Abstract
OBJECTIVES
Nilotinib has very few side effects, including neutropenia, thrombocytopenia, cardiotoxicity, high pancreatic lipase, ischemia, and vascular occlusion. We aimed to investigate whether short-term administration of nilotinib had ototoxic effects in rats.
MATERIALS and METHODS
Wistar-albino rats were categorized into three groups: group C (administered 0.25 mL of distilled water, no nilotinib), group N-20 (administered 20 mg/kg/day of nilotinib dissolved in distilled water), and group N-50 (administered 50 mg/kg/day of nilotinib dissolved in distilled water). A single dose was administered once per day, at the same hour, over 21 days. Auditory brainstem response (ABR) thresholds were recorded on day 0 and day 21.
RESULTS
There were no changes in ABR threshold values obtained on day 0 (baseline) and on day 21 across all three groups. A statistically significant difference was not found in terms of the mean latency of waves V and III, interpeak latency values of waves III–V, and amplitude ratios of waves III–V and V/Va at baseline and on day 21 across all three groups on within-group or between-group evaluation.
CONCLUSION
Consequently, further studies are needed that involve different drug doses, prolonged administration of drugs, as well as distortion otoacoustic emission test for the evaluation of cochlear activation and ABR. Furthermore, histopathological studies are needed to indicate whether the cochlea is affected to prove that nilotinib has definitively no ototoxic effect.
Keywords: Nilotinib, ototoxicity, rat, auditory brainstem response
INTRODUCTION
Tyrosine kinase inhibitors constitute a new group of effective agents for multiple clinical uses. For example they can uses gastrointestinal stromal malignancy, chronic myeloid leukemia, and many other tumors [1]. Since the use of these inhibitors in clinical practice began, the outcomes of patients diagnosed with chronic myeloid leukemia, which has been treated with these inhibitors, have improved, wherein 96% of these patients have survived and have shown no disease progression for 3 years [2]. Results of a pivotal phase III international randomized study on interferon and STI571 (IRIS) conducted with patients who had been followed up for over eight years revealed that the rate of discontinuance of treatment was 5.4% owing to adverse events [3]. Each tyrosine kinase inhibitor has a different safety profile, given that these compounds have different tyrosine kinases as targets. It is well known that they are associated with several short-term side effects; conversely, their long-term side effects have not been clarified yet, particularly those of newer agents [4, 5]. Peripheral and periorbital edema, muscle cramps, gastrointestinal intolerance, skin rashes, congestive cardiac failure, nausea, diarrhea, and fluid retention can be considered adverse effects that are induced by the use of these inhibitors [6].
Nilotinib was accepted as a second-generation tyrosine kinase inhibitor for treating patients with chronic myeloid leukemia, who were resistant to imatinib initially. It is worth noting that this has been a first-line option since 2007 [7]. Although nilotinib has very few side effects, a review of literature has revealed studies indicating that it can cause neutropenia, thrombocytopenia, cardiotoxicity, high pancreatic lipase, ischemia, and vascular occlusion [8–12].
Ototoxicity is a general term referring to the damage of cochlear and vestibular organs resulting from exposure to various therapeutic agents or chemical substances [13]. The sensitivity of the inner ear toward numerous chemical substances has been known for many centuries [14]. Currently, antibiotics, diuretics, anti-inflammatory drugs, antineoplastic agents, antimalarial drugs, and other drugs are known to cause ototoxicity [15]. Numerous agents may cause ototoxicity; currently, many new drugs have been developed to treat the disease with support from the rapidly improving drug industry. Symptoms of ototoxicity occur only after long-term clinical use of newly developed treatment agents. Therefore, it should be always kept in mind that all newly available drugs might be potential ototoxic agents [16, 17].
Although the ototoxic mechanisms of certain drugs are not thoroughly known, many are known to also show ototoxic effects, such as neurotoxicity, increased oxidative stress, and vascular occlusion; for example, ischemia may play an important role in developing aminoglycoside ototoxicity. However, only a limited number of studies have examined the direct interaction between ischemia and aminoglycoside ototoxicity [18, 19]. Similarly, it is known that the ototoxic effect of cisplatin may be associated with increased oxidative stress [20].
Nilotinib is a new second-generation tyrosine kinase inhibitor and has value in the treatment of chronic myeloid leukemia. The use of nilotinib has been reported to be associated with vascular adverse events, such as peripheral arterial occlusive disease and ischemic heart disease, and has also been known to cause metabolic disturbances, such as dyslipidemia [21, 22]. It is known that hyperlipidemia, ischemia, and vascular occlusion play roles in the etiology of sensorineural hearing loss [23, 24]. Hence, we formulated a hypothesis: “could nilotinib have a negative effect on hearing?” From this point, we came across two studies upon literature review in the Pubmed database using the keywords “nilotinib and hearing loss” and “nilotinib and hearing,” the first being included in a study by Sabha et al., [25], a study demonstrating that nilotinib can be effective for vestibular schwannoma in in vitro cell culture, and it does not mention that the drug has impacts hearing loss. Kapur et al., [26] conducted the second study, in which they indicated that there were cases of secondary sensorineural hearing loss associated with imatinib treatment in the literature. However, they also stated that no such side effects were previously reported; therefore, it was difficult to state whether nilotinib impacted hearing loss or intracranial hemorrhage among the cases included in their study.
In conclusion, we were unable to find any study that supports our hypothesis. The fact that previously conducted studies also involved case reports indicating that imatinib likely causes hearing loss has directed us to investigate whether second-generation tyrosine kinase inhibitors have such an effect [27, 28]. Therefore, using the ABR test, we aimed to investigate whether short-term administration of nilotinib on rats had any ototoxic effects.
MATERIALS AND METHODS
Experimental Animals
The trials were conducted in accordance with the National Institute of Health (NIH) Guide for the care and use of Laboratory Animals (NIH Publications No. 80–23 Revised 1996). The protocol of the study was approved by the Institutional Review and Animal Ethics Use Committee of Sivas Cumhuriyet University School of Medicine, and the study was conducted based on the accepted guidelines on the care and use of laboratory animals.
This randomized experimental study followed the aforementioned protocol. The external auditory canals and eardrums of 30 rats anesthetized with 3 mg/kg Xylazine SC and 90 mg/kg Ketamine HCL SC were examined under a surgical microscope (OPMİ Vario; Zeiss, Jena, Germany) prior to being selected for the study. Those with external and middle ear pathologies were excluded from the study.
Male, 16–18 week-old Wistar-albino rats with an average body weight of 230±10 gm were included in this study. The rats were maintained under standard laboratory conditions (12 h light/dark cycles, 24±2°C, 35%–60% humidity).
The literature review did not reveal an experimental model that was used for investigating the effect of nilotinib on the auditory nerve. Thus, two doses were administered based on doses mentioned in studies considering the toxic effects of nilotinib on rats in the literature [29, 30]. The rats (n=24) were categorized into three groups via randomization: group C (0.25 mL of distilled water, no nilotinib n=8), group N-20 (20 mg/kg/day of nilotinib dissolved in distilled water, n=8), and group N-50 (50 mg/kg/day of nilotinib dissolved in distilled water, n=8). The researchers decided to include eight rats into each group considering α=0.05, β= 0.20, and (1-β)=0.80; the power of the test was found to be p=0.80942. The aforementioned nilotinib dosages according to each group were administered to all the rats in experimental groups via gastric gavage as a single dose once per day, at the same hour, over 21 days.
It was planned to exclude rats that died, regardless of the cause, in an experimental protocol during experimentation; however, no rat died. Each animal was weighed individually prior to auditory evaluation, upon which drug-induced weight loss and changes in the overall health were recorded. No significant weight loss or general condition disorder was observed in any of the 24 rats in every three groups during the experiment.
Drug and Chemicals
Nilotinib (Novartis Pharma AG, Basle, Switzerland) was dissolved in distilled water, and the purity of all chemical reagents was at least analytical grade.
Study Protocol and Hearing Examination
After rats included in the study were anesthetized with 3 mg/kg Xylazine SC and 90 mg/kg Ketamine HCL SC, their auditory brainstem response (ABR) threshold values were recorded in a silent room using Neuro-Audio 0710 VX (Neurosoft Ltd., 5, Voronin str. Ivanovo, 153032, Russian) device on day 0 (baseline; before administration of any drug or distilled water via gastric gavage) and day 21 (after administration of the drug or distilled water). Measurements of ABR of all rats were performed from their right ear. The ABR responses were recorded using a Natus Ultra Subdermal Needle Electrode (Stainless Steel Needle DIN 42802 Connector, Natus Neurology Incorporated 3150 Pleasant, View Road Middleton, WI. USA. 53562, Indonesia). The active electrode was placed on the vertex, earth electrode was placed on the contralateral mastoid, and reference electrode was placed on the ipsilateral mastoid. The stimuli were delivered with an insert earphone. ABR was evaluated by providing 8, 12, and 16 kHz tone bursts at 10, 30, 50, and 70 dB stimuli. The stimuli were presented at an alternate polarity: a filter of 30–2000 Hz; repetition rate of 37/sec; and time window of 20 msec, wherein 1,024 samples were taken to obtain the average signal. The impedance value was verified and accepted as being <1.0 kΩ.
The ABR threshold was defined as the lowest intensity level, at which the V wave of ABR is visible. A hearing threshold of >10 dB was accepted as the inclusion criterion of the study.
Thresholds, latency, and amplitude may be obtained from five ABR peaks. Latency was measured in milliseconds. The time between respective peaks was referred to as the interpeak latency. Interpeak latencies are generally used to measure the conduction time of the central auditory pathway. The interpeak latency of waves I–III, III–V, and I–V reflects the time to traverse in the caudal, rostral, and whole brainstem, respectively. A lesion affecting central auditory processing is characterized by a prolonged interpeak latency [31]. Therefore, the study evaluated the absolute latency of waves III and V at 50 and 70 dB and that of wave V at 30 and 10 dB. The stimulus intensity of III–V interpeak latency was measured at 50 and 70 dB. Moreover, the amplitude ratios of waves III/V and waves V/Va were also recorded.
Statistical analysis
The Statistical Package for the Social Sciences (SPSS) (IBM Corp.; Armonk, NY, USA) for Windows, version 23.0, was used to analyze the data. The Kolmogorov–Smirnov, Wilcoxon signed-rank, and Kruskal–Wallis tests were used to assess the data obtained from this study because the data did not meet parametric test assumptions. A value of 0.05 was accepted as the level of statistical significance.
RESULTS
Wave V at 8, 12, and 16 kHz frequencies and a stimulus intensity of 10 dB was observed for all the rats included in the study on ABR evaluation conducted at baseline and on day 21.
Wave Latencies
ABR latency is another indicator for auditory sensitivity. ABR latencies between stimulus onset and waveform valley were measured.
The latencies were analyzed in terms of tone burst stimulus intensity at 30, 50, and 70 dB SPL for wave V and at 50 and 70 dB SPL for wave III [32].
Table 1 presents the mean latencies of wave V for rats in Group C, Group N-20, and Group N-50 at 8, 12, and 16 kHz and at 30, 50, and 70 dB SPL measured at baseline and on day 21. Base line and 21 days V wave mean latency values in all three groups during the within-group evaluation there was no statistically significant difference in all three frequencies and stimulus intensity level (p>0.05).
Table 1.
Wave V mean latency of the rats in Group C, Group N-20, and Group N-50 at baseline and on day 21 of ABR evaluation
| Group | Wave V Latency | ||||
|---|---|---|---|---|---|
| Frequency | dB SPL | Day | Mean±SD (msn) | p | |
| Group C | 8 kHZ | 70 | Baseline | 1.89±0.20 | 0.05 |
| Day 21 | 1.78±0.11 | ||||
| 50 | Baseline | 2.08±0.22 | 0.08 | ||
| Day 21 | 1.93±0.13 | ||||
| 30 | Baseline | 2.35±0.19 | 0.05 | ||
| Day 21 | 2.16±0.21 | ||||
| 12 kHZ | 70 | Baseline | 1.84±0.11 | 0.31 | |
| Day 21 | 1.78±0.09 | ||||
| 50 | Baseline | 2.03±0.13 | 0.41 | ||
| Day 21 | 1.93±0.15 | ||||
| 30 | Baseline | 2.25±0.17 | 0.87 | ||
| Day 21 | 2.25±0.26 | ||||
| 16 kHZ | 70 | Baseline | 1.81±0.29 | 0.57 | |
| Day 21 | 1.85±0.10 | ||||
| 50 | Baseline | 2.15±0.19 | 0.45 | ||
| Day 21 | 2.04±0.18 | ||||
| 30 | Baseline | 2.44±0.20 | 0.08 | ||
| Day 21 | 2.35±0.21 | ||||
| Group N-20 | 8 kHZ | 70 | Baseline | 1.86±0.14 | 0.45 |
| Day 21 | 1.80±0.08 | ||||
| 50 | Baseline | 2.00±0.14 | 0.48 | ||
| Day 21 | 1.96±0.15 | ||||
| 30 | Baseline | 2.21±0.21 | 0.44 | ||
| Day 21 | 2.14±0.18 | ||||
| 12 kHZ | 70 | Baseline | 1.92±0.19 | 0.21 | |
| Day 21 | 1.78±0.12 | ||||
| 50 | Baseline | 2.08±0.19 | 0.16 | ||
| Day 21 | 1.95±0.13 | ||||
| 30 | Baseline | 2.30±0.24 | 0.11 | ||
| Day 21 | 2.14±0.19 | ||||
| 16 kHZ | 70 | Baseline | 1.91±0.17 | 0.46 | |
| Day 21 | 1.85±0.18 | ||||
| 50 | Baseline | 2.11±0.23 | 0.74 | ||
| Day 21 | 2.09±0.30 | ||||
| 30 | Baseline | 2.35±0.31 | 0.61 | ||
| Day 21 | 2.30±0 | 33 | |||
| Group N-50 | 8 kHZ | 70 | Baseline | 1.89±0.11 | 0.07 |
| Day 21 | 1.79±0.14 | ||||
| 50 | Baseline | 1.87±0.41 | 0.46 | ||
| Day 21 | 1.81±0.39 | ||||
| 30 | Baseline | 2.25±0.15 | 0.05 | ||
| Day 21 | 2 10±0.18 | ||||
| 12 kHZ | 70 | Baseline | 1.91±0 10 | 0.15 | |
| Day 21 | 1.78±0.19 | ||||
| 50 | Baseline | 2.07±0.13 | 0.24 | ||
| Day 21 | 1.95±0.17 | ||||
| 30 | Baseline | 2.30±0.14 | 0.07 | ||
| Day 21 | 2.09±0.20 | ||||
| 16 kHZ | 70 | Baseline | 1.96±0.17 | 0.09 | |
| Day 21 | 1.79±0.10 | ||||
| 50 | Baseline | 2.12±0.13 | 0.18 | ||
| Day 21 | 1.97±1.15 | ||||
| 30 | Baseline | 2.41±0.18 | 0.06 | ||
| Day 21 | 2.22±0.16 | ||||
kHZ: kilohertz; dB SPL: Decibel sound pressure level
Table 2 presents the mean latencies of wave III for the rats in Group C, Group N-20, and Group N-50 at 8, 12, and 16 kHz and at 50 and 70 dB SPL on days 0 or 21. No statistically significant difference was determined in terms of mean latencies of wave III at either of the three frequencies and stimulus intensity levels at baseline and on day 21 across all three groups during the within-group evaluation (p>0.05).
Table 2.
Wave III mean latency of the rats in Group C, Group N-20, and Group N-50 at baseline and on day 21 of ABR evaluation
| Group | Wave III Latency | ||||
|---|---|---|---|---|---|
| Frequency | dB SPL | Day | Mean±SD (msn) | p | |
| Group C | 8 kHZ | 70 | Base line | 0.97±0.16 | 0.06 |
| Day 21 | 0.80±0.10 | ||||
| 50 | Base line | 1 07±0.22 | 0.05 | ||
| Day 21 | 0.97±0.25 | ||||
| 12 kHZ | 70 | Base line | 0.98±0.15 | 0.21 | |
| Day 21 | 0.76±0.16 | ||||
| 50 | Base line | 1.11±0.18 | 0.11 | ||
| Day 21 | 0.92±0.19 | ||||
| 16 kHZ | 70 | Base line | 0.95±0.32 | 0.29 | |
| Day 21 | 0.83±0.19 | ||||
| 50 | Base line | 1.06±0.36 | 0.83 | ||
| Day 21 | 1 03±0.22 | ||||
| Group N-20 | 8 kHZ | 70 | Base line | 0.80±0.24 | 0.35 |
| Day 21 | 0.74±0.13 | ||||
| 50 | Base line | 1.05±0.28 | 0.07 | ||
| Day 21 | 0.86±0.19 | ||||
| 12 kHZ | 70 | Base line | 0.79±0.22 | 0.45 | |
| Day 21 | 0.72±0.14 | ||||
| 50 | Base line | 0.99±0.27 | 0.57 | ||
| Day 21 | 0.89±0.21 | ||||
| 16 kHZ | 70 | Base line | 0.87±0.20 | 0.18 | |
| Day 21 | 0.70±0.16 | ||||
| 50 | Base line | 1.09±0.25 | 0.08 | ||
| Day 21 | 0.89±0.17 | ||||
| Group N-50 | 8 kHZ | 70 | Base line | 0.75±0.15 | 0.67 |
| Day 21 | 0.79±0.13 | ||||
| 50 | Base line | 1.13±0.45 | 0.27 | ||
| Day 21 | 1.03±0.39 | ||||
| 12 kHZ | 70 | Base line | 0.77±0.17 | 0.74 | |
| Day 21 | 0.73±0.13 | ||||
| 50 | Base line | 0.98±0.11 | 0.21 | ||
| Day 21 | 0.89±0.17 | ||||
| 16 kHZ | 70 | Base line | 0.85±0.13 | 0.89 | |
| Day 21 | 0.82±0.18 | ||||
| 50 | Base line | 1.00±0.12 | 0.83 | ||
| Day 21 | 1.02±0.17 | ||||
kHZ: kilohertz; dB SPL: Decibel sound pressure level
No statistically significant difference was obtained on between-group statistical analysis of the mean latencies of wave V at 8, 12, and 16 kHz and 30, 50, and 70 dB SPL on day 0 and 21 of ABR evaluation (p>0.05).
Similarly, no statistically significant difference was obtained on between-group statistical analysis that was performed in terms of mean latencies of wave III at 8, 12, and 16 kHz and 50 and 70 dB SPL on day 0 and 21 of ABR evaluation (p>0.05).
Interpeak Intervals
Table 3 presents the mean III–V interpeak latency at different frequency values (8, 10, and 12 kHz) and stimulus intensity levels (50 and 70 dB) of the rats in Group C, Group N-20, and Group N-50 at on day 0 and 21. A statistically significant difference was not determined in terms of mean interpeak latencies of waves III–V across all three groups (p>0.05). Similarly, there was no statistically significant difference between mean interpeak latencies of waves III–V at on day 0 and 21 on between-group evaluation (p>0.05).
Table 3.
Mean III–V interpeak latency of the rats in Group C, Group N-20, and Group N-50 at baseline and on day 21 of ABR evaluation
| Group | III–V Interpeak Latency | ||||||
|---|---|---|---|---|---|---|---|
| Frequency | dB SPL | Day | Mean±SD (msn) | Minimum (msn) | Maximum (msn) | p | |
| Group C | 8 kHZ | 70 | Baseline | 0.91±0.17 | 0.71 | 1.15 | 0.35 |
| Day 21 | 0.96±0.12 | 0.71 | 1.07 | ||||
| 50 | Baseline | 0.94±0.18 | 0.71 | 1.27 | 0.55 | ||
| Day 21 | 0.91±0.16 | 0.67 | 1.11 | ||||
| 12 kHZ | 70 | Baseline | 0.85±0.09 | 0.79 | 1.03 | 0.05 | |
| Day 21 | 1.01±0.16 | 0.75 | 1.23 | ||||
| 50 | Baseline | 0.90±0.12 | 0.79 | 1.11 | 0.18 | ||
| Day 21 | 0.99±0.15 | 0.75 | 1.19 | ||||
| 16 kHZ | 70 | Baseline | 0.94±0.24 | 0.71 | 1.47 | 0.39 | |
| Day 21 | 1.02±0.20 | 0.75 | 1.39 | ||||
| 50 | Baseline | 0.42±0.99 | 0.01 | 2.87 | 0.99 | ||
| Day 21 | 0.98±0.15 | 0.71 | 1.19 | ||||
| Group N-20 | 8 kHZ | 70 | Baseline | 1.04±0.21 | 0.75 | 1.43 | 0.73 |
| Day 21 | 1.06±0.07 | 0.95 | 1.19 | ||||
| 50 | Baseline | 0.95±0.25 | 0.6 | 1.35 | 0.15 | ||
| Day 21 | 1.10±0.13 | 0.95 | 1.35 | ||||
| 12 kHZ | 70 | Baseline | 1.12±0.30 | 0.87 | 1.83 | 0.99 | |
| Day 21 | 1.05±0.06 | 0.95 | 1.11 | ||||
| 50 | Baseline | 1.08±0.28 | 0.79 | 1.63 | 0.83 | ||
| Day 21 | 1.06±0.10 | 0.87 | 1.19 | ||||
| 16 kHZ | 70 | Baseline | 1.05±0.11 | 0.87 | 1.23 | 0.12 | |
| Day 21 | 1.16±0.21 | 0.95 | 1.63 | ||||
| 50 | Baseline | 1.05±0.17 | 0.87 | 1.31 | 0.18 | ||
| Day 21 | 1.19±0.23 | 0.95 | 1.71 | ||||
| Group N-50 | 8 kHZ | 70 | Baseline | 1.14±0.21 | 0.91 | 1.55 | 0.12 |
| Day 21 | 0.99±0.11 | 0.79 | 1.15 | ||||
| 50 | Baseline | 1.06±0.16 | 0.79 | 1.31 | 0.44 | ||
| Day 21 | 1.01±0.14 | 0.83 | 1.27 | ||||
| 12 kHZ | 70 | Baseline | 1.13±0.21 | 0.87 | 1.43 | 0.27 | |
| Day 21 | 1.04±0.23 | 0.83 | 1.59 | ||||
| 50 | Baseline | 1.08±0.17 | 0.83 | 1.31 | 0.31 | ||
| Day 21 | 1.04±0.23 | 0.67 | 1.47 | ||||
| 16 kHZ | 70 | Baseline | 1.10±0.15 | 0.91 | 1.31 | 0.24 | |
| Day 21 | 0.96±018 | 0.67 | 1.19 | ||||
| 50 | Baseline | 1.06±0.14 | 0.91 | 1.35 | 0.08 | ||
| Day 21 | 0.96±0.13 | 0.71 | 1.07 | ||||
kHZ: kilohertz; dB SPL: Decibel sound pressure level
Wave Amplitude Ratio
Table 4 presents the mean III–V amplitude ratios at different frequency values (8, 10, and 12 kHz) and stimulus intensity levels (50 and 70 dB) of the rats in Group C, Group N-20, and Group N-50 at on day 0 and 21 of ABR evaluation. A statistically significant difference was not determined in terms of mean amplitude ratios of waves III–V across all three groups during the within-group evaluation (p>0.05). Similarly, there was no statistically significant difference between mean amplitude ratios of waves III–V on between-group evaluation (p>0.05).
Table 4.
Mean III/V amplitude ratio of the rats in Group C, Group N-20, and Group N-50 at baseline and on day 21 of ABR evaluation
| Group | III/V Amplitude Ratio | ||||||
|---|---|---|---|---|---|---|---|
| Frequency | dB SPL | Day | Mean±SD (msn) | Minimum (msn) | Maximum (msn) | p | |
| Group C | 8 kHZ | 70 | Baseline | 0.69±0.99 | 0.03 | 2.32 | 0.67 |
| Day 21 | 1.36±0.21 | 0.09 | 0.69 | ||||
| 50 | Baseline | 0.17±0.14 | 0.03 | 0.47 | 0.89 | ||
| Day 21 | 0.22±0.18 | 0.01 | 0.56 | ||||
| 12 kHZ | 70 | Baseline | 0.16±0.12 | 0.01 | 0.33 | 0.09 | |
| Day 21 | 0.48±0.40 | 0.01 | 1.12 | ||||
| 50 | Baseline | 0.10±0.06 | 0.01 | 0.18 | 0.12 | ||
| Day 21 | 0.38±0.25 | 0.01 | 0.65 | ||||
| 16 kHZ | 70 | Baseline | 0.08±0.05 | 0.03 | 0.17 | 0.06 | |
| Day 21 | 0.30±0.32 | 0.08 | 1.06 | ||||
| 50 | Baseline | 0.42±0.99 | 0.01 | 2.87 | 0.34 | ||
| Day 21 | 0.21±0.15 | 0.02 | 0.41 | ||||
| Group N-20 | 8 kHZ | 70 | Baseline | 0.46±0.30 | 0.12 | 0.95 | 0.78 |
| Day 21 | 0.48±0.19 | 0.26 | 0.76 | ||||
| 50 | Baseline | 0.41±0.0.31 | 0.06 | 0.79 | 0.58 | ||
| Day 21 | 0.36±0.18 | 0.16 | 0.68 | ||||
| 12 kHZ | 70 | Baseline | 0.72±0.68 | 0.03 | 1.78 | 0.18 | |
| Day 21 | 0.35±0.17 | 0.08 | 0.55 | ||||
| 50 | Baseline | 0.56±0.86 | 0.01 | 2.67 | 0.67 | ||
| Day 21 | 0.31±0.14 | 0.05 | 0.48 | ||||
| 16 kHZ | 70 | Baseline | 0.44±0.37 | 0.08 | 1.07 | 0.27 | |
| Day 21 | 0.26±0.13 | 0.03 | 0.44 | ||||
| 50 | Baseline | 0.26±0.36 | 0.01 | 1.12 | 0.23 | ||
| Day 21 | 0.60±0.0.63 | 0.12 | 2.04 | ||||
| Group N-50 | 8 kHZ | 70 | Baseline | 0.50±0.31 | 0.08 | 0.98 | 0.89 |
| Day 21 | 0.46±0.29 | 0.12 | 0.94 | ||||
| 50 | Baseline | 0.39±0.32 | 0.10 | 0.90 | 0.76 | ||
| Day 21 | 0.27±0.71 | 0.05 | 0.55 | ||||
| 12 kHZ | 70 | Baseline | 0.61±0.77 | 0.06 | 2.38 | 0.99 | |
| Day 21 | 0.42±0.26 | 0.11 | 0.88 | ||||
| 50 | Baseline | 0.90±1.60 | 0.02 | 4.72 | 0.67 | ||
| Day 21 | 0.30±028 | 0.01 | 0.76 | ||||
| 16 kHZ | 70 | Baseline | 0.41±0.43 | 0.05 | 1.40 | 0.39 | |
| Day 21 | 0.53±0.64 | 0.08 | 1.94 | ||||
| 50 | Baseline | 0.63±0.82 | 0.03 | 2.56 | 0.61 | ||
| Day 21 | 0.76±1.24 | 0.13 | 3.56 | ||||
kHZ: kilohertz; dB SPL: Decibel sound pressure level
Table 5 presents the mean V/Va amplitude ratios at different frequencies (8, 10, and 12 kHz) and stimulus intensity levels (50 and 70 dB) in the rats in Group C, Group N-20, and Group N-50 at on day 0 and 21 of ABR evaluation. No statistically significant difference was determined in the mean V/Va amplitude ratios at all three frequencies and stimulus intensity levels at on day 0 and 21 in all three groups on within-group evaluation (p>0.05). Similarly, no statistically significant difference was found between the mean V/Va amplitude ratios at on day 0 or on day 21 on the between-group evaluation (p>0.05).
Table 5.
Mean V/Va amplitude ratio of rats in Group C, Group N-20, and Group N-50 at baseline and on day 21 of ABR evaluation
| Group | V/Va Amplitude Ratio | ||||||
|---|---|---|---|---|---|---|---|
| Frequency | dB SPL | Day | Mean±SD (msn) | Minimum (msn) | Maximum (msn) | p | |
| Group C | 8 kHZ | 70 | Baseline | 2.19±1.57 | 0.37 | 5.39 | 0.13 |
| Day 21 | 2.00±0.71 | 1.29 | 3.43 | ||||
| 50 | Baseline | 1.32±0.43 | 0.79 | 2.04 | 0.48 | ||
| Day 21 | 1.76±1.15 | 0.78 | 3.66 | ||||
| 12 kHZ | 70 | Baseline | 2.40±1.72 | 1.00 | 6.22 | 0.07 | |
| Day 21 | 1.30±0.65 | 0.63 | 2.60 | ||||
| 50 | Baseline | 1.21±0.41 | 0.56 | 1.85 | 0.05 | ||
| Day 21 | 0.79±0.31 | 0.28 | 1.16 | ||||
| 16 kHZ | 70 | Baseline | 1.92±1.41 | 0.96 | 5.9 | 0.99 | |
| Day 21 | 1.20±0.67 | 0.42 | 2.62 | ||||
| 50 | Baseline | 1.11±0.57 | 0.01 | 2.02 | 0.12 | ||
| Day 21 | 0.75±0.26 | 0.32 | 1.19 | ||||
| Group N-20 | 8 kHZ | 70 | Baseline | 1.09±0.57 | 0.58 | 2.22 | 0.40 |
| Day 21 | 1.36±0.68 | 0.54 | 2.83 | ||||
| 50 | Baseline | 0.86±0.47 | 0.19 | 1.51 | 0.04 | ||
| Day 21 | 1.43±0.88 | 0.07 | 3.18 | ||||
| 12 kHZ | 70 | Baseline | 0.82±0.48 | 0.23 | 1.85 | 0.05 | |
| Day 21 | 1.74±1.63 | 0.14 | 5.58 | ||||
| 50 | Baseline | 0.95±0.57 | 0.05 | 1.84 | 0.48 | ||
| Day 21 | 1.16±0.88 | 0.13 | 3.15 | ||||
| 16 kHZ | 70 | Baseline | 1.01±0.62 | 0.27 | 1.96 | 0.40 | |
| Day 21 | 1.36±0.83 | 0.26 | 3.19 | ||||
| 50 | Baseline | 0.74±0.44 | 0.07 | 1.42 | 0.78 | ||
| Day 21 | 0.69±0.43 | 0.01 | 1.49 | ||||
| Group N-50 | 8 kHZ | 70 | Baseline | 1.43±0.55 | 0.77 | 2.49 | 0.58 |
| Day 21 | 1.32±0.58 | 0.42 | 2.33 | ||||
| 50 | Baseline | 1.15±0.65 | 0.43 | 2.40 | 0.53 | ||
| Day 21 | 1.20±0.31 | 0.48 | 1.50 | ||||
| 12 kHZ | 70 | Baseline | 1.96±1.45 | 0.25 | 4.14 | 0.13 | |
| Day 21 | 1.23±0.42 | 0.31 | 1.70 | ||||
| 50 | Baseline | 1.08±0.66 | 0.31 | 2.22 | 0.78 | ||
| Day 21 | 0.99±0.48 | 0.13 | 1.72 | ||||
| 16 kHZ | 70 | Baseline | 1.18±0.38 | 0.62 | 1.71 | 0.87 | |
| Day 21 | 1.06±0.50 | 0.06 | 1.56 | ||||
| 50 | Baseline | 0.78±0.61 | 0.16 | 1.84 | 0.99 | ||
| Day 21 | 0.63±0.42 | 0.02 | 1.29 | ||||
kHZ: kilohertz; dB SPL: Decibel sound pressure level
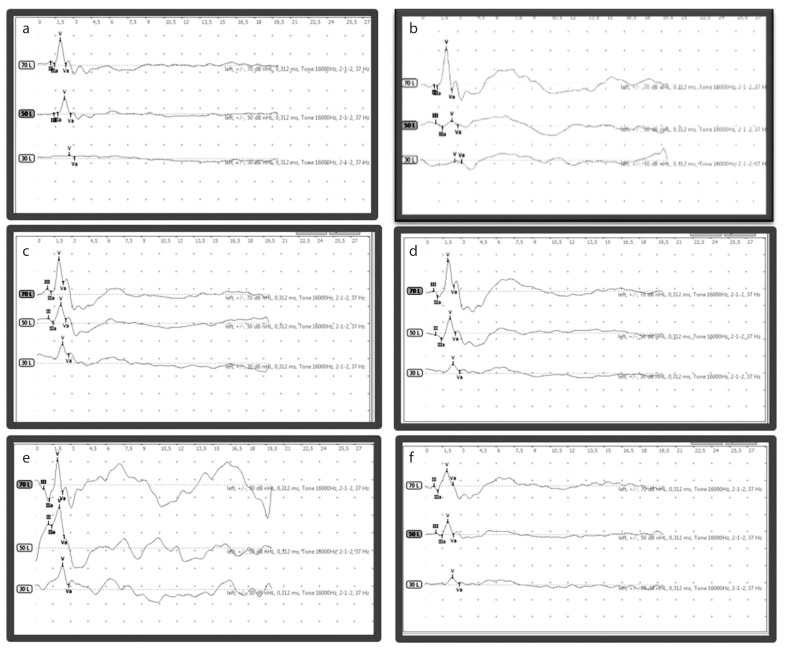
To conclude, no statistically significant difference was determined in terms of the mean latency of waves V and III, interpeak latency of waves III–V, and amplitude ratios of waves III–V or V/Va at on day 0 or on day 21 in either of the three groups on within-group and between-group evaluations. Figure 1 shows ABR at on day 0 and 21 in all three groups.
Figure 1. a–f.
Auditory brainstem response at baseline and on day 21 in all three groups. Response a) at baseline of Group C, b) on day 21 of Group C, c) at baseline of Group N-20, d) on day 21 of Group N-20, e) at baseline of Group N-50, and f) on day 21 of Group N-50.
DISCUSSION
No significant difference was observed in the latency, interpeak latencies, and amplitude ratio of ABR between rats in Group N-20 or Group N-50 and in the control group. To the best of our knowledge, no study has investigated the potential ototoxic effects of the short-term use of nilotinib and other tyrosine kinase inhibitors yet.
In accordance with our current knowledge, ototoxicity is described as chemical substances/drugs structurally damaging or rendering the cochlea, cochlear nerve, and vestibular system dysfunctional. Generally, there are four mechanisms playing a role in the development of ototoxicity: 1) Magnesium was required for oxidative phosphorylation. Binding phosphatidylinositol prevent the transport of magnesium and cause cell damage and death.; 2) As a result of removal of trace elements such as zinc, copper, which are necessary for oxidative metabolism in cochlea,; 3) development of degenerative changes in the basal fold causing vasoconstriction in the capillary bed in the spiral ligament, basilar membrane, and stria vascularis; and 4) decreased receptor current owing to external hairy cell damage. Decrease in the receptor current owing to external hairy cell damage was emphasized as the main mechanism leading to the development of antineoplastic drug-induced ototoxicity [33].
Pure tone audiometry is frequently used to evaluate ototoxicity. Hearing shift associated with ototoxicity is defined as 1) a ≥20 dB change at one frequency; 2) ≥10 dB change at ≥2 consecutively tested frequencies; or 3) loss of response at three consecutively tested frequencies [34].
Although the distortion product otoacoustic emission is often used to evaluate ototoxicity and pure tone audiometry, measuring ABR is a test used for monitoring ototoxicity [35]. Because it is essential to determine ototoxicity via objective measures, such as ABR, is necessary because many patients become incapacitated at some point during treatment and cannot undergo the rigors of a hearing test.
Waves obtained on ABR evaluation are known to be important to show lesion levels. Wave I is produced by the 8th nerve, wave III is produced mainly by auditory nerves in cochlear nucleus, and wave V is produced by auditory nerves in the lateral lemniscus and/or inferior colliculus nucleus found in more central or rostral regions of the brain stem. Therefore, although waves I and III are influenced by pathology in the peripheral or caudal regions of the brain stem, wave V is influenced by pathology in both peripheral and central regions. Although amplitudes of waves decrease owing to the pathology of these sites, latencies still are prolonged [36, 37]. Interpeak latencies are generally considered for measuring the conduction time of the central auditory pathway [31]. The III–V interpeak interval reflects neural conduction at more central (or rostral) regions of the brainstem [38]. Interpeak latencies (I–III, III–V, and I–V) of ABR are a simple method used to roughly localize lesions in the auditory pathway [39]. The amplitude of the waveform is supported by synchronously activated neurons [31]. On considering all these characteristics associated with ABR, the latency, amplitude ratios, and interpeak latency durations were evaluated to reveal whether the 8th nerve was influenced by nilotinib administration in this study.
Tyrosine kinase inhibitors are one of the several options for treating chronic myeloid leukemia [40]. Nilotinib is also a strong second-generation tyrosine kinase inhibitor used to treat leukemia [41]. The literature includes various case reports on monitoring cases of secondary sensorineural hearing loss during imatinib treatment [27, 28]. Only one case report concerning the correlation between nilotinib and hearing loss was conducted by Kapur et al., [26]. However, in this case report, it has been pointed out that hearing loss is caused by primary nilotinib or it is difficult to say whether it is a result of a cerebrovascular event. The current study investigated whether short-term administration of nilotinib causes an ototoxic effect in rats, given that ischemia and vascular occlusion are among the side effects of nilotinib. Furthermore, there is a case report in the literature that suggests that nilotinib leads to hearing loss. The results of the current study showed that a 21-day nilotinib exposure did not cause any change in ABR III wave latency, V wave latency, III–V wave interpeak latency, V/Va and III/V amplitude ratios among the groups of rats receiving various doses of drugs. In the literature where we can compare and interpret our results, we could not reach the clinical or animal study, which was done with any of tyrosine kinase inhibitors. For this reason, we only share our results with the literature.
Conversely, this study had some limitations. Because no other study on tyrosine kinase inhibitors and ototoxicity exists, important limitations include that there is no reference study for any of the dosage ranges or that the administration time of drugs was likely to be insufficient in terms of revealing these side effects, even though tyrosine kinase inhibitors are used by patients with chronic leukemia throughout their life. Another major limitation was that only the ABR method was used to evaluate ototoxicity; thus, the evaluation of the inner ear functions was limited, which meant that a histopathological evaluation of the cochlea could not be performed properly.
However, despite these limitations, we hope that this study would encourage further studies.
CONCLUSION
To conclude, further studies are needed that involve different drug doses, prolonged administration of drugs, distortion otoacoustic emission test for the evaluation of cochlear activation and ABR. Furthermore, histopathological studies are needed to indicate whether the cochlea is affected to reveal that nilotinib definitively has no ototoxic effect.
Acknowledgements
We would like to thank Selim Çam for his contribution to statistical evaluation.
Footnotes
This study was presented at the “13. International Ear Nose Throat and Head Neck Surgery Congress, S056, 5–7 April 2018”, “Ankara, Turkey”.
Ethics Committee Approval: Experiments were performed at the Experimental Trial and Animal Laboratory of Cumhuriyet University, Faculty of Medicine in accordance with the Care and Use of Laboratory Animals Guideline of National Institute of Health (NIH) (NIH Publications No. 80-23 Revised 1996), by obtaining the approval of Animal Experiments Local Ethics Committee of Cumhuriyet University (date 06. 07. 2017 and no. 65202830-050.04.04-71).
Informed Consent: N/A.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - A.B., K.D., E.E.A.; Design A.B., E.E.A.; Supervision – E.E.A., H.T.; Resource – A.B., H.T.; Materials - A.B., K.D., E.E.A.; Data Collection and/or Processing - A.B., K.D., E.E.A; Analysis and/or Interpretation - A.B., K.D., E.E.A.; Literature Search - A.B., K.D., E.E.A., H.T.; Writing – A.B., E.E.A.; Critical Reviews - A.B., K.D., E.E.A., H.T.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Johnson JR, Bross P, Cohen M, Rothmann M, Chen G, Zajicek A, et al. Approval summary: imatinib mesylate capsules for treatment of adult patients with newly diagnosed Philadelphia chromosome-positive chronic myelogenous leukemia in chronic phase. Clin Cancer Res. 2003;9:1972–9. [PubMed] [Google Scholar]
- 2.Castagnetti F, Di Raimondo F, De Vivo A, Spitaleri A, Gugliotta G, Fabbiano F, et al. A population-based study of chronic myeloid leukemia patients treated with imatinib in first line. Am J Hematol. 2017;92:82–7. doi: 10.1002/ajh.24591. [DOI] [PubMed] [Google Scholar]
- 3.Deininger M, O’Brien SG, Guilhot F, Goldman JM, Hochhaus A, Hughes TP, et al. International Randomized Study of Interferon Vs STI571 (IRIS) 8-Year Follow up: Sustained Survival and Low Risk for Progression or Events in Patients with Newly Diagnosed Chronic Myeloid Leukemia in Chronic Phase (CML-CP) Treated with Imatinib. Blood. 2009;114:462. [Google Scholar]
- 4.Druker BJ, Guilhot F, O’Brien SG, Gathmann I, Kantarjian H, Gattermann N, et al. Five-year follow-up for patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–17. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 5.Caldemeyer L, Dugan M, Edwards J, Akard L. Long-term side effects of tyrosine kinase inhibitors in chronic myeloid leukemia. Curr Hematol Malig Rep. 2016;11:71–9. doi: 10.1007/s11899-016-0309-2. [DOI] [PubMed] [Google Scholar]
- 6.Deininger MWN, O’Brien SG, Ford JM. Practical management of patients with chronic myeloid leukemia receiving imatinib. J Clin Oncol. 2003;21:1–11. doi: 10.1200/JCO.2003.11.143. [DOI] [PubMed] [Google Scholar]
- 7.Kantarjian HM, Hochhaus A, Saglio G, De Souza C, Flinn IW, Stenke L, et al. Nilotinib versus imatinib for the treatment of patients with newly diagnosed chronic phase, Philadelphia chromosome-positive, chronic myeloid leukaemia: 24-month minimum follow-up of the phase 3 randomised ENESTnd trial. Lancet Oncol. 2011;12:841–51. doi: 10.1016/S1470-2045(11)70201-7. [DOI] [PubMed] [Google Scholar]
- 8.Damrongwatanasuk R, Fradley MG. Cardiovascular Complications of Targeted Therapies for Chronic Myeloid Leukemia. Curr Treat Options Cardiovasc Med. 2017;19:24. doi: 10.1007/s11936-017-0524-8. [DOI] [PubMed] [Google Scholar]
- 9.Giles FJ, Mauro MJ, Hong F, Ortmann CE, McNeill C, Woodman RC, et al. Rates of peripheral arterial occlusive disease in patients with chronic myeloid leukemia in the chronic phase treated with imatinib, nilotinib, or non-tyrosine kinase therapy: A retrospective cohort analysis. Leukemia. 2013;27:1310–5. doi: 10.1038/leu.2013.69. [DOI] [PubMed] [Google Scholar]
- 10.Kim TD, Rea D, Schwarz M, Grille P, Nicolini FE, Rosti G, et al. Peripheral artery occlusive disease in chronic phase chronic myeloid leukemia patients treated with nilotinib or imatinib. Leukemia. 2013;27:1316–21. doi: 10.1038/leu.2013.70. [DOI] [PubMed] [Google Scholar]
- 11.Montemurroa M, Schoffskib P, Reichardtc P, Gelderblomd H, Schuttee J, Hartmannf JT, et al. Nilotinib in the treatment of advanced gastrointestinal stromal tumours resistant to both imatinib and sunitinib. Eur J Cancer. 2009;45:2293–7. doi: 10.1016/j.ejca.2009.04.030. [DOI] [PubMed] [Google Scholar]
- 12.Jager NG, Stuurman FE, Baars JW, Opdam FL. Cerebrovascular events during nilotinib treatment. Neth J Med. 2014;72:113–4. [PubMed] [Google Scholar]
- 13.Mutlu C. Ototoksisite. In: Çelik O, editor. Otorhinolaryngology and head and neck surgery. İstanbul: Turgut Publishing; 2002. pp. 257–70. [Google Scholar]
- 14.Schacht J, Hawkins JE. Sketches of otohistory. Part 11: ototoxicity: drug-induced hearing loss. Audiol Neurootol. 2006;11:1–6. doi: 10.1159/000088850. [DOI] [PubMed] [Google Scholar]
- 15.Francis SP, Cunningham LL. Non-autonomous Cellular Responses to Ototoxic Drug-Induced Stress and Death. Front Cell Neurosci. 2017;11:252. doi: 10.3389/fncel.2017.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henley CM, Rybak LP. Developmental ototoxicity. Otolaryngol Clin North Am. 1993;26:857–71. [PubMed] [Google Scholar]
- 17.Crumling MA, King KA, Duncan RK. Cyclodextrins and Iatrogenic Hearing Loss:New Drugs with Significant Risk. Front Cell Neurosci. 2017;11:355. doi: 10.3389/fncel.2017.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin CD, Wei IH, Tsai MH, Kao MC, Lai CH, Hsu CJ, et al. Changes in guinea pig cochlea after transient cochlear ischemia. Neuroreport. 2010;21:968–75. doi: 10.1097/WNR.0b013e32833da3c3. [DOI] [PubMed] [Google Scholar]
- 19.Lin CD, Kao MC, Tsai MH, Lai CH, Wei IH, Tsai MH, et al. Transient ischemia/hypoxia enhances gentamicin ototoxicity via caspase-dependent cell death pathway. Lab Invest. 2011;91:1092–106. doi: 10.1038/labinvest.2011.69. [DOI] [PubMed] [Google Scholar]
- 20.Huang T, Cheng AG, Stupak H, Liu W, Kim A, Staecker H, et al. Oxidative stress-induced apoptosis of cochlear sensory cells: otoprotective strategies. Int J Dev Neurosci. 2000;18:259–70. doi: 10.1016/S0736-5748(99)00094-5. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki K, Yamamoto J, Kakeda S, Takamatsu S, Miyaoka R, Kitagawa T, et al. Vessel wall magnetic resonance imaging findings and surgical treatment in nilotinib-associated cerebrovascular disease: A case report. Mol Clin Oncol. 2019;10:239–43. doi: 10.3892/mco.2018.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rea D, Mirault T, Cluzeau T, Gautier JF, Guilhot F, Dombret H, et al. Early onset hypercholesterolemia induced by the 2nd-generation tyrosine kinase inhibitor nilotinib in patients with chronic phase-chronic myeloid leukemia. Haematologica. 2014;99:1197–203. doi: 10.3324/haematol.2014.104075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marcucci R, Alessandrello Liotta A, Cellai AP, Rogolino A, Berloco P, Leprini E, et al. Cardiovascular and thrombophilic risk factors for idiopathic sudden sensorineural hearing loss. J Thromb Haemost. 2005;3:929–34. doi: 10.1111/j.1538-7836.2005.01310.x. [DOI] [PubMed] [Google Scholar]
- 24.Shi WY, Li KJ, Li Q. [Analysis of risk factors for recurrent sudden sensorineural hearing loss] Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2018;32:976–8. doi: 10.13201/j.issn.1001-1781.2018.13.004. [DOI] [PubMed] [Google Scholar]
- 25.Sabha N, Au K, Agnihotri S, Singh S, Mangat R, Guha A, Zadeh G. Investigation of the in vitro therapeutic efficacy of nilotinib in immortalized human NF2-null vestibular schwannoma cells. PLoS One. 2012;7:e39412. doi: 10.1371/journal.pone.0039412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kapur S, Wax M, Miles L, Hussain A. Permanent sensorineural deafness in apatient with chronic myelogenous leukemia secondary to intracranial hemorrhage. Case Rep Hematol. 2013;2013:894141. doi: 10.1155/2013/894141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Attili VSS, Bapsy PP, Anupama G, Lokanatha D. Irreversible sensorineural hearing loss due to Imatinib. Leuk Res. 2008;32:991–2. doi: 10.1016/j.leukres.2007.11.039. [DOI] [PubMed] [Google Scholar]
- 28.Lin HW, Roberts DS, Kay J, Stankovic KM. Sensorineural hearing loss following imatinib (Gleevec) administration. Otolaryngol Head Neck Surg. 2012;146:335–7. doi: 10.1177/0194599811415008. [DOI] [PubMed] [Google Scholar]
- 29.Nader MA, Wagih HM. Nilotinib, a tyrosine kinase inhibitor exhibits protection against acute pancreatitis-induced lung and liver damage in rats. Naunyn Schmiedebergs Arch Pharmacol. 2017;390:291–300. doi: 10.1007/s00210-016-1327-2. [DOI] [PubMed] [Google Scholar]
- 30.El-Agamy DS. Anti-allergic effects of nilotinib on mast cell-mediatedanaphylaxis like reactions. Eur J Pharmacol. 2012;680:115–21. doi: 10.1016/j.ejphar.2012.01.039. [DOI] [PubMed] [Google Scholar]
- 31.Smit JV, Jahanshahi A, Janssen MLF, Stokroos RJ, Temel Y. Hearing assessment during deep brain stimulation of the central nucleus of the inferior colliculus and dentate cerebellar nucleus in rat. PeerJ. 2017;5:e3892. doi: 10.7717/peerj.3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cui J, Zhu B, Fang G, Smith E, Brauth SE, Tang Y. Effect of the Level of Anesthesia on the Auditory Brainstem Response in the Emei Music Frog (Babina daunchina) PLoS One. 2017;12:e0169449. doi: 10.1371/journal.pone.0169449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rybak LB, Touliatos J. Ototoxicity. In: Snow JB Jr, Ballenger JJ, editors. Ballenger’s Otolaryngology Head and Neck Surgery. 16th ed. BC Decker Inc; 2003. pp. 374–80. [Google Scholar]
- 34.American Speech-Language-Hearing Association (ASHA) Audiologic management of individuals receiving cochleotoxic drug therapy [Guidelines] ASHA. 1994;36:11–9. Available from: www.asha.org/policy. [Google Scholar]
- 35.Dille MF, Ellingson RM, McMillan GP, Konrad-Martin D. ABR obtained from time-efficient train stimuli for cisplatin ototoxicity monitoring. J Am Acad Audiol. 2013;24:769–81. doi: 10.3766/jaaa.24.9.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shim HJ, An YH, Kim DH, Yoon JE, Yoon JH. Comparisons of auditory brainstem response and sound level tolerance in tinnitus ears and non-tinnitus ears in unilateral tinnitus patients with normal audiograms. PLoS One. 2017;12:e0189157. doi: 10.1371/journal.pone.0189157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang ZD, Chen C. Short-term outcome of functional integrity of the auditory brainstem in term infants who suffer perinatal asphyxia. J Neurol Sci. 2017;376:219–24. doi: 10.1016/j.jns.2017.03.036. [DOI] [PubMed] [Google Scholar]
- 38.Jiang ZD. Evoked potentials in pediatric brainstem lesions. In: Galloway G, editor. Clinical Neurophysiology in Pediatrics: A Practical Approach to Neurodiagnostic Testing and Management. New York: Demos Medical Publishing, LLC; 2015. pp. 187–213. (in press) [DOI] [Google Scholar]
- 39.Otto D, Hudnell K, Boyes W, Janssen R, Dyer R. Electrophysiological measures of visual and auditory function as indices of neurotoxicity. Toxicology. 1988;49:205–18. doi: 10.1016/0300-483X(88)90001-7. [DOI] [PubMed] [Google Scholar]
- 40.Abbott BL. Dasatinib: from treatment of imatinib-resistant or - intolerant patients with chronic myeloid leukemia to treatment of patients with newly diagnosed chronic-phase chronic myeloid leukemia. Clin Ther. 2012;34:272–81. doi: 10.1016/j.clinthera.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 41.Golemovic M, Verstovsek S, Giles F, Cortes J, Manshouri T, Manley PW, et al. AMN107, a novel aminopyrimidine inhibitor of Bcr-Abl, has in vitro activity against imatinib-resistant chronic myeloid leukaemia. Clin Cancer Res. 2005;11:4941–7. doi: 10.1158/1078-0432.CCR-04-2601. [DOI] [PubMed] [Google Scholar]