Abstract
OBJECTIVES
This study aimed to understand if videos of the patients’ nystagmus recorded by themselves during the attacks can help in the diagnosis of Meniere’s disease (MD).
MATERIALS and METHODS
Sixty patients (age range 32–78 years) who had vestibular attacks and hearing complaints admitted to Çukurova University Hospital Otolaryngology Department and a private office between September 2013 and January 2017 were included in this randomized clinical trial study. Two groups with 30 patients each were formed. The first group was asked to send eye-videos recorded during the attack, while the patients in the second group were followed with conventional methods. Twenty-six patients in the first group were able to send satisfactory eye movement videos; four patients were excluded due to repeated recording faults. Twenty-seven patients in the second group could be followed; three patients were lost to follow-up. The number of attacks and time needed to diagnose both groups were compared.
RESULTS
The video group could be diagnosed in a shorter period compared to the control group. The diagnosis was made within two attacks (38 days) in the video group and within four attacks (92 days) in the control group.
CONCLUSION
This study shows that cell phone camera recordings of nystagmus of the patients are very helpful to diagnose MD. These recordings can also be used as an adjunct to understand the pathophysiology of the disease.
Keywords: Diagnosis of Meniere’s disease, nystagmus, vertigo attack, pathophysiology, mobile phone camera recordings, randomized clinical trial
INTRODUCTION
Meniere’s disease (MD) is one of the common disorders of the inner ear and vestibular system. Its major symptoms are episodic vertigo, fluctuating hearing loss, tinnitus, and aural fullness [1]. Natural history of the disease is characterized by variable periods of exacerbation and remission of symptoms. Treatment of MD includes many drugs including diuretics, betahistine, and even antidepressives [2, 3].
The American Academy of Otolaryngology–Head and Neck Surgery (AAO-HNS) developed a specific guideline for the diagnosis of MD in 1995 [4]. These guidelines had been used in MD as diagnostic criteria for many years; in 2015, Equilibrium Committee revised the 1995 AAO-HNS Guidelines for the Definition of MD due to some difficulties in clinical practice [5, 6].
In spite of the guidelines and improvements in vestibular tests, diagnosis of MD is a challenge. Vertigo is a major symptom in diagnosis of MD, but it’s a subjective symptom based on patient’s statement. The only objective finding of vertigo is nystagmus; so evaluation of nystagmus during attacks is essential in diagnosis of MD. Nystagmus is an objective finding that is always present during vertiginous attacks [7]. Videonystagmography devices are very useful to record and evaluate nystagmus, but most of the patients cannot attend a clinic during their vertigo attacks. Accordingly, nystagmus cannot be evaluated during the attacks, but it is probably the most important diagnostic criterion for this disorder. This situation is a challenge in diagnosis and treatment of patients with MD. Recording of nystagmus during the attack can be a very useful adjunct not only in diagnosis but also in brightening the pathophysiology of the disease.
These days, most of the population have access to mobile phones. We thought that patients or accompanying people could use their mobile phone cameras to record the nystagmus during attacks and share the recordings with their doctors. Accordingly, physicians can use these data to evaluate patients more accurately.
In many patients, the diagnosis of MD is based on history, but mostly the descriptions of the patients are not satisfactory. This study aimed to understand if videos of the eye movements recorded during the attacks can help in the diagnosis of MD. Some of these recordings were also used for a discussion on the physiopathology of the disease.
MATERIALS AND METHODS
Sixty patients with similar ages and gender who had vestibular complaints and hearing loss admitted to Çukurova University Hospital Otolaryngology Department and a private office between September 2013 and January 2017 were included in this study. The local ethics committee of the Çukurova University School of Medicine approved the study. Informed consent was obtained from all patients. All patients underwent a comprehensive otoneurologic examination, and audiological and audiovestibular tests. Magnetic resonance imaging was also obtained to rule out other identifiable causes for their complaints. Otomicroscopy and tympanometry were observed as normal in all subjects. Patients with known MD were not included in the study. All patients had sensorineural hearing loss; the average loss was 35 dB (25–70 dB). The hearing loss was bilateral in two cases. Two groups with 30 patients were formed totaling 60 patients. The first group of patients was asked to send their eye-videos recorded during the attack; and the second group of patients, the control group, was followed through conventional methods. All video recordings were assessed by the same experienced otolaryngologist according to 1-horizontal, vertical, or rotatory and 2-beating left or right. Patients in the video group were also asked to determine the direction of their vertigo during the Meniere’s attacks. If the spinning feeling of vertigo changes direction, they were asked to record again.
Twenty-six patients in the first group were able to send satisfactory eye movement videos; four patients were excluded due to repeated recording problems. Twenty-seven patients in the second group could be followed with conventional methods; three patients were lost to follow-up. There were 26 patients (14 males and 12 females) in the video group with a mean age of 50.2 (between 35 and 75) years. The control group consisted of 27 patients (14 males and 13 females) with a mean age of 48.8 (between 32 and 78) years. The data of both groups are listed in Tables 1 and 2.
Table 1.
Age, sex, number of attacks till diagnosis (attacks needed)/(total number of videos), days needed for diagnosis (time), the direction of nystagmuses (direction), and the diagnosis of the patients in the video group are listed
| Patient | Age | Sex | Attacks needed | Time | Direction | Diagnosis |
|---|---|---|---|---|---|---|
| 1 | 64 | Male | 2/(30) | 12 | Both | Meniere |
| 2 | 33 | Male | 2/(3) | 45 | Both | Meniere |
| 3 | 35 | Female | -/(2) | - | - | Migraine |
| 4 | 46 | Female | 2 | 50 | Contralateral | Meniere |
| 5 | 52 | Male | 2 | 38 | Ipsilateral | Meniere |
| 6 | 64 | Male | 2 | 61 | Contralateral | Meniere |
| 7 | 50 | Female | -/(3) | - | - | Anxiety |
| 8 | 62 | Male | 2 | 31 | Contralateral | Meniere |
| 9 | 41 | Female | -/(4) | - | - | Migraine |
| 10 | 39 | Female | 2 | 28 | Ipsilateral | Meniere |
| 11 | 75 | Female | 2 | 54 | Contralateral | Meniere |
| 12 | 42 | Male | 2 | 27 | Ipsilateral | Meniere |
| 13 | 51 | Female | 2 | 29 | Contralateral | Meniere |
| 14 | 61 | Male | -/(5) | - | - | Anxiety |
| 15 | 70 | Male | 2 | 32 | Contralateral | Meniere |
| 16 | 52 | Female | 2 | 50 | Ipsilateral | Meniere |
| 17 | 56 | Male | 2 | 11 | Contralateral | Meniere |
| 18 | 40 | Female | 2 | 82 | Both | Meniere |
| 19 | 39 | Male | 2 | 30 | Contralateral | Meniere |
| 20 | 34 | Male | 2 | 45 | Contralateral | Meniere |
| 21 | 61 | Male | 2 | 26 | Ipsilateral | Meniere |
| 22 | 57 | Female | 2 | 33 | Contralateral | Meniere |
| 23 | 42 | Female | -/(3) | - | - | Migraine |
| 24 | 39 | Female | 2 | 57 | Contralateral | Meniere |
| 25 | 41 | Male | 2 | 30 | Contralateral | Meniere |
| 26 | 60 | Male | 2 | 71 | Contralateral | Meniere |
Table 2.
Age, sex, number of attacks till diagnosis (attacks needed), days needed for diagnosis (time), and the diagnosis of the patients in the video group are listed
| Patient | Age | Sex | Attacks needed | Time | Diagnosis |
|---|---|---|---|---|---|
| 1 | 54 | Male | 6 | 64 | Meniere |
| 2 | 44 | Male | 4 | 103 | Meniere |
| 3 | 39 | Female | 6 | 100 | Meniere |
| 4 | 54 | Female | 5 | 125 | Meniere |
| 5 | 41 | Male | 5 | 118 | Meniere |
| 6 | 78 | Male | 3 | 130 | Meniere |
| 7 | 51 | Female | - | - | Migraine |
| 8 | 42 | Male | 4 | 99 | Meniere |
| 9 | 49 | Female | 5 | 111 | Meniere |
| 10 | 55 | Female | 3 | 86 | Meniere |
| 11 | 64 | Female | 2 | 78 | Meniere |
| 12 | 70 | Male | - | - | Migraine |
| 13 | 42 | Female | 5 | 120 | Meniere |
| 14 | 59 | Male | 4 | 111 | Meniere |
| 15 | 61 | Male | 4 | 110 | Meniere |
| 16 | 48 | Female | 2 | 90 | Meniere |
| 17 | 40 | Male | 5 | 139 | Meniere |
| 18 | 40 | Female | 5 | 89 | Meniere |
| 19 | 46 | Male | 2 | 92 | Meniere |
| 20 | 32 | Male | 4 | 78 | Meniere |
| 21 | 55 | Male | 5 | 85 | Meniere |
| 22 | 67 | Female | 2 | 92 | Meniere |
| 23 | 44 | Female | 4 | 80 | Meniere |
| 24 | 38 | Female | 5 | 100 | Meniere |
| 25 | 43 | Male | 6 | 87 | Meniere |
| 26 | 51 | Male | 6 | 160 | Meniere |
| 27 | 67 | Female | 5 | 99 | Meniere |
In the video group, patients or their relatives were asked to record their eye movements during the attacks while looking midline, 30 degrees right, and 30 degrees left. The recording duration was 20 s per each position totaling 60 s. Total number of attacks needed to diagnose definite MD was evaluated in both groups. Because the frequency of attacks in MD can vary widely, the number of attacks the patient had until the diagnosis was accepted to be the criterion while comparing both groups. This was based on the assumption that a time unit such as days would be misleading in the comparison of the time needed for diagnosis between the two groups because of the unpredictable time course of the disease. The diagnosis can be said to be faster in patients who could be diagnosed with fewer attacks. The time required to diagnose the patient was also noted.
This study aimed to prove that patients followed with video records could be diagnosed in a shorter period when compared to the control group. The number of vertigo attacks till the diagnosis of MD was compared in two groups.
Statistical Analysis
All analyses were performed using the The Statistical Package for the Social Sciences (SPSS) Version 20.0 (IBM Corp.; Armonk, NY, USA) statistical software package. Categorical variables were expressed as numbers and percentages, whereas continuous variables were summarized as mean and standard deviation. Chi-square test was used to compare categorical variables between the groups. For comparison of age and the duration needed for the diagnosis between two groups, the Student’s t-test was used. The statistical level of significance for all tests was considered to be 0.05.
RESULTS
In total, 88 satisfactory video recordings during Meniere’s attack were evaluated from 26 cases in the video group. Horizontal nystagmus was observed in 71 videos (21 cases), and no nystagmus was present in 17 videos (five cases). There was no obvious rotatory or vertical nystagmus seen in the recordings though two or three downbeating vertical nystagmus were seen while the patients were looking absentminded and unfocused. The directions of nystagmus were toward the healthy ear (contralateral) in 13 cases, toward the diseased ear (ipsilateral) in five cases, and to both sides (changed direction) in three cases. If more than one video were sent in the same attack, they were considered as one recording. The 17 videos without nystagmus belonged to five cases who were requested to send more recordings due to absence of nystagmus. After consultation with neurology and psychiatry departments, two of the cases were diagnosed with anxiety and the other three were diagnosed with migraine. Of the 26 cases with video recordings, 21 had a diagnosis of MD within two episodes (mean 40.1 days) (Table 3). Apart from the 17 videos of five cases, one patient sent 3 videos and one patient sent 30 videos (due to direction change in vertigo feeling) in the video group. The nystagmus report of these two patients is listed below to help discussion on pathophysiology of MD.
Table 3.
The gender, age, number of attacks, and time needed to diagnose in both groups
| Video group (n=21) | Conventionale group (n=25) | p | |
|---|---|---|---|
| Gender Female/Male | 8/13 | 12/13 | 0.500 |
| Age-Mean±SD | 51.3±12.3 | 50.1±11.1 | 0.737 |
| Number of attack needed | |||
| 2 | 21 (100%) | 4 (16%) | <0.001 |
| 3 | 0 (0%) | 2 (8%) | |
| 4 | 0 (0%) | 6 (24%) | |
| 5 | 0 (0%) | 9 (36%) | |
| 6 | 0 (0%) | 4 (16%) | |
| Time needed for the diagnosis (day) –mean±SD | 40.1±18.1 | 101.8±21.6 | <0.001 |
(Only patients with a diagnosis of Meniere’s disease in both groups.)
Patient 1: A 64-year-old male patient with right-sided hearing loss had 30 attacks in five months. All his nystagmus were toward right at the first month, ninth attack was very hard, and the tenth attack had a nystagmus toward left. Following 20 days, he had nine attacks all with nystagmus beating left. He had side reversal at the 20th and 21st attacks. At the 20th attack, the nystagmus was toward left at beginning and changed direction toward right at the end, he confirmed that the side reversal of his turning sensation happened for the first time during the same attack. The nystagmus was again toward left at the beginning and reversed to right at the 21st attack. The beating side of nystagmus in the following attacks were as follows; 22nd and 23rd to left, 24th to 26th to right, and 27th to 30th to left. No direction change was observed during these last ten attacks.
Patient 2: A 33-year-old male with left-sided hearing loss sent three videos. He had two attacks in two months with nystagmus beating to right the third attack occurred one month later with nystagmus beating right for four hours and changed direction to left and lasted for 45 min, following this nystagmus the patient declared he felt very well.
In 27 cases without video recordings, a mean of 4.04 vertigo attacks (2–9 episodes) were needed to diagnose the patient according to patients’ descriptions, histories, or otoneurological examination performed at various centers at the time of seizure (Table 3). The average duration of diagnosis in this patient group was 101.8 days. All the patients except two (diagnosed with migraine) in this group were diagnosed to have MD though 21 of 26 cases were diagnosed with MD in the video group. This change may be due to over diagnose in the control group (Table 4).
Table 4.
The gender, age, number of attacks, and time needed to diagnose in both groups
| Video group (n=21) | Conventionale group (n=25) | p | |
|---|---|---|---|
| Gender Female/Male | 12/14 | 13/14 | 0.999 |
| Age-Mean±SD | 50.2±11.9 | 50.9±11.4 | 0.838 |
| Diagnosis – n (%) | |||
| Meniere | 21 (80.8%) | 25 (92.6%) | 0.192 |
| Anxiety | 2 (7.7%) | 0 (0%) | |
| Migraine | 3 (11.5%) | 2 (7.4%) | |
| Diagnosis – n (%) | |||
| Meniere | 21 (80.8%) | 25 (92.6%) | 0.250 |
| Not Meniere | 5 (19.2%) | 2 (7.4%) | |
As a result, the video group could be diagnosed in a shorter period compared to the control group concerning the number of attacks. The diagnosis was made within two attacks with a mean of 40.1 days in the video group and within four (mean 4.28) attacks with a mean of 101.8 days in the control group (p<0.001) (Table 3).
DISCUSSION
The diagnosis of MD is dependent on hearing loss, details of the vertigo attack, and excluding other pathologies [8]. Though many tests can be used as an adjunct to diagnose patients with MD, examining the patient during the attack is more valuable [9–16]. However, it is difficult to see the patient during vertigo attack. On the other hand, accepting the patient’s subjective vertigo complaints may mislead the physician and may prolong the diagnosis process. The most important examination during a vertigo attack is nystagmus, which can be recorded and shared by mobile phone cameras. Apart from helping diagnosis, camera recordings may provide very important information concerning the nature of the disease.
Our study has shown clearly that diagnosis of patients with objective documentation of vertigo during the attack with a camera is more accurate and faster than the diagnosis based on subjective patient discourse. As far as we know, this is the first study demonstrating the utility of cell phone camera to make a diagnosis of MD.
Another result of this study is that the diagnosis of recurrent vertigo with hearing loss is not always, but most likely is MD. Of 27 patients in the control group, 25 were diagnosed with MD, while of 26 cases in the video group, only 21 were diagnosed with MD. This finding may be due to the over diagnosis of patients whose vertigo complaints were thought to be due to hydrops as the patient had hearing loss. This finding supports our suggestion of recording the eye movements during the attack and uncertainty of vertigo history of the patient.
Nystagmus data of two cases are presented in the results as an adjunct to propose that these video recordings can be useful while discussing the pathophysiology of MD. These results are preliminary to support the idea that cell phone cameras can help to understand the nature of the disease.
Three periods of Meniere’s attack have been shown: an initial short “irritative” phase beating toward the diseased ear, then toward the healthy ear “paralytic” phase, and at the end “recovery” phase beating toward the diseased side again. This classic triad was not seen among our cases. We did not notice any irritative nystagmus, may it be due to the fact that this period is very short or patients aren’t able to feel it. We have noticed direction-changing nystagmus during three attacks; these nystagmus are compatible with the names paralytic and recovery in our cases because all nystagmus were toward the healthy ear at the beginning (paralytic) and changed to the diseased ear at the end (recovery). Also, patients declared that the recovery period was shorter, and they felt much better after this direction changing when compared to unidirectional attacks.
Nystagmus in attacks without direction change were sometimes toward the healthy ear (paralytic) or toward the diseased ear (irritative or recovery). We think it is irrational to dub these attacks as irritative, paralytic, or recovery. During irritative and recovery nystagmus, ipsilateral vestibular nuclei are predominant; and during paralytic nystagmus, contralateral vestibular nuclei are predominant. Defining nystagmus in MD using the names ipsilateral (toward the diseased ear) or contralateral (toward the healthy ear) seems more logical.
To classify a nystagmus as paralytic, one needs to show a VOR reduction during the attack. The reduction of the VOR during Meniere’s attack has been shown during the paralytic phase of nystagmus in a study [17], and this is a strong evidence for the paralytic phase of this disease. Though there was also an irritative phase lasting for 5 min in this case, no VOR recordings were made during this period. In the recovery period direction of the nystagmus changed toward the diseased ear for the last 30 min, while VOR values normalized. If a paralytic nystagmus changes direction at the end of the attack, this nystagmus can be named recovery. Our patients’ statements about feeling unusually well after the end of this reversal also support the entity of recovery nystagmus. As said before, we don’t think that all nystagmus seen in these videos can be strictly classified into irritative, paralytic, and recovery. Because some of the nystagmus were beating toward the diseased ear for the whole attack and such a long irritative nystagmus is not frequent. We can only say that this is an ipsilateral nystagmus. In the two cases that were presented in our study, all nystagmus that changed direction began toward the healthy ear and then changed to beat toward the diseased side; such attacks can be said to be paralytic and recovery. Otherwise, if any unidirectional nystagmus persists for the entire Meniere’s attack without changing direction, it can be classified as ipsilateral or contralateral.
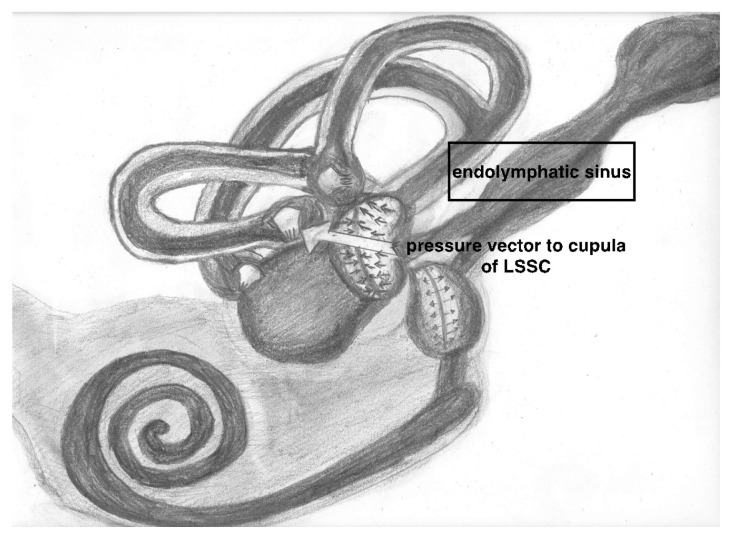
We did not observe any irritative nystagmus in our cases, as in some other studies [18]. We can speculate that there is no irritative nystagmus during Meniere’s attack by using the mechanical theory, which was an explanation for hearing loss in endolymphatic hydrops [19]. According to this theory, the nystagmus begins toward the healthy ear (no irritative nystagmus) when endolymphatic hydrops reaches the cupula of the lateral semicircular canal from the utriculus side, it will displace the cupula away from the utriculus toward the canal side, this situation will be detected like an ampullafugal (utriculofugal) flow which will lead to a nystagmus toward the healthy ear and will be named contralateral (paralytic) nystagmus. The cupula of the ampulla of lateral semicircular canal is perpendicular to the vectorial force of pressure coming from endolymphatic sinus during Meniere’s attack as seen in Figure 1 (arrow). This force may deviate the cupula away from the utriculus and lead to a contralateral horizontal nystagmus. This vectorial position of cupula of lateral canal ampulla seen in Figure 1 (contrary to superior and posterior canal) may explain the horizontal nystagmus seen in our cases.
Figure 1.
The cupula of the ampulla of lateral semicircular canal is perpendicular to the vectorial force of pressure coming from endolymphatic sinus during Meniere’s attack as seen in Figure 1 (arrow). This force may deviate the cupula away from the utriculus and lead to a contralateral horizontal nystagmus. This vectorial position of cupula of lateral canal ampulla seen in Figure 1 (contrary to superior and posterior canal) may explain the horizontal nystagmus seen in our cases.
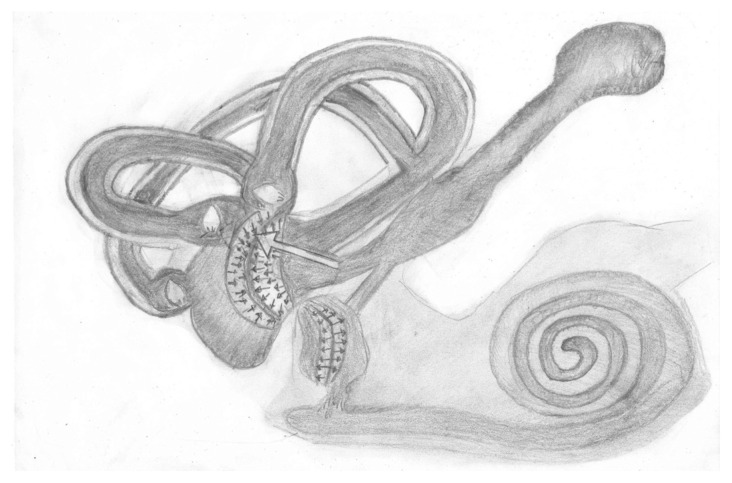
McClure et al. [18] showed that nystagmus was toward the healthy ear (contralateral: paralytic) in most of the attacks and reversed in some. This seems logical as the hydrops is mostly expected to come from utricular side, which is more related with endolymphatic duct system. Our findings support this thesis; the directions of nystagmus in our series were toward the healthy ear (contralateral) in 13 cases, and toward the diseased ear (ipsilateral) in five cases. The hydrops coming from endolymphatic duct will lead to bulging of utricular side of cupula and will deviate the cupula toward the canalicular side. If the attack is long enough, later the displacement of utriculus will finish and the adaptation of central nervous system that tries to equalize the input from both labyrinths to depress the crisis will lead to a recovery nystagmus beating to the diseased side because of the centrally accumulated reducing effect on the input coming from the healthy side. The statements of our two patients support this recovery thesis; they say that reverse of nystagmus happened after heavy attacks and lasted for about 20 min and they felt well after this reversal. This mechanical explanation is in accordance with our cases and with the cadaveric study by Morito and Paparella [20]. The unidirectional attacks of our cases with ipsilateral nystagmus (toward the diseased ear) need more attention; a VOR testing during this crisis is expected to show that the diseased labyrinth is dominant during this attack. Funabiki et al. [21] support our idea. They showed that VOR gain toward the affected side was higher than that toward the intact side during ipsilateral nystagmus in patients with MD. It is not possible to explain this ipsilateral nystagmus with a pressure force coming from the canal side toward the ampulla. We can speculate that these attacks may be due to paradoxical central inhibition of the healthy side (as in recovery nystagmus) or due to pressure change coming from endolymphatic sinus and affecting the kinocillia of utriculus (Figure 2). The hair cells on opposite sides of the striola in utriculus have opposing morphological polarizations. Accordingly, the pressure coming acutely from endolymphatic system may affect the hair cells in utriculus and lead to horizontal nystagmus with a direction decided by the involved utricular hair cells and can have a direction to both sides. Manzari et al. [22] proposed that unilateral utricular dysfunction may cause horizontal spontaneous nystagmus.
Figure 2.
The hair cells on opposite sides of the striola in utriculus have opposing morphological polarizations. Accordingly, the pressure coming acutely from endolymphatic system may affect the hair cells in utriculus and lead to horizontal nystagmus with a direction decided by the involved utricular hair cells and can have a direction to both sides.
Some reports support the mechanical theory for vertigo attacks in Meniere. Brown et al. [23] investigated the changes in guinea pig perilymphatic space and reported that membrane rupture theory cannot explain the nystagmus direction as they never witnessed irritative nystagmus at the beginning of the attacks. They also argue that mechanical theory for MD seems more logical. Bill Gibson suggested a similar hypothetical mechanism for vertigo in MD: The endolymph flows into the utricle, stretches the cristae of the semicircular canals, which causes the vertigo [24].
Another fact to consider about this video study is that patients can define the direction of the vertigo. Otolaryngologists should ask for the side of the vertigo to the patients. If answered, this information is a very valuable adjunct for both diagnosis and pathophysiology of the disease.
CONCLUSION
This study shows that cell phone camera recordings are very helpful to diagnose MD. These recordings can also be used to investigate the nature of the disease. One important issue is that our cases are patients with early MD, and the discussion of physiopathology is restricted with early cases; MD physiopathology may change in patients with a long-term disease. This is a preliminary study to support the use of cell phone cameras in MD, and the pathophysiologic ideas will be supported in our prospective studies.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from Çukurova University Faculty of Medicine Local Ethics Committee (Approval No: 73/8/2018).
Informed Consent: Written informed consent was obtained from the patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – M.K.; Design – M.K.; Supervision – M.K., M.D; Resource – M.K., M.D.; Materials – M.K., M.D.; Data Collection and/or Processing – M.K.; Analysis and/or Interpretation – M.K., M.D.; Literature Search – M.K., M.D.; Writing – M.K., M.D.; Critical Reviews – M.K., M.D.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Claes GM, De Valck CF, Van de Heyning P, Wuyts FL. The Ménière’s Disease Index: an objective correlate of Ménière’s disease, based on audiometric and electrocochleographic data. Otol Neurotol. 2011;32:887–92. doi: 10.1097/MAO.0b013e318219ff9a. [DOI] [PubMed] [Google Scholar]
- 2.Kıroğlu O, Sürmelioğlu Ö, Kıroğlu M. Effects of Selective Seratonine Re-Uptake Inhibitors on Meniere’s Disease. J Int Adv Otol. 2017;13:276–8. doi: 10.5152/iao.2017.3042. [DOI] [PubMed] [Google Scholar]
- 3.Kıroğlu MM, Dağkıran M, Özdemir S, Sürmelioğlu Ö, Tarkan Ö. The Effects of Betahistine and Dimenhydrinate on Caloric Test Parameters; Slow-Phase Velocity of Nystagmus. J Int Adv Otol. 2014;10:68–71. doi: 10.5152/iao.2014.015. [DOI] [Google Scholar]
- 4.Committee on Hearing and Equilibrium guidelines for the diagnosis and evaluation of therapy in Meniére’s disease. American Academy of Otolaryngology-Head and Neck Foundation, Inc. Otolaryngol Head Neck Surg. 1995;113:181–5. doi: 10.1016/S0194-5998(95)70102-8. [DOI] [PubMed] [Google Scholar]
- 5.Lopez-Escamez JA, Carey J, Chung WH, Goebel JA, Magnusson M, Mandalà M, et al. Diagnostic criteria for Menie’re’s disease. J Vestib Res. 2015;25:1–7. doi: 10.3233/VES-150549. [DOI] [PubMed] [Google Scholar]
- 6.Goebel JA. 2015 Equilibrium Committee Amendment to the 1995 AAOHNS Guidelines for the Definition of Meniere’s Disease. Otolaryngology-Head and Neck Surgery. 2016;154:403–4. doi: 10.1177/0194599816628524. [DOI] [PubMed] [Google Scholar]
- 7.Monsell EM. New and revised reporting guidelines from the Committee on Hearing and Equilibrium. American Academy of Otolaryngology-Head and Neck Surgery Foundation, Inc. Otolaryngol Head Neck Surg. 1995;113:176–8. doi: 10.1016/S0194-5998(95)70100-1. [DOI] [PubMed] [Google Scholar]
- 8.Nevoux J, Franco-Vidal V, Bouccara D, Parietti-Winkler C, Uziel A, Chays A, et al. Diagnostic and therapeutic strategy in Menière’s disease. Guidelines of the French Otorhinolaryngology-Head and Neck Surgery Society (SFORL) Eur Ann Otorhinolaryngol Head Neck Dis. 2017;134:441–4. doi: 10.1016/j.anorl.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 9.De Waele C, Huy PT, Diard JP, Freyss G, Vidal PP. Saccular dysfunction in Meniere’s disease. Am J Otol. 1999;20:223–32. [PubMed] [Google Scholar]
- 10.Winters SM, Campschroer T, Grolman W, Klis SF. Ocular vestibular evoked myogenic potentials in response to air-conducted sound in Meniere’s disease. Otol Neurotol. 2011;32:1273–80. doi: 10.1097/MAO.0b013e31822e5ac9. [DOI] [PubMed] [Google Scholar]
- 11.Murofushi T, Nakahara H, Yoshimura E, Tsuda Y. Association of airconducted sound oVEMP findings with cVEMP and caloric test findings in patients with unilateral peripheral vestibular disorders. Acta Otolaryngol. 2011;131:945–50. doi: 10.3109/00016489.2011.580003. [DOI] [PubMed] [Google Scholar]
- 12.Skalabrin TA, Mangham CA. Analysis of the glycerin test for Meniere’s disease. Otolaryngol Head Neck Surg. 1987;96:282–8. doi: 10.1177/019459988709600310. [DOI] [PubMed] [Google Scholar]
- 13.Yacovino DA, Hain TC, Musazzi M. Fluctuating Vestibulo-Ocular Reflex in Meniere’s Disease. Otol Neurotol. 2017;38:244–7. doi: 10.1097/MAO.0000000000001298. [DOI] [PubMed] [Google Scholar]
- 14.Manzari L, Burgess AM, MacDougall HG, Bradshaw AP, Curthoys IS. Rapid fluctuations in dynamic semicircular canal function in early Meniere’s disease. Eur Arch Otorhinolaryngol. 2011;268:637–9. doi: 10.1007/s00405-010-1442-5. [DOI] [PubMed] [Google Scholar]
- 15.Wu Z, Zhang S, Zhou N, Yi F, Chen A, Xie S, et al. Significance of some otologic function tests in diagnosis of Meniere’s disease. Lin Chuang Er Bi Yan Hou Ke Za Zhi. 2006;20:433–5. [PubMed] [Google Scholar]
- 16.Moon IJ, Park GY, Choi J, Cho YS, Hong SH, Chung WH. Predictive value of electrocochleography for determining hearing outcomes in Meniere’s disease. Otol Neurotol. 2012;33:204–10. doi: 10.1097/MAO.0b013e318241b88c. [DOI] [PubMed] [Google Scholar]
- 17.Yacovino DDA, Finlay JB. Intra-Attack Vestibuloocular Reflex Changes in Ménière’s. Case Rep Otolaryngol. 2016;2016:2427983. doi: 10.1155/2016/2427983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McClure JA, Copp JC, Lycett P. Recovery nystagmus in ménière’s disease. The Laryngoscope. 1981;91:1727–37. doi: 10.1288/00005537-198110000-00019. [DOI] [PubMed] [Google Scholar]
- 19.Tonndorf J. Endolymphatic hydrops: mechanical causes of hearing loss. Arch Otorhinolaryngol. 1976;212:293. doi: 10.1007/BF00453677. [DOI] [PubMed] [Google Scholar]
- 20.Morita N, Paparella MM. Ménière’s disease. Otol Neurotol. 2008;29:879–80. doi: 10.1097/MAO.0b013e31817d81b0. [DOI] [PubMed] [Google Scholar]
- 21.Funabiki K, Naito Y, Honjo I. Vestibulo-ocular reflex in patients with Meniere’s disease between attacks. Acta Otolaryngol. 1999;119:886–91. doi: 10.1080/00016489950180225. [DOI] [PubMed] [Google Scholar]
- 22.Manzari L, Burgess AM, Curthoys IS. Does unilateral utricular dysfunction cause horizontal spontaneous nystagmus? Eur Arch Otorhinolaryngol. 2012;269:2441–5. doi: 10.1007/s00405-012-2127-z. [DOI] [PubMed] [Google Scholar]
- 23.Brown DH, McClure JA, Downar-Zapolski Z. The membrane rupture theory of Menière’s disease--is it valid? Laryngoscope. 1988;98:599–601. doi: 10.1288/00005537-198806000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Gibson WP. Hypothetical Mechanism for Vertigo in Meniere’s Disease. Otolaryngol Clin North Am. 2010;43:1019–27. doi: 10.1016/j.otc.2010.05.013. [DOI] [PubMed] [Google Scholar]