Abstract
OBJECTIVES
Effects of decreasing auditory activity on speech discrimination ability are not fully understood. To investigate influence of decrease in auditory activity due to conductive and sensorineural components of hearing loss (HL) on speech discrimination ability.
MATERIALS and METHODS
We retrospectively reviewed medical records of patients with suspected HL at Kitasato University Hospital in 2017 and 2018. Patients were divided according to pure-tone audiometry findings: no HL (N-HL), conductive HL (C-HL), sensorineural HL (S-HL), and mixed HL (M-HL) groups.
RESULTS
In total, 149 patients (224 ears) were eligible. The maximum speech discrimination score (SDSmax) for all ears significantly negatively correlated with age (r = −0.29, p<0.0001) and bone conduction (BC) threshold (r = −0.55, p<0.0001). For patients aged <50 years in N-HL and C-HL groups, SDSmax was nearly 100%, with no significant difference. SDSmax was significantly lower for older patients (≥50 years) in the M-HL group than in the S-HL group, even though there were no significant differences in age and BC thresholds between groups.
CONCLUSION
Decrease of auditory activity due to the conductive component of M-HL may worsen speech discrimination ability. Early treatment of M-HL would be desirable for the preservation of auditory function.
Keywords: Conductive component, mixed hearing loss, elderly, maximum speech discrimination score
INTRODUCTION
Aging is one of the factors that significantly influences the development of sensorineural hearing loss (HL) (S-HL). As the world’s population continues to live longer, preventing S-HL while maintaining and restoring hearing is expected to become increasingly critical to people’s good health and well-being. Along these lines, there is increasing evidence to support a link between healthy hearing and healthy aging [1–4]. In fact, many older adults experience difficulty understanding speech, especially in demanding listening conditions, although they can hear speech sounds. There are large individual differences in speech discrimination ability among older adults. Speech discrimination abilities have long been considered affected by peripheral, central auditory, and cognitive systems [5, 6], and the maximum speech discrimination score (SDSmax) derived from speech audiometry findings decreases with the progression of age-related S-HL due to age-related neuronal degeneration [7]. In a clinical setting, speech discrimination tests are indispensable for the evaluation of the type of HL and the regions of functional decline in auditory pathways. Moreover, speech audiometry can help validate the impact of intervention with hearing aids and facilitate the audiological rehabilitation of elderly adults. However, the underlying mechanisms, characteristics, and clinical implications of speech understanding are still not fully understood.
Of note, while the majority of elderly patients may experience age-related S-HL, a subset of elderly individuals might exhibit mixed HL (M-HL), which is caused by a combination of conductive damage to the outer or middle ear and sensorineural damage to the inner ear or central auditory system [8]. Sensorineural damage often leads to difficulties in understanding the speech of other individuals, even if the volume level of the speech is adequately high. Conversely, conductive damage allows the affected individual to understand the speech of other individuals if the volume is adequately high and the background noise is kept at a minimum. However, the relationship between M-HL and speech discrimination ability has received relatively limited attention because of the complexity of M-HL and age-related hearing impairment. Moreover, it is not clear whether the decrease in auditory activity due to the conductive component of M-HL affects the speech discrimination ability.
Accordingly, the aim of the present study was to investigate the influence of decreased auditory activity due to the conductive component of M-HL on the speech discrimination ability and to compare the SDSmax in this context with that of patients showing S-HL with a similar age and bone conduction (BC) threshold. Furthermore, to confirm whether the conductive component of M-HL can cause peripheral damage to the auditory system, we conducted a short-increment sensitivity index (SISI) test to assess the presence of a cochlear lesion.
MATERIALS AND METHODS
Ethical Considerations
The study protocol (B19–141) was approved by the institutional review board at Kitasato University Hospital. The requirement for individual informed consent was formally waived by the institutional review board at Kitasato University Hospital.
Patients
We retrospectively reviewed the medical records of patients with suspected HL who underwent pure-tone audiometry, speech audiometry, and SISI testing at Kitasato University Hospital in 2017 and 2018. Patients with fluctuating or progressive HL, those aged younger than 17 years old, those showing obvious neurological impairment, and those with a history of ear surgery were excluded.
Classification of Patients According to Hearing Function
Pure-tone audiometry was performed using a conventional device (AA-78; Rion, Tokyo, Japan) in a soundproof room. First, the hearing thresholds were obtained through air-conduction (AC) and BC at frequencies of 0.5, 1, 2, and 4 Hz for both ears. To prevent a cross-hearing phenomenon from causing an erroneous measurement result, masking noise was used to occupy the ear not under test while evaluating the other one, if necessary. Briefly, to measure the AC threshold, the necessity of masking was analyzed based on a minimum interaural attenuation level of 40 dB for retesting through AC. To measure the BC threshold, a masking process was applied using ABC methods [9].
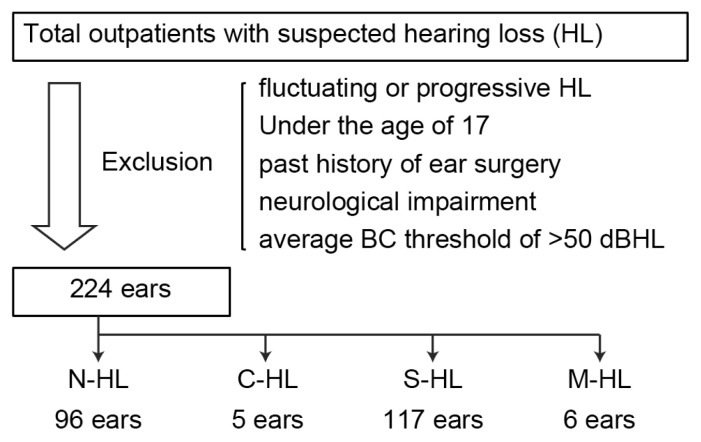
Thresholds were obtained across all frequency octaves from 0.5 to 4 kHz, and the arithmetic average AC and BC thresholds were calculated from the thresholds at 0.5, 1, 2, and 4 kHz. Patients with an average BC threshold of more than 50 dB of HL were excluded, while the remaining patients were divided into the following four groups: the no HL (N-HL) group, having BC threshold of less than 25 dB of HL and air–bone (AB) gap of less than 20 dB of HL; the conductive HL (C-HL) group, having a BC threshold of less than 25 dB of HL and AB gap of 20 dB or greater of HL; the S-HL group, having a BC threshold of 25 dB or greater of HL and AB gap of less than 20 dB of HL; and the M-HL group, having a BC threshold of 25 dB or greater of HL and AB gap of 20 dB or greater of HL. Patient details are presented in Figure 1 and Table 1.
Figure 1.
Selection of the study population.
BC: bone conduction; C-HL: conductive hearing loss; M-HL: mixed hearing loss; N-HL: no hearing loss; S-HL: sensorineural hearing loss.
Table 1.
Clinical characteristics of patients with different types of HL
| N-HL (n=96) | C-HL (n=5) | S-HL (n=117) | M-HL (n=6) | |
|---|---|---|---|---|
| Age (years) (min–max) | 49.7±1.6 (18–83) | 34.2±2.1 (28–41) | 59.2±1.6 (19–83) | 73.8±2.0 (65–79) |
| BC threshold (dB HL) | 13.0±0.7 | 13.5±2.0 | 35.4±0.6 | 40.6±2.3 |
| AC threshold (dB HL) | 17.8±0.9 | 44.8±2.8 | 41.7±0.8 | 65.8±4.8 |
| AB gap (dB HL) | 4.8±0.6 | 31.2±0.6 | 6.3±0.6 | 25.2±3.5 |
| SDSmax (%) | 96.7±0.4 | 97.0±1.2 | 85.9±1.3 | 70.8±7.1 |
AB: air–bone; AC: air-conduction; BC: bone conduction; C-HL: conductive hearing loss; M-HL: mixed hearing loss; N-HL: no hearing loss; SDSmax: maximum speech discrimination score; S-HL: sensorineural hearing loss.
Speech Audiometry
Speech audiometry was performed using the AA-78 audiometer in a soundproof room. For calculating the SDSmax, we used a Japanese monosyllabic word list (67-S) including 20 Japanese monosyllables developed by the Japan Audiological Society [10]. Briefly, the word stimuli were initially presented at a 40 to 50 dB above the average pure-tone threshold. Subsequently, the HL was altered in 10-dB steps until the maximum percentage of correct answers was obtained at a sound level not exceeding 100 dB. The order of test stimuli for each sound level was randomly arranged. The maximum percentage of correct answers for stimuli at a minimum SPL was defined as the SDSmax. Patients attaining the SDSmax at a sound level of 100 dB were excluded because a better SDSmax might be achieved at a sound level of more than 100 dB.
SISI Test
The SISI test was performed to measure the ability of an individual to detect 1 dB increase of intensity modulation in a 20-dB of suprath-reshold tone at frequencies of 1 and 4 kHz. The rate of identification of 20 such increments was calculated. A SISI test score of 70% or higher represents positivity for recruitment, thus indicating the presence of a cochlear lesion.
Statistical Analysis
Study data are presented as means ± standard errors. The correlations of SDSmax with age and BC threshold were calculated using linear regression and Pearson’s correlation. Nonparametric Mann–Whitney U tests were applied to investigate continuous variable prognostic factors. For comparing between more than two groups, a one-way analysis of variance (ANOVA) was used and Tukey’s post-hoc test used to correct for multiple comparisons. The parameters that were statistically significant in the univariate analysis were incorporated in a binary logistic regression analysis for multivariate analysis. Statistical analysis was performed using GraphPad Prism (GraphPad Software Inc., La Jolla, CA, USA) or JMP 14.2 (SAS Institute, Cary, NC, USA). A p less than 0.05 was considered to be statistically significant.
RESULTS
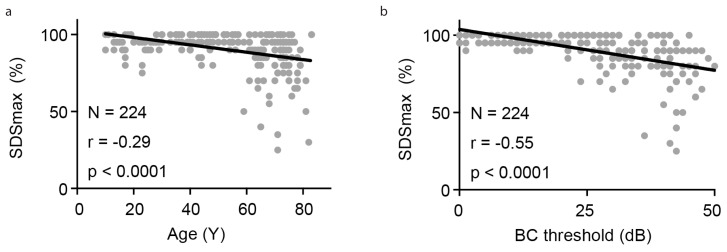
In total, 149 patients and 224 ears were considered eligible for inclusion in this study. The clinical features of the patients in the four groups are presented in Table 1. Of the 224 ears, 96 belonged to the N-HL group, five belonged to the C-HL group, 117 belonged to the S-HL group, and six belonged to the M-HL group. C-HL was caused by cholesteatoma (one ear), ossicular malformations (two ears), or an unknown reason (two ears), while the conductive component of M-HL was caused by cholesteatoma (one ear), chronic otitis media (two ears), otitis media with effusion (one ear), otosclerosis (one ear), or an unknown reason (one ear). Among the four groups, significant differences were observed in terms of average age (one-way ANOVA, F (3,220)=11.32; p<0.0001) (Table 1) and BC thresholds (one-way ANOVA, F (3,220)=213.0; p<0.0001) (Table 1). The average age of patients in the S-HL and M-HL groups was greater than 50 years, and these patients were generally older than those in the N-HL and C-HL groups (N-HL vs. C-HL: p=0.17; N-HL vs. S-HL: p=0.0002; N-HL vs. M-HL: p=0.003; C-HL vs. S-HL: p=0.005; C-HL vs. M-HL: p=0.0005; S-HL vs. M-HL: p=0.15; all determined by Tukey’s post-hoc test). In addition, BC thresholds in the S-HL and M-HL groups were higher than those in the N-HL and C-HL groups due to age-related sensorineural damage (N-HL vs. C-HL: p=0.99; N-HL vs. S-HL: p<0.0001; N-HL vs. M-HL: p<0.0001; C-HL vs. S-HL: p<0.0001; C-HL vs. M-HL: p<0.0001; S-HL vs. M-HL: p=0.25; all determined by Tukey’s post-hoc test). Furthermore, the SDSmax was lower in the S-HL and M-HL groups than in the N-HL and C-HL groups and showed a significant negative correlation with age (r=−0.29; p<0.0001 by Pearson’s correlation) and BC threshold (r=−0.55; p<0.0001 by Pearson’s correlation) (Figure 2). Therefore, for the investigation of the effects of the conductive component of HL on SDSmax, it was necessary to correct for the negative correlation with age and BC threshold prior to pursuing comparisons between the N-HL and C-HL groups or the S-HL and M-HL groups. Thus, each group was further divided into younger (<50 years) and older (≥ 50 years) age groups for correction of the effect of age.
Figure 2. a, b.
SDSmax presented a significant negative correlation with age (a) and BC threshold (b).
BC: bone conduction; SDSmax: maximum speech discrimination score.
First, to investigate the influence of C-HL on speech discrimination ability in younger patients, we compared the SDSmax between the younger patients in the N-HL group and those in the C-HL group (Table 2). These two groups had similar ages (N-HL: 38.6±1.3 years vs. C-HL: 34.2±2.1 years; p=0.16) and BC thresholds (N-HL: 12.1±0.9 vs. C-HL: 13.5±2.0; p=0.41), while the AC threshold and AB gap in the C-HL group were significantly higher than those in the N-HL group (AC: 44.8±2.8 vs. 15.0±1.0 and AB gap: 31.3±2.6 vs. 3.0±0.5; p<0.0001 for both). The SDSmax in both groups was almost 100% (97.7±0.3 vs. 97.0±1.2), with no significant difference noted between the groups (p=0.66), although the sound intensity obtained the SDSmax was significantly higher in the C-HL group than in the N-HL group (78.0±2.0 vs. 49.8±1.2; p<0.0001).
Table 2.
Clinical characteristics of younger patients (<50 years old) with N-HL or C-HL
| N-HL (n=53) | C-HL | (n=5) | p |
|---|---|---|---|
| Age (years) | 38.6±1.3 | 34.2±2.1 | 0.16 |
| BC threshold (dB HL) | 12.1±0.9 | 13.5±2.0 | 0.41 |
| AC threshold (dB HL) | 15.0±1.0 | 44.8±2.8 | <0.0001** |
| AB gap (dB HL) | 3.0±0.5 | 31.3±2.6 | <0.0001** |
| SDSmax (%) | 97.7±0.3 | 97.0±1.2 | 0.66 |
| Sound intensity (dB HL) obtained SDSmax | 49.8±1.2 | 78.0±2.0 | <0.0001** |
Significant difference between groups.
AB: air–bone; AC: air-conduction; BC: bone conduction; C-HL: conductive hearing loss; N-HL: no hearing loss; SDSmax: maximum speech discrimination score; SPL: sound pressure level.
Next, we investigated whether the conductive component of M-HL accelerates the decrease in SDSmax by comparing older patients in the M-HL group with those in the S-HL group (Table 3). Both groups had similar ages (S-HL: 69.2±0.9 vs. M-HL: 73.8±2.0; p=0.11) and BC thresholds (S-HL: 35.8±0.7 vs. M-HL: 40.6±2.3; p=0.14), although the AC threshold and AB gap were significantly higher in the M-HL group than in the S-HL group (AC: 65.8±4.8 vs. 42.2±0.9 and AB gap: 25.2±3.5 vs. 6.4±0.5; p<0.0001). SISI testing revealed positivity for recruitment in more than 80% of patients in both groups, with no significant difference seen between the two groups (S-HL: 84.3% vs. M-HL: 83.3%; p>0.99), supporting that the conductive component of M-HL has no significant impact on the peripheral cochlear region. SDSmax was significantly lower in the M-HL group than in the S-HL group (70.8±7.1 vs. 85.9±1.3; p=0.02), even though age, and BC threshold were similar. The SDSmax related to sound intensity was significantly higher in the M-HL group than in the S-HL group (85.0±2.2 vs. 75.4±1.3; p=0.025). Furthermore, age, BC threshold, and SDSmax were included in the multivariate analysis, which revealed that SDSmax (p=0.03) was significantly lower in the M-HL group than in the S-HL group.
Table 3.
Clinical characteristics of older patients (≥50 years old) with S-HL or M-HL
| S-HL (n=76) | M-HL (n=6) | Univariate | Multivariate | |||
|---|---|---|---|---|---|---|
|
|
|
|||||
| p | p | SE | OR | |||
| Age (years) | 69.2±0.9 | 73.8±2.0 | 0.11 | 0.17 | 0.10 | 0.87 |
|
| ||||||
| BC threshold (dB HL) | 35.8±0.7 | 40.6±2.3 | 0.14 | 0.93 | 0.09 | 0.99 |
|
| ||||||
| AC threshold (dB HL) | 42.2±0.9 | 65.8±4.8 | <0.0001** | |||
|
| ||||||
| AB gap (dB HL) | 6.4±0.5 | 25.2±3.5 | <0.0001** | |||
|
| ||||||
| SISI test (% positive) | 84.3 | 83.3 | >0.99 | |||
|
| ||||||
| SDSmax (%) | 85.9±1.3 | 70.8±7.1 | 0.02* | 0.03* | 0.04 | 1.09 |
|
| ||||||
| Sound intensity (dB HL) obtained SDSmax | 75.4±1.3 | 85.0±2.2 | 0.02* | |||
Significant difference between groups.
AB: air–bone; AC: air-conduction; BC: bone conduction; OR: odds ratio; M-HL: mixed hearing loss; SDSmax: maximum speech discrimination score; SE: standard error; SISI: short-increment sensitivity index; S-HL: sensorineural hearing loss; SPL: sound pressure level.
DISCUSSION
In the present study, we found that the conductive component of M-HL could worsen age-related hearing impairment and speech discrimination ability among older patients.
The conductive component of HL is characterized by the reduced efficiency of sound transmission through the external and middle ear and generally involves a decrease in sound intensity levels or the ability to hear faint sounds. Thus far, the consensus has been that pure C-HL does not affect the SDSmax if the sound intensity of speech is adequately high, whereas S-HL with cochlear lesions results in decreased SDSmax [11, 12]. This is consistent with our results, which showed that the SDSmax among patients with C-HL was similar to that of patients with N-HL, whereas patients with S-HL showed a lower SDSmax relative to that of those with N-HL (Table 1).
The influence of the conductive component of M-HL on SDSmax, however, has received less attention. We found that the conductive component of M-HL resulted in a lower SDSmax than that of S-HL, even though age and BC threshold were comparable between the two groups. Considering that SDSmax reflects both central and peripheral auditory function, the conductive component of M-HL in elderly individuals may not only obstruct sound transmission but also accelerate age-related neuronal damage.
Consistent with our results, previous studies have suggested that chronic C-HL leads to cochlear degeneration and sensorineural damage [13]. The mechanisms underlying cochlear degeneration following C-HL are not well known and further investigation is necessary before the effects of cochlear degeneration can be integrated into the C-HL management protocols in the clinic. In contrast, SISI testing revealed no significant difference of positivity for recruitment, thereby indicating the presence of a cochlear lesion, between the S-HL and M-HL groups. Therefore, this result might indicate that the conductive component of M-HL does not accelerate a cochlear lesion, which is peripheral damage to the auditory system. Other investigators have also argued that long-term C-HL may cause irreversible changes in both the anatomical and functional integrity of central auditory structures such as changes in the relative size of dendrites in the subcortical nuclei or synaptic and spike adaptation disruptions in the auditory cortex [14]. Taken together, decreases in auditory activity due to the conductive component of HL can affect auditory function. Applying fundamental treatment to address the conductive component of M-HL is considered desirable for the prevention of cochlear degeneration and the preservation of auditory function, hearing quality, and speech discrimination ability. Further studies should work to elucidate changes in the auditory pathways following the development of M-HL in elderly individuals.
Treatment strategies and surgical indications for HL are very important in elderly patients. Because presbycusis is an S-HL that cannot be surgically addressed, the most commonly used devices for treating presbycusis are hearing aids and cochlear implants [15]. In contrast, surgical treatment for the conductive component of M-HL may help to reduce the severity of HL, although the underlying sensorineural component will remain. Therefore, treatment for M-HL includes a combination of initial interventions, such as medication or surgery, followed by the placement of hearing aids for correction of the residual HL. Notably, it is often very difficult to make surgical decisions among elderly patients because of a variety of reasons. First, these patients are very likely to have tympanosclerosis and an increased BC threshold probably caused by long-lasting chronic inflammation. As a result, some otolaryngologists may hesitate in recommending elective surgery to this population and generally may select less-aggressive treatments for elderly patients with M-HL than for their younger counterparts. However, most elderly patients with M-HL and positive findings on the SISI test, which indicate the presence of a cochlear lesion, might experience difficulty in being fitted for hearing aids because of the recruitment phenomenon (abnormal loudness growth). Moreover, hearing aids are not necessarily powerful tools for the treatment of M-HL accompanied by a low SDSmax. Therefore, if the patient’s general condition is stable, fundamental surgical treatment for the conductive component of M-HL should be considered first in order to preserve the speech discrimination ability and improve the hearing-related quality of life. This is consistent with previous reports documenting that there is no strong evidence for withholding surgery for chronic otitis media based on the assumption that elderly patients do not show good outcomes or have a high surgical risk [16]. A minimally invasive and safe surgical approach is preferable for elderly patients. Several tools or therapies have recently been introduced for this purpose, including endoscopic ear surgery and vibrant sound bridges.
The findings of this study have clinical implications that are significant in terms of the understanding and management of elderly individuals with M-HL. Although the mechanisms underlying hearing impairment following M-HL are not well known, effects of conductive component mixed with sensorineural component need to be considered in the management of chronic C-HL in the clinic. However, this study has some limitations. First, the disease duration of patients with M-HL is not clear. SDSmax might be progressively decreased as the disease duration of the conductive component of HL increases. Our results showed that the SDSmax of the younger C-HL group was almost 100%, with no significant difference relative to that of the N-HL group, while the SDSmax was significantly lower in the older M-HL group than in the S-HL group, suggesting that the older M-HL group had longer disease duration than the younger C-HL group. Second, in this study, patients obtaining an SDSmax at a sound level of 100 dB, which was maximum output of sound intensity in our clinical setting. were excluded because they might achieve a better SDSmax at a sound level of more than 100 dB. Therefore, further study is necessary to determine the influence of disease duration of M-HL on SDSmax and ascertain the SDSmax of the M-HL group at a sound level higher than 100 dB. Finally, in performing the current research, we conducted a single-hospital, retrospective study, and the sample size of this study was relatively small. Further multicenter studies are necessary involving larger populations.
CONCLUSION
Our findings suggest that a decrease in auditory activity due to the conductive component of M-HL worsens age-related hearing impairment and speech discrimination ability in elderly patients. Therefore, the early comprehensive treatment of the conductive component of M-HL is desirable for the preservation of speech discrimination ability and auditory function.
MAIN POINTS.
Decrease of auditory activity due to the conductive component of mixed hearing loss may worsen speech discrimination ability.
Conductive component of mixed hearing loss in elderly individuals may not only obstruct sound transmission but also accelerate age-related neuronal impairment.
Early treatment of mixed hearing loss would be desirable for the preservation of auditory function.
Footnotes
This study was presented at the 29th Annual Meeting of the Japan Otological Society, October 11, 2019, Yamagata City, Japan.
Ethics Committee Approval: The ethics committee approval for this study was received from the institutional review board at Kitasato University Hospital (B19-141).
Informed Consent: The requirement for individual informed consent was formally waived by the institutional review board at Kitasato University Hospital.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – T.K.; Design – T.K.; Supervision – H.S., T.Y.; Resource – T.K., H.S., S.F..; Materials – T.K., H.S., S.F.; Data Collection and/or Processing – T.K., H.S., S.F.; Analysis and/or Interpretation – T.K., H.S., S.F.; Literature Search – T.K., H.S.; Writing – T.K., H.S.; Critical Reviews – H.S. S.F., T.Y.
Conflict of Interest: The authors declare that they have no conflict of interest.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Fitzpatrick EM, Leblanc S. Exploring the factors influencing discontinued hearing aid use in patients with unilateral cochlear implants. Trends Amplif. 2010;14:199–210. doi: 10.1177/1084713810396511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crowson MG, Semenov YR, Tucci DL, Niparko JK. Quality of Life and Cost-Effectiveness of Cochlear Implants: A Narrative Review. Audiol Neurootol. 2017;22:236–58. doi: 10.1159/000481767. [DOI] [PubMed] [Google Scholar]
- 3.Volter C, Gotze L, Dazert S, Falkenstein M, Thomas JP. Can cochlear implantation improve neurocognition in the aging population? Clin Interv Aging. 2018;13:701–12. doi: 10.2147/CIA.S160517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mosnier I, Vanier A, Bonnard D, Lina-Granade G, Truy E, Bordure P, et al. Long-Term Cognitive Prognosis of Profoundly Deaf Older Adults After Hearing Rehabilitation Using Cochlear Implants. J Am Geriatr Soc. 2018;66:1553–61. doi: 10.1111/jgs.15445. [DOI] [PubMed] [Google Scholar]
- 5.Gordon-Salant S, Fitzgibbons PJ, Yeni-Komshian GH. Auditory Temporal Processing and Aging: Implications for Speech Understanding of Older People. Audiol Res. 2011;1:e4. doi: 10.4081/audiores.2011.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee JY. Aging and Speech Understanding. J Audiol Otol. 2015;19:7–13. doi: 10.7874/jao.2015.19.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maeda Y, Takao S, Sugaya A, Kataoka Y, Kariya S, Tanaka S, et al. Relationship between pure-tone audiogram findings and speech perception among older Japanese persons. Acta Otolaryngol. 2018;138:140–4. doi: 10.1080/00016489.2017.1378435. [DOI] [PubMed] [Google Scholar]
- 8.Acar B, Yurekli MF, Babademez MA, Karabulut H, Karasen RM. Effects of hearing aids on cognitive functions and depressive signs in elderly people. Arch Gerontol Geriatr. 2011;52:250–2. doi: 10.1016/j.archger.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Takeuchi Y. [Masking in bone-conduction testing--proposal of ABC method] Nihon Jibiinkoka Gakkai Kaiho. 1992;95:1744–58. doi: 10.3950/jibiinkoka.95.1744. [DOI] [PubMed] [Google Scholar]
- 10.Noguchi Y, Takahashi M, Ito T, Fujikawa T, Kawashima Y, Kitamura K. Delayed restoration of maximum speech discrimination scores in patients with idiopathic sudden sensorineural hearing loss. Auris Nasus Larynx. 2016;43:495–500. doi: 10.1016/j.anl.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Korsan-Bengtsen M. Distorted speech audiometry. A methodological and clinical study. Acta Otolaryngol Suppl. 1973;310:1–75. [PubMed] [Google Scholar]
- 12.Ryding M, Konradsson K, White P, Kalm O. Hearing loss after “refractory” secretory otitis media. Acta Otolaryngol. 2005;125:250–5. doi: 10.1080/00016480510003183. [DOI] [PubMed] [Google Scholar]
- 13.Liberman MC, Liberman LD, Maison SF. Chronic Conductive Hearing Loss Leads to Cochlear Degeneration. PLoS One. 2015;10:e0142341. doi: 10.1371/journal.pone.0142341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu H, Kotak VC, Sanes DH. Conductive hearing loss disrupts synaptic and spike adaptation in developing auditory cortex. J Neurosci. 2007;27:9417–26. doi: 10.1523/JNEUROSCI.1992-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sprinzl GM, Riechelmann H. Current trends in treating hearing loss in elderly people: a review of the technology and treatment options - a mini-review. Gerontology. 2010;56:351–8. doi: 10.1159/000275062. [DOI] [PubMed] [Google Scholar]
- 16.Shinnabe A, Hara M, Hasegawa M, Matsuzawa S, Kanazawa H, Yoshida N, et al. Clinical characteristics and surgical benefits and problems of chronic otitis media and middle ear cholesteatoma in elderly patients older than 70 years. Otol Neurotol. 2012;33:1213–7. doi: 10.1097/MAO.0b013e31825f24ba. [DOI] [PubMed] [Google Scholar]