Abstract
This study aims to obtain a better understanding of the number and distribution of spiral ganglion cell bodies (SGCBs) in the central modiolar trunk of the human cochlea with normal hearing as well as with hearing loss due to various pathological conditions. A detailed PubMed search was performed using the key words “human spiral ganglion cell population,” “analysis of spiral ganglion cell population,” “survival of human spiral ganglion cells,” “human Rosenthal’s canal,” “human ganglion cell counts,” and “distribution of human spiral ganglion cells” to identify articles published between 1931 and 2019. The articles were included if the number of SGCBs in the four segments of the human cochlea and angular depth distribution of the SGCBs were mentioned. Out of the 237 articles that were initially identified, 20 articles met the inclusion criteria. The presence of SGCBs inside the Rosenthal’s canal (RC) in the modiolar trunk extended to an angular depth of 630°–680°, which is close to the end of the second turn of the cochlea. SGCBs in Segment-IV of the cochlea account for approximately 25–30% of the entire SGCB population, regardless of the cochlear condition (normal vs. pathologic). In normal-hearing subjects, the total number of SGCB cases ranged between 23,910 and 33,702; in patients with hearing loss, the same was between 5,733 and 28,220. This literature review elaborates on the current state of knowledge regarding the number and distribution of SGCBs in the human cochlea.
Keywords: Angular depth, central modiolar trunk, second turn of the cochlea, spiral ganglion cell bodies
INTRODUCTION
Cochlear implant (CI) electrodes are commercially available in various types and array lengths. Whether the CI electrode is stimulating the spiral ganglion cell bodies (SGCBs) that are housed inside the central modiolar trunk or the peripheral nerve dendrites that reaches the Organ of Corti (OC) is still in an ongoing debate, and the answer is not exactly clear. The SGCBs are in Rosenthal’s canal (RC) in the central modiolar trunk. The RC runs by itself in the basal turn and then merges with the modiolar trunk distal to the basal turn. Pre-curved modiolar hugging (MH) implant electrodes-as per their design-are intended to be positioned near the modiolar wall with the aim of directly stimulating the SGCBs. However, in most of the cases implanted with MH electrodes regardless of the manufacturer, the stimulating channels are not actually consistently positioned in closer proximity to the modiolar wall [1]. The straight lateral wall (LW) electrodes would naturally follow the spiral ligament and be positioned right under the OC.
Rosenthal’s canal is protected by a porous layer of bone and covers the outer surface of the modiolar trunk. The size of the porous openings of the modiolar trunk that opens into the scala tympani is approximately 40 μm [2]. If a current can pass through this porous opening to reach the SGCBs, then it is understandable that the peripheral nerve dendrites of the SGCBs in the porous osseous spiral lamina can also receive the electrical stimulus from the implant electrode either directly through the porous osseous spiral lamina [2] [Rask-Andersen, 2006] or via the OC. While the OC can be easily located from the histological slice or through the μCT images of the human cochlea, the presence of SGCB is difficult to follow all the way from the base to the most apical region. Literature studies dating back to 1931 till 2019 have investigated the extension of RC housing the SGCBs and the distribution of SGCBs in the human cochlea [3–23] from the basal to the apical region. A strong positive correlation exists between the SGCB numbers in the 4th (apical) segment with better speech-discrimination scores in individuals with normal hearing, as per Otte et al., 1978 [4]. This apical segment corresponds to an angular depth ranging between 360° and 680°.
Regardless of the CI manufacturer and type of electrode array implanted, patients can perceive the benefit of CI if the cochlear nerve is present. However, the aim of cochlear implantation is to restore the entire spectrum of natural hearing. A clear understanding regarding the distribution and density of SGCBs in the various segments of the cochlea in both normal-hearing subjects and patients with hearing loss can provide useful insights for clinicians and implant developers alike to recognize the impact of electrode insertion depth and improve the design of future electrode arrays. The aim of this review is to collect and extract unbiased information on the number and distribution of SGCBs in the various segments of the human cochlea and the impact of hearing loss on SGCB density in the human cochlea.
METHODS
A PubMed search (https://www.ncbi.nlm.nih.gov/pubmed/) was performed using the following key words: “human spiral ganglion cell population” or “analysis of spiral ganglion cell population” or “survival of human spiral ganglion cells” or “human Rosenthal’s canal” or “human ganglion cell counts” or “distribution of human spiral ganglion cells.”
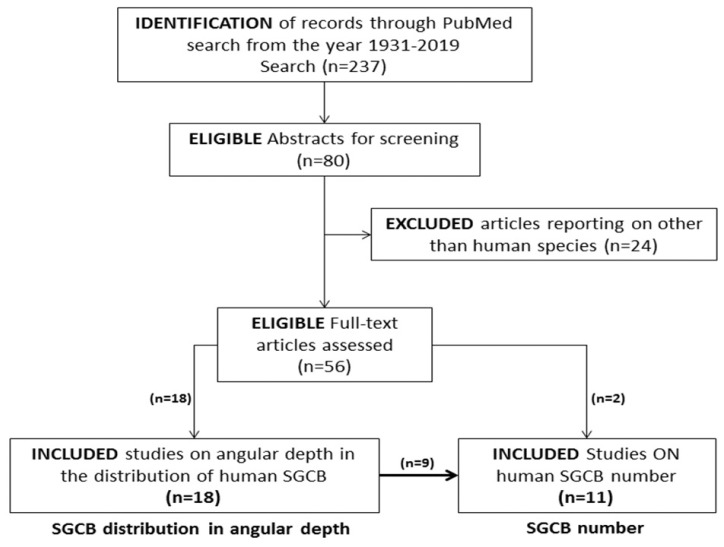
The search included articles published in the English language between 1931 and 2019 that reported the distribution of SGCBs in terms of angular depth in the central modiolar trunk and in terms of numbers in the human cochlea. Articles that reported on species other than human, SGCBs from sites other than the cochlea, and peripheral neural fibers and hair cells (but not on the cell bodies) were excluded. A detailed review of the literature was conducted: Figure 1 shows an outline of the literature review process.
Figure 1.
Identification of studies published between 1931 and 2019 that reported on the number and distribution of human SGCBs.
RESULTS
The PubMed search using the abovementioned key words yielded 237 articles published from 1931 to 2019. Here, 80 studies underwent a full-text review after eliminating articles that did not show any relevance to the key words in its title and abstract. Out of the 80 studies, 56 articles were found to be eligible after filtering out the articles that reported on species other than those involving humans. Further filtering identified 20 studies that discussed the angular depth distribution and/or number of SGCBs. Further, 18 studies described the SGCB distribution with respect to angular depth; out of these, nine discussed SGCB numbers. Two studies separately reported on the SGCB numbers. Table 1 lists the eligible studies.
Table 1.
List of literature studies discussing SGCB numbers and distribution with respect to angular depth
| S.No | Literature | SGCB distribution in angular depth/ SGCB number |
|---|---|---|
| 1 | Guild et al (1931, published online in 2009) | SGCB number and distribution in angular depth |
| 2 | Kerr et al (1968) | SGCB distribution in angular depth |
| 3 | Otte et al (1978) | SGCB distribution in angular depth |
| 4 | Hinojosa et al (1983) | SGCB number and distribution in angular depth |
| 5 | Hinojosa et al (1985) | SGCB number and distribution in angular depth |
| 6 | Schmidt et al (1985) | SGCB number and distribution in angular depth |
| 7 | Pollak et al (1987) | SGCB number and distribution in angular depth |
| 8 | Nadol et al (1988) | SGCB number |
| 9 | Nadol et al (1989) | SGCB number and distribution in angular depth |
| 10 | Ariyasu et al (1989) | SGCB distribution in angular depth |
| 11 | Kawano et al (1996) | SGCB distribution in angular depth |
| 12 | Incesulu et al (1998) | SGCB number and distribution in angular depth |
| 13 | Miura et al (2002) | SGCB number and distribution in angular depth |
| 14 | Glueckert et al (2005) | SGCB distribution in angular depth |
| 15 | Khan et al (2005) | SGCB number and distribution in angular depth |
| 16 | Stakovskaya et al (2007) | SGCB distribution in angular depth |
| 17 | Linthicum et al (2009) | SGCB distribution in angular depth |
| 18 | Ishiyama et al (2011) | SGCB number |
| 19 | Sagers et al (2017) | SGCB distribution in angular depth |
| 20 | Li et al (2019) | SGCB distribution in angular depth |
SGCBs: spiral ganglion cell bodies
Number and Density of SGCB in Various Hearing-Loss Conditions
Kerr and Schuknecht in 1968 [3] described that the numbers of SGCBs could be counted by means of a serial sectioning method. In 2011, Ishiyama et al. [11] reported on the stereology-optical fractionator method of counting the SGCBs, which yielded a 44% higher count as compared to that obtained from the assumption-based Abercrombie method. However, the Abercrombie method has been followed in all the studies in the literature published on SGCB counts. SGCB numbers range between 24,000 and 33,000 [5, 6, 8, 9, 11] in normal-hearing subjects. However, these numbers are reduced and significantly vary with respect to different etiologies of hearing loss [6. 10, 12, 16].
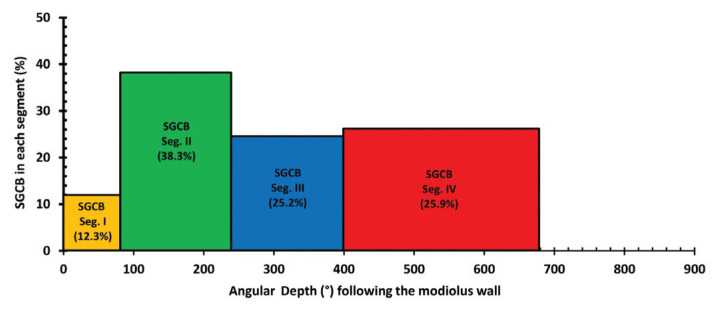
Table 2 summarizes the numbers of SGCBs and provides the percentages of SGCBs in various hearing-loss conditions measured in the four segments of the cochlea, as reported in various studies [5, 6, 8–12, 16]. As described in Nadol et al. [6] in 1989, the basal segment or Segment-I of the SGCB covers the corresponding cochlear duct length for the first 6 mm or 75° of angular depth along the OC. Segment-II of the SGCB covers the next 9–10 mm of the OC length, which is from 75° to 240° of angular depth. Segment-III of the SGCB is short and covers the OC length from 16 to 21 mm, which is from 240° to 400° of angular depth. Segment-IV of the SGCB commences at 21 mm of OC length, which is an angular depth of 400° and extends to the helicotrema. Regardless of the etiology of hearing loss, the SGCBs in Segment-IV account for approximately 25–30% of the entire SGCB population (Table 2). The apex region distal to the 26-mm location on the average OC length houses approximately 10% of the total SGCB population in normal-hearing subjects [6]. Figure 2 shows a pictorial representation of the percentage of SGCBs in various segments and their corresponding angular depths following the modiolar wall.
Table 2.
Numbers and density of SGCBs in both normal-hearing individuals and pathological hearing loss
| Nr | Diagnosis (average age) | Sample size (n) | Total SGCB count | SGCB number & Percentage | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| Seg. I | Seg. II | Seg. III | Seg. IV | |||||
| 1 | Normal hearing (36) | 6 | 28431±3670 | 3105±700 (10.9%) | 11216±1442 (39.4%) | 6401±1428 (22.5%) | 7709±2477 (27.1%) | Nadol 1988 |
| 2 | Normal hearing (39) | 5 | 28418±3675 | 3105±700 (11.0%) | 11214±1421 (39.4%) | 7186±1288 (25.2%) | 6914±1668 (24.3%) | Nadol 1989 |
| 3 | Normal hearing (4) | 16 | 33622±2577 | 3009±627 (8.9%) | 12258±1623 (36.4%) | 12153 ± 1669 (36.1%) | 6234±2151 (18.5%) | Hinojosa 1985 |
| 4 | Normal hearing (55) | 9 | 23910±3693 | 2564±1360 (10.7%) | 9822±1801 (41.0%) | 5090±1077 (21.2%) | 6373±1307 (26.6%) | Pollak 1987 |
| 5 | Normal hearing (50) | 6 | 23110±1865 | Na | Na | Na | Na | Ishiyama 2011 |
| 6 | Normal hearing (24) | 3 | 26421±1296 | Na | Na | Na | Na | Guild 1931 |
| 7 | Congenital infectious disease (1) | 13 | 20523±3892 | 2136±1055 (10.4%) | 8077±1782 (39.3%) | 4803±1240 (23.4%) | 5507±1392 (26.8%) | Miura 2002 |
| 8 | Chromosomal aberration (0.5) | 11 | 18880 ±2088 | 2203±831 (11.6%) | 8116±1248 (42.9%) | 4716±703 (24.9%) | 3845±1,139 (20.3%) | Miura 2002 |
| 9 | Perinatal or postnatal asphyxia (1) | 5 | 14301±5073 | 1370±1025 (9.5%) | 5933±2,621 (41.4%) | 3271±1,643 (22.8%) | 3728±1184 (26.0%) | Miura 2002 |
| 10 | Hereditary anamoly (3) | 21 | 15218±4926 | 1630±803 (10.7%) | 6270±1,998 (41.2%) | 3643±1,519 (23.9%) | 3674±1,486 (24.1%) | Miura 2002 |
| 11 | Sudden hearing loss (67) | 1 | 18096 | 1117 (6.1%) | 6576 (36.3%) | 4836 (26.7%) | 5567 (30.7%) | Khan 2005 |
| 12 | Postnatal virus labyrinthitis (53) | 8 | 7880±5760 | 1281±1,062 (16.2%) | 2649±2,394 (33.6%) | 1865±1,170 (23.6%) | 2084±1677 (26.4%) | Nadol 1989 |
| 13 | Congenital rubella syndrome (12) | 2 | 13127±261 | 2113±86 (16.0%) | 5558±83 (42.3%) | 2876±95 (21.9%) | 2581±162 (19.6%) | Nadol 1989 |
| 14 | Sudden idiopathic deafness (56) | 6 | 21844±11637 | 2403±1289 (11.0%) | 8765±5442 (40.1%) | 5,438±2,935 (24.8%) | 5238±2602 (23.9%) | Nadol 1989 |
| 15 | Congenital syphilis (70) | 2 | 5733±2915 | 684±815 (11.9%) | 1949±1190 (33.9%) | 1503±255 (26.2%) | 1598±655 (27.8%) | Nadol 1989 |
| 16 | Bacterial meningitis (39) | 2 | 16090 | 2141 (13.3%) | 6882 (42.7%) | 4266 (26.5%) | 5600 (34.8%) | Khan 2005 |
| 17 | Bacterial labyrinthitis (71) | 11 | 11968±4367 | 1361±786 (11.3%) | 4471±1955 (37.3%) | 3126±1,143 (26.1%) | 3009±1623 (25.1%) | Nadol 1989 |
| 18 | Temporal bone tumor (45) | 8 | 17620±8385 | 2149±1431 (12.1%) | 6564±3526 (37.2%) | 4529±1,923 (25.7%) | 4379±1743 (24.8%) | Nadol 1989 |
| 19 | Otosclerosis and/or Presbycusis (63) | 3 | 18855±4612 | 1905±1156 (10.1%) | 7320±2003 (38.8%) | 5031±981 (26.6%) | 4629±920 (24.5%) | Nadol 1989 |
| 20 | Otosclerosis (84) | 1 | 9925 | 710 (7.1%) | 2932 (29.5%) | 2428 (24.4%) | 3855 (38.8%) | Khan 2005 |
| 21 | Otosclerosis (83) | 6 | 9714±6207 | 770±373 (7.9%) | 3705±1888 (38.1%) | 2343±1612 (24.1%) | 2897±2402 (29.9%) | Incesulu 1998 |
| 22 | Congenital/genetic cause (40) | 9 | 11197±6823 | 1557±971 (13.9%) | 4968±3028 (44.3%) | 2597±1951 (23.1%) | 2075±1901 (18.5%) | Nadol 1989 |
| 23 | Genetic (74) | 1 | 4646 | 746 (16.0%) | 641 (13.7%) | 1105 (23.7%) | 2154 (46.3) | Khan 2005 |
| 24 | Temporal bone trauma/fracture (57) | 4 | 11468±9152 | 1571±1488 (13.6%) | 4331±3512 (37.7%) | 2747±2398 (23.9%) | 2819±2606 (24.5%) | Nadol 1989 |
| 25 | Temporal bone fracture (67) | 1 | 18936 | 2825 (14.9%) | 6659 (35.1%) | 4033 (21.2%) | 5419 (28.6%) | Khan 2005 |
| 26 | Aminoglycoside ototoxicity (41) | 8 | 21628±5113 | 2522±1728 (11.6%) | 8417±2117 (38.9%) | 5837±1319 (26.9%) | 4852±1115 (22.4%) | Nadol 1989 |
| 27 | Meniere’s disease (74) | 2 | 12726±3080 | 1467±344 (11.5%) | 4797±1247 (37.6%) | 3267±904 (25.6%) | 3195±1273 (25.1%) | Nadol 1989 |
| 28 | Meniere’s disease (69) | 1 | 9233 | 1540 (16.6%) | 3409 (36.9%) | 3031 (32.8%) | 1253 (13.5%) | Khan 2005 |
| 29 | Meniere’s disease (73) | 2 | 13882±2947 | 1598±565 (11.5%) | 5008±1564 (36%) | 3551±844 (25.6%) | 3726±671 (26.9%) | Incesulu 1998 |
| 30 | Ototoxicity (44) | 4 | 15424±11147 | 2194±2119 (14.2%) | 6345±3700 (41.1%) | 3591±2128 (23.3%) | 3294±3235 (21.4%) | Incesulu 1998 |
| 31 | Mondini’s dysplasia | 5 | 11216±3170 | 987±635 (8.7%) | 5194±2089 (46.3%) | 3370±951 (30.0%) | 2776±1863 (24.7%) | Schmidt 1985 |
| 32 | Alport’s syndrome | 2 | 22631±401 | 3441±366 (15.2%) | 8233±436 (36.3%) | 5603±356 (24.7%) | 5355±45 (23.6%) | Schmidt 1985 |
| 33 | Usher’s syndrome | 2 | 20021±2616 | 4253±815 (21.2%) | 10674±490 (53.3%) | 7859±1703 (39.2%) | 6008±465 (30.0%) | Schmidt 1985 |
| 34 | Klipper-Feil’s syndrome | 2 | 28220±1139 | 4253±815 (15.0%) | 10674±490 (37.8%) | 7859±1703 (27.8%) | 5434±741 (19.2%) | Schmidt 1985 |
| 35 | DiGeorge’s syndrome | 4 | 20831±7144 | 2755±1146 (13.2%) | 7966±1583 (38.2%) | 5726±1786 (27.4%) | 5844±2683 (28.0%) | Schmidt 1985 |
| 36 | Down syndrome | 1 | 9612 | 1170 (12.1%) | 3510 (36.5%) | 2430 (25.2%) | 2502 (26.0%) | Schmidt 1985 |
Na: data not available
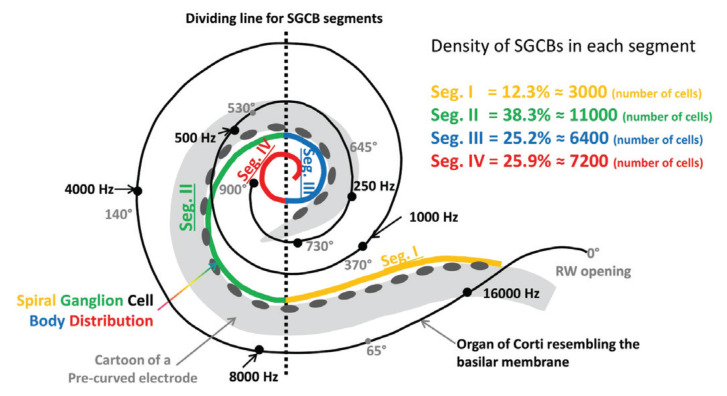
Figure 2.
Density of SGCBs in each segment as a percentage of the entire number of SGCBs (Y axis) vs. angular depth (X axis).
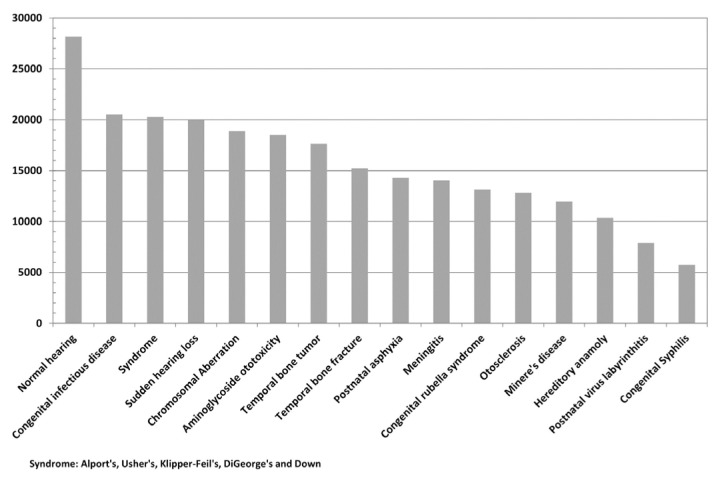
Normal-hearing individuals, as expected, showed significantly higher numbers of SGCBs as compared to subjects with pathological hearing conditions. Pathological conditions including postnatal virus labyrinthitis, congenital syphilis, otosclerosis, genetic, Meniere’s disease, and Down syndrome were associated with significantly reduced number of SGCBs at least from the data available in this study, as shown in Table 2 and summarized in Figure 3.
Figure 3.
Total number of SGCBs for various pathological inner-ear conditions.
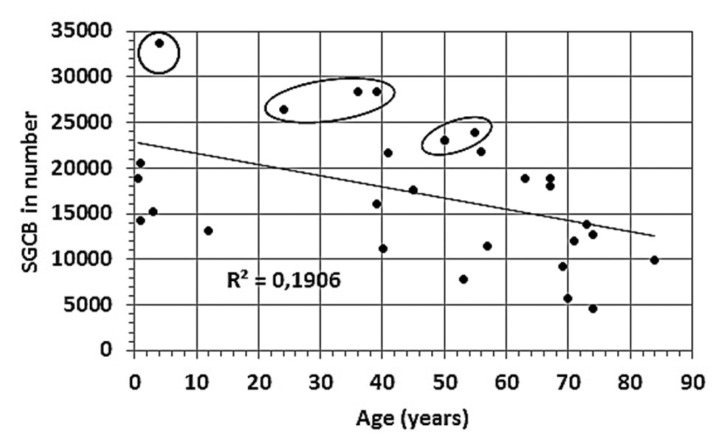
The data extracted from Table 2 show that aging leads to a reduction in the number of SGCBs in both normal-hearing individuals and individuals with various hearing-loss conditions (Figure 4). The circled data points represent the normal-hearing condition.
Figure 4.
Total number of SGCBs vs. age. Circled data points correspond to cochlear samples with normal hearing. Data obtained from Table 2.
SGCB Distribution in Angular Depth in the Human Cochlea
In the basal turn of the cochlea, SGCBs are secured inside the RC within the modiolar trunk. Distal to the basal turn, the RC blends into the modiolar wall and then extends into the upper middle turn [14, 15, 17, 19].
The peripheral axon of the SGCBs extends through the porous modiolar wall into the osseous spiral lamina synapsing with the hair cells in the OC. The central axons of the SGCBs travel in the modiolar trunk and then join to form the cochlear nerve that travels to the cochlear nuclear complex located in the dorsolateral aspect of the brainstem at the junction of the pons and medulla [14]. Considering an average OC length of 35 mm, with the available information that SGCBs are not present in the basal 1% and apical 10% of the OC length, the following discussion will revolve around an average OC length of 33 mm, as shown in Figure 5.
Figure 5.
Adapted from Otte et al., 1978 [4]; Nadol et al., 1989 [6]; Hinojosa et al., 1983 [7]; Pollak et al., 1987 [9]; Schmidt et al., 1985 [13]; and Kawano et al., 1996 [15]; the figure has been redesigned by appending additional information. The OC is represented by the black spline line with the Greenwood frequency distribution along with the angular depth measured from the round window. The SGCB distribution is shown by the prominent colored spline line with the vertical gray dotted line differentiating between the four segments. A pictorial representation of a pre-curved electrode placed over the SGCB distribution showing its electrical coverage from Segments-I–III leaving Segment-IV without any electrical stimulation.
The Organ of Corti begins at the base and reaches the very apex, which is known as the helicotrema. The RC spirals inside the modiolar trunk along the modiolar wall and houses the SGCB up to two turns of the cochlea [14, 15, 17, 19]. For a CI electrode to cover the entire population of SGCBs, this corresponds to an angular insertion depth from 630° to 680°, regardless of the type of electrode used. It is apparent that the SGCB density at the apical end of the cochlea is high, which is consistent with the finding of Otte et al., 1978 [4], of a positive correlation between the improved speech-discrimination scores and the number of SGCBs in Segment-IV in normal-hearing subjects. The distal 3/4 to 1 full rotation of the middle turn in the modiolar trunk beyond the basal 360° houses the SGCBs that cover the mid-low frequencies from approximately 600 to 35 Hz toward the apex [10].
DISCUSSION
This literature review evaluated the current state of knowledge available until the year 2019 (at the time of writing) regarding the number and angular depth distribution of SGCBs in the human cochlea in both normal-hearing individuals and individuals with hearing loss. This is the first study to collate the entire published literature with respect to the number and distribution of SGCBs, and we tried to report on the density of SGCBs in each segment of the cochlea. While the overall numbers of SGCBs were found to be lower in most etiologies of hearing loss as compared to normal-hearing-related cochlea, the distribution pattern of SGCBs remained consistent in all the four segments of the cochlea regardless of the underlying cause of hearing loss.
Considering the data from Table 2 and Figure 3, it is interesting to note that Segment-I, which is very short (length: 6 mm along the OC; angular depth: 75°), has only 11.3% of the total number of SGCBs. Segment-II and Segment-III (length: 15 mm from Segment-I; angular depth: up to 400°) collectively house 63% of the total number of SGCBs. Segment-IV covers the remaining length and reaches an angular depth of up to 680° and it has, on average, almost 25.8% of the total number of SGCBs. A recent report by Li et al. [23], where synchrotron-radiation-based imaging was used, reconfirmed that the SGCBs extend above 630° following the modiolar wall. Every SGCB in each segment is involved in contributing toward the hearing ability of the patient [3]. Therefore, electrically stimulating only Segment-I and a portion of Segment-II with a short electrode array (length: 10 mm; angular depth: 180°) did not permit patients to exploit the full benefit of the CI. This explains why such patients, upon losing low-frequency residual hearing after the initial implantation, would require revision surgery to replace the short electrode array with a conventional-length electrode to obtain better hearing [24, 25].
The length of the RC housing the SGCBs is closely linked to the overall length of the cochlear duct, supporting the opinion within the implant community that differing cochlear sizes need to be accommodated to optimize the hearing outcomes [15]. The fact that approximately 25.8% of the entire SGCB population is located in the apical Segment-IV supports the recent reports from various implant centers worldwide [26–29], which have yielded better hearing performances with increasing electrical coverage when using LW electrodes covering beyond the basal turn of the cochlea, regardless of the CI manufacturer and electrode type. In an electrophysiological study using electrically evoked compound action potential (eCAP) in patients implanted with long LW electrodes, the stimulation in Segment-IV provided evidence of similar electrical responses in comparison to other regions of the cochlea [30].
Considering the angular depth in the context of SGCB density across the different segments of the cochlea, studies that were consistently analyzed demonstrated that the distribution of SGCBs covers the basal turn and extends to the upper middle turn of the cochlea. Figure 5 shows a summary via a graphical representation of the several analyzed studies demonstrating the SGCB distribution pattern along the cochlea.
CONCLUSION
The number of SGCBs significantly varies between the cochleae of normal-hearing individuals and patients with hearing loss due to a variety of pathological conditions. Nevertheless, the percentage of SGCBs in each segment remained the same, regardless of the pathological conditions. SGCBs extend beyond the upper middle turn of the cochlea, which is at an angular insertion depth of 630°–680°. Regardless of the cochlear health status, approximately 25.8% of the total number of SGCBs are in the apical Segment-IV. SGCBs in the apical segment of the cochlea appear to provide a significant contribution to the speech-discrimination performance in normal-hearing subjects. Emerging evidence demonstrates the fact that an increase in the number of stimulated SGCB segments in combination with appropriate temporal coding and stimulation strategies can improve the overall hearing performance effected by cochlear implantation.
MAIN POINTS.
Spiral Ganglion Cell Bodies (SGCBs) are extended beyond the upper middle turn of the cochlea, which is at an angular insertion depth of 630°–680°.
Approximately 25.8% of the total number of SGCBs are in the apical segment.
Footnotes
This study was presented at the 12th Asia Pacific Symposium on Cochlear Implants and Related Sciences, November 27–30, 2019, Tokyo City, Japan.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - A.D., C.N.J., G.R., P.v.d.H.; Design - A.D., C.N.J., G.R., P.v.d.H.; Supervision G.R., P.v.d.H.; Resource - A.D., C.N.J., G.R., P.v.d.H.; Materials - A.D.; Data Collection and/or Processing - A.D., C.N.J., G.R., P.v.d.H.; Analysis and/or Interpretation - A.D., C.N.J., G.R., P.v.d.H.; Literature Search - A.D., C.N.J., G.R., P.v.d.H.; Writing - A.D., G.R.; Critical Reviews - P.v.d.H.
Conflict of Interest: Both Dr. Anandhan Dhanasingh and Dr. Claude Jolly are employed by MED-EL GmbH as Head of Translational Science Communication and Electrodes Expert respectively, which are purely scientific roles with no marketing activities.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Wang J, Dawant BM, Labadie RF, Noble JH. Retrospective evaluation of a technique for patient-customized placement of pre-curved cochlear implant electrode arrays. Otolaryngology-Head and Neck Surgery. 2017;157:107–12. doi: 10.1177/0194599817697298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rask-Andersen H, Schrott-Fischer A, Pfaller K, Glueckert R. Perilymph/Modiolar communication routes in the human cochlea. Ear Hear. 2006;27:457–65. doi: 10.1097/01.aud.0000233864.32183.81. [DOI] [PubMed] [Google Scholar]
- 3.Kerr A, Schuknecht HF. The spiral ganglion in profound deafness. Acta Oto-laryngologica. 1968;65:568–98. doi: 10.3109/00016486809119293. [DOI] [PubMed] [Google Scholar]
- 4.Otte J, Schuknecht H, Kerr A. Ganglion cell populations in normal and pathological human cochleae implications for cochlear implantation. Laryngoscope. 1978;8:1231–47. doi: 10.1288/00005537-197808000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Nadol JB. Quantification of human spiral ganglion cells by serial section reconstruction and segmental density estimates. Am J Otolaryngol. 1988;9:47–51. doi: 10.1016/S0196-0709(88)80007-3. [DOI] [PubMed] [Google Scholar]
- 6.Nadol J, Young Y, Glynn R. Survival of spiral ganglion cells in profound sensorineural hearing loss: Implications for cochlear implantations. Ann Otol Rhinol Laryngol. 1989;98:411–6. doi: 10.1177/000348948909800602. [DOI] [PubMed] [Google Scholar]
- 7.Hinojosa R, Marion M. Histopathology of profound sensorineural deafness. Ann N Y Acad Sci. 1983;405:459–84. doi: 10.1111/j.1749-6632.1983.tb31662.x. [DOI] [PubMed] [Google Scholar]
- 8.Hinojosa R, Seligsohn R, Lerner S. Ganglion Cell Counts in the Cochleae of Patients with Normal Audiograms. Acta Otolaryngol. 1985;99:8–13. doi: 10.3109/00016488509119139. [DOI] [PubMed] [Google Scholar]
- 9.Pollak A, Felix D, Schrott A. Methodological Aspects of Quantitative Study of Spiral Ganglion Cells. Acta Otolaryngol. 1987;(Suppl 437):37–42. doi: 10.3109/00016488709124974. [DOI] [PubMed] [Google Scholar]
- 10.Miura M, Sando I, Hirsch B, Orita Y. Analysis of spiral ganglion cell populations in children with normal and pathological ears. Ann Otol Rhinol Laryngol. 2002;111:1059–65. doi: 10.1177/000348940211101201. [DOI] [PubMed] [Google Scholar]
- 11.Ishiyama G, Geiger C, Lopez IA, Ishiyama A. Spiral and vestibular ganglion estimates in archival temporal bones obtained by design-based stereology and Abercrombie methods. J Neurosci Methods. 2011;196:76–80. doi: 10.1016/j.jneumeth.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Khan A, Handzel O, Damian D, Eddington D, Nadol J. Effect of cochlear implantation on residual spiral ganglion cell count as determined by comparison with the contralateral nonimplanted inner ear in humans. Ann Otol Rhinol Laryngol. 2005;114:381–5. doi: 10.1177/000348940511400508. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt JM. Cochlear neuronal populations in developmental defects of the inner ear. Implications for cochlear implantation. Acta Otolaryngol. 1985;99:14–20. doi: 10.3109/00016488509119140. [DOI] [PubMed] [Google Scholar]
- 14.Glueckert R, Pfaller K, Kinnefors A, Rask-Andersen H, Schrott-Fischer A. The human spiral ganglion: New insights into ultrastructure, survival rate and implications for cochlear implants. Audiol Neurootol. 2005;10:258–73. doi: 10.1159/000086000. [DOI] [PubMed] [Google Scholar]
- 15.Kawano A, Seldon HL, Clark GM. Computer-aided three-dimensional reconstruction in human cochlear maps: measurement of the lengths of organ of Corti, outer wall, inner wall, and Rosenthal’s canal. Ann Otol Rhinol Laryngol. 1996;105:701–9. doi: 10.1177/000348949610500906. [DOI] [PubMed] [Google Scholar]
- 16.Incesulu A, Dadol JB., Jr Correlation of acoustic threshold measures and spiral ganglion cell survival in severe to profound sensorineural hearing loss: implications for cochlea implantation. Ann Otol Rhinol Laryngol. 1998;107:906–11. doi: 10.1177/000348949810701102. [DOI] [PubMed] [Google Scholar]
- 17.Ariyasu L, Galey F, Hilsinger R, Byl F. Computer generated three dimensional reconstruction of the cochlea. Otolaryngol Head Neck Surg. 1989;100:87–91. doi: 10.1177/019459988910000201. [DOI] [PubMed] [Google Scholar]
- 18.Stakhovskaya O, Sridhar D, Bonham H, Leake P. Frequency map for the human cochlear spiral ganglion: implications for cochlear implants. J Assoc Res Otolaryngol. 2007;8:220–33. doi: 10.1007/s10162-007-0076-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nadol J, Burgess B, Reisser C. Morphometric analysis of normal human spiral ganglion cells. Ann Otol Rhinol Laryngol. 1990;90:340–8. doi: 10.1177/000348949009900505. [DOI] [PubMed] [Google Scholar]
- 20.Sagers JE, Landegger LD, Worthington S, Nadol JB, Stankovic KM. Human cochlear histopathology reflects clinical signatures of primary neural degeneration. Sci Rep. 2017;7:4884. doi: 10.1038/s41598-017-04899-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guild SR, Crowe SJ, Bunch CC, Polvogt LM. Correlations of differences in the density of innervation of the organ of corti with differences in the acuity of hearing, including evidence as to the location in the human cochlea of the receptors for certain tones. Acta Oto-laryngologica. 1931;15:269–308. doi: 10.3109/00016483109119096. [DOI] [Google Scholar]
- 22.Linthicum FH, Jr, Fayad JN. Spiral ganglion cell loss is unrelated to segmental cochlear sensory system degeneration in humans. Otol Neurotol. 2009;30:418–22. doi: 10.1097/MAO.0b013e31819a8827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li H, Schart-Moren N, Rohani SA, Ladak HM, Rask-Andersen H, Agrawal S. Synchrotron radiation-based reconstruction of the human spiral ganglion: Implications for cochlear implantation. Ear Hear. 2019;41:173–81. doi: 10.1097/AUD.0000000000000738. [DOI] [PubMed] [Google Scholar]
- 24.Carlson ML, Archibald DJ, Gifford RH, Driscoll CLW, Beatty CW. Reimplantation with a conventional length electrode following residual hearing loss in four hybrid implant recipients. Cochlear Implants Int. 2011;13:148–55. doi: 10.1179/1754762811Y.0000000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fitzgerald MB, Sagi E, Jackson M, Shapiro WH, Roland JT, Jr, Waltzman SB, et al. Reimplantation of hybrid cochlear implant users with a full-length electrode after loss of residual hearing. Otol Neurotol. 2008;29:168–73. doi: 10.1097/MAO.0b013e31815c4875. [DOI] [PubMed] [Google Scholar]
- 26.O’Connell BP, Hunter JB, Haynes DS, Holder JT, Dedmon MM, Noble JH, et al. Insertion depth impacts speech perception and hearing preservation for lateral wall electrodes. Laryngoscope. 2017;127:2352–7. doi: 10.1002/lary.26467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Büchner A, Illg A, Majdani O, Lenarz T. Investigation of the effect of cochlear implant electrode length on speech comprehension in quiet and noise compared with the results with users of electro-acoustic stimulation, a retrospective analysis. PLoS One. 2017;12:e0174900. doi: 10.1371/journal.pone.0174900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Connell BP, Hunter JB, Gifford RH, Rivas A, Haynes DS, Noble JH, et al. Electrode Location and Audiologic Performance after Cochlear Implantation: A Comparative Study between Nucleus CI422 and CI512 Electrode Arrays. Otol Neurotol. 2016;37:1032–5. doi: 10.1097/MAO.0000000000001140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buchman CA, Dillon MT, King ER, Adunka MC, Adunka OF, Pillsbury HC. Influence of Cochlear Implant Insertion Depth on Performance: A Prospective Randomized Trial. Otol Neurotol. 2014;35:1773–9. doi: 10.1097/MAO.0000000000000541. [DOI] [PubMed] [Google Scholar]
- 30.van de Heyning P, Arauz SL, Atlas M, Baumgartner WD, Caversaccio M, Chester-Browne R, et al. Electrically evoked compound action potentials are different depending on the site of cochlear stimulation. Cochlear Implants Int. 2016;17:251–62. doi: 10.1080/14670100.2016.1240427. [DOI] [PubMed] [Google Scholar]