_4. [DOI] [Google Scholar]
- 21.Tustison N.J., Avants B.B. Explicit B-spline regularization in diffeomorphic image registration. Front Neuroinform. 2013;7:39. doi: 10.3389/fninf.2013.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ibanez L., Schroeder W., Ng L., Cates J. Insight Toolkit Kitware, Inc.; New York: 2005. The ITK software guide. [Google Scholar]
- 23.Team R. RStudio Inc; Boston, MA: 2015. RStudio: integrated development for R. [Google Scholar]
- 24.Riyahi S., Choi W., Liu C., Zhong H., Wu A., Mechalakos J. Quantifying local tumor morphological changes with Jacobian map for prediction of pathologic tumor response to chemo-radiotherapy in locally advanced esophageal cancer. Phys Med Biol. 2018;63 doi: 10.1088/1361-6560/aacd22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nadeem S., Marino J., Gu X., Kaufman A. Corresponding supine and prone colon visualization using eigenfunction analysis and fold modeling. IEEE Trans Vis Comput Graph. 2017;23:751–760. doi: 10.1109/TVCG.2016.2598791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hardcastle N., van Elmpt W., De Ruysscher D., Bzdusek K., Tome W.A. Accuracy of deformable image registration for contour propagation in adaptive lung radiotherapy. Radiat Oncol. 2013;8:243. doi: 10.1186/1748-717X-8-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giavarina D. Understanding bland Altman analysis. Biochem Med (Zagreb) 2015;25:141–151. doi: 10.11613/BM.2015.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hazra A. Using the confidence interval confidently. J Thorac Dis. 2017;9(4125–30) doi: 10.21037/jtd.2017.09.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao H., Kelsey C.R., Boyle J., Xie T., Catalano S., Wang X. Impact of esophageal motion on dosimetry and toxicity with thoracic radiation therapy. Technol Cancer Res Treat. 2019;18 doi: 10.1177/1533033819849073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bock M., Eriksson K., Forsgren A., Hardemark B. Toward robust adaptive radiation therapy strategies. Med Phys. 2017;44:2054–2065. doi: 10.1002/mp.12226. [DOI] [PubMed] [Google Scholar]