SUMMARY
Recurrent epidemics of drug-resistant Staphylococcus aureus illustrate the rapid lapse of antibiotic efficacy following clinical implementation. Over the last decade, community-associated methicillin-resistant S. aureus (MRSA) has emerged as a dominant cause of infections, and this problem is amplified by the hyper-virulent nature of these isolates. Herein, we report the discovery of a fungal metabolite, apicidin, as an innovative means to counter both resistance and virulence. Owing to its breadth and specificity as a quorum-sensing inhibitor, apicidin antagonizes all MRSA agr systems in a non-biocidal manner. In skin challenge experiments, the apicidin-mediated abatement of MRSA pathogenesis corresponds with quorum-sensing inhibition at in vivo sites of infection. Additionally, we show that apicidin attenuates MRSA-induced disease by potentiating innate effector responses, particularly through enhanced neutrophil accumulation and function at cutaneous challenge sites. Together, these results indicate that apicidin treatment represents a strategy to limit MRSA virulence and promote host defense.
In Brief
Parlet et al. identified the apicidin family of fungal-derived compounds as potent inhibitors of Staphylococcus aureus agr quorum sensing. In a mouse model of skin infection, apicidin prevented agr activation and MRSA-induced dermonecrosis. Apicidin treatment also induced neutrophil accumulation and function at MRSA challenge sites, aiding host defense to infection.
Graphical Abstract
INTRODUCTION
Antibiotic-resistant pathogens threaten human health and economic stability on a global scale (Laxminarayan et al., 2013; Medina and Pieper, 2016). A common theme among recent reports from the World Health Organization, US Centers for Disease Control and Prevention, the European Centre for Disease Prevention and Control, the NIH, and the World Economic Forum is that the post-antibiotic era looms ever closer as the loss of efficacy among available antibiotics is far outpacing the genera tion of effective replacements (CDC, 2013; NIAID, 2014; O’Neill, 2016; Spellberg et al., 2016). Failure to reverse these trends could lead to an exhaustion of protective interventions against top pathogens. According to the Review on Antimicrobial Resistance, infectious diseases are poised to claim 10 million lives per year and account for 100 trillion dollars of lost economic output by 2050 (O’Neill, 2016). Clearly, the growing specter of antibiotic resistance warrants a reevaluation of long-term infection control strategies for prominent pathogens.
While the pursuit of new antibiotics is a global heath imperative, there is justifiable concern over a research and development enterprise that is narrowly focused upon antibiotic discovery. Given that the efficacy among clinically approved antibiotics is achieved via bacteriostatic or bactericidal mechanisms, extensive use of any given antibiotic therapy will eventually yield resistant populations that emerge as a source of infection (Laxminarayan et al., 2013; Medina and Pieper, 2016). A circumspect approach to infectious disease management mandates the exploration of therapeutic alternatives that are devised to alleviate the selective pressure imposed by antibiotics (Laxminarayan et al., 2013; Medina and Pieper, 2016). Providing a rationale to advance the latter effort, the virulence attributes of many clinically relevant pathogens are regulated through physiological pathways that can be inhibited or ablated without cytotoxic effects. In this way, so-called anti-virulence therapies provide a means of attenuating infectious illness without spurring evolution toward a resistant phenotype (Cegelski et al., 2008; Laxminarayan et al., 2013; Rasko and Sperandio, 2010). Therapeutic targeting of quorum sensing is a paradigmatic anti-virulence strategy that may prove efficacious against some of the most pervasive and problematic bacterial pathogens. Chief among these is Staphylococcus aureus, which remains one of the most frequent causes of both hospital and community-acquired infection (Lowy, 1998; Tong et al., 2015).
The propensity of S. aureus to acquire antibiotic resistance is evidenced by the reoccurring clinical pattern whereby epidemics caused by resistant isolates emerge rapidly after a new antibiotic is introduced for infection control (Chambers and Deleo, 2009). While S. aureus is classified as an opportunistic pathogen, the capacity of highly aggressive USA300 lineages of methicillin-resistant S. aureus (MRSA) to inflict disease among “healthy” community-dwelling individuals has reached pandemic proportions (Chambers and Deleo, 2009; DeLeo et al., 2010; Otto, 2010). The hyper-virulent nature of “community associated” MRSA (CA-MRSA) strains has been attributed to heightened expression of core genome-encoded virulence factors such as α-hemolysin and α phenol soluble modulin (PSMα) peptides, which subvert host defense by exerting cytolytic effects upon immune effector cells (Cheung et al., 2011; Otto, 2010).
Like other S. aureus strains, MRSA utilizes quorum sensing to synchronize virulence factor induction in proportion to prevailing cell density (Novick and Geisinger, 2008; Thoendel et al., 2011). Encoded by the agrBDCA operon, the quorum-sensing signaling apparatus achieves maximal activity at high cell densities when ambient agrBD-derived autoinducing peptides (AIPs) reach a concentration threshold necessary to activate the AgrC-A two component signal transduction system (Figure 1A). There are four different types of AIP signal structures (AIP-I, -II, -III, and -IV), and in turn four types of AgrC receptors, depending on the S. aureus strain. The respective ligand receptor interaction between AIP and the sensor histidine kinase AgrC initiates the signaling cascade via phospho-transfer-mediated activation of the response regulator AgrA. The DNA binding capability of AgrA mediates distinct transcriptional pathways from the systems’ two oppositely oriented promoter units (Queck et al., 2008). Whereas the P2 promoter activates an auto-induction circuit via agrBDCA expression, the P3 promoter exponentially increases the system’s major effector molecule, RNAIII, which regulates >200 virulence factor genes for the purpose of countering host defense and promoting tissue invasion (Kavanaugh and Horswill, 2016; Novick and Geisinger, 2008; Thoendel et al., 2011). Within the context of acute infection, agr-regulated quorum sensing coordinates an explosive outburst of virulence factors that are manifestly harmful to the host (Mayville et al., 1999; Wright et al., 2005). As such, the therapeutic potential of quorum-sensing inhibitors (often termed quorum quenchers) has been intensively investigated (Daly et al., 2015; Figueroa et al., 2014; Gordon et al., 2013; Muhs et al., 2017; Nielsen et al., 2014; Quave et al., 2015; Sully et al., 2014; Wright et al., 2005).
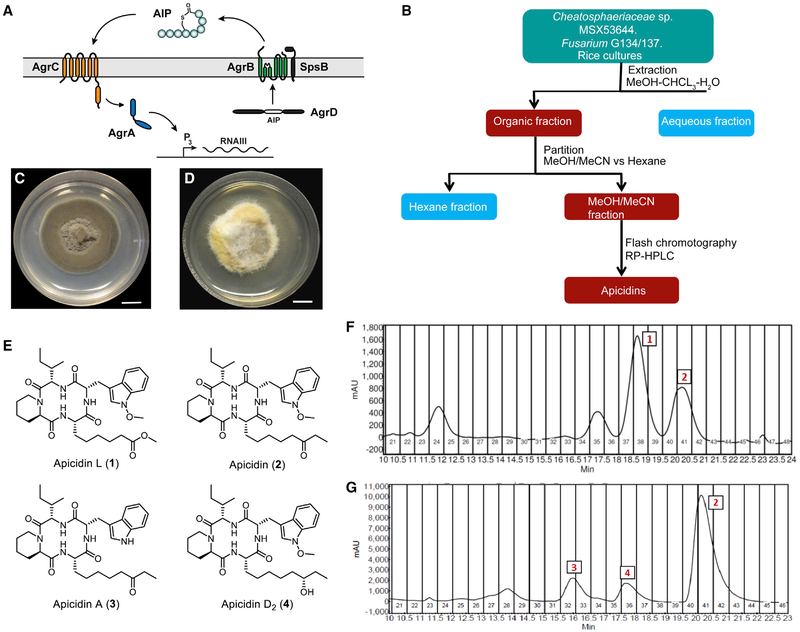
Figure 1. The agr System and Isolation of Apicidin.
(A) Schematic of the agr system.
(B) Flowchart for isolation of apicidins from solid-state culture extracts.
(C) Chaetosphaeriaceae sp. (MSX53644) grown on PDA (scale bar, 10 mm).
(D) Fusarium sp. (G134 and G137) grown on PDA (scale bar, 10 mm).
(E) Apicidin structures.
(F) Preparative chromatogram (λ= 254 nm) of the fraction used to purify compounds 1 and 2 from MSX53644.
(G) Preparative chromatogram (λ = 254 nm) of the fraction used to purify compounds 2–4 from G134 and G137 (each chromatogram is representative of multiple runs).
The overall goal of the present study was to identify quorum-sensing inhibitors that prove efficacious as anti-MRSA interventions. To this end, we discovered the potent activity of the compound apicidin by screening a library of terrestrial, freshwater, and endophytic fungal metabolites, where apicidin was biosynthesized by fungal strains of terrestrial (MSX53644; Chaetosphaeriaceae sp., Chaetosphaeriales, Ascomycota) and endophytic (G134 and G137; Fusarium sp., Nectriaceae, Hypocreales, Ascomycota) origins. In a non-bactericidal manner, apicidin-inhibited quorum-sensing activity across S. aureus isolates, achieving low micromolar IC50s. Importantly, the translatability of apicidin-mediated in vitro activity to efficacy in vivo was confirmed in a cutaneous challenge model that also revealed quorum-sensing interference within the infectious environment. Consistent with the latter finding, apicidin treatment enhanced polymorphonuclear neutrophil (PMN) accumulation and function at cutaneous sites of infection. Together, these results indicate that apicidin-mediated inhibition represents a promising strategy to limit MRSA virulence and promote host defense.
RESULTS
Identification of Apicidin and Related Analogs as S. aureus agr Inhibitors
The exploration of bioactive natural products has emerged as a promising means of pursuing antimicrobial and anti-virulence leads such as S. aureus quorum-sensing inhibitors (Cech and Horswill, 2013; Muhs et al., 2017; Quave and Horswill, 2014; Quave et al., 2015; Todd et al., 2017). This approach recently led to the identification and characterization of ω-hydroxyemodin, an AgrA-antagonizing small molecule derived from the fungus Penicillium restrictum (Daly et al., 2015; Figueroa et al., 2014). In the present study, a class of fungal metabolites, termed apicidins, were uncovered through separate screens of both terrestrial and endophytic fungi. The terrestrial strain, MSX53644 (Chaetosphaeriaceae sp., Chaetosphaeriales, Ascomycota), was cultivated over rice and subjected to purification (Figure 1B) to yield two cyclic tetrapeptides that were identified using HRESIMS and NMR as the known natural product, apicidin (2), and a new natural product analog of apicidin, which was named apicidin L (1) (Figure 1E). In a parallel analysis, endophytic fungi from medicinal plants were studied. LC-MS analysis of endophytes (G135 and G137), which were isolated from the roots of yerba mansa (Anemopsis californica [Nutt.] Hook. and Arn. [Saururaceae]) (Bussey et al., 2015), showed the presence of apicidin by matching retention time, HRESIMS, and tandem mass spectrometry data (El-Elimat et al., 2013) (Figures S1–S3). Considering our interest in this class of compounds, and in an attempt to isolate more structurally related analogs, these samples were further purified using reversed-phase preparative high-performance liquid chromatography (HPLC) to yield more apicidin (2) and two structurally related analogs: apicidin A (3) and apicidin D2 (4) (Figures 1E–1G).
The bioactivity of apicidin and its analogs were screened via in vitro assays that examined the ability of the compounds to suppress S. aureus agr activation in a nonbiocidal manner (Quave and Horswill, 2014). Beginning with well-established agr P3 reporter based assays, time course experiments measuring the dose effects of apicidin and analogs showed potent activity against all agr types with negligible effects upon bacterial growth (Figure 2A; Table S1). Similarly, apicidins’ broad suppression of agr activation was corroborated in parallel experiments measuring inhibition of red blood cell (RBC) lysis. In all cases, apicidin and analogs mediated agr inhibition at IC50 values in low micromolar concentrations that were sub-inhibitory for growth. Interestingly, agr types II and IV represented the most sensitive and resistant alleles to apicidins’ impact, respectively. Relative to its analogs, apicidin (2) showed the most impressive actions as an agr inhibitor (Figure 2), and, therefore, became the focus of our subsequent efforts to evaluate its efficacy as a nontoxic, pan inhibitor of agr activation. Further corroboration of the broad inhibitory effects of this compound on a per cell basis was demonstrated via flow cytometry using the same agr P3 reporter strains (Figure S4).
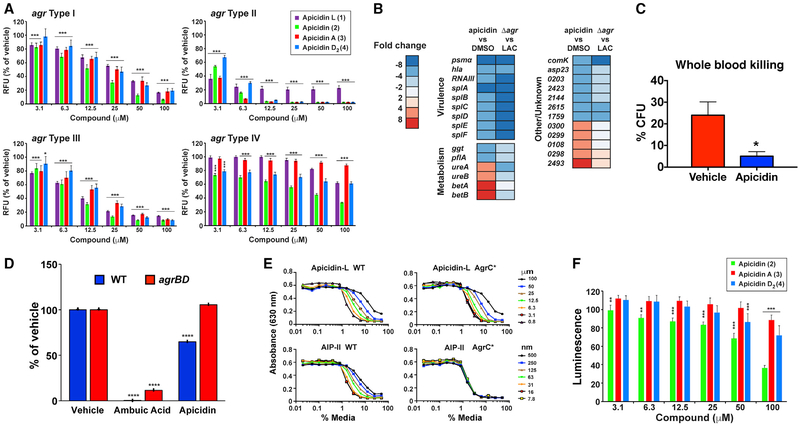
Figure 2. Apicidin Inhibits MRSA agr Function by Targeting AgrA.
(A) Apicidin (apicidin L, apidicin, apicidin A, apicidin D2) mediated suppression of agr-P3 reporters (inhibition extends to all four agr types). Post-test *p < 0.05, **p < 0.01, and ***p < 0.005; n = 10.
(B) USA300 MRSA LAC genes showing >4-fold change in expression after treatment with 100-mM apicidin versus DMSO vehicle control (n = 3), determined using RNA sequencing (RNA-seq). For comparison, gene expression was also measured in the agr mutant treated with DMSO. Numbers for genes of unknown function correspond to locus tags SAUSA300_XXXX.
(C) Heparinized human whole blood was inoculated with MRSA organisms cultured (4 h) in the presence of 100-μm apicidin or vehicle (n = 8). After 1 h, CFUs from inoculated whole blood were plated out and compared with the starting inoculum and reported as percent killing. Error bars represent SEM. Post-test *p < 0.05.
(D) Mass spectrometric measurements comparing ambuic acid and apicidin’s impact on AIP levels in MRSA WT (blue bars, labeled WT) and constitutive AIP-producing (red bars, labeled agrBD) strains. Each compound was tested at 100-μM concentration (n = 3). Statistical analysis was performed using the Student’s t test, ****p < 0.001.
(E) Effect of increasing concentrations of apicidin L on reporter strains for WT AgrC versus constitutive AgrC*. Apicidin L is compared to the AIP-II inhibitor control (representative; also performed with same results on apicidin, n = 3).
(F) Effect of increasing concentrations of apicidin, apicidin A, and apicidin D2 on P3-lux reporter activation using an agr null strain containing an agrA plasmid (n = 4). Post-test *p < 0.05, **p < 0.01, and ***p < 0.005.
To evaluate the impact of apicidin on global transcriptional regulation, we conducted RNA-seq analysis upon USA300 MRSA wild-type (WT) cultures grown in the presence of apicidin or vehicle control. For purposes of comparing the effects of apicidin on the MRSA transcriptome to those of an agr null, we performed parallel RNA-seq analysis of the WT and Δagr preparations. Using a 4-fold cutoff as the threshold for differential gene expression, we found that apicidin altered the expression of thirty genes, with many of these representing prototypical agr targets, such as hla, RNAIII, psmα, and spl genes (Figure 2B; Table S2). Together, these data corroborate and extend upon the inhibitory capability of apicidin by demonstrating a transcriptional signature that is largely confined to agr-regulated transcripts, and most impressively suppressive against cytolytic toxin induction (Figure 2B). Congruent with the apicidin-induced inhibition of agr-regulated virulence factors, we observed enhanced whole blood killing of MRSA when bacteria were cultured in the presence of apicidin prior to inoculation (Figure 2C).
Next, we conducted a series of in vitro assays to systematically assess the relative contribution of each of the core genetic elements of the agrBDCA operon (Figure 1A). The membrane-embedded peptidase AgrB is responsible for the first step, by processing the AgrD pro-peptide into the functional, extracellular AIP signal. To determine if apicidin inhibits agr function at the level of AIP signal generation, we used an established system whereby AIP is constitutively produced in an engineered USA300 MRSA strain (Todd et al., 2017). This engineered strain (labeled agrBD) decouples AIP signal biosyn-thesis from quorum-sensing regulation, enabling quantitative mass spectrometric analysis of culture supernatants to determine AIP levels (Figure 2D). An inhibitor that targets AgrB or another aspect of AIP signal biosynthesis is expected to prevent AIP production in both WT and engineered S. aureus strains, while an inhibitor targeting another competent of the agr system would only prevent AIP production in the WT strain. Consistent with these predictions, ambuic acid, a known agr signal biosynthesis inhibitor (Todd et al., 2017), significantly inhibited AIP peptide production by both strains. In contrast for apicidin, the WT strain was significantly inhibited by 35%, while no statistically significant inhibition was observed for the constitutive AIP-producing strain (Figure 2D). The results of these experiments suggest that apicidin does not target AgrB function or the signal biosynthesis process in general.
Next, we explored the possibility that apicidin interferes with the sensory activity of AIP receptor AgrC. Toward this goal, we employed an R238H construct bearing a point mutation that confers constitutive AgrC activity (Daly et al., 2015; Geisinger et al., 2009), and thereby enables phosphoactivation of AgrA to occur independently of AIP binding. With a hemolysis readout, apicidin L (1) imposed a dose-dependent inhibition of lytic activity on AgrC R238H that mirrored the response of AgrC WT (Figure 2E). In contrast, treatment with noncognate AIP-II, which competitively binds and inhibits AgrC function (Gordon et al., 2013), had no impact on AgrC R238H activation as expected. These results suggest that apicidin mediates its effects downstream of AgrC activation.
Finally, we set out to determine if AgrA serves as the target of apicidin. To this end, we employed an agr P3 lux reporter, where bioluminescence can only be achieved with expression of AgrA (Sully et al., 2014). The dose-dependent inhibition of agr-P3-driven bioluminescence shows that apicidin specifically interferes with AgrA-dependent quorum-sensing activation (Figure 2F). Considering the strain used is agr type I, apicidin (2) worked the best, while apicidin A (3) and D2 (4) were less effective, but still significantly inhibited. Taken together, these mechanistic studies provide compelling evidence that AgrA serves as the molecular target of apicidin.
Apicidin Abates MRSA Pathogenesis
S. aureus causes 76% of skin infections (Moran et al., 2006), more than any other infectious agent. Having demonstrated apicidin’s potent quorum-sensing inhibition in vitro, we next investigated the translatability of this activity within a relevant infectious context in vivo during skin infection using approaches developed by our group (Muhs et al., 2017; Paharik et al., 2017; Todd et al., 2017). For this purpose, an intradermal challenge model was employed by delivering a single 5-mg dose of apicidin as part of the MRSA inoculum suspension. To assess the contribution of differential immune skewing of the host to any apicidin-induced effects, we employed both C57BL/6 and BALB/c mice in our initial challenge experiments. Irrespective of murine strain background, apicidin treatment significantly reduced skin ulceration size and weight loss following MRSA challenge (Figures 3A and 3B). Together, these data show that when applied as an anti-infective, apicidin impressively attenuates MRSA-induced disease following MRSA challenge.
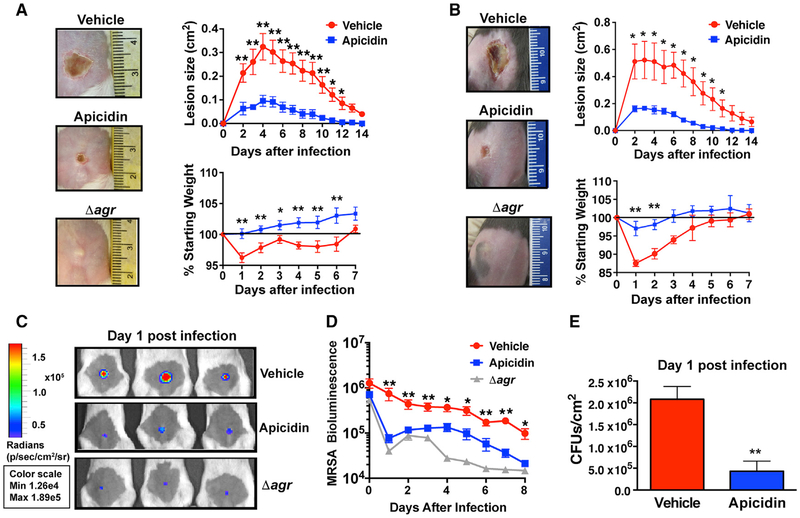
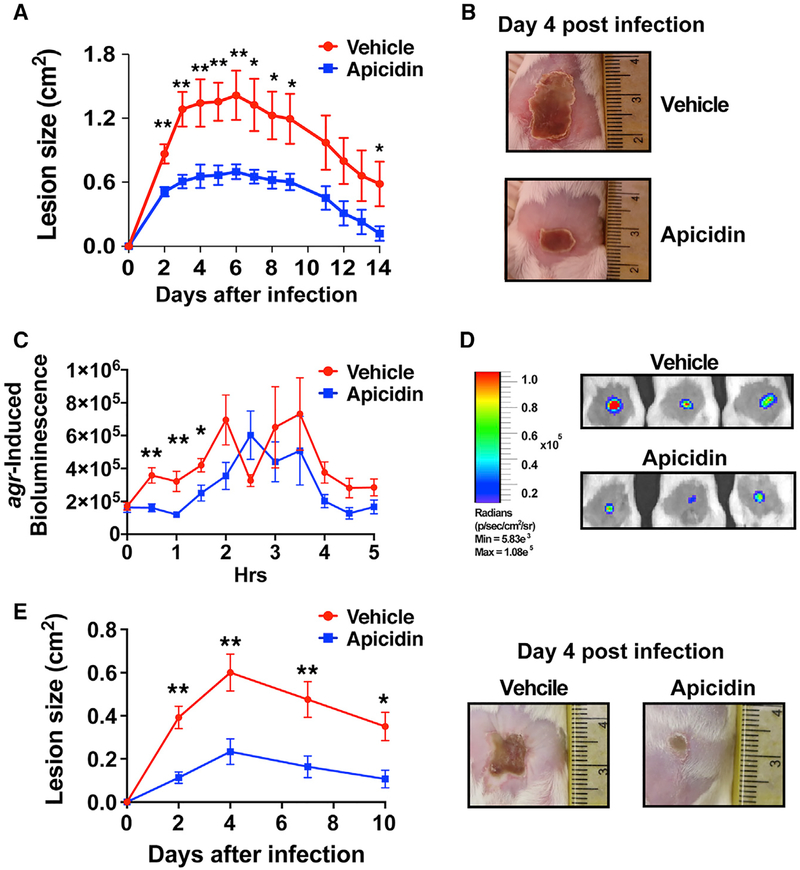
Figure 3. Apicidin Abates MRSA Pathogenesis.
(A and B) Representative images of tissue injury following infection with MRSA WT or Δagr mutant (±5 μg apicidin) and corresponding skin lesion size and weight loss and measurements following infection for the indicated groups in BALB/c (n = 20) (A) and C57BL/6 mice (n = 5) (B). Error bars represent SEM. Post-test *p < 0.05 and **p < 0.01.
(C) Representative images showing decreased bacterial burden in apicidin-treated animals relative to Δagr or vehicle controls 1 day after intradermal challenge with 2 × 107 CFUs MRSA Lux+ or its agr null counterpart.
(D) Corresponding noninvasive, longitudinal measurements of bioluminescence following skin infection with MRSA Lux+ or Δagr Lux+ (n = 8).
(E) CFUs recovered from BALB/c skin lesions 1 day after infection (n = 5).
To determine the impact of apicidin on cutaneous bacterial burden, challenge experiments were conducted with a MRSA Lux+ strain and compared against analogous challenges with its agr deletion construct Δagr Lux+. An advantage of this approach is the noninvasive and longitudinal manner with which bacterial burden can be measured. While an apicidin-induced decrease in bacterial burden was observed throughout the experiment, this effect was most impressively evident one day following infection, where it closely approached values obtained after Δagr Lux+ challenge (Figures 3C and 3D). Corroborating the apicidin-mediated enhancement of bacterial clearance via IVIS imaging, significantly fewer CFUs were recovered from lesional skin one day after infection in apicidin treated mice relative to controls (Figure 3E). In addition, parallel analysis of lesion development in the same apicidin and control mice showed a correspondence between MRSA-driven bioluminescence and dermatopathology.
Apicidin Mediates Quorum Quenching at In Vivo Sites of Infection
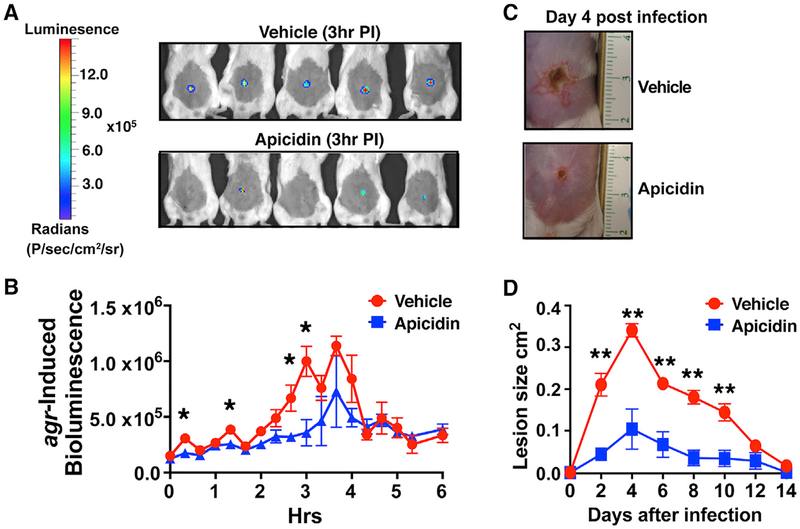
Having demonstrated that apicidin attenuated MRSA-induced illness (e.g., tissue damage, weight loss, bacterial burden), we set out to determine if these therapeutic effects corresponded with quorum-sensing interference in vivo. Based on previous work by Wright et al., which showed that the extent of S. aureus-induced dermatopathology is proportional to the magnitude of agr activation during the first 4 h of infection (Wright et al., 2005), we selected this key time period for in-depth analysis of the apicidin impact against multiple agr types in vivo. By challenging mice with an agr-P3 lux reporter strain and monitoring the early kinetics of agr activation, we showed that the attenuation of MRSA virulence in apicidin-treated animals occurred alongside a significant interference of agr activation in vivo (Figures 4A and 4B). To further demonstrate that the level of agr interference mediated by apicidin corresponded with a hypo-virulent infectious phenotype, we measured the ensuing skin ulcers in these animals over a 14-day period and found a dramatic reduction in cutaneous injury in apicidin-treated animals (Figures 4C and 4D). Altogether, these data demonstrate that the apicidin-mediated attenuation of MRSA pathogenesis corresponds with quorum-sensing inhibition both in vitro and in vivo.
Figure 4. Apicidin-Mediated Quorum-Sensing Inhibition Corresponds with Atten uated Skin Injury.
(A) Images of agr-P3 reporter activity (bioluminescence) 3 h post infection (±5 μg apicidin).
(B) Kinetics of agr activation in apicidin and vehicle-control-treated mice after infection (n = 5).
(C) Representative images of apicidin or control groups at the indicated time points after infection (n = 5).
(D) Skin lesion size measurements at the indicated time points after infection. Error bars represent SEM. Post-test *p < 0.05 and **p < 0.01.
In Vivo Efficacy of Apicidin Extends to agr Type-II Isolates
We next set out to investigate the potential of apicidin to show broad spectrum in vivo efficacy against multiple S. aureus agr types. To this end, the capacity of apicidin to influence the infectious outcome following intradermal challenge with a USA100 MRSA, agr type II, invasive isolate was assessed. As observed in analogous challenge experiments with agr type I strains, apicidin-treated animals exhibited significant attenuation in USA100-induced dermonecrotic injury relative to controls (Figures 5A and 5B). By engineering an agr P3-lux reporter into this agr type II isolate, we showed that the protective effects of apicidin treatment against multiple agr types corresponds with real-time quorum quenching at the cutaneous challenge site (Figures 5C and 5D). Carrying out the P3-lux-infected animals further corresponded with a significant reduction in lesion size at day 4 (Figure 5E), demonstrating the importance of early intervention in agr signaling with the different USA100 lineage.
Figure 5. Apicidin-Mediated Attenuation of MRSA Pathogenesis and In Vivo Quorum Quenching Observed against Multiple agr Types.
(A) Skin lesion measurements following infection with 2 × 107 CFUs of an agr type-II invasive USA100 MRSA isolate (±5 μg apicidin). Error bars represent SEM. Post-test *p < 0.05 and **p < 0.01 (n = 5).
(B) Representative images of tissue injury following infection with agr type-II ± apicidin.
(C) Kinetics and representative images (1-h time point; right) of agr-P3-induced bioluminescence after intradermal challenge with 1 × 107 CFUs of an agr type-II P3-lux reporter (± apicidin; n = 5). Error bars represent SEM. Post-test *p < 0.05 and **p < 0.01.
(D) Corresponding skin lesion size measurements at the indicated time points following infection with strain described in (C) (n = 8). Error bars represent SEM. Post-test *p < 0.05 and **p < 0.01.
(E) Representative images of tissue injury (day 4) following infection with agr type-II reporter described in (C).
Apicidin Treatment Enhances PMN Responses after MRSA Skin Challenge
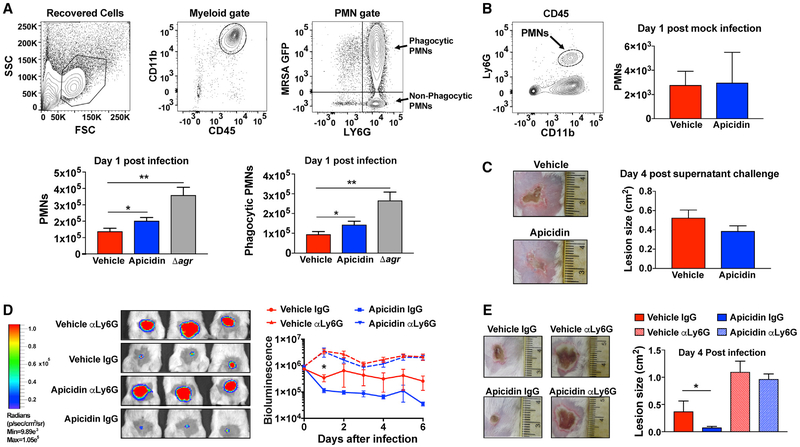
Given that apicidin exposure is nontoxic to MRSA in vitro, the impressive decrease in MRSA burden observed during in vivo infection could be a function of enhanced staphylocidal host immune responses. The recruitment of PMNs to sites of cutaneous S. aureus infection is critical for pathogen clearance (Miller and Cho, 2011; Spaan et al., 2013). Abundant in the circulation, PMNs orchestrate protective anti-S. aureus cutaneous immune responses by extravasating through vascular endothelium proximal to infectious foci where they sequentially accumulate, phagocytose, and clear S. aureus organisms (Spaan et al., 2013). By performing challenge experiments with MRSA GFP+, we were able to show that the apicidin-induced attenuation of MRSA injury and burden corresponded with an increase in the number of phagocytic PMNs accumulating at cutaneous challenge sites (Figure 6A). The frequency of MRSA uptake was unaltered between apicidin and vehicle-exposed PMNs, which matched control experiments that showed no significant impact of apicidin on PMN function (Figure S5). Nonetheless, there was an aggregate increase in the phagocytic capacity of the entire PMN infiltrate during infection with the apicidin-treated group (Figure 6A). Furthermore, the apicidin-induced increase in PMN infiltration was even further surpassed in the setting of agr null challenge, revealing an inverse proportionality between the magnitude of agr activity and the strength of the ensuing PMN response. Meanwhile, the lack of an effect on cutaneous PMN infiltration following mock challenge indicates that apicidin’s impact on PMN responses during infection are largely a consequence of agr interference (Figure 6B).
Figure 6. Apicidin Treatment Enhances PMN Responses after MRSA Skin Challenge.
(A) Gating strategies (top) for identification of PMNs recovered from lesional skin and corresponding PMN accumulation values (bottom) 1 day after intradermal challenge with 2 × 107 MRSA WT expressing GFP (± apicidin) or its agr null counterpart (n = 8). Error bars represent SEM. Post-test *p < 0.05 and **p < 0.01.
(B) Gating strategy (left) and enumerated PMNs (right) 1 day after intradermal saline challenge (± apicidin; n = 8).
(C) Representative skin injuries 4 days after sterile challenge (intradermal) with 30 μL of WT MRSA supernatant at the skin sites intradermally inoculated with saline (± apicidin) 3 h prior (n = 6).
(D) Representative bioluminescence images 1 day after intradermal challenge (2 × 107 CFUs MRSA Lux+) among apicidin-treated and control groups receiving 100 μg of the PMN-depleting anti-Ly6G antibody or isotype control via intraperitoneal injection administered both the day prior and the day of infection. Corresponding time course values (right) of MRSA burden for the indicated groups (n = 6).
(E) Representative images of skin injury (left) and corresponding lesion size measurements 4 days after infection for the indicated groups (n = 5). Error bars represent SEM. Post-test *p < 0.05 and **p < 0.01.
To explore the possibility that apicidin exposure increases the resistance of host cells to agr-regulated virulence factors, we examined the prophylactic effects of apicidin administration against sterile challenge with cytotoxin-containing MRSA super-natants. The similar degree of dermonecrotic injury sustained by apicidin and control groups following MRSA intoxication suggests that apicidin’s ability to attenuate infectious injury is principally achieved by limiting host cell exposure to agr-induced virulence factors (Figure 6C). In view of the correlation between MRSA-induced disease attenuation and cutaneous PMN accumulation, we investigated the dependence of apicidin’s therapeutic effects upon the latter. To this end, we depleted host PMNs via administration of an anti-Ly6G antibody on both the day prior to and the day of infection. The equivalent severity of skin ulceration and bacterial burden within the context of neutropenia demonstrates that apicidin’s therapeutic efficacy requires a functional PMN compartment (Figure 6D). Taken together, these findings indicate that the capacity of apicidin to bolster host defense and improve disease outcome was largely a byproduct of agr inhibition, which in turn sensitizes MRSA for PMN-mediated phagocytic clearance.
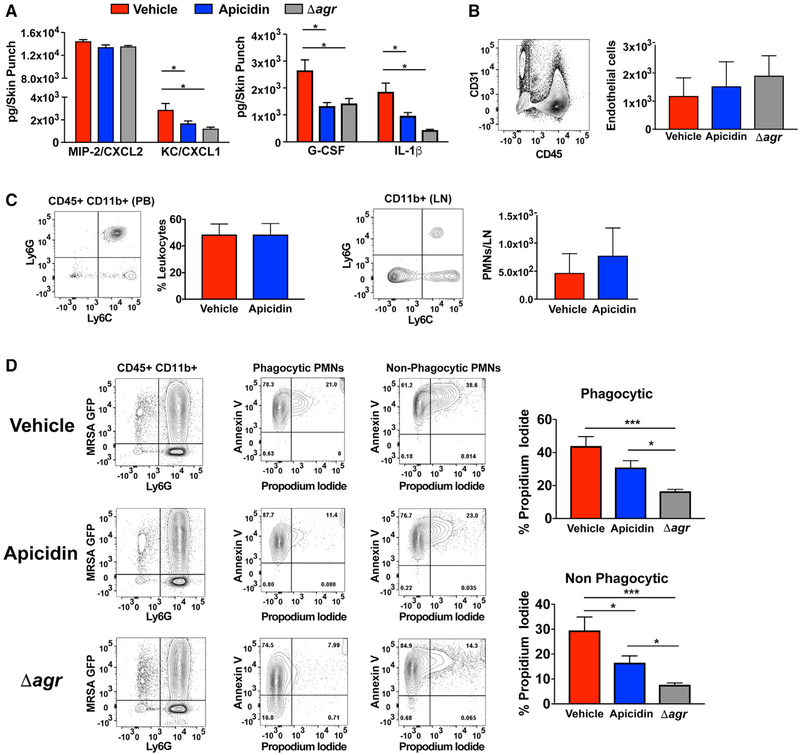
To better understand the mechanistic basis of the apicidin-induced increase in PMN density at cutaneous sites of infection, we assessed the cytokine milieu of lesional tissue one day after infection. Interestingly, the apicidin-induced increase in cutaneous PMNs did not correspond with a commensurate increase in prototypical PMN chemoattractant or granulopoiesis factors. In fact, preparations from apicidin-treated animals showed a decrease in the production of CXCL-1 and granulocyte-colony stimulating factor (G-CSF) relative to controls, suggesting that apicidin’s ability to boost PMN responses does not occur as a result of alleviating a MRSA-induced strain upon factors that mediate extravasation into infected tissue (Figure 7A). In further support of this, equivalent cutaneous endothelial cell recovery and PMN abundance in the peripheral blood (PB) was observed between apicidin-treated and control mice (Figure 7B). While similar PMN counts in the skin-draining lymph nodes (LNs) suggests that altered trafficking to alternative sites known to harbor PMNs after skin infection plays little role in the apicidin-induced increase in cutaneous PMN accumulation (Figure 7C). Together, these data suggest apicidin’s capacity to enhance PMN infiltration is chiefly the result of alterations within the infectious microenvironment.
Figure 7. Apicidin Confers Cytoprotective Effects upon Skin-Infiltrating PMNs after MRSA Skin Challenge.
(A) Supernatants from skin tissue homogenates were collected 1 day after infection and subjected to cytokine array analysis (n = 6).
(B) Gating strategies (left) and enumeration of (right) CD31+ CD45−endothelial cells recovered from lesional tissue 1 day following MRSA challenge (± apicidin) or its agr null counterpart (n = 6).
(C) Gating strategies (left) and enumeration of PMNs (right) in PB and skin-draining LNs 1 day after MRSA skin challenge (n = 6).
(D) Gating strategies (left) and corresponding frequency values (right) for propidium iodide+ phagocytic (GFP+) and non-phagocytic (GFP) PMNs recovered from skin lesions 1 day after intradermal challenge with 2 × 107 MRSA GFP+ (± apicidin) or its agr null counterpart (n = 5). Error bars represent SEM. Post-test *p < 0.05 and ***p < 0.001.
Given that extraordinary expression of agr-regulated cytolytic toxins is a defining feature of USA300 MRSA-induced disease, we explored apicidin’s impact upon PMN persistence within the infectious environment. Because PMN apoptosis-efferocytosis is the cellular fate that is most advantageous to the host, it was important to distinguish the rate of cell death between PMNs that are actively executing the effector function of MRSA uptake (GFP+) and those that have not. To this end, we assessed the frequency of PMNs in late stage apoptosis-necrosis within lesional skin preparations of MRSA GFP+ challenged mice. For the phagocytic and non-phagocytic PMNs, we observed an apicidin-induced decrease in the frequency of PI+, late-stage apoptotic-necrotic cells (Figure 7D), although not quite to the same level as the agr mutant. Together, these data suggest that apicidin impacts the host-pathogen interface by suppressing agr-regulated virulence factors, especially immune cell lysing toxins, and thereby extends cytoprotective effects upon cutaneous PMNs.
DISCUSSION
Since the advent of antibiotic therapy, S. aureus has evolved resistance to every frontline antibiotic used in healthcare settings and done so with devastating human health effects. Considering that ancestral S. aureus is highly sensitive to virtually every antibiotic ever devised, the broad resistance features of today’s most pervasive and problematic isolates suggest that modern medicine’s reliance on antibiotic therapy has spurred resistance development (Chambers and Deleo, 2009; DeLeo and Chambers, 2009; Spellberg et al., 2015). Pandemic CA-MRSA provides an illustrative example of how infectious transmissibility brought on the concurrent evolution of antibiotic resistance and hyper-virulence (Cheung et al., 2011; Otto, 2010). Considering that MRSA is already one of the most pervasive and prob lematic causes of bacterial infection, the continuation of such trends poses a serious threat to public health and highlights the need for new treatments. Through quorum-sensing inhibition and immune boosting, apicidin treatment offers a therapeutic strategy to sensitize MRSA to host-mediated clearance in a resistance-avoiding fashion.
Herein, we report efficacious application of apicidin as an anti-virulence agent. Apicidin’s activity as a pan-quorum-sensing inhibitor was confirmed via assays that demonstrated suppression of agr activation of all types in a non-biocidal manner. Further evidence of agr interference was achieved via RNA-seq analysis, showing that the majority of apicidin-affected transcripts were agr-regulated and included the prototypical cytolytic toxins hla and psms. To determine the apicidin target within the agr machinery, assays specific to each component were employed, surprisingly narrowing the activity to AgrA (Figure 2). Based on the cyclic peptide-like nature of the apicidin structure (Figure 1), we anticipated that the AgrC receptor would be the target, in similar fashion to synthetic AIP derivatives (Gordon et al., 2013, 2016). However, the ability of apicidin to definitely inhibit the constitutive AgrC R235H construct, and also prevent AgrA activation of a P3-lux reporter, strongly indicated that AgrA is the actual target. Thus, apicidin joins savarin and u-hydroxyemodin (which is another fungal metabolite) as the known AgrA inhibitors (Daly et al., 2015; Sully et al., 2014). Additional work will be required to identify the precise AgrA residue(s) that is required for its interaction with apicidin and to further define the mechanism of apicidin inhibition.
The translatability of apicidin’s agr inhibitory activity to in vivo therapeutic efficacy was confirmed using a MRSA intradermal challenge model. As a single-dose intervention, apicidin ameliorated multiple measures of clinical disease severity. While a number of agr inhibitors have shown the ability to attenuate cuta neous ulcer formation following MRSA challenge (Daly et al., 2015; Mayville et al., 1999; Muhs et al., 2017; Quave et al., 2015; Sully et al., 2014), apicidin is first to show that its efficacy extends to multiple agr types using infection models, demonstrating its broad utility as an anti-infective agent. Although, agr type-I alleles, which encompass USA300 isolates, are the most pervasive and problematic clinically (Chambers and Deleo, 2009; DeLeo et al., 2010), other agr types represent a source of human disease (Tong et al., 2015). For instance, isolates bearing agr type-II alleles are the leading cause of hospital-acquired MRSA infection (Limbago et al., 2009). Thus, the applicability of any quorum-targeting therapy is extended by its capacity to inhibit multiple agr types. Consistent with the attenuation of dermonecrotic injury, the single-dose efficacy of apicidin corresponded with significant agr interference within the in vivo infectious environment following cutaneous challenge with both type-I and type-II MRSA isolates. As previously demonstrated by Novick’s group (Wright et al., 2005), the explosive activation of the agr system occurring within the first few hours of in vivo infection largely dictates the severity of MRSA-induced disease. Consistent with this, we recently showed that the potent agr antagonism of S. caprae-derived AIP had only a minor benefit as treatment for established MRSA skin infection (Paharik et al., 2017). With this in mind, pharmacologically abating MRSA pathogenesis via quorum-sensing inhibition will require targeting agr activity during a narrow temporal window that can ensue rapidly after tissue penetration. Thus, it appears that as a potential clinical intervention, the application of apicidin is best suited as a prophylactic agent.
MRSA is a major contributor to the ever-escalating threat of antibiotic resistant bacterial pathogens. Among the core strategic approaches of the National Institutes of Allergy and Infectious Diseases’ antibacterial resistance program are the development of (1) anti-virulence strategies that disarm without directly killing bacteria and (2) immunological interventions that enhance host defenses. The present study demonstrates that these paradigms are complementary in the coordination of host defense, namely, the enhanced effector functionality within the cutaneous PMN network. Both clinical and experimental findings concordantly suggest that the staphylocidal functions of skin-infiltrating PMNs are essential for protective anti-S. aureus immunity (Miller and Cho, 2011; Spaan et al., 2013), in contrast to steady-state skin, which is largely devoid of PMNs. Infectious injury activates cytokine-chemokine circuits that in turn forge an inflammatory milieu replete with PMN recruitment, survival, and differentiation factors. Within the context of S. aureus skin infection, local production of the quintessential inflammatory mediator IL-1β incites cytokine-chemokine pathways that underlie PMN recruitment and function (Miller and Cho, 2011; Spaan et al., 2013). While IL-1β is clearly required for S. aureus host defense, the results from the present study show that increased IL-1β output (as observed in control groups) does not necessarily yield commensurate gains in cutaneous PMNs (Figures 6 and 7). In fact, PMN numbers were greatest in agr-null-challenged tissue where IL-1β production was the lowest. These findings suggest that protective anti-MRSA effector responses may be optimal in conditions where local inflammation is finely tuned. Consistent with this notion, apicidin treatment augmented cutaneous PMN responses and hastened MRSA clearance, while subduing inflammatory cytokine production. The inverse relationship between PMN accumulation and the bacterial burden resulting from apicidin treatment is reminiscent of prior work demonstrating the therapeutic efficacy of S. caprae AIP. Using a similar model of intradermal challenge, Paharik and Parlet et al. showed that one of the most striking alterations imposed upon the host-pathogen interface via pharmacological targeting of MRSA quorum sensing is increased PMN amassment at sites of infection (Paharik et al., 2017). The present study further clarifies the mechanistic basis for this occurrence by showing that quorum-sensing inhibition confers cytoprotective effects to skin-infiltrating PMNs. When viewed together, these results are consistent with the following interpretation: By disabling S. aureus quorum sensing, the resultant suppression of immune-toxigenic virulence factors (e.g., PSMs and α-toxin) increases the survival of PMN effectors that mediate pathogen clearance, the manifestations of which are attenuated tissue damage and accelerated bacterial clearance.
In conclusion, we report the efficacious application of the fungal derivative apicidin as an anti-virulence intervention against MRSA-induced disease. As the threat posed by antibiotic-resistant pathogens continues to grow, there is an urgent need for innovative approaches that are mechanistically distinct from conventional antibiotic therapy, and are tailored for the pathophysiology of specific infectious agents. Apicidin exemplifies such a pioneering treatment modality. By inhibiting virulence factor expression, apicidin impacts host-pathogen interactions in a manner that favors immune clearance of the pathogen and blocks disease progression.
STAR★METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and request for resources should be forwarded to and will be fulfilled by the Lead Contact, Dr. Alexander R. Horswill (alexander.horswill@ucdenver.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Bacterial strains and plasmids
For all experiments, S. aureus cultures were grown in a tryptic soy broth (TSB) at 37°C with shaking at 200 rpm. Chloramphenicol (Cam) was added to the media as needed at 10 mg/mL. Bacterial strains used are listed specifically in each method section and in the Key Resource Table.
KEY RESOURCES TABLE
| REAGENT or RESOURCE Antibodies | SOURCE | IDENTIFIER | |
|---|---|---|---|
| Antibodies | |||
| anti-mouse Ly6G (1A8) | Biolegend | Cat# 127614 | |
| anti-mouse Ly6C (HK1.4) | Biolegend | Cat# 128016 | |
| anti-mouse CD45 (30-F11) | Biolegend | Cat# 103134 | |
| anti-mouse CD31 (390) | Biolegend | Cat# 102412 | |
| anti-mouse/human CD11b (M1/70) | Biolegend | Cat# 101226 | |
| rat anti-mouse CD16/32 FcγRIII/II (2.4G2) | Dr. Thomas Waldschmidt, Univ. of Iowa | N/A | |
| Bacterial and Virus Strains | |||
| Chaetosphaeriaceae sp. [Sordariomycetes, Ascomycota] | Mycosynthetix | MSX53644 | |
| Fusarium sp. | This work | G134 | |
| Fusarium sp. | This work | G137 | |
| S. aureus Restriction deficient cloning host | Lab collection | RN4220 | |
| S. aureus MN EV, agr type IV | Lab collection | AH407 | |
| S. aureus 502a + pDB59 cmR, YFP reporter, agr type II | Lab collection | AH430 | |
| S. aureus MW2, agr type III | Lab collection | AH843 | |
| S. aureus USA300 agr type I | Boles et al., 2010 | AH845 | |
| S. aureus USA300 CA-MRSA ErmS (LAC*) | Boles et al., 2010 | AH1263 | |
| LAC* Δagr::tet | Kiedrowski et al., 2011 | AH1292 | |
| AH845 + pDB59 cmR, YFP reporter, agr type I | Kirchdoerfer et al., 2011 | AH1677 | |
| MW2 + pDB59 cmR, YFP reporter, agr type III | Kirchdoerfer et al., 2011 | AH1747 | |
| MN EV + pDB59 cmR; YFP reporter, agr type IV | Kirchdoerfer et al., 2011 | AH1872 | |
| LAC* agrP3::luxABCDE | Figueroa et al., 2014 | AH2759 | |
| LAC* Δagr::tet + pCM29 cmR (GFP) | Paharik et al., 2017 | AH2768 | |
| LAC* Δagr::tet, φ11::LL29 sarAP1-agrBD | Todd etal., 2017 | AH2989 | |
| RN4220 Δagr::ermB agrP3::luxABCDE + pEPSA5-agrA | Sully etal., 2014 | AH3048 | |
| JE2 agrC::ΦNΣ, φ11::LL29 + pRMC2-agrBDCA | Daly et al., 2015 | AH3469 | |
| JE2 agrC::ΦNΣ, φ11::LL29 + pRMC2-agrBDCA (agrC R238H) | Daly et al., 2015 | AH3470 | |
| LAC* + pCM29 cmR (GFP) | Pang et al., 2010 | AH3669 | |
| S. aureus USA100 MRSA blood isolate IA116 | Daniel Diekema | AH3684 | |
| LAC* Lux | Todd etal., 2017 | AH3700 | |
| LAC* Δagr::tet Lux | Paharik et al., 2017 | AH3774 | |
| USA 100 IA116 agrP3::luxABCDE | This work | AH4390 | |
| Biological Samples | |||
| Rabbit defibrinated blood 250 mL | HemoStat Laboratories | Cat# DRB250 | |
| Chemicals, Peptides, and Recombinant Proteins | |||
| Trypsin | VWR Life Sciences | Cat# 90002-07-7 | |
| Collagenase Type II | Gibo | Cat# 9001-12-1 | |
| Propidium iodide | Biolegend | Cat# 421301 | |
| Annexin V | Biolegend | Cat# 640919 | |
| Chloramphenicol | Sigma | Cat# C0378 | |
| Ambuic acid | Apidogen Life Sciences | Cat# AG-CN2–0129-M001 | |
| S. aureus AIP-II | Anaspec | Peptide# 61088 | |
| Apicidin | Sigma | Cat# A8851 | |
| RNAprotect | QIAGEN | Cat# 76526 | |
| Lysostaphin | Sigma | Cat# L7386 | |
| Formic Acid (Optima Grade) | Fisher Scientific | Cat# A117–50 | |
| Acetonitrile (Optima Grade) | Fisher Scientific | Cat# A955–4 | |
| Acetonitrile (HPLC Grade) | Fisher Scientific | Cat# A998SK-4 | |
| Water (Optima Grade) | Fisher Scientific | Cat# W6–4 | |
| Stemsol (DMSO) | Protide Pharmaceuticals | Cat# PP1350 | |
| Critical Commercial Assays | |||
| Mouse magnetic Luminex Assay | R&D Systems | Custom | |
| RNeasy Mini kit | QIAGEN | Cat# 74104 | |
| Turbo DNA-free kit | Fisher Scientific | Cat# AM1907 | |
| Ribo-Zero rRNA Removal Kit (Gram-Positive Bacteria) | Illumina | Cat# MRZGP126 | |
| TruSeq Stranded mRNA Library Prep Kit | Illumina | Cat# RS-122–2103 | |
| Deposited Data | |||
| RNaseq data | This work | GenBank: SRP182661 | |
| Chaetosphaeriaceae sp. ITS spacer and 28S rRNA | This work | GenBank: MF374619 | |
| Fusarium sp. G134 ITS spacer | This work | GenBank: KM816766 | |
| Fusarium sp. G137 ITS spacer | This work | GenBank: KM816768 | |
| Experimental Models: Organisms/Strains | |||
| C57BL/6N mice | Charles River Laboratories | Strain code 027 | |
| BALB/c mice | Charles River Laboratories | Strain code 028 | |
| Oligonucleotides | |||
| CTTGGTCATTTAGAGGAAGTAA | IDT | ITS1F | |
| TCCTCCGCTTATTGAT ATGC | IDT | ITS4 | |
| Software and Algorithms | |||
| Living Image Software | Xenogen | Version 4.4 | |
| Flow Jo | TreeStar | Version 10.4 | |
| FACSDiva | BD | Version 8 | |
| CellQuest | BD | Version 5.1 | |
| ImageJ | NIH | N/A | |
| Galaxie Chromatography Workstation | Varian | Version 1.9.3.2 | |
| SeqMan NGen | DNASTAR | Version 14 | |
| ArrayStar | DNASTAR | Version 14 | |
| PRISM | GraphPad | Version 7.0a | |
| Other | |||
| TECAN plate reader | TECAN LifeSciences | Infinite M200 | |
| Stuart SI505 Microplate Shaking Incubator | Cole-Parmer | Item # EW-79520–08 | |
| Xenogen in vivo imaging system (IVIS) | Caliper Life Sciences | IVIS200 | |
| CombiFlash RF system | Teledyne-Isco | Unit ID: 625230006 | |
| Q Exactive Plus orbitrap mass spectrometer | Thermo Fisher | N/A | |
| Acquity UPLC | Waters | Product No: Photodiode Array eλ Detector −186015033 | |
| Acquity UPLC | Waters | Column Manager - 800000247 | |
| Acquity UPLC | Waters | Sample Manager −186015006 | |
| Acquity UPLC | Waters | Binary Solvent Manager - 186015001 | |
| Acquity BEH C18 UPLC column | Waters | 186002350 | |
| Prostar HPLC | Varian | N/A | |
| Gemini-NX C18 (analytical) | Phenomenex | 00G-4435-E0 | |
| Gemini-NX C18 (preparative) | Phenomenex | 00G-4435-P0-AX | |
| 130 g Redisep RF C18 column | Teledyne-Isco | Cat# 69-2203-337 | |
| LTQ Orbitrap XL mass spectrometer | Thermo Fisher | N/A | |
| ECA-500 NMR Spectrometer | JEOL | N/A | |
| Luminex 200 | Luminex | N/A | |
| FACS LSR II | BD | N/A | |
Mice
C57BL/6N or BALB/c mice (equal male and female numbers) were purchased from the Charles River and housed in specific pathogen free facilities at the University of Iowa. Prior to their inclusion in the study, mice were allowed to acclimate to the ABSL-2 animal housing facility at the University of Iowa for at least seven days. For in vivo studies, 8 to 20-week old age-matched, sex-matched mice were randomly assigned to treatment groups and at experimental end points, mice were humanely euthanized using carbon dioxide inhalation. The animal studies were reviewed and protocol approved by the University of Iowa Institutional Animal Care and Use Committee. The University of Iowa is AAALAC accredited, and the centralized facilities meet and adhere to the standards in the “Guide and Care of Laboratory Animals.”
Plant material
Plant material of yerba mansa [Anemopsis californica (Nutt.) Hook. & Arn. (Saururaceae)] was collected with permission by Amy Brown of Apache Creek Ranch in Santa Fe, NM (35 35’ 56.40”N, 105 50’ 27.22”W). A voucher specimen (NCU602027) was deposited in the University of North Carolina Herbarium. The specimen was authenticated by Amy Brown.
Fungal strains
Fungal strain MSX53644 from Mycosynthetix library was stored, fermented, and extracted via well-established methods. Based on GenBank BLAST search and molecular phylogenetic analysis using the internal transcribed spacers (ITS1-5.8S-ITS2) region and the D1–D2 variable portion of the 28S rRNA large subunit gene of the nuclear RNA operon, as detailed previously (Raja et al., 2017), MSX53644 was identified as Chaetosphaeriaceae sp. [Sordariomycetes, Ascomycota] (Figure S2D). The sequence data were deposited in GenBank under accession numbers: MF374619, MF374620, MF374621, MF374622.
The endophytic fungal strains G134 and G137 were isolated from surface sterilized fresh roots of yerba mansa [Anemopsis californica (Nutt.) Hook. & Arn. (Saururaceae)] using methods reported in detail previously (Bussey et al., 2015). Strains G134 and G137 were found to be two isolates for the same strain and were identified as a Fusarium sp. by sequencing the internal transcribed spacer region of the ribosomal RNA gene (ITS) using molecular methods (Raja et al., 2017). The ITS sequences for G134 and G137 were deposited in GenBank (accession number KM816766 for G134 and KM816768 for G137).
METHOD DETAILS
Quenching assays with reporter strains:
Apicidin was tested for quorum quenching activity against all four agr types using P3-YFP reporter strains AH1677 (type I), AH430 (type II), AH1747 (type III), and AH1872 (type IV) (Hall et al., 2013; Kirchdoerfer et al., 2011). Overnight cultures of reporter strains that were grown in TSB supplemented with Cam were inoculated at a dilution of 1:250 into fresh TSB containing Cam. 100 μL aliquots were added to 96-well microtiter plates containing 100 μL aliquots of TSB containing Cam and 2-fold serial dilutions of apicidin. Readings were recorded at 30 min increments using a Tecan Systems Infinite M200 plate reader.
Hemolytic activity
A rabbit red blood cell (RBC) hemolysis assay based on previously described methods was used (Daly et al., 2015; Pang et al., 2010). Overnight cultures of agr strain types I, II, III, and IV were inoculated 1:500 into 5 mL of TSB (in 17×150 mm culture tubes) containing apicidin at concentrations of 100, 50, 25, 12.5, and 6.25 μg/mL. For assessing the impact on constitutive AgrC, strains AH3469 and AH3470 were used in parallel with apicidin treatment as previously described (Daly et al., 2015). All cultures were incubated at 37 C with shaking (250 rpm), and growth was monitored by periodically transferring 100 μL of culture to a 96-well microtiter plate and reading OD600 in a Tecan Systems Infinite M200 plate reader. Following incubation, 600 μL of each culture was filter sterilized using cellulose acetate SpinX 0.22 μm filters. To quantify hemolytic activity, the filter sterilized culture supernatants from apicidin treated cultures were diluted, and 50 μL aliquots were dispensed in quadruplicate into 96-well microtiter plates. Rabbit erythrocytes, were added to the microtiter plates at 50 μL per well. The erythrocytes and culture supernatants were mixed and incubated statically at room temperature for 2 hr. Hemolysis was detected by the loss of turbidity as measured at OD630 using a Tecan Infinite M200 plate reader.
Mass Spectrometric Measurements of AIP
Experiments to evaluate the impact of apicidin on signal biosynthesis by MRSA were conducted as described previously (Todd et al., 2017). Briefly, 250 μL cultures of strains AH2989 (AIP constitutively producing strain (Todd et al., 2017)) and AH1263 (wild-type, USA300 LAC (Boles et al., 2010)) were treated with 5 mL of 5 μM apicidin in DMSO, for a final concentration of 100 μM in the culture media. The known signal biosynthesis inhibitor ambuic acid (also at 100 μM) served as a positive control, and vehicle (2% DMSO in TSB) served as negative control. AIP I levels produced by both strains after 18 hr were measured directly from spent media using ultraperformance liquid chromatography (Waters Acquity UPLC) coupled to high resolution mass spectrometry (Q-Exactive Orbitrap, Thermo).
Fermentation, extraction and isolation
For chemistry studies, a 2.8-L Fernbach flask (Corning, Inc., Corning, NY, USA) containing 150 g rice and 300 mL H2O was inoculated with as seed culture this strain that was grown in YESD medium. After incubation for 14 days at r.t., the solid culture was extracted by addition of a 500 mL mixture of 1:1 MeOH/CHCl3. Using a spatula, the culture was chopped into small pieces and left to shake at 125 rpm at r.t., followed by filtration. The solid residues were then washed with 100 mL of 1:1 MeOH/CHCl3. To the combined filtrates, 900 mL CHCl3 and 1500 mL H2O were added so that the final ratio of CHCl3/MeOH/H2O was 4:1:5 and left to stir for 30 min. The mixture was then transferred into as a separatory funnel and the organic bottom layer was drawn off and evaporated to dryness. The dried organic phase was then re-constituted in 100 mL of 1:1 MeOH/CH3CN and 100 mL of hexanes and transferred into a separatory funnel. The MeOH/CH3CN layer was drawn off and evaporated to dryness under vacuum. The defatted crude material (1.2 g) was dissolved in a mixture of CHCl3/MeOH, adsorbed onto Celite 545, and fractionated via normal phase flash chromatography using a gradient solvent system of hexane/CHCl3/MeOH at a 40 mL/min flow rate and 53.3 column volumes over 63.9 min to afford five fractions. Fraction 4 (300 mg) was subjected to preparative reversed-phase HPLC over a Phenomenex Gemini-NX C18 preparative column using a gradient system of 40:60 to 70:30 over 30 min of CH3CN/H2O (acidified with 0.1% formic acid) at a flow rate of21.24 mL/min (Figure 1F) to yield compounds 1 (4.1 mg) and 2 (3.0 mg) which eluted at 18.0 and 19.5 min, respectively. The HRMS (Figure S1A) and 1H and 13C NMR data (Figure S1B) for compounds 1 and 2 were in agreement with the literature.
Fermentation, extraction and isolation of compounds from strains G134 and G137
Strains G134 and G137 were fermented and extracted using techniques akin to those described for strain MSX53644 above. The extracts from G134 and G137 were combined, as the LC-MS analysis of the extracts showed similar chemical profiles, the two cultures showed similar morphological characteristics, and decisively, the two isolates showed similar sequences of the internal transcribed spacer region of the ribosomal RNA gene (ITS). The combined defatted extracts of G134 and G137 (125 mg) were then fractionated using normal phase flash chromatography using a gradient solvent system of hexane/CHCl3/MeOH at a 18 mL/min flow rate and 68.1 column volumes over 18.2 min to afford five fractions. Fraction 5 (35 mg) was found to contain apicidin as evidenced by LC MS analysis and hence was subjected to preparative reversed-phase HPLC purification over a Phenomenex Gemini-NX C18 preparative column using a gradient system of 40:60 to 70:30 over 30 min of CH3CN/H2O (acidified with 0.1% formic acid) at a flow rate of 21.24 mL/min (Figure 1F) to yield compounds 2 (8.9 mg), 3 (1.7 mg), and 4 (1.5 mg), which eluted at 19.5, 15.5, and 17.5 min, respectively. The HRMS (Figure S1A) and 1H and 13C NMR data (Figure S1B) for compounds 2, 3 and 4 were in agreement with the literature.
NMR methods
NMR data were collected using a JEOL ECA-500 NMR spectrometer operating at 500 MHz for 1H and 125 MHz for 13C (JEOL Ltd., Tokyo, Japan). Residual solvent signals were utilized for referencing. High resolution mass spectra (HRMS) were obtained using a Thermo LTQ Orbitrap XL mass spectrometer equipped with an electrospray ionization source (Thermo Fisher Scientific, San Jose, CA, USA). Phenomenex Gemini-NX C18 analytical (5 μm; 250 × 4.6 mm) and preparative (5 μm; 250 × 21.2 mm) columns (Phenomenex, Torrance, CA, USA) were used on a Varian Prostar HPLC system equipped with ProStar 210 pumps and a Prostar 335 photodiode array detector (PDA), with data collected and analyzed using Galaxie Chromatography Workstation software (version1.9.3.2, Varian Inc.). Flash chromatography was conducted on a Teledyne ISCO CombiFlash Rf using Silica Gold columns and monitored by UV and evaporative light-scattering detectors (both from Teledyne Isco, Lincoln, NE, USA).
S. aureus skin infections
At D0, age, strain and sex matched C57BL/6 or BALB/c mice were anesthetized with isoflurane, abdominal skin was carefully shaved and exposed skin was cleansed by wiping with an alcohol prep pad. For inoculum preparation, a USA300 MRSA LAC strain (AH1263) (Boles et al., 2010), and strains engineered for constitutive bioluminescence via lux operon insertion (AH3700) or its agr null counterpart (AH2989), or the LAC WT engineered to constitutively express green fluorescent protein (AH3669) or its agr null counterpart (AH2768), were grown to mid-log-phase, pelleted and resuspended in DPBS to achieve 50 mL inoculum mixtures that contained either 2 ×107 CFUs ± 5 μg apicidin (diluted in neat DMSO). Apicidin or DMSO containing inoculums were injected intradermally into abdominal skin. Baseline body weights of mice were measured before infection and every day thereafter for a period of 7–14 days. For determination of lesion size, digital photos of skin lesions were taken daily and analyzed via ImageJ software. For the USA100 experiments, strain AH3684 (IA116, kindly provided by Dr. Daniel Diekema) was used. The plasmid for agr-P3 lux was transformed into this strain for monitoring agr kinetics during infection (new strain AH4390).
Measurement of quorum quenching in vivo
Inoculum preparation for assessing quorum quenching in vivo. An MRSA LAC reporter strain engineered to couple agr activation with bioluminescence, called agr-P3 lux (AH2759) (Figueroa et al., 2014), was grown in TSB medium containing chloramphenicol overnight at 37 C in a shaking incubator set to 200 rpm. Overnight cultures were diluted 1:100 TSB with chloramphenicol and subcultured to an optical density of 0.1 at 600nm (≈ hr). Bacterial cells were then pelleted and resuspended in sterile saline. 50 μL inoculum suspensions containing 1×107 CFUs and 5 μg of apicidin diluted in DMSO or DMSO alone were injected intradermally into abdominal skin using 0.3 mL/31 gauge insulin syringe (as a technical control several mice were injected in the same manner with 50 μL of sterile saline only. For all infections, challenge dose was confirmed by plating serial dilutions of inoculum on TSA and counting ensuing colonies after overnight culture. Beginning immediately after infection, mice were imaged under isoflurane inhalation anesthesia (2%). Photons emitted from luminescent bacteria were collected during a 2 min exposure using the Xenogen IVIS Imaging System and living image software (Xenogen, Alameda, CA). Bioluminescent image data are presented on a pseudocolor scale (blue representing least intense and red representing the most intense signal) overlaid onto a gray-scale photographic image. Using the image analysis tools in living image software, circular analysis windows (of uniform area) were overlaid onto abdominal regions of interest, and the corresponding bioluminescence values (total flux) were measured and plotted versus time after infection.
Evaluation of skin and blood immune cells
For skin: abdominal skin ulcerations were excised and incubated in trypsin (0.6% in PBS) for 75 min at 37 C; then cut into small pieces and incubated in Collagenase type II (0.5 mg/mL RPMI) for 90 min at 37 C. Skin cell suspensions were generated by serial passage of skin fragments through 18 and 20 gauge syringes. LN cell suspensions were generated mincing inguinal LNs with a razor blade before serial passage of LN fragments through 18 and 20 gauge syringes. For whole blood: blood was collected, washed and re-suspended in Tris-buffered ammonium chloride to lyse red blood cells. All tissue preps were passed through a 70 μm filter before staining.
Whole blood killing assays
For killing assays: PB was collected in sodium heparin tubes, transferred into round bottomed 96 well plates (Corning, NY). LAC cultures were grown in the presence of apicidin (100 μM) or DMSO for 4 hr at 37 C with shaking (250 rpm). After culture, cells were washed in saline and resuspended to a concentration of ≈1×105 CFUs/μL. Cells exposed to Apicidin or control were then mixed with heparinized PB in at a concentration of 1×106 CFUs/150 μL PB, the infected blood was incubated at 37 C in a 37 C with shaking (250 rpm). After 1 h, serial dilutions in saline were performed to determine the endpoint numbers of CFU, which were compared to the initial bacterial load to determine the viable percentage of the initial inoculum.
RNA Seq
RNaseq was essentially performed as previously described (Crosby et al., 2016). Briefly, cultures of LAC and LACΔagr were grown in TSB with DMSO alone or 100 μM apicidin (diluted in neat DMSO) in triplicate in a 48-well plate. Cultures were grown to optical density of 4 at 600 nm, and cells were harvested and treated with RNAProtect Bacteria Reagent (QIAGEN). Cells were lysed using lysostaphin and RNA purified using the RNeasy mini kit (QIAGEN). The samples were treated with DNA-free (Ambion) and sample quality was affirmed via Bioanalyzer (Agilent). Ribosomal RNA was depleted using Ribo-Zero rRNA Removal Kit for gram positive bacteria (Illumina). cDNA libraries were generated at the University of Iowa Genomics Division using the TruSeq Stranded mRNA Library Prep kit (Illumina). Sample were barcoded, pooled and sequenced in 125×125 paired-end reads on an Illumina HiSeq 2000 sequencer. The resulting reads were aligned to an updated S. aureus USA300 genome file containing annotations for small RNAs (Carroll et al., 2016) using SeqMan NGen (DNASTAR) and reads were quantified using ArrayStar (DNASTAR). Genes were considered differentially expressed if they showed a ≥ 4-fold change in expression with 95% confidence as evaluated by Student’s t test with a false discovery rate (FDR) correction applied for multiple t tests.
Flow cytometry
For staining of skin, LN and whole blood preparations, the following antibodies: Anti-Ly6G (1A8), Anti-Ly6C (HK1.4), -CD11b (M1/70), CD45 (30-F11) were purchased from BioLegend. To block nonspecific binding, cells were incubated with rat anti-mouse CD16/32 FcγRIII/II (2.4G2) and vortexed prior to surface staining. In all experiments, cells were collected on a LSR II using Diva software, and analyzed using FlowJo software. Dead cells were excluded by low forward-scatter and side-light scatter. Spectral overlaps between fluorochrome channels were corrected by automated compensation on singly stained, positive controls for each fluoro-chrome. In general, 50,000 cells were collected/tube. Flow cytometric analyses of bacterial cultures were conducted by incubating MRSA subcultures in 100 μM apicidin, for the 4 or 7 hr in a Stuart incubator set to 37 C, shaking at 1000 RPM. After, washing and resuspending cultures in DPBS, samples were collected on a FACS LSR II using Diva software.
Cytokine Measurements from Tissue Homogenates
Biopsy punches (4 mm), obtained from the center of skin ulcerations 1 day after infection, were homogenized with in sterile RPMI with proteinase inhibitor cocktail. The supernatants from these preparations were incubated with a multiplex bead array for IL-1β, G-CSF, KC (CXCL1), and CXCL2. Data were collected on a Luminex 200 (Luminex, Austin, TX, USA) and analyzed with Bio-plex manager software 6.0.
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistics
For each experiment, the total number of replicates (n) and the statistical tests performed can be found in the figure legends. With the exception of flow cytometry and bioluminescent imaging data, all analyses were performed using GraphPad PRISM software version 7 (GraphPad Software, Inc. La Jolla, CA). Throughout this work, significance was defined as p < 0.05. The P values for comparisons between experimental groups were calculated by use of an unpaired Student’s t test. Error is presented as standard error of the mean (SEM) unless otherwise noted.
For the RNaseq experiments, expression data was analyzed with ArrayStar (DNASTAR) and genes were considered differentially expressed if they showed a ≥ 4-fold change with 95% confidence as evaluated by Student’s t test with a false discovery rate (FDR) correction applied for multiple t tests.
DATA AND SOFTWARE AVAILABILITY
The RNaseq results with LAC (+/−apicidin treatment) and the Dagr mutant were uploaded to the NCBI GEO database. Accession information: https://www.ncbi.nlm.nih.gov/sra?term=SRP182661.
Supplementary Material
Highlights.
Apicidin is a fungal-derived agr quorum-sensing inhibitor of S. aureus
Apicidin prevents agr activation and MRSA-induced dermonecrosis during skin infection
Treatment with apicidin enhances host defense to MRSA by augmenting innate immunity
ACKNOWLEDGMENTS
C.P.P. was supported by NIH T32 Training Awards AI007511 and AI007343. A.R.H. was supported by a merit award (I01 BX002711) from the Department of Veteran Affairs and by NIH Public Health Service Grant AI083211 (Project 3). H.A.C. was supported by NIH T32 AI007511 and American Heart Association Postdoctoral Fellowship 15POST25720016. The libraries of fungal extracts and pure compounds were assembled with partial support from the North Carolina Biotechnology Center (2015-BRG-1208). The authors thank the flow cytometry, microscopy, and genomics core facilities at the University of Iowa for technical assistance, as well as Michael Shay for his technical assistance with the collection of cytokine measurements from infected skin. Mass spectro-metric data were collected in the Triad Mass Spectrometry Facility at the University of North Carolina at Greensboro. The authors also thank Dr. Ronan Carroll for providing an annotated USA300 genome for RNA-seq analysis and Dr. Daniel Diekema for providing strains.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information can be found online at https://doi.org/10.1016/j.celrep.2019.03.018.
DECLARATION OF INTERESTS
The authors have no financial or competing interests to declare. A patent has been filed by A.R.H., J.S.K., C.P.P., T.E.E., and N.H.O. on the biological activity of apicidin.
REFERENCES
- Boles BR, Thoendel M, Roth AJ, and Horswill AR (2010). Identification of genes involved in polysaccharide-independent Staphylococcus aureus biofilm formation. PLoS One 5, e10146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey RO 3rd, Kaur A, Todd DA, Egan JM, El-Elimat T, Graf TN, Raja HA, Oberlies NH, and Cech NB (2015). Comparison of the chemistry and diversity of endophytes isolated from wild-harvested and greenhouse-cultivated yerba mansa (Anemopsis californica). Phytochem. Lett 11, 202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll RK, Weiss A, Broach WH, Wiemels RE, Mogen AB, Rice KC, and Shaw LN (2016). Genome-wide annotation, identification, and global transcriptomic analysis of regulatory or small RNA gene expression in Staphylococcus aureus. MBio 7, e01990–e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (2013). Antibiotic resistance threats in the United States, 2013. (Report from the Centers for Disease Control). https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf.
- Cech NB, and Horswill AR (2013). Small-molecule quorum quenchers to prevent Staphylococcus aureus infection. Future Microbiol. 8, 1511–1514. [DOI] [PubMed] [Google Scholar]
- Cegelski L, Marshall GR, Eldridge GR, and Hultgren SJ (2008). The biology and future prospects of antivirulence therapies. Nat. Rev. Microbiol 6, 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers HF, and Deleo FR (2009). Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol 7, 629–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung GY, Wang R, Khan BA, Sturdevant DE, and Otto M (2011). Role of the accessory gene regulator agr in community-associated methicillin-resistant Staphylococcus aureus pathogenesis. Infect. Immun 79, 1927–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby HA, Schlievert PM, Merriman JA, King JM, Salgado-Pabón W, and Horswill AR (2016). The Staphylococcus aureus global regulator MgrA modulates clumping and virulence by controlling surface protein expression. PLoS Pathog. 12, e1005604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly SM, Elmore BO, Kavanaugh JS, Triplett KD, Figueroa M, Raja HA, El-Elimat T, Crosby HA, Femling JK, Cech NB, et al. (2015). u-Hydroxyemodin limits staphylococcus aureus quorum sensing-mediated pathogenesis and inflammation. Antimicrob. Agents Chemother 59, 2223–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLeo FR, and Chambers HF (2009). Reemergence of antibiotic-resistant Staphylococcus aureus in the genomics era. J. Clin. Invest 119, 2464–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLeo FR, Otto M, Kreiswirth BN, and Chambers HF (2010). Community-associated meticillin-resistant Staphylococcus aureus. Lancet 375, 1557–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Elimat T, Figueroa M, Ehrmann BM, Cech NB, Pearce CJ, and Oberlies NH (2013). High-resolution MS, MS/MS, and UV database of fungal secondary metabolites as a dereplication protocol for bioactive natural products. J. Nat. Prod 76, 1709–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa M, Jarmusch AK, Raja HA, El-Elimat T, Kavanaugh JS, Horswill AR, Cooks RG, Cech NB, and Oberlies NH (2014). Polyhydroxyanthraquinones as quorum sensing inhibitors from the guttates of Penicillium restrictum and their analysis by desorption electrospray ionization mass spec-trometry. J. Nat. Prod 77, 1351–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisinger E, Muir TW, and Novick RP (2009). agr receptor mutants reveal distinct modes of inhibition by staphylococcal autoinducing peptides. Proc. Natl. Acad. Sci. USA 106, 1216–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon CP, Williams P, and Chan WC (2013). Attenuating Staphylococcus aureus virulence gene regulation: a medicinal chemistry perspective. J. Med. Chem 56, 1389–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon CP, Olson SD, Lister JL, Kavanaugh JS, and Horswill AR (2016). Truncated autoinducing peptides as antagonists of Staphylococcus lugdunensis quorum sensing. J. Med. Chem 59, 8879–8888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall PR, Elmore BO, Spang CH, Alexander SM, Manifold-Wheeler BC, Castleman MJ, Daly SM, Peterson MM, Sully EK, Femling JK, et al. (2013). Nox2 modification of LDL is essential for optimal apolipoprotein B-mediated control of agr type III Staphylococcus aureus quorum-sensing. PLoS Pathog. 9, e1003166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanaugh JS, and Horswill AR (2016). Impact of environmental cues on Staphylococcal quorum sensing and biofilm development. J. Biol. Chem 291, 12556–12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiedrowski MR, Kavanaugh JS, Malone CL, Mootz JM, Voyich JM, Smeltzer MS, Bayles KW, and Horswill AR (2011). Nuclease modulates biofilm formation in community-associated methicillin-resistant Staphylococcus aureus. PLoS One 6, e26714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchdoerfer RN, Garner AL, Flack CE, Mee JM, Horswill AR, Janda KD, Kaufmann GF, and Wilson IA (2011). Structural basis for ligand recognition and discrimination of a quorum-quenching antibody. J. Biol. Chem 286, 17351–17358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxminarayan R, Duse A, Wattal C, Zaidi AK, Wertheim HF, Sumpradit N, Vlieghe E, Hara GL, Gould IM, Goossens H, et al. (2013). Antibiotic resistance—the need for global solutions. Lancet Infect. Dis 13, 1057–1098. [DOI] [PubMed] [Google Scholar]
- Limbago B, Fosheim GE, Schoonover V, Crane CE, Nadle J, Petit S, Heltzel D, Ray SM, Harrison LH, Lynfield R, et al. (2009). Characterization of methicillin-resistant Staphylococcus aureus isolates collected in 2005 and 2006 from patients with invasive disease: a population-based analysis. J. Clin. Microbiol 47, 1344–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy FD (1998). Staphylococcus aureus infections. N. Engl. J. Med 339, 520–532. [DOI] [PubMed] [Google Scholar]
- Mayville P, Ji G, Beavis R, Yang H, Goger M, Novick RP, and Muir TW (1999). Structure-activity analysis of synthetic autoinducing thiolactone peptides from Staphylococcus aureus responsible for virulence. Proc. Natl. Acad. Sci. USA 96, 1218–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina E, and Pieper DH (2016). Tackling threats and future problems of multidrug-resistant bacteria. Curr. Top. Microbiol. Immunol 398, 3–33. [DOI] [PubMed] [Google Scholar]
- Miller LS, and Cho JS (2011). Immunity against Staphylococcus aureus cutaneous infections. Nat. Rev. Immunol 11, 505–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, McDougal LK, Carey RB, Talan DA, et al. (2006). Methicillin-resistant S. aureus infections among patients in the emergency department. N. Engl. J. Med 355, 666–674. [DOI] [PubMed] [Google Scholar]
- Muhs A, Lyles JT, Parlet CP, Nelson K, Kavanaugh JS, Horswill AR, and Quave CL (2017). Virulence inhibitors from Brazilian peppertree block quorum sensing and abate dermonecrosis in skin infection models. Sci. Rep 7, 42275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIAID (2014). NIAID’s Antibacterial Resistance Program: Current status and future directions. Report of the National Institute of Allergy and Infectious Diseases https://www.niaid.nih.gov/sites/default/files/arstrategicplan2014.pdf.
- Nielsen A, Månsson M, Bojer MS, Gram L, Larsen TO, Novick RP, Frees D, Frøkiær H, and Ingmer H (2014). Solonamide B inhibits quorum sensing and reduces Staphylococcus aureus mediated killing of human neutrophils. PLoS One 9, e84992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick RP, and Geisinger E (2008). Quorum sensing in staphylococci. Annu. Rev. Genet 42, 541–564. [DOI] [PubMed] [Google Scholar]
- O’Neill J (2016). Tackling Drug-Resistant Infections Globally. (Wellcome Trust). [Google Scholar]
- Otto M (2010). Basis of virulence in community-associated methicillin-resistant Staphylococcus aureus. Annu. Rev. Microbiol 64, 143–162. [DOI] [PubMed] [Google Scholar]
- Paharik AE, Parlet CP, Chung N, Todd DA, Rodriguez EI, Van Dyke MJ, Cech NB, and Horswill AR (2017). Coagulase-negative Staphylococcal strain prevents Staphylococcus aureus colonization and skin infection by blocking quorum sensing. Cell Host Microbe 22, 746–756.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang YY, Schwartz J, Thoendel M, Ackermann LW, Horswill AR, and Nauseef WM (2010). agr-dependent interactions of Staphylococcus aureus USA300 with human polymorphonuclear neutrophils. J. Innate Immun 2, 546–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quave CL, and Horswill AR (2014). Flipping the switch: tools for detecting small molecule inhibitors of staphylococcal virulence. Front Microbiol. 5, 706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quave CL, Lyles JT, Kavanaugh JS, Nelson K, Parlet CP, Crosby HA, Heilmann KP, and Horswill AR (2015). Castanea sativa (European Chestnut) Leaf Extracts Rich in Ursene and Oleanene Derivatives Block Staphylococcus aureus Virulence and Pathogenesis without Detectable Resistance. PLoS One 10, e0136486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queck SY, Jameson-Lee M, Villaruz AE, Bach TH, Khan BA, Sturdevant DE, Ricklefs SM, Li M, and Otto M (2008). RNAIII-independent target gene control by the agr quorum-sensing system: insight into the evolution of virulence regulation in Staphylococcus aureus. Mol. Cell 32, 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raja HA, Miller AN, Pearce CJ, and Oberlies NH (2017). Fungal identification using molecular tools: A primer for the natural products research community. J. Nat. Prod 80, 756–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasko DA, and Sperandio V (2010). Anti-virulence strategies to combat bacteria-mediated disease. Nat. Rev. Drug Discov 9, 117–128. [DOI] [PubMed] [Google Scholar]
- Spaan AN, Surewaard BG, Nijland R, and van Strijp JA (2013). Neutrophils versus Staphylococcus aureus: a biological tug of war. Annu. Rev. Micro-biol 67, 629–650. [DOI] [PubMed] [Google Scholar]
- Spellberg B, Bartlett J, Wunderink R, and Gilbert DN (2015). Novel approaches are needed to develop tomorrow’s antibacterial therapies. Am. J. Respir. Crit. Care Med 191, 135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellberg B, Srinivasan A, and Chambers HF (2016). New societal approaches to empowering antibiotic stewardship. JAMA 315, 1229–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sully EK, Malachowa N, Elmore BO, Alexander SM, Femling JK, Gray BM, DeLeo FR, Otto M, Cheung AL, Edwards BS, et al. (2014). Selective chemical inhibition of agr quorum sensing in Staphylococcus aureus promotes host defense with minimal impact on resistance. PLoS Pathog. 10, e1004174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoendel M, Kavanaugh JS, Flack CE, and Horswill AR (2011). Peptide signaling in the staphylococci. Chem. Rev 111, 117–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd DA, Parlet CP, Crosby HA, Malone CL, Heilmann KP, Horswill AR, and Cech NB (2017). Signal biosynthesis inhibition with ambuic acid as a strategy to target antibiotic-resistant infections. Antimicrob Agents Chemother 61, e00263–00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong SY, Davis JS, Eichenberger E, Holland TL, and Fowler VGJ Jr. (2015). Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev 28, 603–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JS 3rd, Jin R, and Novick RP (2005). Transient interference with staphylococcal quorum sensing blocks abscess formation. Proc. Natl. Acad. Sci. USA 102, 1691–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNaseq results with LAC (+/−apicidin treatment) and the Dagr mutant were uploaded to the NCBI GEO database. Accession information: https://www.ncbi.nlm.nih.gov/sra?term=SRP182661.