Abstract
OBJECTIVES
Malignant (necrotizing) otitis externa (MOE) is an aggressive form of skin inflammation of the external ear with a tendency to spread infection to the temporal bone. The study aimed to evaluate a causal relationship between treatment responses and clinical features in patients with MOE.
MATERIALS and METHODS
In a retrospective, descriptive section study, the database was analyzed between January 2008 and December 2018 in our department, all patients with diagnosed MOE were identified.
RESULTS
A total of 30 patients were evaluated, of which 27 men and 3 women. The youngest patient was 52 years old while he was eldest 88 years, (mean-71 years old). As the most common comorbidity, diabetes mellitus was found in 23 (76%) subjects. Median duration of symptoms was about 3 months. The most common isolated pathogen was Pseudomonas aeruginosa (47%). Patients with facial nerve palsy and erosion of temporal bone find on computerized tomography affect prolonged stationary treatment (Mean, SD 29.2±8.5 and 26,7±11.6 days), while 80% of patients with facial nerve palsy had recurrence of disease (p=0.005) with mean duration of clinical remission of 60±17.3 days. Overall length of treatment is also increased in the presence of comorbidities as well as in patients with cranial nerve involvement.
CONCLUSION
Patients with cranial nerve involvement, erosion of temporal bone and presence of comorbidities affect prolonged treatment and adverse prognosis. Early diagnosis and initiation of aggressive therapy are essential for stopping the further spread of the disease and prevention of serious complications.
Keywords: Malignant otitis externa, Pseudomonas aeruginosa, facial nerve palsy, diabetes mellitus
INTRODUCTION
Malignant (necrotizing) otitis externa (MOE) is an aggressive form of skin inflammation of the external ear with a tendency to spread the infection to the temporal cortical bone, leading to potential skull base osteomyelitis [1]. The condition was first reported by Toulmouche in 1838 [2] and is mentioned as the first case report of this disease. The most common causative pathogen is Pseudomonas aeruginosa, especially in immunocompromised patients with diabetes, HIV, leukemia, granulocytopenia, anemia, on immunosuppressive therapy. In addition, some species of fungi have been described as a causative agent, such as Aspergillus and Candida species. Several cases of methicillin-resistant Staphylococcus aureus (MRSA) have been described, and there is an ongoing increase in the MOE causative agents, such as Klebsiella and Proteus mirabilis [3].
The mechanism of tissue damage involves coagulation tissue necrosis because of microangiopathy of small blood vessels. The most common clinical findings are severe otalgia, otorrhea, impaired hearing, and granulation polyps. The facial nerve is the most commonly involved cranial nerve, but glossopharyngeal, vagus, accessory, or hypoglossal nerves could also be affected.
Diagnosis was based on anamnesis; clinical examination; audiological assessment; microbiological analysis of ear swab; and CT (computed tomography) scan of the temporal bone, skull base, and endocranium [4]. Notably, the diagnostic criteria have changed over time. A recent systematic review described 27 different sets of diagnostic criteria for this condition [5].
The primary treatment of MOE is long-term antimicrobial therapy. Other treatment strategies include close follow-up of blood glucose levels and inflammation markers and repeated local debridement of necrotic tissue. Lately, increased use of the hyperbaric oxygen chamber has been noted as one of the therapeutic modalities. Notably, surgery has a limited role in the treatment of MOE. Despite MOE being recognized for decades, the literature reveals a low level of evidence supporting its management [6]. Therefore, in the absence of strong evidence to guide decision-making, clinicians depend on their individual experience and judgment [7].
Our study aimed to evaluate the causal relationship between treatment responses and clinical features of this disease.
MATERIALS AND METHODS
The database was searched for all patients with MOE who were treated between 2008 and 2018 in our department. In this series, a diagnosis of MOE was made if all the following main criteria listed in Cohen and Friedman were met: clinical findings of severe otalgia, edema, and exudate in the external auditory canal (EAC) or granulation tissue in EAC, positive finding on CT scan of the temporal bone, failure of local treatment after more than 1 week, histological exclusion of other causes of external otitis like tumors and cholesteatomas, and microabscesses on pathological samples obtained during surgery. Anamnestic data were used from medical history, laboratory findings, CT scans, and magnetic resonance imaging. Clinical findings were recorded along with comorbidities for each patient. Predictors of treatment response were defined as the duration of inpatient admission, length of antibiotic treatment, and disease-related hospital readmission.
Patients with incomplete medical documentation were excluded. The study was approved by the Institutional Ethics Committee. Informed consent was obtained, where appropriate.
Statistical Analysis
All the results were analyzed using the Statistical Packages for the Social Sciences (SPSS) Statistics for Windows, version 21.0 (IBM Corp., Armonk, NY, USA). Absolute numbers and percentages described the data. Numerically continuous data were expressed using mean±standard deviation. Comparison of categorical variables was performed using the chi-square test and the Fisher’s test. A P value of less than 0.05 was considered statistically significant. Analysis of variance was used to check if the means of two or more independent samples were significantly different from each other. The Mann–Whitney U test was used for non-normally distributed variables.
RESULTS
Overall, 30 patients were analyzed, including 27 men and 3 women. The youngest patient was 52 years old, and the oldest was 88 years old (mean age 71±8.8 years). Notably, 24 patients had two or more symptoms. The most common symptoms were otorrhea and otalgia (Table 1).
Table 1.
Signs and symptoms of patients with malignant otitis externa
| Clinical characteristics | All patients (30) | % |
|---|---|---|
| Otalgia | 23 | 76 |
| Otorrhea | 24 | 79 |
| Edema and granulation in EAC | 10 | 31 |
| Edema in EAC | 14 | 45 |
| Chronic suppurative otitis media | 4 | 14 |
| Polypus in EAC | 5 | 17 |
| Edema of the preauricular and retroauricular regions | 3 | 12 |
| Peripheral paralysis of the facial nerve | 5 | 17 |
EAC: External auditory canal
The mean duration of symptoms was about 3 months. The minimum and maximum duration of stay in our department were 5 and 44 days, respectively (mean: 17.8±11.7 days).
In almost 50% of patients, urea was elevated. Regarding inflammatory markers, leukocytes and CRP (C-reactive protein) were within normal limits in most patients. Erythrocyte sedimentation rate (ESR) was elevated in 30% (n=9), with values ranging between 40–105 mm/h. No relationship was observed between ESR and the degree of disease spread on the CT scan. Blood glucose level was elevated in 46% (n=14), with the mean glycemic value of 10.1 mmol/L. The most common isolated pathogen was P. aeruginosa. Fungi were isolated in five patients (Table 2). Among the five swab findings, several pathogens were isolated, but no relationship with other variables was observed.
Table 2.
Types of microbiological findings
| Swab finding | No of patients | % |
|---|---|---|
| Pseudomonas aeruginosa | 14 | 47 |
| Staphylococcus aureus | 3 | 10 |
| Candida | 5 | 17 |
| Enteroccocus | 1 | 3 |
| Escherichia coli | 2 | 7 |
| Proteus mirabilis | 1 | 3 |
| Streptococcus pyogenes | 1 | 3 |
| Normal findings | 8 | 27 |
Mixed hearing loss and conductive hearing loss were observed in 14 and 15 patients, respectively. A biopsy was obtained in 15 patients. Pathohistological findings described non-specific granulation tissue and inflammation. Conservative therapy was initiated in 20% of patients, and 80% of patients underwent surgery in combination with antibiotic therapy. Notably, resultant otologic complications were observed in 15 patients.
Involvement of the Cranial Nerves
Paralysis of the facial nerve, one of the most common otologic complications, was documented in five patients, three infected with Pseudomonas and two non-Pseudomonas-infected patients. Erosion of temporal bone on CT scan was described in 12 patients, with 50% of them having cranial nerve involvement and Pseudomonas positive swab.
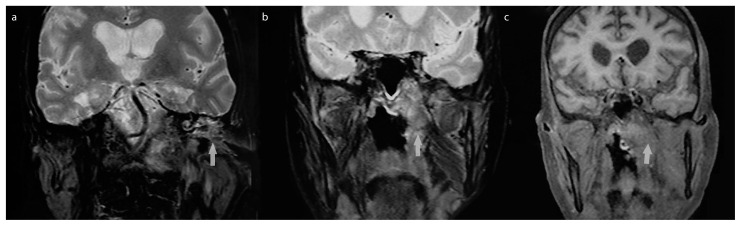
Bilateral laryngeal paralysis and paralysis of the soft palate was observed in one patient because of the probable expansion of the infective process to the skull base, affecting the ninth and tenth nerves (Figure 1). In addition, these nerves, along with the seventh cranial nerve, were affected in another patient who had infection spreading in the area of the infratemporal and pterygopalatine fossa, clivus, sphenoidal sinuses with infiltration of adjacent meninges. Moreover, the hypoglossal nerve was affected in one patient with advanced disease.
Figure 1. a–c.
Coronal MRI images of patient who had expansion of the process in soft tissue. a) spread of process in the left tympanic cavity and soft tissue swelling in the infratemporal space. b) left pharyngeal wall swelling. c) The spread of infection in left parapharyngeal space and affected reccurent nerve.
Comorbidities
Diabetes mellitus type 2 was observed in 23 patients (76%), 13 of them on therapy with insulin, and was the most common comorbidity, as observed in 36% infected with Pseudomonas, 23% non-Pseudomonas infected patients, and 17% with normal swab findings. Complications with diabetes were documented in 23% of patients, including polyneuropathies, renal insufficiency, and peripheral occlusive arterial disease.
Regarding other comorbidities, arterial hypertension was documented in 13 patients, stroke in 3 patients, and myocardial infarction in 1 patient. Three patients were defined as immunocompromised, one with bladder cancer and on chemotherapy and the other two diagnosed with secondary anemia.
MOE Management
The typical duration of antimicrobial treatment is at least 4–6 weeks. Depending on the antibiogram findings, all patients were treated with aggressive antibiotic therapy, most commonly systemic quinolones, such as ciprofloxacin, ceftriaxone, meropenem, or clindamycin. Fluconazole was the first treatment option in the case of fungal infection. In 21 patients, different types of surgical treatment were performed, including tympanomastoidectomy in 38%. Only one patient required radical temporal bone trepanation because of skull base osteomyelitis. When clinical findings demonstrated recovery, the treatment was stopped, and a CT scan was performed a few days later if needed. Negative findings on the CT scan confirmed complete recovery. Sometimes several CT scans were required to be performed in some of our patients until disease remission was confirmed.
Predictive Values
The contribution of patient clinical characteristics on readmission to our department is presented in Table 3. Facial nerve paralysis with the involvement of other cranial nerves and erosion of temporal bone were identified as factors that could influence the length of stay (LOS). We observed a statistically significant correlation between the presence of these factors and LOS (Table 4). The mean value of LOS for patients with facial nerve paralysis was 29.2 days, and that for temporal bone erosion was 26.7 days.
Table 3.
Hospital readmission in relation to different variables associated variables
| Hospital readmission | p | ||
|---|---|---|---|
| yes | no | ||
| Facial nerve paralysis | 4 | 1 | 0.005 |
| Erosion of temporal bone | 5 | 7 | 0.067 |
| Pseudomonas aeruginosa | 4 | 9 | 0.405 |
| Cranial nerve involvement | 5 | 4 | 0.083 |
| Diabetes | 5 | 18 | 0.532 |
| Surgical treatment | 6 | 18 | 0.567 |
| Comorbidities | 5 | 12 | 0.326 |
Table 4.
LOT and LOS in relation to different variables
| associated variables | LOT (weeks) Mean, SD | LOS (days) Mean, SD |
|---|---|---|
| Facial nerve paralysis | p=0.024 31.6±29.7 | p=0.012 29.2±8.5 |
| Erosion of temporal bone | p=0.074 22.3±23.8 | p<0.001 26.7±11.6 |
| Pseudomonas aeruginosa | p=0.199 20±23.5 | p=0.819 18±13.2 |
| Diabetes mellitus | p=0.980 15.5±18.9 | p=0.484 18.3±12 |
| Cranial nerve involvement | p=0.012 24.0±23.2 | p<0.001 24.6±10.7 |
| Comorbidities | p=0.048 19.82±20.5 | p=0.502 19.12±11.0 |
LOT: Length of treatment; LOS: length of hospital stay; SD: Standard Deviation
In some cases, the disease was persistent and aggressive despite prolonged treatment. In this subgroup, two patients died from complications of the disease. We documented disease recurrence rate of 23% after complete remission, involving 71% of patients having P. aeruginosa infection and temporal bone erosion and 60% with facial nerve paralysis. The mean duration of clinical remission was 55.7±27.7 days. However, in patients with facial nerve palsy and temporal bone erosion, the duration of clinical remission was 60±17.3 and 52±20.9 days, respectively.
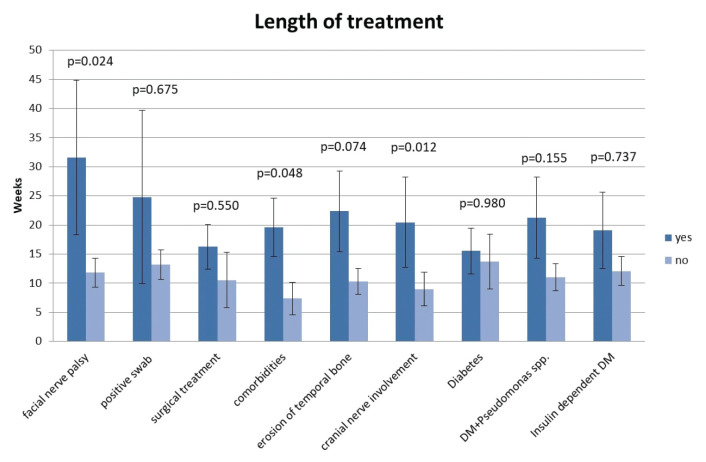
Regarding the length of treatment (LOT), some patients were treated in the outpatient setting as well, so we have identified several variables that affect slower therapy response. The mean duration of overall treatment was 15±18.4 weeks. Nevertheless, patients were treated for a significantly longer time if they had comorbidities and cranial nerve involvement (Figure 2).
Figure 2.
Influence of different variables on the length of treatment.
DISCUSSION
Our series of cases over the last 10 years recorded successful management of patients with MOE, with an observed mortality rate of 7% directly attributed to MOE that is concordant with values from some previous studies, 17.8% and 21% [6, 8]. The recurrence rate of MOE after treatment completion was 23% compared with the 10% reported in the literature [9].
The link with diabetes was well established, with recent studies reporting the prevalence of diabetes in MOE to be between 65% and 85% [10,11]. We documented 23 patients with diabetes mellitus type 2 (76%). As reported by Joshua et al. [12] MOE was associated with diabetes and a history of diabetic complications, which led to significantly longer treatment and shorter survival periods. In contrast, some authors reported that the presence of diabetes was not a significant prognostic factor in itself [10, 13].
The most frequent symptoms were otorrhea, otalgia, edema, and granulations in the EAC. Other symptoms were polypus in the EAC and edema of the preauricular area. In one study, all patients had severe otalgia and chronic otorrhea, and 56% of them had granulations in the EAC [14]. Laboratory findings did not reveal elevated values of inflammatory markers, such as leukocytes and CRP. ESR levels were elevated in nine patients. Most publications have revealed elevated ESR and considered it a non-specific inflammatory marker for the diagnosis of disease [15,16]. Bhat et al. [17] reported elevated ESR in all patients except one. Moreover, they observed the total leukocyte count was within normal limits in 14 patients, and only 1 patient had an elevated total leukocyte count of 12,700/mm3. The mean value of ESR level in the study of Lee et al. [18] was 96.1±30.8 mm/hr for the uncontrolled group of patients who either remained alive with the disease or died from the progression of the disease.
In our series, 14 patients (46%) had isolated P. aeruginosa, which was the most commonly isolated microbiological agent. In a study conducted by Shavit et al. [19] P. aeruginosa was isolated in 39 (44.3%) of 88 patients and S. aureus in 7 (8%) patients. In our study, the most common fungal pathogen was Candida albicans. Nevertheless, an increasing number of normal swab findings could be observed, which makes it imperative to consider MOE in non-diabetic patients with normal swab findings.
Overall, 12 patients had erosion of temporal bone. The facial nerve was the most commonly affected cranial nerve. Nevertheless, only one patient had nerve function recovery after therapy completion. Some authors compared patients with facial paralysis and without facial paralysis who were diagnosed with MOE and observed no significant difference in survival [20, 21]. In a study conducted by Franco-Vidal et al. [10] 20% of 46 patients had facial nerve involvement. According to Stevens et al. [8] facial nerve palsy defines a severe form of MOE and correlated with increased mortality. Facial nerve involvement (palsy or paresis) was documented in 15.5% of patients and was associated with a significantly longer mean LOS of 12.9±19.6 days, compared with the unaffected patients.
Despite biopsy being considered the only form of surgical approach by some authors in the available medical literature, we demonstrated different results. Surgery was performed in 70% of all cases. Failure of local treatment and poor antibiotic response were probably responsible for that high percentage of surgical treatment. Notably, recent studies have observed an increased resistance for fluoroquinolones, with resistance rates of 20%–54% [22–24]. Furthermore, with the increasing frequency of non-pseudomonal MOE, ciprofloxacin may not always be an effective treatment because it has poor gram-positive coverage and is ineffective against MRSA [25]. The role of surgery is to debride the necrotic material in cases with extensive disease. Mastoidectomy, as part of debridement, may be needed to clear the disease in severe cases [17]. However, a growing trend toward surgical intervention is observed in severe cases. Glikson et al. [11] performed surgery on 68% of patients (n=17), with five patients undergoing mastoidectomy under general anesthesia. In another study, surgical interventions were performed on 50% of patients who responded poorly to intravenous antibiotic treatment [26].
Factors like facial nerve paralysis and temporal bone erosion significantly contributed to the increase in the number of days spent in our department and readmission. In a systematic review by Hatch et al. [21] patients with weight loss, diabetes with chronic complications, CHF, coagulopathy, and liver disease were noted to have a significantly prolonged hospitalization compared with the other patients. Loh et al. [6] reported that the presence of major findings on initial CT scans was not predictive of outcome (33.3% in the poor outcome group and 36.4% in the good outcome group had major findings; p=1.00). As reported by Soudry et al. [20] facial nerve involvement indicated progression of MOE, but did not, by itself, worsen the prognosis. Some other studies determined that facial nerve palsy affected the overall patient mortality risk [19]. According to the analysis by Kaya et al. [15] uncontrolled diabetes mellitus and older age are associated with the LOS. Moreover, the age of 70 years and above, diabetes mellitus, facial nerve palsy, and positive findings on the CT scan at diagnosis were significant factors, according to Shavit et al. [19].
Notably, surgical debridement and parenteral or oral antibiotic therapy for 4–6 weeks is the recommended treatment for osteomyelitis of peripheral bones [27]. Park et al. [28] suggested that 4–6 weeks may be inadequate for patients at high risk of recurrent infections, such as those with infection because of MRSA and end-stage renal disease. Nonetheless, the optimal length of therapy for osteomyelitis in MOE remains unclear, warranting further research in this field and a more accurate therapy protocol.
CONCLUSION
Patients with cranial nerve involvement, erosion of temporal bone, and the presence of comorbidities require prolonged treatment and have an adverse prognosis.
Early diagnosis and initiation of aggressive therapy based on microbiological cultures can prevent severe complications and disease spread, thereby resulting in better outcomes and reduced mortality. Moreover, it may be suggested that the duration of therapy be not less than 6 weeks, especially in the sensitive group of patients at high risk of recurrent infections.
In addition, regular check-ups are recommended in preventing the recurrence of the disease. Nevertheless, the treatment regimen should be individualized, with multidisciplinary cooperation among ENT specialists, infectious diseases, and others.
Nonetheless, this study had some limitations. The study was a descriptive, retrospective, observational study, and the available records limited the study data. Therefore, further analysis of a larger number of respondents will provide a better understanding of this unpredictable illness and perhaps compensate for the lack of clarity, especially in terms of etiopathogenesis and diagnosis.
MAIN POINTS.
Malignant (necrotizing) otitis externa (MOE) is an aggressive form of skin inflammation of the external ear especially in immunocompromised patients.
Despite MOE being recognized for decades, the literature reveals a low level of evidence supporting its management
There is a growing trend toward surgical intervention as part of comprehensive MOE treatment.
Duration of antibiothic therapy should not be less than 6 weeks, especially in the sensitive group of patients at high risk of recurrent infections.
Patients with cranial nerve involvement, erosion of temporal bone, and the presence of comorbidities require prolonged treatment and have an adverse prognosis.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Ethics Committee of Clinical Center of Serbia.
Informed Consent: Informed consent was obtained, where appropriate.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – N.R., A.R.; Design N.R., Z.D.; Supervision N.A., S.B.; Resource S.J., L.C.; Materials N.R., Z.D.; Data Collection and/or Processing N.R., N.A., Z.D.; Analysis and/or Interpretation L.C.; Literature Search N.R., L.C.; Writing N.R., N.A.; Critical Reviews J.S., S.B.;
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Peled C, Kraus M, Kaplan D. Diagnosis and treatment of necrotising otitis externa and diabetic foot osteomyelitis – similarities and differences. J Laryngol Otol. 2018:1–5. doi: 10.1017/S002221511800138X. [DOI] [PubMed] [Google Scholar]
- 2.Toulmouche MA. Observations on cerebral otorrhea: latest considerations. Gaz Med Paris. 1838;6:422–6. [Google Scholar]
- 3.Bovo R, Benatti A, Ciorba A, Libanore M, Borrelli M, Martini A. Pseudomonas and Aspergillus interaction in malignant external otitis: risk of treatment failure. Acta Otorhinolaryngol Ital. 2012;32:416–9. [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen D, Friedman P. The diagnostic criteria of malignant external otitis. J Laryngol Otol. 1987;101:216–21. doi: 10.1017/S0022215100101562. [DOI] [PubMed] [Google Scholar]
- 5.Mahdyoun P, Pulcini C, Gahide I, Raffaelli C, Savoldelli C, Castillo L, et al. Necrotizing otitis externa: a systematic review. Otol Neurotol. 2013;34:620–9. doi: 10.1097/MAO.0b013e3182804aee. [DOI] [PubMed] [Google Scholar]
- 6.Loh S, Loh WS. Malignant otitis externa an Asian perspective on treatment outcomes and prognostic factors. Otolaryngol Head Neck Surg. 2013;148:991–6. doi: 10.1177/0194599813482107. [DOI] [PubMed] [Google Scholar]
- 7.Chawdhary G, Pankhania M, Douglas S, Bottrill I. Current management of necrotising otitis externa in the UK: survey of 221 UK otolaryngologists. Acta Otolaryngol. 2017;137:818–22. doi: 10.1080/00016489.2017.1295468. [DOI] [PubMed] [Google Scholar]
- 8.Stevens SM, Lambert PR, Baker AB, Meyer TA. Malignant Otitis Externa: A Novel Stratification Protocol for Predicting Treatment Outcomes. Otol Neurotol. 2015;36:1492–8. doi: 10.1097/MAO.0000000000000839. [DOI] [PubMed] [Google Scholar]
- 9.Sharma S, Corrah T, Singh A. Management of necrotizing otitis externa: Our experience with forty-three patients. J Int Adv Otol. 2017;13:394–8. doi: 10.5152/iao.2017.4399. [DOI] [PubMed] [Google Scholar]
- 10.Franco-Vidal V, Blanchet H, Bebear C, Dutrone H, Darrouzet V. Necrotizing external otitis: a report of 46 cases. Otol Neurotol. 2007;28:771–3. doi: 10.1097/MAO.0b013e31805153bd. [DOI] [PubMed] [Google Scholar]
- 11.Glikson E, Sagiv D, Wolf M, Shapira Y. Necrotizing otitis externa: diagnosis, treatment, and outcome in a case series. DiagnMicrobiol Infect Dis. 2017;87:74–8. doi: 10.1016/j.diagmicrobio.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 12.Joshua BZ, Sulkes J, Raveh E, Bishara J, Nageris BI. Predicting outcome of malignant external otitis. Otol Neurotol. 2008;29:339–43. doi: 10.1097/MAO.0b013e3181661879. [DOI] [PubMed] [Google Scholar]
- 13.Chen JC, Yeh CF, Shiao AS, Tu TY. Temporal bone osteomyelitis: The relationship with malignant otitis externa, the diagnostic dilemma, and changing trends. ScientificWorldJournal. 2014;2014:591–714. doi: 10.1155/2014/591714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hutson KH, Watson GJ. Malignant otitis externa, an increasing burden in the twenty-first century: review of cases in a UK teaching hospital, with a proposed algorithm for diagnosis and management. J Laryngol Otol. 2019:1–7. doi: 10.1017/S0022215119000604. [DOI] [PubMed] [Google Scholar]
- 15.Kaya I, Sezgin B, Eraslan S, Öztürk K, Göde S, Bilgen C, et al. Malignant Otitis Externa: A Retrospective Analysis and Treatment Outcomes. Turk Arch Otorhinolaryngol. 2018;56:106–10. doi: 10.5152/tao.2018.3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tarazi AE, Al-Tawfiq JA, Abdi RF. Fungal malignant oti-tis externa: pitfalls, diagnosis, and treatment. Otol Neurotol. 2012;33:769–73. doi: 10.1097/MAO.0b013e3182565b46. [DOI] [PubMed] [Google Scholar]
- 17.Bhat V, Aziz A, Bhandary SK, Aroor R, Kamath PSD, Saldanha M. Malignant otitis externa - A retrospective study of 15 patients treated in a tertiary healthcare center. Int Adv Otol. 2015;11:72–6. doi: 10.5152/iao.2015.430. [DOI] [PubMed] [Google Scholar]
- 18.Lee SK, Lee SA, Seon SW, Jung JH, Lee JD, Choi JY, et al. Analysis of Prognostic Factors in Malignant External Otitis. Clin Exp Otorhinolaryngol. 2017;3:228–35. doi: 10.21053/ceo.2016.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stern Shavit S, Soudry E, Hamzany Y, Nageris B. Malignant external otitis: Factors predicting patient outcomes. Am J Otolaryngol. 2016;37:425–30. doi: 10.1016/j.amjoto.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Soudry E, Joshua BZ, Sulkes J, Nageris BI. Characteristics and prognosis of malignant external otitis with facial paralysis. Arch Otolaryngol Head Neck Surg. 2007;133:1002–4. doi: 10.1001/archotol.133.10.1002. [DOI] [PubMed] [Google Scholar]
- 21.Hatch JL, Bauschard MJ, Nguyen Shaun A, Lambert PR, Meyer TA, McRackan TR, et al. Malignant Otitis Externa Outcomes: A Study of the University HealthSystem Consortium Database. Ann Otol Rhinol Laryngol. 2018;127:514–20. doi: 10.1177/0003489418778056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Master RN, Clark RB, Karlowsky JA, Ramirez J, Bordon JM. Analysis of resistance, cross-resistance and antimicrobial combinations for Pseudomonas aeruginosa isolates from 1997 to 2009. Int J Antimicrob Agents. 2011;38:291–95. doi: 10.1016/j.ijantimicag.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 23.Saeed M, Rasheed F, Afzal RK, Hussain S, Riaz S, Ahmad A. Pseudomonas aeruginosa: Evaluation of Pathogen Burden and Drug-Resistance Trends in a Tertiary Care Hospital. J Coll Physicians Surg Pak. 2018;28:279–83. doi: 10.29271/jcpsp.2018.04.279. [DOI] [PubMed] [Google Scholar]
- 24.Lister PD, Wolter DJ, Hanson ND. Antibacterial-Resistant Pseudomonas aeruginosa: Clinical Impact and Complex Regulation of Chromosomally Encoded Resistance Mechanisms. Clin Microbiol Rev. 2009;22:582–610. doi: 10.1128/CMR.00040-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hobson C, Moj JK, Byers KE, Raz J, Hirsch BE, et al. Malignant otitis externa: Evolving pathogens and implications for diagnosis and treatment. Otolaryngol Head and Neck Surg. 2014;151:112–6. doi: 10.1177/0194599814528301. [DOI] [PubMed] [Google Scholar]
- 26.Lee JE, Song JJ, Oh SH, Chang SO, Kim CH, Lee JH. Prognostic value of extension patterns on follow-up magnetic resonance imaging in patients with necrotizing otitis externa. Arch Otolaryngol Head Neck Surg. 2011;137:688–93. doi: 10.1001/archoto.2011.98. [DOI] [PubMed] [Google Scholar]
- 27.Courson AM, Vikram HR, Barrs DM. What are the criteria for terminating treatment for necrotizing (malignant) otitis externa? Laryngoscope. 2014;124:361–2. doi: 10.1002/lary.24093. [DOI] [PubMed] [Google Scholar]
- 28.Park KH, Cho OH, Lee JH, Park JS, Ryu KN, Park SY, et al. Optimal duration of antibiotic therapy in patients with hematogenous vertebral osteomyelitis at low risk and high risk of recurrence. Clin Infect Dis. 2016;62:1262–9. doi: 10.1093/cid/ciw098. [DOI] [PubMed] [Google Scholar]