Abstract
Introduction
Deficiencies in innate immune responses may contribute to the increased susceptibility to infection in preterm infants. In vivo cytokine profiles in response to sepsis in very preterm infants are not fully understood.
Aims
To characterise plasma pro- and anti-inflammatory cytokine concentrations and pre-defined ratios in very preterm infants with late-onset sepsis (LOS).
Methods
In this observational study, peripheral blood samples were collected at the time of evaluation for suspected LOS from 31 preterm infants (<30 weeks gestational age). Plasma cytokine concentrations were determined by 12-plex immunoassay.
Results
IL-10, IFN-γ, IL-12p70, IP-10, IL-6 and CCL2 were elevated in the majority infants with LOS (n = 12) compared to those without LOS (n = 19). There was no difference in TNF-α, IL-1β, IL-17AF, IL-8 and IL-15 concentrations between groups. IL-10/TNF-α ratios were increased, while CCL2/IL-10 and IL-12p70/IL-10 ratios were decreased in infants with LOS compared to those without.
Conclusion
Very preterm infants have a marked innate inflammatory response at the time of LOS. The increase in IL-10/TNF-α ratio may indicate early immune hypo-responsiveness. Longitudinal studies with a larger number of participants are required to understand immune responses and clinical outcomes following LOS in preterm infants.
Introduction
Globally, approximately 15 million infants are born preterm (<37 weeks gestational age, GA) each year, of which ~15% are born less than 32 weeks GA. [1] Preterm infants are susceptible to invasive bacterial infections and the risk is associated with lower GA and birthweight. [2] Despite advances in neonatal care, late-onset sepsis (LOS; occurring >72 hours after birth) is a frequent complication in preterm infants, affecting up to 40% of infants born <28 weeks GA. [2] Infection-related inflammation is associated with increased mortality and morbidity, particularly long-term adverse neurodevelopmental outcomes. [3]
The mechanisms underpinning the distinct neonatal immune responses to bacterial infections are incompletely understood. Pro- and anti-inflammatory cytokines are critical key initiators and regulators of inflammation and host response to bacterial infection. [4] In adults, both pro- and anti-inflammatory cytokines are simultaneously produced during the early stages of sepsis and correlate with disease progression and mortality. [5] Elevated ratios of anti- and pro-inflammatory cytokines (e.g. IL-10/TNF-α and IL-6/IL-10) are associated with multiple organ failure and are proposed markers of sepsis-induced immunosuppression in adult patients following sepsis. [6, 7] Recovery from sepsis may depend on the balance of pro- and anti-inflammatory immune responses to achieve homeostasis. [8]
It is unclear whether the immediate response of preterm infants with LOS is hyper- or hypo-inflammatory. [9] Cytokine concentrations at the time of neonatal sepsis are inconsistent, partly as studies often include both LOS and early-onset sepsis (EOS; occurring <72 hours after birth), [10–12] and a wide range of GA, [11, 13–18] making interpretation of cytokine responses challenging. We therefore aimed to characterise key cytokines [5] in plasma at the time of evaluation for suspected LOS in a homogeneous cohort of very preterm infants (born <30 weeks GA) enrolled in an observational cohort study of innate immune ontogeny. We also investigated whether infants with LOS have increased pro- and anti-inflammatory cytokine responses and elevated pre-defined cytokine ratios, as observed in adults, compared to neonates without LOS.
Materials and methods
Study participants
This study was approved by the Women and Newborn Health Services Human Research Ethics Committee, Perth, Australia (1627/EW). Written, informed consent was obtained by the Principal Investigator or delegate from the parent(s)/legal guardian prior to study participation. Infants born less than 30 weeks GA without birth defects or genetic abnormalities who were admitted to the Neonatal Intensive Care Unit (NICU) at King Edward Memorial Hospital, Perth, Australia were eligible to participate in this prospective observational cohort study of innate immune system ontogeny, with recruitment from July 2009 to October 2011. Samples taken at the time of evaluation for suspected LOS were collected opportunistically during normal working hours from enrolled infants. Blood culture was performed on infants if they had clinical signs suggestive of LOS, including increased ventilation or oxygen requirements, lethargy, decreased perfusion, temperature instability, vomiting, feed intolerance, increased apnoea and/or bradycardia. For ethical reasons only one blood sample was collected at the time of sepsis evaluation, when the clinical decision was made to investigate sepsis and commence antibiotic therapy. Infants who had episodes of EOS (n = 2) or necrotising enterocolitis (NEC) prior to the episode of suspected LOS (n = 1) were excluded. Placental histology were analyzed using an adaptation of a widely accepted semi-quantitative scoring system, as previously described. [19] Haematological parameters were recorded close to the time of blood culture sampling (mean ± SD, 124 ± 177 min).
Definition of late-onset sepsis
‘Confirmed LOS’ was retrospectively defined as a positive blood culture with a single organism and a C-reactive protein (CRP) of >15mg/L within 72hrs of blood culture sampling and antibiotic therapy for ≥ 5 days, as per the local NICU guidelines during the study period. Episodes without evidence for LOS, referred to as ‘no LOS’, were defined as a negative blood culture. Four infants were considered to have contaminating blood cultures (based on the presence of Gram-positive commensal bacteria, lack of an inflammatory response with two or more CRP measurements <15mg/L and absence of clinical features of sepsis) were excluded from analysis.
Blood sampling and processing for cytokine analyses
Approximately 0.5ml of peripheral blood was collected by venepuncture into Lithium-heparin tubes (BD Biosciences, North Ryde, Australia) at the time of blood culture (68% of samples) or close to the time of blood culture sampling (mean ± SD, 1.2 ± 2.6 hr). Sample processing and analysis was performed at the Children’s Clinical Research Facility in Perth, Australia. Samples were centrifuged at 6,000 x g for 2 minutes, and plasma extracted and stored at -80°C until cytokine measurement.
Multiplex immunoassay
Plasma cytokine concentrations were quantified using a custom 12-plex magnetic bead-based immunoassay kit (ProcartaPlex Biosystems eBioscience, San Diego, California) following the manufacturer’s instructions. The kit provided a mixture of magnetic beads conjugated with primary antibodies against IL-1β, IL-6, IL-12p70, IL-15, IL-17AF, IL-8, IL-13, IL-10, TNF-α, interferon (IFN)-γ, inducible protein (IP)-10 and chemokine (C-C motif) ligand-2 (CCL2). Fluorescence for each cytokine bead region was acquired electronically in real time on the BioPlex 200 System (Bio-Rad, Gladesville, Australia) and analysed using BioPlex Manager 5.0 Software. Cytokine concentrations, in pg/mL, were generated from a seven-point, five-parameter (four-parameter for IL-13) logistic standard curve. All samples analysed were run as a single sample on one plate. Concentrations above the highest standard were repeated at a dilution that fell within the standard curve. Concentrations below the lowest standard were assigned a value of half the lowest standard for statistical analysis.
Statistical analysis
Continuous data were summarised with median and interquartile ranges (IQR, 25th-75th) and categorical data with frequency distributions. All cytokine data were tested for normality using the Shapiro-Wilk normality test and presented using nonparametric data summaries, medians and IQR (25th-75th percentiles). Comparisons between the ‘confirmed LOS’ and ‘No LOS’ groups were by the Mann-Whitney test for continuous data and Chi-square tests for categorical data. All statistical analysis was performed using Prism 8 software (Graphpad Software, San Diego, California). P values <0.05 were considered significant.
Results
Study subject characteristics
There were no differences in the basic demographic and clinical details between the infants with confirmed LOS (n = 12) and no LOS (n = 19), details are shown in Table 1. One infant from the confirmed LOS group (sampled at 16 days postpartum) died aged 44 days of respiratory failure, and two infants in the no LOS group (both sampled at day 7 postpartum) died aged 19 and 56 days of NEC and respiratory failure, respectively. No other infants in the no LOS group developed infections or NEC prior to discharge from the NICU.
Table 1. Basic demographic and clinical parameters of study cohorta.
| Confirmed LOS (n = 12) | No LOS (n = 19) | P value | |
|---|---|---|---|
| Gestational age (weeks) | 26.7 (24.5–28.4) | 25.6 (24.6–26.6) | 0.383 |
| Birthweight (grams) | 835 (669–1101) | 705 (555–895) | 0.194 |
| Male | 4 (33.3) | 12 (63.2) | 0.149 |
| Caesarean section | 5 (41.7) | 11 (57.9) | 0.473 |
| Membranes rupture >24h before delivery | 4 (33.3) | 9 (47.4) | 0.484 |
| Antenatal steroidsb | 11/12 (91.7) | 18/18 (100) | 0.400 |
| Histological chorioamnionitisb | 5/11 (45.5) | 9/16 (56.3) | 0.704 |
| Mechanical ventilation | 12 (100) | 19 (100) | >0.999 |
| Duration (hours) | 241 (26.3–842) | 394 (115–1091) | 0.417 |
| CPAP | 12 (100) | 18 (94.7) | >0.999 |
| Duration (hours) | 1003 (771–1284) | 1057 (563–1298) | 0.723 |
| IVH (grade III/IV) | 0 (0) | 2 (10.5) | 0.510 |
| ROP (stage III/IV) | 0 (0) | 1 (5.3) | >0.999 |
| Length of NICU stay (days) | 83 (60–109) | 109 (94–149) | 0.150 |
aData are expressed as median (IQR) or n (%), as appropriate.
bFrom available reports. CPAP, continuous positive airway pressure; IVH, intraventricular haemorrhage; ROP, retinopathy of prematurity
The clinical parameters at the time of sepsis evaluation are shown in Table 2. For the 12 episodes of confirmed LOS, coagulase-negative staphylococci (CoNS) were the most common infectious agents isolated (10 cases, 83%), consistent with local epidemiology. [20] All infants recovered from the LOS episode.
Table 2. Clinical parameters at the time of sepsis evaluation a.
| Confirmed LOS (n = 12) | No LOS (n = 19) | P value | |
|---|---|---|---|
| Age (days) | 12 (9–14) | 10 (7–15) | 0.404 |
| Gram-positive organisms | |||
| CoNS | 10 (83.4) | - | - |
| Bacillus sphaericus | 1 (8.3) | - | - |
| Enterococcus faecalis | 1 (8.3) | - | - |
| CRP (mg/L) | |||
| Highest within 72hr blood culture | 59 (26–91) | 17 (5–30) | 0.0008 |
| At blood culture samplingb | 20 (7–36) | 12 (5–24) | 0.106 |
| White blood cell count (x109/L)c | 14.5 (9.4–18.1) | 24.8 (15.0–31.5) | 0.095 |
| Neutrophil count (x109/L)c | 11.1 (5.0–15.4) | 16.7 (9.6–20.0) | 0.244 |
| Platelet count (x109/L)c | 137 (102–219) | 215 (118–332) | 0.253 |
| Mechanical ventilation commenced | 4 (33.3) | 5 (26.3) | 0.704 |
| Inotrope use | 0 (0) | 0 (0) | >0.999 |
aData are expressed as median (IQR) or n (%), as appropriate.
bAt or before (mean ± SD, 2.5 ± 2.5 hr) blood culture sampling.
cData available for n = 11 confirmed LOS and n = 18 no LOS. CoNS, coagulase-negative staphylococci; CRP, C-reactive protein.
Circulating cytokine concentrations at the time of late-onset sepsis evaluation
Overall, infants with confirmed LOS had markedly higher concentrations of IL-10, CCL2, IFN-γ, IL-12p70, IL-13, IL-6 and IP-10 compared to no LOS, whereas concentrations of TNF-α, IL-15, IL-1β, IL-17AF and IL-8 were similar in the two groups (Table 3). For IL-1β, IL-17AF and IL-13, fewer than 25%, 15% and 10%, respectively, of concentrations were above the lowest limit of detection, and results should be interpreted accordingly.
Table 3. Circulating cytokine concentrations at the time of late-onset sepsis evaluationa.
| Multiplex pg/mL range | Confirmed LOS (n = 12 episodes) | No LOS (n = 19 episodes) | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Minimum | Maximum | Median | IQR | Range | Median | IQR | Range | ||
| IL-6 | 9.2 | 37,800 | 231 | 62.8–922 | 4.6–4309 | 4.6 | 4.6–149 | 4.6–2881 | 0.017 |
| IFN-γ | 12.4 | 50,700 | 347 | 65.7–712 | 6.2–1512 | 6.2 | 6.2–6.2 | 6.2–949 | 0.001 |
| IL-12p70 | 6.9 | 28,100 | 32.8 | 3.4–72.7 | 3.4–85.7 | 3.4 | 3.4–24.4 | 3.4–39.3 | 0.049 |
| TNF-α | 7.2 | 29,500 | 9.1 | 6.1–32.2 | 3.6–49 | 6.1 | 3.6–14.0 | 3.6–23.2 | 0.168 |
| IL-15 | 3.1 | 12,500 | 43.8 | 1.5–383 | 1.5–576 | 218 | 1.5–276 | 1.5–670 | 0.676 |
| IL-1βb | 2.0 | 8,250 | 1.0 | 1.0–9.7 | 1.0–164 | 1.0 | 1.0–3.8 | 1.0–33.1 | 0.861 |
| IL-17AFb | 6.1 | 25,000 | 3.1 | 3.1–332 | 3.1–960 | 3.1 | 3.1–3.1 | 3.1–347 | 0.172 |
| IL-8 | 2.2 | 8,900 | 234 | 137–1103 | 51.1–5138 | 174 | 84.2–707 | 33.7–6628 | 0.164 |
| IP-10 | 2.2 | 8,800 | 13079 | 8348–16026 | 2661–18013 | 1066 | 602–1498 | 57.6–16033 | <0.0001 |
| CCL2 | 4.8 | 19,500 | 3407 | 2428–7627 | 1780–15105 | 1350 | 473–2588 | 184–7701 | 0.002 |
| IL-10 | 2.3 | 9,250 | 194 | 46.8–255 | 22.9–487 | 6.5 | 1.1–22.9 | 1.1–529 | <0.0001 |
| IL-13b | 3.3 | 13,400 | 1.6 | 1.6–3.8 | 1.6–16.5 | 1.6 | 1.6–1.6 | 1.6–1.6 | 0.049 |
aData are expressed as median, IQR (25th-75th) and range in pg/mL.
bFewer than 25% of data points in both groups are above the lowest detectable standard. LOS, late-onset sepsis; IL, interleukin; IFN, interferon; IP, inducible protein, CCL2, chemokine (C-C motif) ligand-2; TNF, tumour necrosis factor; ns, not statistically significant. Level of significance measured by Mann-Whitney test.
Analysis of anti- and pro-inflammatory ratios in infants with suspected late-onset sepsis
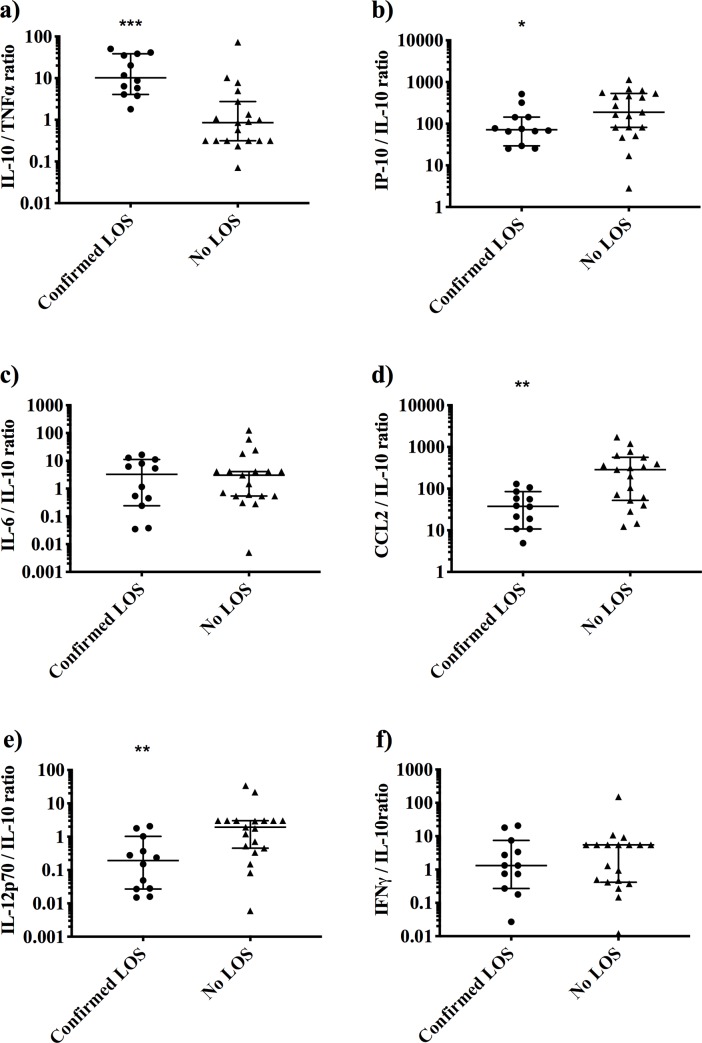
We next evaluated whether the ratios of anti- and pro-inflammatory cytokine concentrations, namely IL-10/TNF-α, IL-6/IL-10 and IP-10/IL-10, differed between groups, as previously reported in adults [6] and infants. [12–14] Infants with confirmed LOS had a markedly elevated IL-10/TNF-α and IP-10/IL-10 ratio compared to infants with no LOS (median (IQR) 10 (5–38) vs 0.9 (0.3–3), and 72 (38–144) vs 188 (82–533), respectively; Fig 1A and 1B), yet IL-6/IL-10 was similar across groups (Fig 1C).
Fig 1. Cytokine ratio at the time of late-onset sepsis evaluation.
Comparison of a) IL-10/TNF-α, b) IP-10/IL-10, c) IL-6/IL-10, d) CCL2/IL-10, e) IL-12p70/IL-10 and f) IFN-γ/IL-10 from infants with confirmed LOS (n = 12 episodes, closed circles) and no LOS (n = 19 episodes, closed triangles). Cytokines were measured by bead-based multiplex assay. All data shown on a log scale with median with 95% confidence intervals. Symbols depict level of significance between confirmed LOS and no LOS groups (*P = 0.048; **P = 0.003; ***P = 0.0002) by Mann-Whitney test. LOS, late-onset sepsis; IL, interleukin; IFN, interferon; IP, inducible protein; CCL2, chemokine (C-C motif) ligand-2.
As concentrations of the pro-inflammatory cytokine IFN-γ, IL-12p70 and chemokine CCL2 were associated with confirmed LOS, we evaluated the ratios of CCL2 to IL-10, IFN-γ to IL-10 and IL-12p70 to IL-10 to determine if an anti- or pro-inflammatory response predominated. A low ratio of CCL2/IL-10 and IL-12p70/IL-10 were strongly associated with confirmed LOS compared to the no LOS group (median (IQR) 38 (13–78) vs 286 (52–563) and 0.2 (0.03–0.9) vs 1.9 (0.5–3.0), respectively. Fig 1D and 1E), but there was no difference in the IFN-γ/IL-10 ratio between the patient groups (Fig 1F).
Discussion
In this study, we report that very preterm infants with microbially confirmed LOS had elevated concentrations of both pro-inflammatory cytokines, IFN-γ, IP-10, IL-12p70, IL-6, CCL2 and the anti-inflammatory cytokine IL-10, compared to those without LOS. TNF-α levels were relatively reduced in infants with LOS. Furthermore, we observed an elevated IL-10/TNF-α ratio and decreased CCL2/IL-10 and IL-12p70 ratios in infants with confirmed LOS, compared to the no LOS group.
Elevated circulating cytokine concentrations are associated with late-onset sepsis
Elevated plasma concentrations of IL-10, IFN-γ, IP-10, IL-12p70, IL-6 and CCL2, were strongly associated with confirmed LOS compared to no LOS; consistent with other preterm infant sepsis studies. [10, 13, 14, 16, 18]. Concentrations of TNF-α, IL-1β, IL-17AF and IL-8 were similar between the two groups. Published concentrations of TNF-α, IL-1β, IL-17AF and IL-8 in infants with sepsis are inconsistent, with some studies reporting results similar to ours, [10–12, 15, 17] and others reporting higher cytokine concentrations in septic infants. [14–17] The reasons underlying these discrepant results are unclear. Several factors may affect cytokine concentrations, including GA, [10] EOS vs LOS (which generally have different aetiologies), [12] sepsis definition (culture proven vs clinical sepsis) [15] and inclusion of Gram-negative, fungal and NEC-related infections (which may elicit different cytokine responses). [13] To our knowledge, this is the first study to measure IL-15 in neonates with and without sepsis. IL-15 has anti-apoptotic properties that may prevent sepsis-induced apoptosis of immune cells in septic patients, [21]. Similar to adult sepsis studies, [22] we did not observe a difference in IL-15 between preterm infants with and without sepsis.
Cytokine ratios as potential indicators of neonatal late-onset sepsis
The elevated ratio of IL-10/TNF-α in infants with confirmed LOS may indicate early immune hypo-responsiveness. Elevated ratio of IL-10/TNF-α has been observed in adult sepsis data and suggested to indicate sepsis-induced immunosuppression, [6]; it may therefore indicate relative immunosuppression in preterm infants with sepsis. The balance between these cytokines may be important in sepsis pathogenesis and susceptibility to infections in preterm infants, as described in other children and adults with sepsis. [6, 23] Other studies report markedly lower levels of LPS-, R848-, and LPS/ATP-induced TNF-α/IL-10 ratios in neonatal cord blood compared to adult peripheral blood, and an increased LPS-stimulated IL-10/TNF-α ratio in cord blood from infants born very preterm compared to moderate and late preterm. [24, 25] Further, peripheral blood from infants with LOS had a markedly higher IL-10/TNF-α ratio following stimulation with live Staphylococcus epidermidis compared to uninfected infants, indicating infected infants have an impaired immune response to bacteria. [26] This suggests that a marked anti-inflammatory response may be characteristic of infants and particularly those who are very preterm. Our results of elevated IL-10/TNF-α and decreased IP-10/IL-10 ratios are similar to other studies looking at septic neonates with disseminated intravascular coagulation (DIC). [13, 14]
We did not observe a difference in the IL-6/IL-10 ratio between groups. Previous studies report an elevated IL-6/IL-10 ratio in septic infants, [12] and specifically in those with disseminated intravascular coagulation, which is often associated with fungal and Gram-negative bacterial infections that are known to elicit different cytokine responses. [13, 14] The infants with LOS in our study had Gram-positive bacterial LOS and did not have DIC, which may explain why an elevated ratio was not observed in our cohort. Further studies with well-defined sepsis definitions are needed to resolve these discrepant findings.
The elevated CCL2 and IL-12p70 observed in LOS infants is consistent with data on neonatal [11, 14, 16] and adult sepsis. [5] To our knowledge, this is the first study to report lower ratios of CCL2/IL-10 and IL-12p70/IL-10 in infants with LOS compared to infants without LOS. The role of CCL2, a member of the chemokine CC subfamily with potent monocyte chemotactic activity, in sepsis is unclear. In a murine model of caecal ligature and puncture-induced infection, higher levels of CCL2 modulates peritoneal bacterial clearance, suggesting CCL2 is important in antimicrobial defence. [27] In contrast, adult studies report that sepsis mortality is associated with increased CCL2, [5] potentially due to chemotaxis of innate leukocytes and marked production of pro-inflammatory cytokines that contribute to organ failure, septic shock and death. [28] Elevated levels of IL-12p70, an IFN-γ inducing pro-inflammatory cytokine, have been reported in other preterm infant sepsis studies. [18] Further studies investigating the role of CCL2 and IL-12p70 in LOS in preterm infants are warranted.
Study strengths and limitations
This is the first study to show a skewed anti-inflammatory response in very preterm infants with only Gram-positive LOS. Our study focused on a well-defined population of very preterm infants with LOS, which may reduce some of the inter-individual variability seen in other neonatal studies. We acknowledge some limitations, including modest sample size, which precluded investigation of rarer Gram-negative and fungal associated LOS and infrequent outcomes, such as septic shock and death. We were unable to collect serial samples over the course of the episode and therefore lack important kinetic data. Lastly, it was not considered ethical to take blood samples from very preterm infants without clinical indication, so the no LOS group had clinical signs that led to investigations for sepsis at the time of sample collection. This group, therefore, may not be representative of responses in otherwise healthy very preterm infants.
Conclusions
Very preterm infants with LOS have elevated concentrations of both pro- and anti-inflammatory cytokines with a prominent shift to an anti-inflammatory immune response. These results suggest that neonatal LOS maybe associated with a hypo-responsiveness, similar to sepsis-induced immunosuppression observed in adults. Consistently reported patterns of cytokines production associated with sepsis may lead new diagnostic methods, prognostic tools and highlight possible therapeutic targets. Future studies should further explore the kinetic profile of circulating cytokines in preterm infants with sepsis by serial sampling over the course of the septic episode and ideally would be powered to evaluate associations between cytokine profile and disease severity and outcomes.
Acknowledgments
Authors would like to acknowledge the assistance of Gail Abernethy, Annie Chang, Chooi Heen Kok and the nursing staff at King Edward Memorial Hospital NICU for recruitment and sample collection. We would like to thank Professor Dorota Doherty and Ms Liz Nathan (both of University of Western Australia) for statistical advice. Lastly, we would like to thank all the study participants and their families.
Data Availability
All relevant data are within the paper and Supporting Information files.
Funding Statement
This study was supported by the National Health and Medical Research Council (NHMRC) of Australia (572548, www.nhmrc.gov.au). TS is supported by a Raine Medical Research Foundation Clinician Research Fellowship (rainefoundation.org.au). DB is supported by NHMRC Research Fellowship (www.nhmrc.gov.au). JH is supported by a University Postgraduate Award (www.education.gov.au). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–72. 10.1016/S0140-6736(12)60820-4 [DOI] [PubMed] [Google Scholar]
- 2.Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110:285–91. 10.1542/peds.110.2.285 [DOI] [PubMed] [Google Scholar]
- 3.Strunk T, Inder T, Wang X, Burgner D, Mallard C, Levy O. Infection-induced inflammation and cerebral injury in preterm infants. Lancet Infect Dis. 2014;14:751–62. 10.1016/S1473-3099(14)70710-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaudhry H, Zhou J, Zhong Y, Ali MM, McGuire F, Nagarkatti PS, et al. Role of cytokines as a double-edged sword in sepsis. In Vivo. 2013;27:669–84. [PMC free article] [PubMed] [Google Scholar]
- 5.Bozza FA, Salluh JI, Japiassu AM, Soares M, Assis EF, Gomes RN, et al. Cytokine profiles as markers of disease severity in sepsis: a multiplex analysis. Crit Care. 2007;11:R49 10.1186/cc5783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gogos CA, Drosou E, Bassaris HP, Skoutelis A. Pro- versus anti-inflammatory cytokine profile in patients with severe sepsis: a marker for prognosis and future therapeutic options. J Infect Dis. 2000;181:176–80. 10.1086/315214 [DOI] [PubMed] [Google Scholar]
- 7.Loisa P, Rinne T, Laine S, Hurme M, Kaukinen S. Anti-inflammatory cytokine response and the development of multiple organ failure in severe sepsis. Acta Anaesthesiol Scand. 2003;47:319–25. 10.1034/j.1399-6576.2003.00004.x [DOI] [PubMed] [Google Scholar]
- 8.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13:862–74. 10.1038/nri3552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hibbert JE, Currie A, Strunk T. Sepsis-Induced Immunosuppression in Neonates. Front Pediatr. 2018;6:357 10.3389/fped.2018.00357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Segura-Cervantes E, Mancilla-Ramirez J, Gonzalez-Canudas J, Alba E, Santillan-Ballesteros R, Morales-Barquet D, et al. Inflammatory Response in Preterm and Very Preterm Newborns with Sepsis. Mediators Inflamm. 2016;2016:6740827 10.1155/2016/6740827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leal YA, Alvarez-Nemegyei J, Lavadores-May AI, Giron-Carrillo JL, Cedillo-Rivera R, Velazquez JR. Cytokine profile as diagnostic and prognostic factor in neonatal sepsis. J Matern Fetal Neonatal Med. 2018:1–7. [DOI] [PubMed] [Google Scholar]
- 12.Ye Q, Du LZ, Shao WX, Shang SQ. Utility of cytokines to predict neonatal sepsis. Pediatr Res. 2017;81:616–21. 10.1038/pr.2016.267 [DOI] [PubMed] [Google Scholar]
- 13.Ng PC, Li K, Wong RP, Chui K, Wong E, Li G, et al. Proinflammatory and anti-inflammatory cytokine responses in preterm infants with systemic infections. Arch Dis Child Fetal Neonatal Ed. 2003;88:F209–13. 10.1136/fn.88.3.F209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ng PC, Li K, Leung TF, Wong RP, Li G, Chui KM, et al. Early prediction of sepsis-induced disseminated intravascular coagulation with interleukin-10, interleukin-6, and RANTES in preterm infants. Clin Chem. 2006;52:1181–9. 10.1373/clinchem.2005.062075 [DOI] [PubMed] [Google Scholar]
- 15.Silveira-Lessa AL, Quinello C, Lima L, Redondo ACC, Ceccon M, Carneiro-Sampaio M, et al. TLR expression, phagocytosis and oxidative burst in healthy and septic newborns in response to Gram-negative and Gram-positive rods. Hum Immunol. 2016;77:972–80. 10.1016/j.humimm.2016.07.230 [DOI] [PubMed] [Google Scholar]
- 16.Sugitharini V, Prema A, Berla Thangam E. Inflammatory mediators of systemic inflammation in neonatal sepsis. Inflamm Res. 2013;62:1025–34. 10.1007/s00011-013-0661-9 [DOI] [PubMed] [Google Scholar]
- 17.Tosson AMS, Glaser K, Weinhage T, Foell D, Aboualam MS, Edris AA, et al. Evaluation of the S100 protein A12 as a biomarker of neonatal sepsis. J Matern Fetal Neonatal Med. 2018:1–191. [DOI] [PubMed] [Google Scholar]
- 18.Sherwin C, Broadbent R, Young S, Worth J, McCaffrey F, Medlicott NJ, et al. Utility of interleukin-12 and interleukin-10 in comparison with other cytokines and acute-phase reactants in the diagnosis of neonatal sepsis. Am J Perinatol. 2008;25:629–36. 10.1055/s-0028-1090585 [DOI] [PubMed] [Google Scholar]
- 19.Redline RW, Faye-Petersen O, Heller D, Qureshi F, Savell V, Vogler C. Amniotic infection syndrome: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2003;6:435–48. 10.1007/s10024-003-7070-y [DOI] [PubMed] [Google Scholar]
- 20.Isaacs D, Barfield C, Clothier T, Darlow B, Diplock R, Ehrlich J, et al. Late-onset infections of infants in neonatal units. J Paediatr Child Health. 1996;32:158–61. 10.1111/j.1440-1754.1996.tb00914.x [DOI] [PubMed] [Google Scholar]
- 21.Inoue S, Unsinger J, Davis CG, Muenzer JT, Ferguson TA, Chang K, et al. IL-15 prevents apoptosis, reverses innate and adaptive immune dysfunction, and improves survival in sepsis. J Immunol. 2010;184:1401–9. 10.4049/jimmunol.0902307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jekarl DW, Kim JY, Ha JH, Lee S, Yoo J, Kim M, et al. Diagnosis and Prognosis of Sepsis Based on Use of Cytokines, Chemokines, and Growth Factors. Dis Markers. 2019;2019:1089107 10.1155/2019/1089107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Westendorp RG, Langermans JA, Huizinga TW, Elouali AH, Verweij CL, Boomsma DI, et al. Genetic influence on cytokine production and fatal meningococcal disease. Lancet. 1997;349:170–3. 10.1016/s0140-6736(96)06413-6 [DOI] [PubMed] [Google Scholar]
- 24.Speer EM, Dowling DJ, Ozog LS, Xu J, Yang J, Kennady G, et al. Pentoxifylline inhibits TLR- and inflammasome-mediated in vitro inflammatory cytokine production in human blood with greater efficacy and potency in newborns. Pediatr Res. 2017;81:806–16. 10.1038/pr.2017.6 [DOI] [PubMed] [Google Scholar]
- 25.Dembinski J, Behrendt D, Martini R, Heep A, Bartmann P. Modulation of pro- and anti-inflammatory cytokine production in very preterm infants. Cytokine. 2003;21:200–6. 10.1016/s1043-4666(02)00498-2 [DOI] [PubMed] [Google Scholar]
- 26.Strunk T, Hibbert J, Doherty D, Nathan E, Simmer K, Richmond P, et al. Impaired cytokine responses to live Staphylococcus epidermidis in preterm infants precede Gram-positive late-onset sepsis. Clin Infect Dis. 2020. [DOI] [PubMed] [Google Scholar]
- 27.Gomes RN, Teixeira-Cunha MG, Figueiredo RT, Almeida PE, Alves SC, Bozza PT, et al. Bacterial clearance in septic mice is modulated by MCP-1/CCL2 and nitric oxide. Shock. 2013;39:63–9. 10.1097/SHK.0b013e31827802b5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aziz M, Jacob A, Yang WL, Matsuda A, Wang P. Current trends in inflammatory and immunomodulatory mediators in sepsis. J Leukoc Biol. 2013;93:329–42. 10.1189/jlb.0912437 [DOI] [PMC free article] [PubMed] [Google Scholar]