Abstract
Despite scientific interest in animal empathy, and growing public concern for farm animal welfare, the empathic abilities of farm animals remain under researched. In this study, we investigated empathic responses of young Holstein dairy calves to conspecifics recovering from hot-iron disbudding, a painful procedure common on dairy farms. A combination of social approach and place conditioning was used. First, ‘observer’ calves witnessed two ‘demonstrator’ calves recover from either a painful procedure (hot-iron disbudding and sedation) or a sham procedure (sedation alone) in distinct pens. Observer calves spent more time in proximity and paid more attention to calves recovering from the painful procedure compared to sham calves (proximity: 59.6 ± 4.3%; attention: 54.3 ± 1.5%). Observers were then tested for conditioned place aversion (in the absence of demonstrators) at 48h, 72h and 96h after the second demonstration; observers tended to avoid the pen associated with conspecific pain during the second of the three tests, spending 34.8 ± 9.6% of their time in this pen. No strong evidence of pain empathy was found, but our tentative results encourage further research on empathy in animals.
Introduction
Assessing animal empathy is not straightforward, in part because the definition of empathy is subject to disagreement [1–3]. In this study, we consider empathy in its broadest sense: a multi-layered sensitivity to a conspecific’s state, ranging from basic mimicry to more complex perspective-taking (i.e. ‘imagining yourself in the physical or mental place of another’) [4–6]. Many approaches have been adopted in the study of empathy, from consolation in primates after conflict [7–9], to rats freeing mates from traps [10–12]. Farm animals have rarely been studied [5,6], and to our knowledge no work on cattle has been published. Most farm animals are gregarious, including cattle, so the social environment is likely to be relevant [13]. Moreover, cattle are routinely subjected to painful procedures, including hot-iron disbudding for dairy calves [14,15], providing an opportune model to explore empathic responses to pain.
This study had three aims. First, to investigate whether calves preferentially associate with a conspecific in pain compared to an unaffected conspecific (Objective 1). Based on previous observations in mice [16,17], we predicted calves would preferentially approach a conspecific in pain. Second, in an effort to examine a more complex empathic process (defined as ‘true empathy’ by Edgar et al. [18]), we tested whether the empathic response was valenced (i.e. positive or negative) by using conspecific state as a conditioning stimuli in a place conditioning paradigm [19] (Objective 2). As previously shown in mice, we predicted that a conspecific state of pain would result in conditioned place aversion [17]. Finally, we examined whether calf empathic responses are dependent on ‘pain-related’ behaviours [15] commonly used in the assessment of calf pain following disbudding. We predicted that calves showing more pain behaviours would be more attractive as a social partner and elicit stronger conditioned aversion.
Methods
Ethics statement
This research was conducted at the University of British Columbia’s Dairy Education and Research Center in Agassiz, Canada. All procedures were approved by the university’s Animal Care Committee (under protocol A16-0310).
Animals and housing
Female Holstein calves were housed in group pens (4.9 x 7.3 m) of 8 to 10 animals. Calves had been living in the same pen since they were 7 days old. Calves (n = 36) were enrolled as trios (n = 12) coming from the same pen: one ‘observer’ (n = 12) and two ‘demonstrators’ (n = 24). The average age and weight at enrollment were 47 (± 5.0) days and 75.0 (± 10.2) kg, with an age difference within the trio of 3 (± 2.8) days.
Apparatus
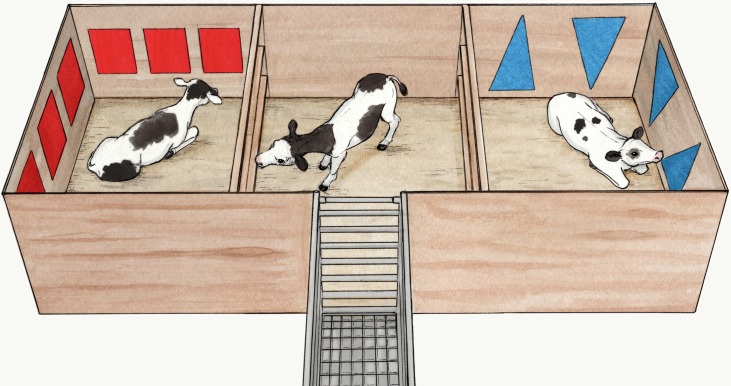
The apparatus was a 2.1 x 6.0 m area divided in three 2.1 x 2.0 m pens connected by removable gates that calves could see and interact through (horizontal fences, made of three 38 x 89 mm wood studs). The outermost sections were treatment pens, with distinct visual cues on the walls (either two blue triangles or three red squares) to help calves make the association between pen and treatment. Calves entered the apparatus through the central pen accessed via a chute acting as a start-box (Fig 1).
Fig 1. Experimental apparatus.
During demonstrations, observer calves were restricted in the central pen and demonstrators were restricted in treatment pens (with either red squares or blues triangles on the walls). One demonstrator was recovering from a painful procedure (sedation, local anesthesia and hot-iron disbudding) and the other from a sham procedure (sedation alone). During test sessions gates were removed, and observers were assessed by themselves for conditioned place aversion. Illustration by Ann Sanderson.
Protocol
Pre-exposure
Observer calves were individually pre-exposed to the apparatus. Observers were led from their home pen to the start-box where they received a small (0.3 L) milk reward. They were then let into the apparatus, with gates removed, allowing free access to all three sections. Time spent in each section (with both front legs in the pen) was recorded throughout the 15 min trial, and calves were then returned to their home pen. To reduce the risk of pre-existing avoidance bias, two calves that did not enter all pens during pre-exposure were not enrolled.
Demonstrations
Each trio (one observer and two demonstrators) were subjected to two demonstrations 24 h and 72 h after observer pre-exposure. During demonstrations, gates separating the pens were in place such that the observer calf was restricted to the central pen and demonstrators were each confined to one of the two treatment pens (as illustrated in Fig 1). Over the course of the 6 h trial, the observer could see and have limited physical contact (i.e. head contact through gates) with demonstrators recovering from two different treatments: ‘sham’ calves that had been sedated with xylazine (0.2 mg/kg BW, Rompun, 20 mg/mL, Bayer, Leverkusen, Germany), and ‘pain’ calves that had received the same sedation, as well as local anesthesia (5 mL of Lido-2; lidocaine 2%, Epinephrine 1:100,000, Rafter8, Calgary, Canada; injected in the lateral canthus of each eye) and hot-iron disbudding (X30 Rhinehart, Spencerville, IN, USA; heated to approximately 500°C and applied to horn buds for approximately 15 s, 10 min after local anesthesia). During the second demonstration (which also lasted 6 h), the observer was again restricted to the central pen but demonstrators were placed in the opposite pen and were assigned the opposite treatment (i.e. a demonstrator that first received the ‘pain’ treatment would next receive the ‘sham’ treatment and vice-versa). This design was chosen to balance the social preferences of observers for specific demonstrators. Colour of the treatment pen associated with the ‘pain’ procedure was balanced across trios (n = 6 in red squares, n = 6 in blue triangles). Preferences observed during pre-exposure were also balanced across treatments.
All observers had previously been disbudded (following the same ‘pain’ procedure that demonstrators experienced) two days before enrollment.
Tests
Observers were tested for conditioned place aversion 48h, 72h and 96h after the second demonstration. Gates were removed so observers could freely explore the apparatus until they chose to lie down for at least one minute, or 60 min had passed (which ever occurred first); calves were then returned to their home pen.
Measures and statistical analysis
All calves were video recorded during demonstration and test trials (camera: WV-CP310, Panasonic Canada, Ontario). Videos were analysed using Geovision’s viewlog software (Vision Systems, Saint-Laurent, Canada) by one blinded and one non-blinded observer. Proximity, attention and contact of the observer with the demonstrators were recorded. Pain behaviours displayed by demonstrators were also recorded (see Table 1 for details). Inter-rater agreements were calculated for proximity, attention, interaction and pain behaviours based on over 100 observations, using R’s ‘agree’ function [20]. During tests, time spent in each pen by the observer was continuously recorded, as well as where observers chose to lie down.
Table 1. Behaviours recorded during demonstrations (where an ‘observer’ calf would witness two ‘demonstrators’ recover from either a painful or a sham procedure) and during tests (where observers were tested for conditioned place aversion of the pens associated with the two procedures).
| Measure | Session | Subject calf | Description | Sampling method |
|---|---|---|---|---|
| Proximity | Demonstration | Observer | Which half of the central pen the calf placed her front legs | Instantaneous scans every 5 min |
| Attention | Demonstration | Observer | Which demonstrator the calf’s head was oriented towards | Instantaneous scans every 5 min |
| Contact | Demonstration | Demonstrator | Number of physical contacts between observer and demonstrator | 1 min scans every 5 min |
| Pain behaviours | Demonstration | Demonstrator | Number of ear flicks, head rubs and head shakes | 1 min scans every 5 min |
| Conditioned place aversion | Test | Observer | Time spent in each pen, where the calf lay down | Continuous |
Preferences in proximity, attention and contact for the conspecific recovering from disbudding were calculated for each observer as a ratio of the number of scans directed towards the ‘pain’ demonstrator compared to the total number of scans directed towards both demonstrators (i.e. the sum of scans directed towards ‘pain’ demonstrators and ‘sham’ demonstrators). A difference from the null expectation of 50% preference was calculated with a one-sample Student t-test. Similarly, during conditioned place aversion tests preference for the pen associated with conspecific pain was calculated as a ratio of time spent in the ‘pain’ pen compared to the total amount of time spent in both treatment pens. A difference from the null expectation of 50% preference was again tested with a one-sample t-test. Differences in where calves chose to lie down were analysed with Pearson χ² tests.
Due to low counts, pain behaviours were summed to calculate the total numbers of pain behaviours displayed in the ‘pain’ and ‘sham’ pen for each trio. Pain behaviours were analysed with a t-test to confirm that these behaviors differed with treatment. To test if observer preferences (during demonstrations and place aversion tests) were associated with these behaviors, we calculated the difference in the pain behaviours witnessed by the observer (i.e. the total in the pain pen minus the total is the sham pen, across the two demonstrator sessions) and compared this with preference using Pearson correlation. Data were graphically scrutinized for normality and outliers.
Results
Inter-rater agreement was satisfactory for all measures (position: 96%, attention: 83%, interaction: 100%, pain behaviours: 96%).
Objective 1: Do calves preferentially associate with a conspecific in pain?
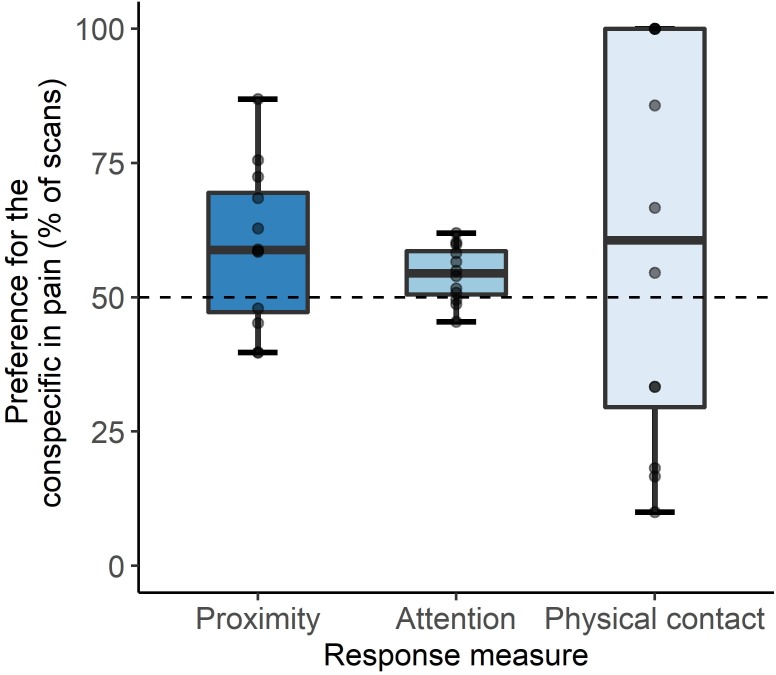
During demonstrations, observers spent more time in proximity and paid more attention to conspecifics recovering from disbudding compared to what could be expected by chance (Fig 2; mean ± SE proximity: 59.6 ± 4.3% of scans, t11 = 2.2, P = 0.05; attention: 54.3 ± 1.5% of scans, t11 = 2.9, P = 0.01). Physical contact occurred infrequently (on average just 5.2 ± 1.1 contacts per trio); 59.9 ± 10.5% of these contacts were with the painful calf (t11 = 0.9, P = 0.4).
Fig 2. Preferences of proximity, attention and contact of observer calves towards conspecifics in pain (recovering from sedation, local anesthesia and hot-iron disbudding) compared to sham conspecifics (recovering from sedation only).
Values above 50% represent a preference for the conspecific in pain.
Objective 2: Does observing a conspecific in pain lead to conditioned place aversion?
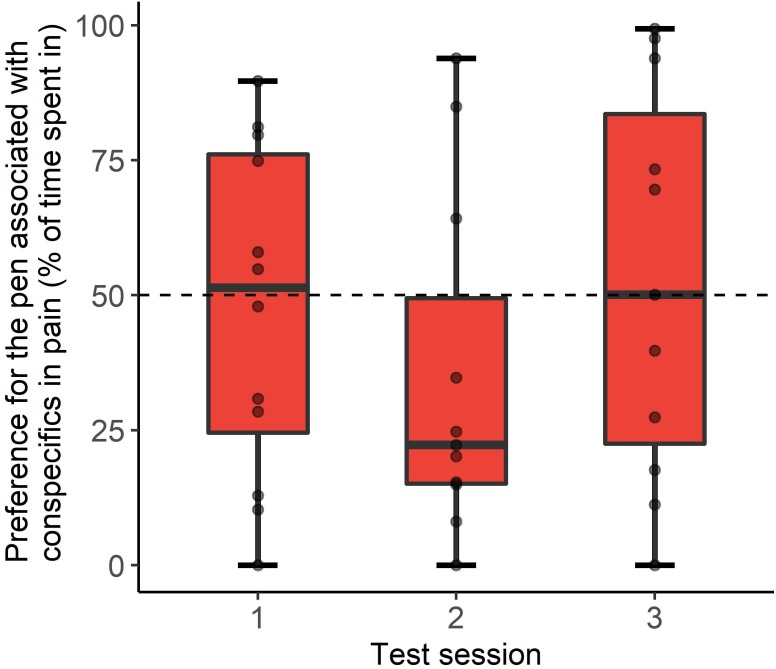
Observers tended to spend less time in the pen associated with the conspecific in pain compared to what could be expected by chance, but only during the second aversion test (Fig 3; 34.8 ± 9.6% of time spent in treatment pens, t10 = -1.6, P = 0.1) with no difference detected during the first and third tests (first test: 47.4 ± 8.8%, t11 = -0.3, P = 0.8; third test: 52.7 ± 10.9%, t10 = 0.2, P = 0.8).
Fig 3. Conditioned place aversion results.
Preference in time spent by observer calves in the pen where they previously observed pen mates recover from the ‘pain’ procedure (sedation, local anesthesia and hot-iron disbudding) compared to the ‘sham’ procedure (sedation alone). Values under 50% represent an aversion to the ‘pain’ pen. Test sessions 1, 2 and 3 took place 48 h, 72 h and 96 h after the last demonstration.
Out of the 36 tests (12 calves x 3 sessions), calves did not lie down within the 60 min period provided on 10 occasions, and lay down in the central pen in six of the sessions. Out of the remaining 20 sessions, calves lay down 11 times in the ‘sham’ pen and 9 times in the ‘pain’ pen, χ² = 0.2, P = 0.7). In all cases calves lay down for at least one minute, ending the session.
Objective 3: Does observer response vary in relation to the demonstrators’ pain-related behaviour?
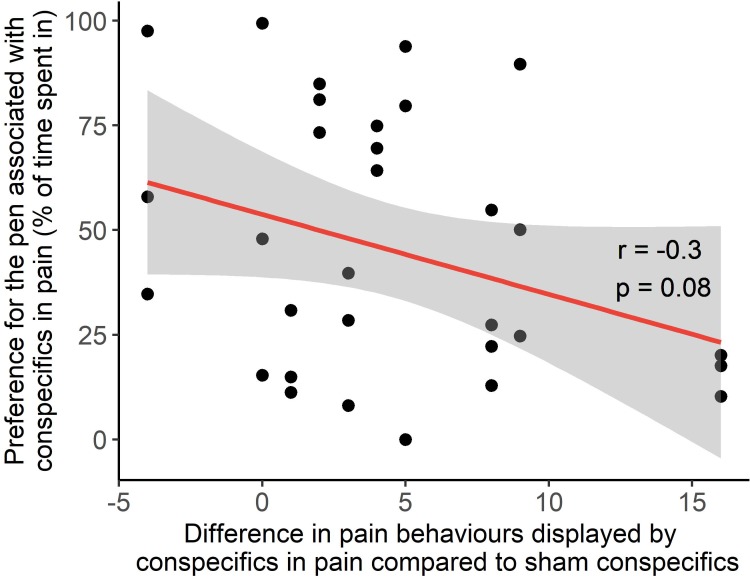
Demonstrators recovering from disbudding displayed more pain behaviours compared to calves recovering from the ‘sham’ procedure (4.8 ± 1.5 more pain behaviours; t11 = 3.2, P = 0.008). The difference in pain behaviours displayed by ‘pain’ demonstrators compared to ‘sham’ demonstrators was not correlated with preference in position, attention or contact of the observer (position: r = -0.2, P = 0.6; attention: r = 0.03, P = 0.9; contact: r = -0.09, P = 0.8). During place aversion tests, observers tended to show stronger avoidance of the pen associated with pain if more pain behaviours were displayed (Fig 4; r = -0.3, P = 0.09).
Fig 4. Correlation between the difference of pain behaviours displayed by demonstrators in the ‘pain’ procedure (sedation, local anesthesia and hot-iron disbudding) and ‘sham’ (sedation alone) procedures, versus observer calf place aversion of the pen associated with pain.
The shaded area represents the 95% confidence interval of the regression.
Discussion
Objective 1: Do calves preferentially associate with a conspecific in pain?
Our results indicate that dairy calves are socially attracted to animals in pain, as evidenced by the increased time spent in proximity and attention to demonstrators recovering from disbudding, compared to demonstrators recovering from sedation alone. These results are consistent with those of Langford et al. [16] and Watanabe et al. [17] who reported that mice are more likely to approach cage-mates in pain.
Objective 2: Does observing a conspecific in pain lead to conditioned place aversion?
Place aversion results were mixed, making it difficult to draw strong conclusions. We found no difference in time spent between treatment pens in the first session. We expected aversion to the pain pen to be strongest during the first test, based upon our previous observations of calf avoidance of a pen associated with their own disbudding [21]. However, in the current study observers were restricted to the central pen during demonstrations, so calves may have been motivated to explore the unfamiliar test pens when provided the opportunity in the first test session, allowing for treatment effects to emerge in the second session when calves were less motivated to explore. We encourage future studies to provide calves with habituation sessions to the test apparatus before the observer sessions to diminish any effects of novelty.
The finding from the second session (i.e. that calves tended to avoid the pen associated with conspecific pain), warrants further study on the idea that the affective state of another calf can act as a conditioning stimulus. During the third and last test, the lack of place aversion was predicted and is consistent with previous work on conditioned pain preference in calves [21], as pens were expected to lose their association to treatment with repeated, unreinforced test sessions.
Objective 3: Does observer response vary in relation to the demonstrators’ pain-related behaviour?
Demonstrators recovering from disbudding displayed more pain-related behaviours than sham demonstrators, a result that is consistent with the considerable research on hot-iron disbudding of calves [14]. We found no evidence that the expression of these behaviors was associated with observer proximity, attention or contact with the demonstrators, but calves that observed demonstrators showing stronger pain responses tended to show stronger place aversion to the pen associated with disbudding.
Previous reports of animal empathic processes are ambiguous: increased contact of ewes with a lamb in pain was correlated with the lamb’s pain behaviours [22], but ‘fear conditioning by proxy’ of rats (i.e. learning to fear a tone by being exposed to demonstrators who had previously associated the tone with an electric shock) was not dependent on the number of fear behaviours displayed by demonstrators [23]. Similarly, observer mice did not require visual cues to develop hyperalgesia when placed in a room with another mouse in pain [24]. Langford et al. [16] reported a negative correlation between the proximity of observers and the number of pain behaviours expressed by demonstrators. The type of cues relevant to different species likely varies among species (e.g. rodents may rely less on visual cues as they tend to have a poorer eyesight). Our results suggest that calves are able to identify a conspecific in pain but may do so using cues other than the pain behaviors we measured in the current study.
General discussion
Emotional contagion is a process by which observers experience similar states to those experienced by demonstrators, as illustrated, for example, by mice becoming hyperalgesic in the presence of other mice in pain [24]; this is considered a basic form of empathy [5,6]. In the case of emotional contagion, we would not expect observers to preferentially approach demonstrators in pain as they are the source of their own discomfort. As we observed approach towards the conspecific in pain, our results indicate that the empathic response of calves is not limited to basic motor mimicry or emotional contagion (or ‘primal empathy’ [1]) but rather includes a component of perspective taking, implying the capacity to separate the conspecific’s situation from their own. The tendency of observers to also avoid a place associated with conspecific pain suggests that calves might be able to identify that a conspecific is in a negative state and use this information to learn about their surroundings.
There are many limitations to the current study. We used a relatively low sample size for both ethical (calves were not provided with drugs to control post-operative pain) and practical reasons. Moreover, the experimental design restricted social contact between observers and demonstrators to only head contact. Calves are motivated for full social contact [25], meaning that the restriction imposed in this study might have impaired calves empathic process. This restriction might also explain the low number of physical contacts observed. It is also unclear what, if any, effect the smell of disbudding may have had on the observer’s response. Finally, the removal of demonstrators during tests may have affected the conditioned aversion response of observers.
Additional factors should be considered in future work. We explored the relationship between young, unrelated animals but it is reasonable to predict a greater empathic response from animals with better established bonds, such as a dam towards her offspring [26]; other work has reported increased empathic responses with increased kinship and familiarity [8,16,27,28]. A sex effect has been observed in mice, with males showing less evidence of empathy [16]. Only female calves were enrolled in the current study preventing any inferences regarding sex. Finally, observer calves had all been previously disbudded, potentially affecting their response; previous experience of pain and distress has been indicated to influence empathic response in other species [11,29].
The focus of this study was the effect of the conspecific’s state on the observer, but it would be interesting to explore whether the observers’ response (or simply social presence) affects demonstrators in their recovery from a painful procedure. We speculate that social presence will facilitate recovery from pain; evidence of ‘social buffering’ has previously been described in humans, primates, rodents and birds [30], including effects specific to pain mitigation in humans, rats, mice and goats [31].
Conclusion
Calves spent more time in proximity and paid more attention to a conspecific in pain compared to a sham treated calf, and tended to avoid the pen associated with conspecific pain, especially when more ‘pain-related’ behaviours were shown by the calf. These results are suggestive of an empathic processes that warrants further study.
Supporting information
(CSV)
(CSV)
(DOCX)
(R)
Acknowledgments
We thank the staff of the UBC Dairy Research and Education Center and students from the UBC’s Animal Welfare Program for their help in running the study. We are particularly grateful to Philippine Coeugnet and Ashley Cate for their help in assisting procedures and collecting data.
Data Availability
Data and R code are freely accessible in supplementary materials.
Funding Statement
This study was funded by a Discovery grant (RGPIN-2016-04620) from Canada’s Natural Science and Engineering Research Council to D.M.W. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Panksepp J, Panksepp JB. Toward a cross-species understanding of empathy. Trends Neurosci. 2013;36: 489–496. 10.1016/j.tins.2013.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jean Decety, Ben-Ami Bartal Inbal, Florina Uzefovsky, Ariel Knafo-Noam. Empathy as a driver of prosocial behaviour: highly conserved neurobehavioural mechanisms across species. Philos Trans R Soc B Biol Sci. 2016;371: 20150077 10.1098/rstb.2015.0077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vasconcelos M, Hollis K, Nowbahari E, Kacelnik A. Pro-sociality without empathy. Biol Lett. 2012;8: 910–912. 10.1098/rsbl.2012.0554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Preston SD, Waal FBM de. Empathy: Its ultimate and proximate bases. Behav Brain Sci. 2002;25: 1–20. 10.1017/s0140525x02000018 [DOI] [PubMed] [Google Scholar]
- 5.de Waal FBM, Preston SD. Mammalian empathy: behavioural manifestations and neural basis. Nat Rev Neurosci. 2017;18: 498–509. 10.1038/nrn.2017.72 [DOI] [PubMed] [Google Scholar]
- 6.Sivaselvachandran S, Acland EL, Abdallah S, Martin LJ. Behavioral and mechanistic insight into rodent empathy. Neurosci Biobehav Rev. 2018;91: 130–137. 10.1016/j.neubiorev.2016.06.007 [DOI] [PubMed] [Google Scholar]
- 7.Fraser ON, Stahl D, Aureli F. Stress reduction through consolation in chimpanzees. Proc Natl Acad Sci. 2008;105: 8557–8562. 10.1073/pnas.0804141105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romero T, Castellanos MA, Waal FBM de. Consolation as possible expression of sympathetic concern among chimpanzees. Proc Natl Acad Sci. 2010;107: 12110–12115. 10.1073/pnas.1006991107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clay Z, Waal FBM de. Bonobos Respond to Distress in Others: Consolation across the Age Spectrum. PLOS ONE. 2013;8: e55206 10.1371/journal.pone.0055206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rice GE, Gainer P. “Altruism” in the albino rat. J Comp Physiol Psychol. 1962;55: 123–125. 10.1037/h0042276 [DOI] [PubMed] [Google Scholar]
- 11.Sato N, Tan L, Tate K, Okada M. Rats demonstrate helping behavior toward a soaked conspecific. Anim Cogn. 2015;18: 1039–1047. 10.1007/s10071-015-0872-2 [DOI] [PubMed] [Google Scholar]
- 12.Bartal IB-A, Decety J, Mason P. Empathy and Pro-Social Behavior in Rats. Science. 2011;334: 1427–1430. 10.1126/science.1210789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rault J-L. Friends with benefits: Social support and its relevance for farm animal welfare. Appl Anim Behav Sci. 2012;136: 1–14. 10.1016/j.applanim.2011.10.002 [DOI] [Google Scholar]
- 14.Winder CB, Miltenburg CL, Sargeant JM, LeBlanc SJ, Haley DB, Lissemore KD, et al. Effects of local anesthetic or systemic analgesia on pain associated with cautery disbudding in calves: A systematic review and meta-analysis. J Dairy Sci. 2018. 10.3168/jds.2017-14092 [DOI] [PubMed] [Google Scholar]
- 15.Herskin MS, Nielsen BH. Welfare effects of the use of a combination of local anesthesia and NSAID for disbudding analgesia in dairy calves—Reviewed across different welfare concerns. Front Vet Sci. 2018;5 10.3389/fvets.2018.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langford DJ, Tuttle AH, Brown K, Deschenes S, Fischer DB, Mutso A, et al. Social approach to pain in laboratory mice. Soc Neurosci. 2010;5: 163–170. 10.1080/17470910903216609 [DOI] [PubMed] [Google Scholar]
- 17.Watanabe S. Distress of mice induces approach behavior but has an aversive property for conspecifics. Behav Processes. 2012;90: 167–173. 10.1016/j.beproc.2012.01.001 [DOI] [PubMed] [Google Scholar]
- 18.Edgar JL, Nicol CJ, Clark CCA, Paul ES. Measuring empathic responses in animals. Appl Anim Behav Sci. 2012;138: 182–193. 10.1016/j.applanim.2012.02.006 [DOI] [Google Scholar]
- 19.Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56: 613–672. 10.1016/s0301-0082(98)00060-4 [DOI] [PubMed] [Google Scholar]
- 20.Gamer M, Lemon J, Singh IFP. irr: Various Coefficients of Interrater Reliability and Agreement. 2019. Available: https://CRAN.R-project.org/package=irr [Google Scholar]
- 21.Ede T, Lecorps B, Keyserlingk MAG von, Weary DM. Calf aversion to hot-iron disbudding. Sci Rep. 2019;9: 5344 10.1038/s41598-019-41798-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hild S, Clark CCA, Dwyer CM, Murrell JC, Mendl M, Zanella AJ. Ewes are more attentive to their offspring experiencing pain but not stress. Appl Anim Behav Sci. 2011;132: 114–120. 10.1016/j.applanim.2011.04.003 [DOI] [Google Scholar]
- 23.Bruchey AK, Jones CE, Monfils M-H. Fear conditioning by-proxy: Social transmission of fear during memory retrieval. Behav Brain Res. 2010;214: 80–84. 10.1016/j.bbr.2010.04.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith ML, Hostetler CM, Heinricher MM, Ryabinin AE. Social transfer of pain in mice. Sci Adv. 2016;2: e1600855 10.1126/sciadv.1600855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holm L, Jensen MB, Jeppesen LL. Calves’ motivation for access to two different types of social contact measured by operant conditioning. Appl Anim Behav Sci. 2002;79: 175–194. 10.1016/S0168-1591(02)00137-5 [DOI] [Google Scholar]
- 26.Flower FC, Weary DM. Effects of early separation on the dairy cow and calf:: 2. Separation at 1 day and 2 weeks after birth. Appl Anim Behav Sci. 2001;70: 275–284. 10.1016/s0168-1591(00)00164-7 [DOI] [PubMed] [Google Scholar]
- 27.Edgar JL, Lowe JC, Paul ES, Nicol CJ. Avian maternal response to chick distress. Proc R Soc B Biol Sci. 2011;278: 3129–3134. 10.1098/rspb.2010.2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edgar JL, Paul ES, Harris L, Penturn S, Nicol CJ. No Evidence for Emotional Empathy in Chickens Observing Familiar Adult Conspecifics. PLOS ONE. 2012;7: e31542 10.1371/journal.pone.0031542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wechkin S, Masserman JH, Terris W. Shock to a conspecific as an aversive stimulus. Psychon Sci. 1964;1: 47–48. 10.3758/BF03342783 [DOI] [Google Scholar]
- 30.Takefumi Kikusui, Winslow James T, Mori Yuji. Social buffering: relief from stress and anxiety. Philos Trans R Soc B Biol Sci. 2006;361: 2215–2228. 10.1098/rstb.2006.1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McMillan FD. The psychobiology of social pain: Evidence for a neurocognitive overlap with physical pain and welfare implications for social animals with special attention to the domestic dog (Canis familiaris). Physiol Behav. 2016;167: 154–171. 10.1016/j.physbeh.2016.09.013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(CSV)
(CSV)
(DOCX)
(R)
Data Availability Statement
Data and R code are freely accessible in supplementary materials.