Abstract
OBJECTIVES
To assess the incidence and onset of cochlear obliteration after translabyrinthine and retrosigmoid vestibular schwannoma surgery.
MATERIALS and METHODS
We retrospectively identified a consecutive series of eighty ears in eighty vestibular schwannoma patients who were treated via a translabyrinthine or retrosigmoid approach by a single neuro-otological surgical team in a tertiary referral center from May 2011 to January 2018. Postoperative, high-resolution T2-weighted turbo spin echo three-dimensional magnetic resonance (MR) images of the posterior fossa were evaluated at the level of the membranous labyrinth and internal auditory canal. Perilymphatic patency of the vestibule, basal, and apical cochlear turns were scored and classified as patent, hypointense, partially obliterated, or completely obliterated.
RESULTS
Twenty-five vestibular schwannomas were treated with surgery via a translabyrinthine approach, and fifty-five were treated using a retrosigmoid approach; of these, 8% and 65%, respectively, showed no signs of perilymphatic alterations in the basal or apical turns, while 84% and 20%, respectively, showed partial or complete obliteration in the basal or apical turns with a mean postoperative interval of 127 and 140 days, respectively. All the patients who underwent multiple MR scans and had a completely patent perilymphatic system on the first postoperative scan remained patent during subsequent scans; 16% of the patients showed worsened perilymphatic appearance. The onset of cochlear obliteration occurred within 2–7 months in most translabyrinthine patients.
CONCLUSION
These findings may support the need for simultaneous cochlear electrode or dummy implantation in translabyrinthine surgery. Second-stage implantation could be feasible in cases where a retrosigmoid approach is used; however, the implantation should be considered within the initial months to avoid cochlear obliteration. Findings on the first postoperative MR could indicate the need for intensified MR follow-up and may even predict the occurrence of cochlear obliteration.
Keywords: Cochlear obliteration, cochlear patency, retrosigmoid, translabyrinthine, vestibular schwannoma
INTRODUCTION
The presence of a vestibular schwannoma (VS) as well as its surgical treatment, via either a translabyrinthine or a retrosigmoid approach, may lead to micro-histological cochlear alterations, retro-cochlear or cochlear sensorineural hearing loss, and cochlear ossification. Retro-cochlear type of sensorineural hearing loss is most likely to be caused by cochlear nerve compression and atrophy; Cochlear type of sensorineural hearing loss could be caused by compromised cochlear microcirculation and cochlear hypoxia or by ototoxic signaling proteins that are released by the schwannoma itself [1–5]. Surgically induced hemorrhage could in turn lead to hyalinization and atrophy of the stria vascularis, causing cochlear fibrosis and ossification [6,7]. All these histological alterations may lead to secondary spiral ganglion cell and cochlear nerve degeneration. However, a large population of spiral ganglion cells may survive many after years of surgery. Thus, cochlear implantation may be successful, given that the cochlear nerve is spared [8–13]. Ossification can compromise future cochlear electrode implantation; therefore, cochlear implantation is ideally considered before any ossification is initiated. It can be argued that VS patients with expected postoperative deafness and chances of postoperative cochlear obliteration should be offered simultaneous cochlear electrode or placeholder implantation [14, 15]. This may especially be indicated in patients with contralateral deafness or bilateral VS. It may even be indicated in patients with potential unilateral postoperative deafness because cochlear implantation can also improve sound localization and speech perception in these cases [16–18]. In this study, we decided to assess the incidence and onset of cochlear obliteration after translabyrinthine and retrosigmoid VS surgery. The main objective was to determine if and when there is a potential need for simultaneous or second-stage placeholder or electrode implantation.
MATERIALS AND METHODS
Inclusion and Exclusion Criteria
A retrospective cohort study was performed in our tertiary referral center after obtaining approval from the Institutional Review Board. Patients with VS who were treated using a translabyrinthine or retrosigmoid approach were identified retrospectively. The choice of surgical approach was based on the size of the VS, degree of extension within the internal acoustic meatus, and patient-related factors, such as residual preoperative hearing, comorbidity, and age. All patients without adequate postoperative, high-resolution T2 TSE 3D MR imaging of the posterior fossa were excluded. This mainly included recently operated patients or those who underwent different postoperative MR imaging at their referring center. Revision cases, patients with neurofibromatosis, and patients who underwent simultaneous surgery that may have affected the cochlear patency, such as cochlear electrode or placeholder implantation, were also excluded. Finally, 80 ears consecutively operated by a single neuro-otological surgical team from May 2011 to January 2018 were included in this study.
Diagnostic Imaging Protocol
Two observers independently reviewed the pre- and postoperative high-resolution T2-weighted turbo spin echo three-dimensional magnetic resonance (T2 TSE 3D MR) images of the posterior fossa. Images were acquired from a 3-Tesla magnetic resonance scanner (MAGNETOM Skyra-Fit; Siemens, Erlangen, Germany) using a 32-channel array head coil. Images were obtained in the axial plane at the level of the membranous labyrinth and internal auditory canal. These images allow for a detailed visualization of the membranous labyrinth, with high sensitivity, specificity, and predictive value in the assessment of cochlear patency, mainly in early types of obliteration [19,20]. Perilymphatic patency was evaluated in the vestibule, basal turn, and apical turns. Perilymphatic patency in these subunits was classified as either patent, decreased radiodensity, partially obliterated or completely obliterated. A subunit was considered patent if it showed hyperintense radiodensity comparable to that of the unaffected contralateral side, and if a perilymphatic subunit showed decreased hyperintensity, it was described as such (Figure 1). Perilymphatic filling defects or zones with an absence of hyperintense radiodensity in the perilymphatic subunit were considered as being partially obliterated (Figure 2). The subunit was considered to be completely obliterated in the total absence of any hyperintensity (Figure 3).
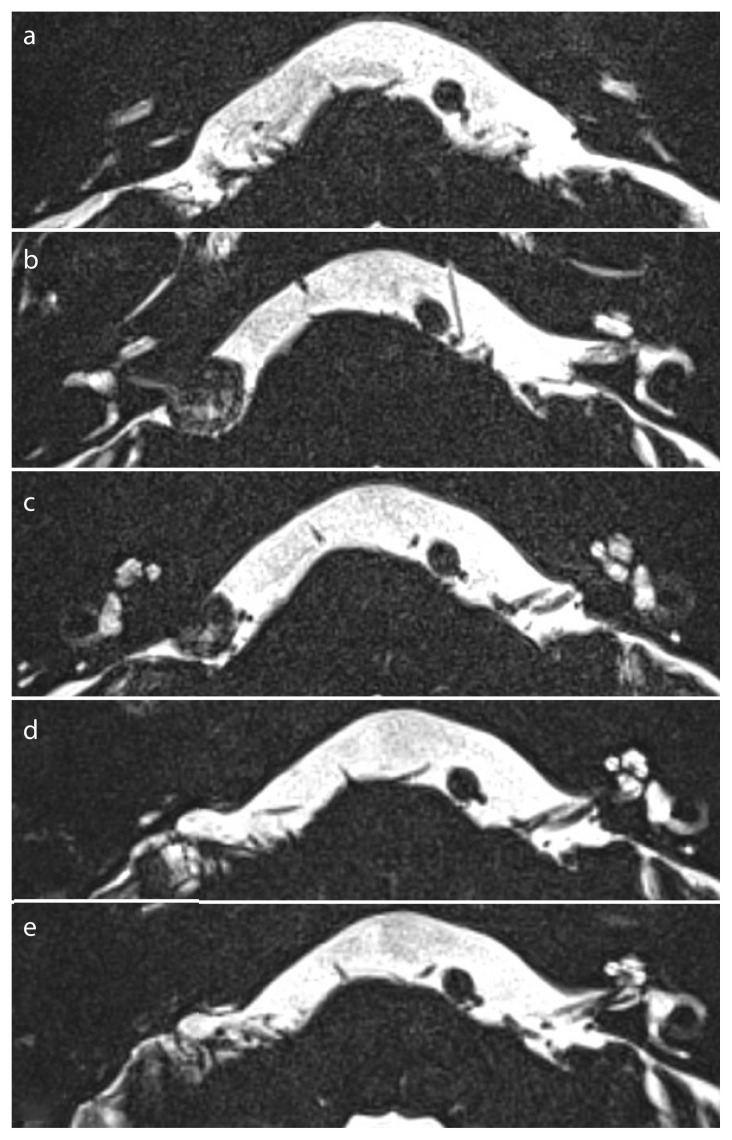
Figure 1. a–f.
Imaging before and after surgery via a translabyrinthine approach for a left-sided VS. a) Preoperative patency in the basal turn; b, c) Preoperative patency in the apical turns; d) Postoperative partial obliteration in the basal turn; e, f ) Postoperative decreased radiodensity in the apical turns.
Figure 2. a–e.
Imaging before and after surgery via a translabyrinthine approach for a right-sided VS. a) Preoperative patency in the basal turn; b, c) Preoperative patency in the apical turns; d) Postoperative partial obliteration in the basal turn; e) Postoperative complete obliteration in the apical turns.
Figure 3. a–d.
Imaging after surgery via a retrosigmoid approach for a left-sided VS. a) Preoperative patency of the basal turn; b, c) Preoperative patency of the apical turns; d) postoperative complete obliteration.
Statistical Analysis
All the data were entered in a Microsoft Excel spreadsheet, and statistical analyses were conducted using the Statistical Packages for the Social Sciences (SPSS) 24.0 (IBM Corp.; Armonk, NY, USA). Interobserver agreement was assessed using kappa analysis for categorical data. Sample t-test and two-tailed Fisher’s exact test were used for the statistical analyses. Categorical data are presented as frequencies and percentages. p<0.05 was considered to indicate a statistically significant difference.
RESULTS
Eighty ears in eighty VS patients undergoing surgery via a translabyrinthine or retrosigmoid approach were included in this study, and eleven VS patients undergoing surgery via similar approaches were excluded. Total 48 (60%) subjects were women, and 32 (40%) were men. The percentage of those operated on the left side (56%; N=45) was slightly lower than that of those operated on the right side (44%; N=35). Twenty-five VS patients (31%) were treated through a translabyrinthine approach and fifty-five (69%) through a retrosigmoid approach. The mean time until the first postoperative MR follow-up was 150 days (SD 134), and the mean time until the last MR follow-up was 824 days (SD 705). Total 72% (N=57) of the patients underwent their first postoperative scan within 4 months, and 71% (N=56) had already undergone two or more postoperative scans at the time of data collection. Most patients (75%) with the first postoperative MRI within 4 months had already undergone a second or third postoperative MR scan at the time of data collection.
After comparison of all the observations (patent, hypointense, partial obliteration, or total obliteration) of both observers in subsequent scans, the inter observer agreement of independent observations showed a measure of agreement with a kappa of 0.927 (p=0.000). When the difference in the observations were discussed case-bycase, the observers agreed on a classification for all the cases, and scoring was adjusted accordingly for further analyses. The findings were as follows: 100% (N=29) of the patients with multiple postoperative scans and a completely patent perilymphatic system on the first postoperative scan remained patent during the subsequent scans. The mean duration till the first postoperative MRI in these cases was 158 days (SD 138). Further, 16% (N=9) of the patients with multiple postoperative T2 TSE 3D MR scans showed worsened perilymphatic appearance at the subsequent scans (e.g., hypo-intensity advancing toward partial obliteration or partial obliteration advancing toward complete obliteration). We also observed that 6.6% (N=2) of the ears showing patent cochlear turns with hypo-intensity or partial obliteration in the vestibule showed obliteration of the cochlear turns at a later stage. Two of six ears (33%) with multiple postoperative scans and hypo-intensity anywhere in the perilymph without signs of obliteration on the first postoperative scan progressed to partial or total obliteration in the cochlear turns. In both these cases, a retrosigmoid approach was used. Further, these cases had different duration till the first postoperative scan (81 vs. 489 days) and a very different timespan until the obliteration first occurred on the scan (501 vs. 1896 days).
Total 8% (N=2) of those in the translabyrinthine approach group did not show any obliteration or decreased radiodensity in the cochlear turns at the most recent postoperative MR scan. In contrast, in the retrosigmoid approach group, 65% (N=36) did not show any obliteration or decreased radiodensity in the cochlear turns at the most recent MR scan. Further, 84% (N=21) of those in the translabyrinthine approach group showed partial or complete obliteration of the basal or apical turns with a mean duration of 127 days (SD 78.4) until the first documented signs of perilymphatic obliteration. Only 20% (N=11) of the retrosigmoid approach group showed partial or complete obliteration in the basal or apical turns, with a mean duration of 410 days (SD 536) until the first documented signs of perilymphatic obliteration (Table 1).
Table 1.
Findings on the most recent postoperative T2 TSE 3D MR, translabyrinthine versus retrosigmoid approach
| Translabyrinthine (N=25) | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Basal or apical turns | Basal turn | Apical turn | ||||
|
|
|
|
||||
| Open | Partial/Total obliteration | Hypointense radiodensity | Partial/Total obliteration | Hypointense radiodensity | Partial/Total obliteration | Hypointense radiodensity |
| 2 (8%) | 21 (84%) | 7 (28%) | 20 (80%) | 3 (12%) | 16 (64%) | 5 (20%) |
|
| ||||||
| Retrosigmoïd (N=55) | ||||||
|
| ||||||
| Basal or apical turns | Basal turn | Apical turn | ||||
|
|
|
|
||||
| Open | Partial/Total obliteration | Hypointense radiodensity | Partial/Total obliteration | Hypointense radiodensity | Partial/Total obliteration | Hypointense radiodensity |
|
| ||||||
| 36 (65%) | 11 (20%) | 14 (25%) | 9 (16%) | 8 (15%) | 7 (13%) | 10 (18%) |
DISCUSSION
Complete loss of cochlear function can occur after VS surgery with a translabyrinthine, retrosigmoid, or middle fossa approach [21–23]. Simultaneous cochlear implantation has increasingly been performed in surgeries performed via a translabyrinthine approach during the previous two decades owing to the high chance of hearing loss and risk of cochlear obliteration [14, 15, 17, 24]. Simultaneous cochlear electrode implantation might be indicated in surgeries performed via any approach that includes a high risk of profound postoperative sensorineural hearing loss and cochlear obliteration. Based on our data and previous findings, the onset of cochlear obliteration could occur until several years after VS surgery [25, 26]; however, signs of perilymphatic alterations are commonly observed during the early stages; absence of these signs appears to predict a long-term patent cochlea. In our series, all patients (N=32) with eventual perilymphatic obliteration in the basal or apical turns showed partial perilymphatic obliteration or decreased perilymphatic radiodensity at their first postoperative scan. Alternatively, all patients with a completely patent perilymphatic system at the first postoperative scan remained patent during the subsequent scans (N=29). The onset of obliteration of the membranous labyrinth causes potential loss of a chance for cochlear implantation. Hearing revalidation after VS surgery with cochlear implantation is possible, and simultaneous cochlear electrode or placeholder implantation seems to be indicated in certain cases. To benefit postoperative sound localization and speech perception it might even be indicated in cases with expected postoperative unilateral deafness [16–18]. The preservation of the hearing ability after translabyrinthine VS surgery has occasionally been reported [27–29]; however, this is very unlikely due to damage to the membranous labyrinth that usually leads to loss of cochlear function, even if the vascular supply and the cochlear nerve are preserved. According to our data, a large number of ears for which a translabyrinthine approach has been used will not only loose the hearing ability, but also show an onset of cochlear obliteration within 2–7 months. This might hinder cochlear implantation, and if implantation in an obliterated cochlea is achieved, the hearing outcome might not be as good as expected [30]. Hence, simultaneous cochlear electrode implantation might be chosen during surgery via a translabyrinthine approach. When financial issues or the need for MR imaging follow-up (e.g., after partial tumor removal) do not allow direct cochlear electrode implantation, a placeholder could be used to prevent obliteration and facilitate future electrode implantation. The disadvantages of using a placeholder could be that it would not stimulate the spiral ganglion cells and fail to prevent diminishment of the spiral ganglion cell population. After all, the number of functional ganglion cells at the time of implantation might be an important factor influencing the functional outcome [31–34]. After performing surgery via a retrosigmoid approach, second-stage implantation might be indicated immediately on observation of postoperative deafness. This should then be considered within the initial postoperative months to prevent cochlear obliteration; however, it is difficult to determine a recommended time interval for implantation based on our data. We found that obliteration is unlikely once a completely patent perilymphatic system is observed on the first postoperative MR scan.
CONCLUSION
Partial loss of signal or obliteration in the vestibule and cochlear perilymph occurred in the majority (84%) of surgeries performed using a translabyrinthine approach. However, this effect was observed in 1/5th of the surgeries performed using a retrosigmoid approach. Considering the expected postoperative sensorineural hearing loss, these findings support the need for simultaneous cochlear implantation in VS surgeries using a translabyrinthine approach. Implantation could be performed with direct cochlear implant electrode insertion or with the use of a placeholder. Second-stage cochlear implantation might be considered in surgeries performed via a retrosigmoid approach, resulting in documented profound postoperative sensorineural hearing loss; especially if a functioning cochlear nerve is observed on perioperative intra-cochlear or intracranial nerve examination. Findings on the first postoperative MR could show the need for intensified MR follow-up and may even predict the occurrence of cochlear obliteration. If cochlear implantation is considered, it should be done within the initial postoperative months to prevent cumbersome insertion and suboptimal functional outcome due to cochlear obliteration.
Footnotes
Ethics Committee Approval: Ethics committee approval was received from the Medical Ethics Committee of GZA Hospitals (5-2-2019).
Informed Consent: Informed consent is not necessary due to the retrospective nature of this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - H.F.v.W., E.L.; Design - H.F.v.W.; Supervision - T.S., T.V.H.; Materials - H.F.v.W.; Data Collection and/or Processing - H.F.v.W., E.L.; Analysis and/or Interpretation - H.F.v.W., T.S.; Literature Search - H.F. van Waegeningh; Writing - H.F.v.W.; Critical Reviews - T.S., E.L., T.V.H.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Eckermeier L, Pirsig W, Mueller D. Histopathology of 30 non-operated acoustic schwannomas. Arch Otorhinolaryngol. 1979;222:1–9. doi: 10.1007/BF00456332. [DOI] [PubMed] [Google Scholar]
- 2.Johnsson LG, Hawkins JE, Jr, Rouse RC. Sensorineural and vascular changes in an ear with acoustic neurinoma. Am J Otolaryngol. 1984;5:49–59. doi: 10.1016/S0196-0709(84)80020-4. [DOI] [PubMed] [Google Scholar]
- 3.Bozorg Grayeli A, Refass A, Smail M, Elgarem H, Kalamarides M, Bouccara D, et al. Diagnostic value of auditory brainstem responses in cerebellopontine angle tumours. Acta Otolaryngol. 2008;128:1096–100. doi: 10.1080/00016480701881803. [DOI] [PubMed] [Google Scholar]
- 4.Roosli C, Linthicum FH, Jr, Cureoglu S, Merchant SN. Dysfunction of the cochlea contributing to hearing loss in acoustic neuromas: an underappreciated entity. Otol Neurotol. 2012;33:473–80. doi: 10.1097/MAO.0b013e318248ee02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dilwali s, Landegger LD, Soares VYR, Deschler DG, Stankovic KM. Secreted Factors from Human Vestibular Schwannomas Can Cause Cochlear Damage. Sci Rep. 2015;5:18599. doi: 10.1038/srep18599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olivetto E, Simoni E, Guaran V, Astolfi L, Martini A. Hearing loss and ischemic injury: Development of animal models to assess vascular and oxidative effects. Hear Res. 2015;327:58–68. doi: 10.1016/j.heares.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Belal A. Pathology of vascular sensorineural hearing impairment. Laryngoscope. 1980;90:1831–9. doi: 10.1288/00005537-198011000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Belal A. Is Cochlear Implantation Possible after Acoustic Tumor Removal? Otol Neurotol. 2001;22:497–500. doi: 10.1097/00129492-200107000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Chen DA, Linthicum FH, Rizer FM. Cochlea histopathology in the labyrinthectomized ear: implications for cochlea implantation. Laryngoscope. 1988;98:1170–2. doi: 10.1288/00005537-198811000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Shin YJ, Fraysse B, Sterkers O, Bouccara D, Rey A, Lazorthes Y. Hearing restoration in posterior fossa tumors. Am J Otol. 1998;19:649–53. [PubMed] [Google Scholar]
- 11.Hoffman RA, Kohan D, Cohen NL. Cochlear implants in the management of bilateral acoustic neuromas. Am J Otol. 1992;13:525–8. [PubMed] [Google Scholar]
- 12.Hulka GF, Bernard EJ, Pillsburg HC. Cochlear implantation in a patient after removal of an acoustic neuroma. Arch Otolaryngo Head Neck Surg. 1995;121:465–8. doi: 10.1001/archotol.1995.01890040083014. [DOI] [PubMed] [Google Scholar]
- 13.Fayad JN, Linthicum FH., Jr Multichannel cochlear implants: relation of histopathology to performance. Laryngoscope. 2006;116:1310–20. doi: 10.1097/01.mlg.0000227176.09500.28. [DOI] [PubMed] [Google Scholar]
- 14.Arístegui M, Denia A. Simultaneous cochlear implantation and translabyrinthine removal of vestibular schwannoma in an only hearing ear: report of two cases (neurofibromatosis type 2 and unilateral vestibular schwannoma) Otol Neurotol. 2005;26:205–10. doi: 10.1097/00129492-200503000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Ahsan S, Telischi F, Hodges A, Balkany T. Cochlear implantation concurrent with translabyrinthine acoustic neuroma resection. Laryngoscope. 2003;113:472–4. doi: 10.1097/00005537-200303000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Buss E, Dillon MT, Rooth MA, King ER, Deres EJ, Buchman CA, et al. Effects of Cochlear Implantation on Binaural Hearing in Adults with Unilateral Hearing Loss. Trends Hear. 2018;22:1–15. doi: 10.1177/2331216518771173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanna M, Medina MD, Macak A, Rossi G, Sozzi V, Prasad SC. Vestibular schwannoma resection with ipsilateral simultaneous cochlear implantation in patients with normal contralateral hearing. Audiol Neurootol. 2016;21:286–95. doi: 10.1159/000448583. [DOI] [PubMed] [Google Scholar]
- 18.Hassepass F, Arndt S, Aschendorff A, Laszig R, Wesarg T. Cochlear implantation for hearing rehabilitation in single-sided deafness after translabyrinthine vestibular schwannoma surgery. Eur Arch Otorhinolaryngol. 2016;273:2373–83. doi: 10.1007/s00405-015-3801-8. [DOI] [PubMed] [Google Scholar]
- 19.Trimble K, Blaser S, James AL, Papsin BC. Computed tomography and/ or magnetic resonance imaging before pediatric cochlear implantation? Developing an investigative strategy. Otol Neurotol. 2007;28:317–24. doi: 10.1097/01.mao.0000253285.40995.91. [DOI] [PubMed] [Google Scholar]
- 20.Isaacson B, Booth T, Kutz JW, Jr, Lee KH, Roland PS. Labyrinthitis ossificans: how accurate is MRI in predicting cochlear obstruction? Otolaryngol Head Neck Surg. 2009;140:692–6. doi: 10.1016/j.otohns.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 21.Ginzkey C, Scheich M, Harnisch W, Bonn V, Ehrmann-Müller D, Shehata-Dieler W, et al. Eur Arch Otorhinolaryngol. 2013;270:1209–16. doi: 10.1007/s00405-012-2074-8. [DOI] [PubMed] [Google Scholar]
- 22.Colletti V, Fiorino F. Middle fossa versus retrosigmoid-transmeatal approach in vestibular schwannoma surgery: a prospective study. Otol Neurotol. 2003;24:927–34. doi: 10.1097/00129492-200311000-00018. [DOI] [PubMed] [Google Scholar]
- 23.Meyer TA, Canty PA, Wilkinson EP, Hansen MR, Rubinstein JT, Gantz BJ. Small acoustic neuromas: surgical outcomes versus observation or radiation. Otol Neurotol. 2006;27:380–92. doi: 10.1097/00129492-200604000-00015. [DOI] [PubMed] [Google Scholar]
- 24.Zanetti D, Campovecchi CB, Pasini S, Nassif N. Simultaneous translabyrinthine removal of acoustic neuroma and cochlea implantation. Auris Nasus Larynx. 2008;35:562–9. doi: 10.1016/j.anl.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 25.Beutner C, Mathys C, Turowski B, Schipper J, Klenzner T. Cochlear obliteration after translabyrinthine vestibular schwannoma surgery. Eur Arch Otorhinolaryngol. 2015;272:829–33. doi: 10.1007/s00405-013-2877-2. [DOI] [PubMed] [Google Scholar]
- 26.Delgado-Vargas B, Medina M, Polo R, Lloris A, Vaca M, Pérez C, et al. Cochlear obliteration following a translabyrinthine approach and its implications in cochlear implantation. Acta Otorhinolaryngol Ital. 2018;38:56–60. doi: 10.14639/0392-100X-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tringali S, Ferber-Viart C, Gallégo S, Dubreuil C. Hearing preservation after translabyrinthine approach performed to remove a large vestibular schwannoma. Eur Arch Otorhinolaryngol. 2009;266:147–50. doi: 10.1007/s00405-008-0634-8. [DOI] [PubMed] [Google Scholar]
- 28.Smith PG, Bigelow DC, Kletzker GR, Leonetti JP, Pugh BK, Mishler ET. Hearing preservation following a transtemporal resection of an acoustic schwannoma: a case report. Am J Otol. 1993;14:434–6. doi: 10.1097/00129492-199309000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Springborg LK, Springborg JB, Thomsen J. Hearing preservation after classical translabyrinthine removal of a vestibular schwannoma: case report and literature review. J Laryngol Otol. 2007;121:76–9. doi: 10.1017/S0022215106003598. [DOI] [PubMed] [Google Scholar]
- 30.Wang L, Zhang D. Surgical methods and postoperative results of cochlear implantation in 79 cases of ossified cochlea. Acta Otolaryngol. 2014;134:1219–24. doi: 10.3109/00016489.2014.947656. [DOI] [PubMed] [Google Scholar]
- 31.Fayad J, Linthicum FH, Jr, Otto SR, Galey FR, House WF. Cochlear implants: histopathologic findings related to performance in 16 human temporal bones. Ann Otol Rhinol Laryngol. 1991;100:807–11. doi: 10.1177/000348949110001004. [DOI] [PubMed] [Google Scholar]
- 32.Xu J, Shepherd RK, Millard RE, Clark GM. Chronic electrical stimulation of the auditory nerve at high stimulus rates: a physiological and histopathological study. Hear Res. 1997;105:1–29. doi: 10.1016/S0378-5955(96)00193-1. [DOI] [PubMed] [Google Scholar]
- 33.El-Kashlan HK, Ashbaugh C, Zwolan T, Telian SA. Cochlear implantation in prelingually deaf children with ossified cochleae. Otol Neurotol. 2003;24:596–600. doi: 10.1097/00129492-200307000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Durisin M, Arnoldner C, Stöver T, Lenarz T, Lesinski-Schiedat A. Audiological performance in cochlear implanted patients deafened by meningitis depending on duration of deafness. Eur Arch Otorhinolaryngol. 2008;265:381–8. doi: 10.1007/s00405-008-0584-1. [DOI] [PubMed] [Google Scholar]