Abstract
Background
HIV testing contributes to the prevention and control of the HIV epidemic in the general population. South Africa has made strides to improve HIV testing towards reaching the first of the UNAIDS 90–90–90 targets by 2020. However, to date no nationally representative analysis has examined temporal trends and factors associated with HIV testing among youth and adults in the country.
Aim
This study aimed to examine the trends and associations with ever having tested for HIV among youth and adults aged 15 years and older in South Africa using the 2005, 2008, 2012 and 2017 nationally representative population-based household surveys.
Methods
The analysis of the data collected used multi-stage stratified cluster randomised cross-sectional design. P-trend chi-squared test was used to identify any significant changes over the four study periods. Bivariate and multivariate logistic regression analysis was conducted to determine factors associated with HIV testing in each of the survey periods.
Results
Ever having tested for HIV increased substantially from 2005 (30.6%, n = 16 112), 2008 (50.4%, n = 13 084), 2012 (65.5%, n = 26 381), to 2017 (75.2%, n = 23 190). Those aged 50 years and older were significantly less likely to ever have tested for HIV than those aged 25–49 years. Those residing in rural areas were significantly less likely to have tested for HIV as compared to people from urban areas.
There was a change in HIV testing among race groups with Whites, Coloureds and Indian/Asians testing more in 2005 and 2008 and Black Africans in 2017. Marriage, education and employment were significantly associated with increased likelihood of ever testing for HIV. Those who provided a blood specimen for laboratory HIV testing in the survey rounds and were found to have tested positive were more likely to have ever tested for HIV previously.
Conclusion
The results show that overall there has been an increase in ever having an HIV test in the South African population over time. The findings also suggest that for South Africa to close the testing gap and reach the first of the UNAIDS 90–90–90 targets by 2020, targeted programmes aimed at increasing access and utilization of HIV testing in young people, males, those not married, the less educated, unemployed and those residing in rural areas of South Africa should be prioritised.
Introduction
HIV testing is a critical step in the HIV treatment cascade, which includes diagnosis, linkage to care, engagement in care, initiation of antiretroviral therapy, retention in care, and sustained viral load suppression [1]. HIV testing also contributes to the prevention and control of the HIV epidemic in the general population, since people diagnosed with HIV can make decisions that potentially lower their risk of HIV transmission and re-infection, while those who test negative can make informed decisions to protect themselves from getting infected [2].
Evidence shows that decision-making and practices related to HIV-testing could be influenced by several factors including accurate knowledge about HIV transmission, perceived risk of HIV infections, attitudes and perceptions of HIV-testing services, and previous history and experiences of HIV-testing [3]. HIV testing uptake may also be influenced by individual level factors such as gender, age and marital status, and socio-economic characteristics such as urban or rural residence, education attainment, and employment status among others [3, 4]. Understanding these associations is important for making effective interventions aimed at containing the HIV epidemic, particularly as countries aim to attain the UNADS 90–90–90 targets. These targets are aimed to increase knowledge of HIV‐positive status, initiation of antiretroviral therapy (ART) and viral suppression by 2020 [1, 5, 6]. Changes in HIV testing overtime has implications for the UNAIDS targets and for evaluating the impact of national policies [7].
In South Africa, policy initiatives aligned to meet UNAIDS 90-90-90 targets have an impact on HIV testing [8]. The Government has embarked on a deliberate effort to scale up HIV testing services (HTS) by increasing availability of quality HTS and its uptake in all public health facilities [9]. Scaling up of HIV testing HIV testing has the potential to affect the first ‘90’ of the UNAIDS 90-90-90 targets.
Due to the high burden of HIV overtime, the country has experienced demographic, socio-economic and behavioural change because of the epidemiological transition [10, 11]. It is therefore important to monitor changes in HIV testing and important indicators in order to inform policy. This study examined temporal trends and factors associated with HIV testing in the South Africa population aged 15 years and older using data from the 2005, 2008, 2012, and 2017 nationally representative population-based household surveys.
Methods
All youth and adults who agreed to participate were required to provide written or verbal (where respondent was illiterate). Written Informed consent for participating in the survey and for the collection of the dried blood spot specimens were obtained or participants 18 years and older. For those less than 18 years informed consent was obtained from parents or guardians and assent obtained from the participants. Where people could not write verbal consent was obtained and fieldwork supervisor signed as witness. The four survey protocols were approved by the Human Sciences Research Council Research Ethics Committee (REC approval numbers: 5/24/06/04; 2/23/10/07; 5/17/11/10 and 4/18/11/15).
Data
Data from the 2005, 2008, 2012 and 2017 South African national household-based HIV Prevalence, Incidence and Behaviour surveys were used for this study [12–15]. The methodology for these surveys has been the same across the different survey waves, hence allowing this comparison of temporal trends [12–15]. The surveys used a multi-stage stratified cluster randomised cross-sectional design. In each survey a systematic probability sample of 15 households was randomly chosen from 1 000 enumeration areas (EAs) which were randomly selected from 86 000 EAs based on the national sampling frame released by Statistics South Africa in 2001 and updated in 2011 [16–18]. The sampling of EAs was stratified by province and locality type (urban formal, urban informal, rural formal—including commercial farms and rural informal localities).
Study participants
In the 2005 and 2008 surveys, in each household a maximum of three people were selected randomly to participate in the study, each representing the 2–14 years, 15–24 years and 25 years and older age groups. In the 2012 and 2017 surveys, all household members were eligible to participate in the survey. In all the surveys age appropriate questionnaires were administered to solicit information on socio-demographic characteristics, sexual practices and behaviour, knowledge, attitudes and perceptions, testing of tuberculosis and HIV, exposure to HIV media campaigns, alcohol and substance use and general health related characteristics. The current analysis focused on individuals aged 15 years and older who responded to the question on ever tested for HIV.
HIV testing
Dried blood spots’ (DBS) specimens were collected from consenting individuals for HIV testing. Samples were tested for HIV using an enzyme immunoassay (EIA) (Vironostika HIV Uni-Form II plus O, Biomeriux, Boxtel, The Netherlands), and samples which tested positive were retested using a second EIA (Advia Centaur XP, Siemens Medical Solutions Diagnostics, Tarrytown, New York, USA). Any samples with discordant results on the first two EIAs were tested with a third EIA (Roche Elecys 2010 HIV Combi, Roche Diagnostics, Mannheim, Germany).
Measures
Primary outcome
In all four surveys, participants who responded to the question “Have you ever been tested for HIV?” are included in the analysis. The response was dichotomized for the primary outcome (yes = 1 and no = 0).
Explanatory variables
Covariate socio-demographic variables such as age, grouped into 15–24 years, 25–49 years, 50 years and older, sex (male, female), race (Black Africans, White, Coloured, Indian/Asian), current marital status (not married, married), level of education (no education, primary, secondary, tertiary), employment status (unemployed, employed), and locality type (urban areas, rural informal, rural formal areas) were included.
Other covariates included HIV behavioural variables such as ever had sexual intercourse (no, yes), age of sexual debut (had sex before the age of 15 years, had sex aged 15 years and older), age of sexual partner (partner more than five years younger, partner within five years of age, partner more than five years older), multiple sexual partners in the last 12 months (one partner, two or more partners), condom use at last sex (yes, no), the Alcohol Abuse Disorder Identification Test (AUDIT) score (abstainers, low risk (with scores ranging from1–7), risky/hazardous level (8–15), high risk/harmful (16–19), very high risk (20+), [19]. Accurate knowledge about preventing the sexual transmission of HIV was based on responses to five prompted questions; ‘Can a person reduce the risk of getting HIV by using a condom every time they have sex?’ ‘Can a person reduce the risk of HIV by having fewer sexual partners?’, ‘Can AIDS be cured?’ ‘Can a person get HIV by sharing food with someone who is infected?’ ‘Can a healthy-looking person have HIV? Accurate knowledge of preventing the sexual transmission of HIV and rejection of misconceptions about HIV transmission was scored 1 ‘yes’ if all five items were correctly answered, whereas if they answered any incorrectly, they scored 0 ‘no’. Self-perceived risk of HIV infection (yes, no) and antibody detected HIV status (HIV positive, HIV negative) were also included.
Statistical analysis
Descriptive statistics of socio-demographic variables and HIV risk behaviours by HIV testing were generated for each of the study waves, using frequencies and proportions. P-trend chi-squared test was used to identify any significant change over the four study periods. Percentage differences in HIV testing between the 2005 and 2017 surveys were also calculated. Bivariate logistic regression analysis was used to assess factors associated with having ever been tested for HIV for each study wave. All statistically significant variables were entered into a final multivariate logistic regression model. Crude Odds ratios (OR) and adjusted (aOR) for the bivariate and multivariate models, with 95% Confidence intervals (CI) and a p-value ≤ 0.05 were considered statistically significant. Separate models were fitted for each of the survey waves. Sample weights were introduced to all models to account for the complex survey design and non-response using the ‘svy’ command. Statistical analyses were done using Stata statistical software, Release 15.0 (College Station, TX: Stata Corporation).
Results
Trends in ever having tested for HIV in 2005–2017
Table 1 shows trends in ever having tested for HIV by socio-demographic characteristics among youth and adults aged 15 years and older. Overall the percentage of those ever been tested for HIV increased from 30.6% in 2005 to 75.2% in 2017 (a 44.6% point increase). There was a significant increase in the percentages of respondents who had ever been tested for HIV across all socio-demographic characteristics from 2005 to 2017 (p<0.001 for all variables). The largest increase was among those aged 50 and older changing from 18.3% in 2005 to 69.7% in 2017 (a 51.4% point increase), Black Africans from 26.2% in 2005 to 76.5% in 2017 (a 50.3% point increase), those with no education or who had only attained primary school education from 18.4% in 2005 to 71.9% in 2017 (a 53.5% point increase), those unemployed from 23.2% in 2005 to 70.7% in 2017 (a 47.5% point increase), and those residing in rural informal areas from 19.4% to 70.3% in 2017 (a 50.9% point increase).
Table 1. Trend in ever having tested for HIV by socio-demographic characteristics of the study participants age 15 years and older in South Africa by survey period from 2005–2017.
| 2005 | 2008 | 2012 | 2017 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | n | % | 95% CI | n | % | 95% CI | n | % | 95% CI | n | % | 95% CI | Percentage point increase | p-value* |
| Age categories | ||||||||||||||
| Total | 16 112 | 30.6 | 29.1–32.1 | 13 084 | 50.4 | 48.9–51.8 | 26 381 | 65.5 | 64.2–66.7 | 23 190 | 75.2 | 74.0–76.4 | 44.6 | <0.001 |
| 15–24 years | 5 615 | 19.3 | 17.7–20.9 | 4 192 | 37.3 | 35.1–39.6 | 7 121 | 50.6 | 48.5–52.7 | 5 921 | 58.8 | 56.6–61.1 | 39.5 | <0.001 |
| 25–49 years | 6 764 | 43.1 | 40.8–45.5 | 5 606 | 65.1 | 63.0–67.2 | 11 553 | 78.2 | 76.6–79.8 | 10 674 | 85.0 | 83.6–86.2 | 41.9 | <0.001 |
| 50+ years | 3 733 | 18.3 | 16.4–20.4 | 3 286 | 34.1 | 31.6–36.6 | 7 707 | 54.8 | 52.7–57.0 | 6 595 | 69.7 | 68.0–71.4 | 51.4 | <0.001 |
| Sex of respondent | ||||||||||||||
| Male | 6 193 | 27.6 | 25.5–29.8 | 5 193 | 44.2 | 42.0–46.4 | 11 403 | 59.0 | 57.2–60.8 | 9 762 | 70.9 | 69.2–72.5 | 43.3 | <0.001 |
| Female | 9 919 | 33.0 | 31.3–34.7 | 7891 | 55.8 | 54.1–57.4 | 14 978 | 71.5 | 70.1–72.9 | 13 428 | 79.3 | 78.0–80.5 | 46.3 | <0.001 |
| Race of respondent | ||||||||||||||
| Black African | 9 515 | 26.2 | 24.6–27.8 | 7 844 | 49.0 | 47.3–50.7 | 15 166 | 65.8 | 64.3–67.2 | 15 255 | 76.5 | 75.1–77.9 | 50.3 | <0.001 |
| White | 1 888 | 53.2 | 48.5–57.9 | 1 570 | 59.0 | 55.4–62.5 | 2 823 | 62.7 | 58.8–66.4 | 1 634 | 69.4 | 65.9–72.8 | 16.2 | <0.001 |
| Coloured | 2 949 | 36.2 | 33.1–39.4 | 2 348 | 51.0 | 48.1–54.0 | 4 911 | 67.8 | 65.4–70.1 | 4 182 | 73.8 | 71.7–75.7 | 37.6 | <0.001 |
| Indian/Asian | 1 728 | 44.7 | 39.6–49.8 | 1 294 | 51.6 | 46.0–57.2 | 3 419 | 60.6 | 55.7–65.3 | 2 119 | 61.8 | 56.7–66.7 | 17.1 | <0.001 |
| Marital status | ||||||||||||||
| Not married | 10 160 | 25.5 | 24.0–27.1 | 8 392 | 47.4 | 45.6–49.1 | 16 707 | 62.4 | 60.8–64.0 | 7 391 | 81.1 | 79.6–82.6 | 55.6 | <0.001 |
| Married | 5 917 | 39.1 | 36.6–41.8 | 4 649 | 56.1 | 53.8–58.4 | 9 276 | 73.2 | 71.3–75.0 | 15 794 | 72.8 | 71.4–74.2 | 33.7 | <0.001 |
| Level of education | ||||||||||||||
| No education/Primary | 4 537 | 18.4 | 16.4–20.5 | 3 291 | 35.8 | 33.4–38.3 | 4 285 | 58.4 | 56.0–60.7 | 3 626 | 71.9 | 69.8–74.0 | 53.5 | <0.001 |
| Secondary | 9 955 | 31.7 | 29.9–33.5 | 8 384 | 52.3 | 50.5–54.1 | 15 883 | 66.2 | 64.6–67.8 | 11 266 | 80.4 | 79.0–81.7 | 48.7 | <0.001 |
| Tertiary | 1 571 | 61.4 | 57.6–65.1 | 1 330 | 72.7 | 68.7–76.4 | 2 248 | 81.8 | 78.2–84.8 | 2 471 | 85.2 | 82.9–87.3 | 23.8 | <0.001 |
| Employment status | ||||||||||||||
| Unemployed | 10 822 | 23.2 | 21.9–24.7 | 8 163 | 43.3 | 41.8–44.9 | 14 298 | 61.3 | 59.7–62.9 | 14 796 | 70.7 | 69.2–72.0 | 47.5 | <0.001 |
| Employed | 5 223 | 46.0 | 43.0–49.1 | 4 854 | 62.3 | 59.8–64.7 | 9 658 | 75.2 | 73.2–77.0 | 8 070 | 83.7 | 82.1–85.2 | 37.7 | <0.001 |
| Locality type | ||||||||||||||
| Urban areas | 11 011 | 38.9 | 36.9–40.9 | 9 370 | 53.7 | 51.9–55.5 | 18 271 | 68.0 | 66.4–69.6 | 15 082 | 77.4 | 76.0–78.7 | 38.5 | <0.001 |
| Rural informal areas | 3 682 | 19.4 | 17.3–21.5 | 2 837 | 43.1 | 40.6–45.6 | 5 602 | 61.6 | 59.6–63.5 | 5 432 | 70.3 | 68.0–72.4 | 50.9 | <0.001 |
| Rural formal areas | 1 419 | 21.0 | 16.7–26.1 | 877 | 50.4 | 45.6–55.1 | 2 508 | 63.0 | 57.4–68.2 | 2 676 | 71.2 | 67.2–74.9 | 50.2 | <0.001 |
* p-value ≤ 0.05 was considered statistically significant
Table 2 shows trends in ever having tested for HIV by risk behaviour characteristics among youth and adults aged 15 years and older. There was a significant increase in the percentages of respondents who had ever been tested for HIV across all HIV risk behaviour characteristics in 2005 to 2017 (p<0.001 for all variables). The largest increase was among those who ever had sex from 34.6% in 2005 to 81.1% in 2017 (a 46.5% point increase), those with sexual partners 5 years and older than themselves, from 29.7% in 2005 to 89.9% in 2017 (a 60.2% point increase), those with two or more sexual partners from 35.5% in 2005 to 83.0% in 2017 (a 47.5% point increase), among those who reported no alcohol consumption from 27.0% in 2005 to 73.6 in 2017 (a 46.6% point increase) and among those who tested HIV positive in the survey, from 36.7% in 2005 to 87.1% in 2017 (a 50.4% point increase).
Table 2. Trend in every having tested for HIV by HIV risk behaviour characteristics of the study participants age 15 years and older in South Africa by survey period from 2005–2017.
| 2005 | 2008 | 2012 | 2017 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | n | % | 95% CI | n | % | 95% CI | n | % | 95% CI | n | % | 95% CI | Percentage point increase | p-value* |
| Sexual activity | ||||||||||||||
| Never had sex | 2 596 | 8.3 | 6.4–10.6 | 1 910 | 15.8 | 13.3–18.7 | 3 317 | 28.4 | 25.6–31.4 | 3 655 | 43.4 | 40.5–46.3 | 35.1 | <0.001 |
| Had sex | 13 148 | 34.6 | 33.0–36.3 | 9 586 | 55.2 | 53.7–56.8 | 22 360 | 71.0 | 69.7–72.3 | 18 319 | 81.1 | 80.0–82.2 | 46.5 | <0.001 |
| Sexual debut | ||||||||||||||
| Sex before aged 15 | 248 | 25.4 | 19.0–32.9 | 192 | 44.5 | 34.9–54.4 | 391 | 57.9 | 50.3–65.2 | 387 | 66.1 | 59.6–72.0 | 40.7 | <0.001 |
| Sex at aged 15 and older | 5 367 | 19.0 | 17.3–20.7 | 3 911 | 36.8 | 34.5–39.2 | 6 696 | 50.0 | 47.9–52.2 | 5 513 | 58.1 | 55.8–60.4 | 39.1 | <0.001 |
| Age of sexual partner | ||||||||||||||
| Partner more than 5 years younger | 1 624 | 36.2 | 32.6–40.1 | 1 138 | 52.1 | 47.5–56.6 | 2 659 | 72.8 | 69.7–75.7 | 2 21 | 81.6 | 79.1–83.9 | 45.4 | <0.001 |
| Partner within five years | 5 329 | 39.4 | 36.9–42.1 | 3 997 | 60.1 | 57.8–62.4 | 9 739 | 73.2 | 71.4–74.9 | 6 874 | 84.1 | 82.7–85.5 | 44.7 | <0.001 |
| Partner more than 5 years older | 5 943 | 29.7 | 27.8–31.8 | 1 448 | 65.9 | 62.2–69.5 | 3 241 | 86.3 | 84.3–88.1 | 2 728 | 89.9 | 88.3–91.3 | 60.2 | <0.001 |
| Number of sexual partners in the past 12 months | ||||||||||||||
| 1 sexual partner | 8 444 | 39.5 | 37.5–41.6 | 648 | 47.8 | 41.8–54.0 | 14 183 | 77.0 | 75.7–78.3 | 10 845 | 84.9 | 83.8–86.0 | 45.4 | <0.001 |
| 2 or more sexual partners | 674 | 35.5 | 29.6–41.8 | 7 159 | 61.8 | 60.0–63.6 | 1 487 | 67.8 | 64.0–71.4 | 1 049 | 83 | 79.8–85.9 | 47.5 | <0.001 |
| Condom use at last sex in the past 12 months | ||||||||||||||
| No condom use | 6 215 | 39.7 | 37.3–42.2 | 4 900 | 59.9 | 57.5–62.1 | 10 663 | 75.7 | 74.0–77.4 | 7 657 | 84.6 | 83.2–85.9 | 44.9 | <0.001 |
| Yes condom use | 2 961 | 38.2 | 35.5–41.0 | 2 887 | 61.0 | 58.4–63.6 | 4 707 | 76.0 | 73.9–78.0 | 4 144 | 85 | 83.4–86.4 | 46.8 | <0.001 |
| AUDIT Score | ||||||||||||||
| Abstainers | 10 979 | 27.0 | 25.4–28.6 | 8 892 | 48.0 | 46.4–49.7 | 14 638 | 64.3 | 62.8–65.8 | 15 168 | 73.6 | 72.0–75.1 | 46.6 | <0.001 |
| Low risk (1–7) | 2 905 | 39.4 | 36.3–42.6 | 2 826 | 59.6 | 56.7–62.5 | 6 326 | 68.3 | 65.8–70.6 | 4 067 | 77.6 | 75.5–79.6 | 38.2 | <0.001 |
| Risky/hazardous level (8–15) | 805 | 36.5 | 31.4–41.9 | 936 | 47.3 | 42.1–52.5 | 1 855 | 63.9 | 59.7–67.8 | 1 473 | 79.9 | 76.4–82.9 | 43.4 | <0.001 |
| High risk/harmful (16–19) | 143 | 33.4 | 24.5–43.6 | 120 | 37.4 | 25.2–51.4 | 340 | 62.1 | 53.6–70.0 | 253 | 75.7 | 66.7–83.0 | 42.3 | <0.001 |
| High risk (20+) | 1 124 | 38.6 | 34.3–43.0 | 128 | 50.2 | 37.4–63.1 | 295 | 65.7 | 56.3–73.9 | 263 | 71.5 | 62.5–79.0 | 32.9 | <0.001 |
| Correct HIV knowledge and myth rejection | ||||||||||||||
| No knowledge | 9 184 | 28.4 | 26.7–30.2 | 8 902 | 48.3 | 46.7–49.9 | 18 716 | 64.4 | 62.9–65.8 | 14 652 | 74.1 | 72.7–75.4 | 45.7 | <0.001 |
| Yes knowledge | 6 904 | 33.4 | 31.3–35.6 | 4 138 | 55.0 | 52.4–57.4 | 7 555 | 68.9 | 67.0–70.7 | 8 492 | 77.3 | 75.7–78.9 | 43.9 | <0.001 |
| Self–perceived risk of HIV infection | ||||||||||||||
| No risk | 11 151 | 28.6 | 26.8–30.5 | 10 081 | 48.0 | 46.3–49.6 | 4 904 | 75.1 | 73.1–77.1 | 18 383 | 72.3 | 70.9–73.7 | 43.7 | <0.001 |
| Yes risk | 4 914 | 34.1 | 31.9–36.3 | 2 923 | 56.2 | 53.7–58.8 | 21 240 | 62.3 | 60.8–63.8 | 2 836 | 79.3 | 76.8–81.5 | 45.2 | <0.001 |
| HIV status | ||||||||||||||
| HIV Negative | 10 510 | 29.3 | 27.6–31.0 | 8 929 | 48.2 | 46.5–49.8 | 17 872 | 62.3 | 60.8–63.8 | 13 193 | 74.7 | 73.4–75.9 | 45.4 | <0.001 |
| HIV Positive | 1 328 | 36.7 | 32.9–40.7 | 1 227 | 65.2 | 61.2–69.0 | 2 605 | 81.7 | 79.2–84.0 | 2 604 | 87.1 | 84.7–89.2 | 50.4 | <0.001 |
* p-value ≤ 0.05 was considered statistically significant
Determinants of ever having tested for HIV in 2005–2017
All variables in the bivariate logistic regression analysis were statistically significant and therefore they were all controlled for in the multivariate logistic regression analysis. Fig 1 shows coefficient plots of the final multiple logistic regression models of determinants of ever being tested for HIV in each survey wave. The increased likelihood of ever being tested for HIV was significantly associated with those aged 25–49 years rather than those aged 15–24 years, in 2005 [aOR = 1.33 (95% CI: 1.06–1.66), p = 0.013], 2008 [aOR = 2.08 (95% CI: 1.75–2.47), p<0.001], 2012 [aOR = 2.5 (95% CI: 2.1–3.0) p<0.001], and 2017 [aOR = 2.5 (95% CI: 2.1–3.0), p<0.001]. Females were significantly more likely to have ever tested for HIV than males, in 2005 [aOR = 1.74 (95% CI: 1.44–2.10), p<0.001], 2008 [aOR = 1.83 (95% CI: 1.59–2.11), p<0.001], 2012 [aOR = 2.3 (95% CI: 2.0–2.6), p<0.001], and 2017 [aOR = 2.05 (95% CI: 1.88–2.23), p<0.001]. Whites were significantly more likely to have ever tested for HIV than Black Africans, in 2005 [aOR = 2.64 (95% CI: 1.99–3.49), p<0.001] and in 2008 [aOR = 1.31 (95% CI: 1.02–1.68), p = 0.031]. Similarly, Indians [aOR = 1.57 (95% CI: 1.2–2.1), p<0.001], and Coloureds [aOR = 1.61 (95% CI: 1.3–2.0), p<0.001], were significantly more likely to ever have tested for HIV in 2005 than Black Africans. Married respondents were significantly more likely to have ever tested for HIV than those unmarried: in 2005 [aOR = 1.24 (95% CI: 1.04–1.47), p = 0.018], in 2008 [aOR = 1.23 (95% CI: 1.06–1.44), p = 0.008], in 2012 [aOR = 1.60 (95% CI: 1.3–1.9), p<0.001], and in 2017 [aOR = 1.58 (95% CI: 1.41–1.76), p<0.001].
Fig 1. Multivariate logistic regression models of determinates of having ever been tested for HIV in South Africa by survey period from 2005–2017.
The increased likelihood of ever being tested for HIV was also significantly associated with attainment of a secondary school level of education compared to those who had no education or those who only had attained primary school education, in 2005 [aOR = 1.56 (95% CI:1.26–1.93, p<0.001], 2008 [aOR = 1.80 (95% CI:1.52–2.12), p<0.001], 2012 [aOR = 1.5 (95% CI:1.3–1.7), p<0.001], and 2017 [aOR = 1.76 (95% CI: 1.58–1.96), p<0.001]. Tertiary level education had higher likelihood of ever being tested for HIV, in 2005 [aOR = 3.74 (95% CI:2.74–5.12), p<0.001], 2008 [aOR = 3.74 (95% CI:2.74–5.11), p<0.001], 2012 [aOR = 2.7 (95% CI:1.9–4.0), p<0.001], and 2017 [aOR = 2.66 (95% CI:2.21–3.20), p<0.001] compared to the same referent. Employed respondents were significantly more likely to have ever tested for HIV than those not employed, in 2005 [aOR = 1.50 (95% CI: 1.27–1.78), p<0.001], 2008 [aOR = 1.55 (95% CI: 1.30–1.83), p<0.001], 2012 [aOR = 1.3 (95% CI: 1.2–1.5), p<0.001], and 2017 [aOR = 1.34 (95% CI: 1.22–1.48, p<0.001]. Those with accurate knowledge of preventing the sexual transmission of HIV and rejection of misconceptions about HIV transmission were significantly more likely to have ever tested for HIV, in 2005 [aOR = 1.17 (95% CI: 1.00–1.36), p = 0.052], 2012 [aOR = 1.2 (95% CI: 1.1–1.4), p = 0.006], and 2017[aOR = 1.15 (95% CI: 1.05–1.25), p = 0.002]. The respondents who perceived themselves as being at risk of HIV infection were significantly more likely to have ever tested for HIV, in 2005 [aOR = 1.23 (95% CI: 1.05–1.44), p = 0.009], 2008 [aOR = 1.30 (95% CI: 1.10–1.52), p = 0.002], and 2017 [aOR = 1.40 (95% CI: 1.24–1.57), p<0.001], except in 2012 [aOR = 0.7 (95% CI: 0.6–0.8), p<0.001]. Those who tested HIV positive were significantly more likely to have ever tested for HIV, in 2005 [aOR = 1.27 (95% CI: 1.03–1.57), p = 0.027], 2008 [aOR = 1.57 (95% CI: 1.27–1.94), p<0.001], and 2012 [aOR = 1.7 (95% CI: 1.4–2.0), p<0.001], and in 2017 [aOR = 1.28 (95% CI: 1.13–1.54), p<0.001].
The decreased likelihood of being tested for HIV was significantly associated with those aged 50 years and older years than those aged 15–24 years, in 2005 [aOR = 0.43 (95% CI: 0.34–0.56), p<0.001], and 2008[aOR = 0.70 (95% CI: 0.57–0.86), p<0.001]. The decreased likelihood of ever having tested for HIV was significantly associated with being white in 2012 [aOR = 0.7 (95% CI: 0.6–0.9), p = 0.011], and 2017 [aOR = 0.49 (95% CI: 0.40–0.61), p<0.001] than Black Africans. In 2012, Indians/Asians were significantly less likely to ever have tested for HIV than Black Africans [aOR = 0.34 (95% CI: 0.28–0.40), p<0.001] and in 2017 [aOR = 0.34 (95% CI: 0.28–0.40), p<0.001]. Coloureds were significantly less likely to ever have tested for HIV than Black Africans in 2017 [aOR = 0.81 (95% CI: 0.71–0.92), p<0.001]. Those residing in rural informal areas were significantly less likely to have ever have tested for HIV in 2005 [aOR = 0.63(95% CI: 0.51–0.78), p<0.001], and 2017 [aOR = 0.80 (95% CI: 0.73–0.89), p<0.001] including those residing in rural formal areas in 2017 [aOR = 0.70 (95% CI: 0.61–0.79), p<0.001] compared to respondents from urban areas.
Discussion
This study reports results on HIV testing trends for those who have ever tested across four nationally representative surveys in the South African population aged 15 years and older.
The observed overall significant increase in youth and adults 15 years and older who have ever been tested for HIV is encouraging, especially among Black Africans. This suggests that the national HIV testing programmes are gradually reaching these two groups that are still the most vulnerable to HIV infection. There is also an indication that over time (between the study waves) the national HTS programme is reaching high-risk groups, especially those who are sexually active, those with older sexual partners and those who tested positive in the survey rounds. Scaling up access and outreach to testing among most at risk populations remains an important goal for universal access to treatment and support in South Africa [20].
Understanding the associations between ever having tested for HIV and these identified factors may help improve HIV testing policy and increase testing utilization, which together will lead to better control of the HIV epidemic in South Africa. The current findings revealed that over the past decade people aged 25–49 years were more likely to test for HIV than people aged 15–24 years. These findings are similar to the findings from other studies [21, 22]. This is not surprising, as older people would have had more opportunities to have ever tested compared to those who are younger. What is concerning is that younger people represent the largest proportion of new HIV cases in South Africa [15]. Providing increased access to HIV testing in educational settings will increase awareness of HIV status among the youth [23]. Studies have also shown that by including youth in the planning and development of HIV testing strategies and by providing youth friendly environments for HIV testing, increases the uptake of HIV testing in this age group [23, 24].
The findings also show that the proportion of people who have ever tested for HIV was lower among those residing in rural areas compared to residing in urban areas [25]. This could reflect poor programme outreach in rural areas due to the focus on urban areas as a result of the high prevalence of HIV in the urban areas. This disparity in service delivery is well documented in other studies [26]. There is need to expand services in rural areas in order to increase access to testing services for the rural population. Community-based approaches such as mobile health clinics and door-to-door campaigns have been shown to be successful in reaching populations that do not present at health facilities and where there are inadequate fixed facilities to provide HIV testing services [27–29].
The findings showed that HIV testing has increased substantially among Black Africans. This may be due to the targeted and/or focussed national testing efforts. This may reflect the success of the national HIV testing and counselling campaign launched in 2010, and the revitalised HIV testing services which focused on getting more people to test for HIV [8, 20]. However, there is a growing concern with regard to the Coloured race group, as there seems to be a brewing HIV epidemic among this population [15]. The changing racial dynamics over time suggest that efforts to get people to test have been uneven and need to be addressed. HIV testing services therefore need to be inclusive to prevent the oversight of other racial groups in providing this critical service across the board [30]. The proportion of Whites and Indian/Asians ever having tested for HIV increased like every other race group but not to the same extent, a 16.2% and 17.1% point increase respectively as compared to a 37.6% point increase for Coloureds and a 50.3% point increase for Black Africans. Previous research shows that other race groups are often reluctant to test, as they perceive themselves as not being at risk of HIV [14, 15].
In addition, the findings showed that marriage, education, and employment is associated with an increase in HIV testing. This is similar to other studies [31–33]. In South Africa, associations have also been shown between an individual’s socio-economic background and the likelihood to test for HIV [14, 15]. The findings indicate that there is a need to target people with no or little formal education and those not employed.
These findings also showed that those who tested positive in the survey rounds were more like to have tested for HIV. This is encouraging as awareness of HIV status among those who are HIV positive is essential to ensure that people living with HIV are supported and receive treatment.
Limitations
Some key limitations for our study include that data on HIV testing, socio-demographic and HIV related risk behaviours were collected using self-reports, and so were subject to social desirability and recall biases. Furthermore, each survey wave was cross-sectional in nature rather than longitudinal. The study is therefore limited to assessing the associations between HIV testing and potential determinants as one cannot infer causality among the variables studied. Nevertheless, the study provides nationally representative data on trends in ever testing for HIV that can be inferred to the general youth and adult population in South Africa.
Conclusion
It is encouraging that HIV testing uptake has increased significantly from 2005 to 2017 in South Africa. HIV testing programmes have reached those most at risk of HIV infection. However, more HIV testing opportunities in different settings that prompts both provider and client initiated approaches are required to ensure that low HIV testing groups are reached. The findings inform the need for a comprehensive strategy targeting young people, those living in rural areas, the never married, those with no formal education or low educational attainment, and the unemployed. Box 1 outline key challenges identified and recommendations for improving HIV testing.
Box 1. Key challenges to HIV testing and policy recommendations.
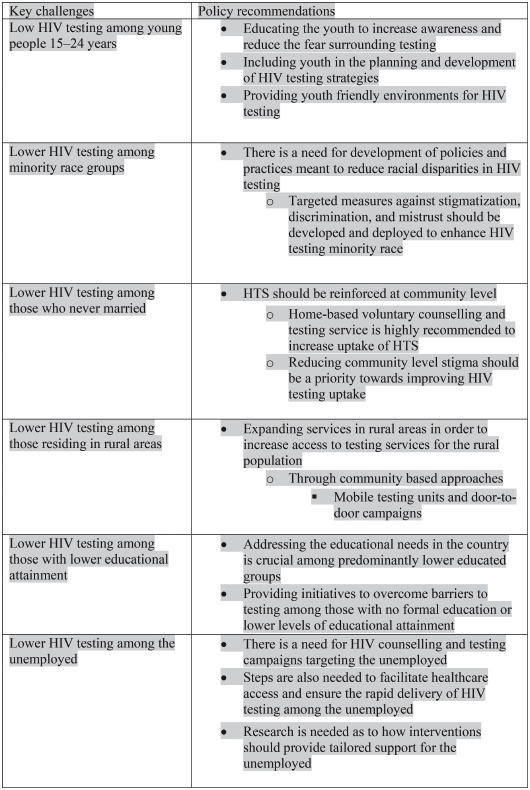 |
Supporting information
(ZIP)
Acknowledgments
We would like to thank study participants who willingly opened their doors and hearts to give us private information about themselves for the sake of contributing to a national effort to contain the spread of HIV. We are grateful to our international partners and our research consortium members for unwavering support. Finally yet importantly, we would like to thank the project team that made this survey a success.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The study was funded by the President’s Emergency Plan for AIDS Relief (PEPFAR) under the terms of 5U2GGH000570. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.UNAIDS. Ending AIDS: Progress Towards the 90-90-90 Targets. Geneva: Joint United Nations Programme on HIV/AIDS, 2017.
- 2.Hayes R, Sabapathy K, Fidler S. Universal testing and treatment as an HIV prevention strategy: research questions and methods. Current HIV research. 2011;9(6):429–45. Epub 2011/09/. 10.2174/157016211798038515 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adebayo OW, Gonzalez-Guarda RM. Factors Associated With HIV Testing in Youth in the United States: An Integrative Review. The Journal of the Association of Nurses in AIDS Care: JANAC. 2017;28(3):342–62. Epub 2016/12/21. 10.1016/j.jana.2016.11.006 . [DOI] [PubMed] [Google Scholar]
- 4.Detsis M, Tsioutis C, Karageorgos SA, Sideroglou T, Hatzakis A, Mylonakis E. Factors Associated with HIV Testing and HIV Treatment Adherence: A Systematic Review. Current pharmaceutical design. 2017;23(18):2568–78. Epub 2017/03/31. 10.2174/1381612823666170329125820 . [DOI] [PubMed] [Google Scholar]
- 5.UNAIDS. The Gap Report. Geneva: Joint United Nations Programme on HIV/AIDS: Joint United Nations Programme on HIV/AIDS, 2014.
- 6.UNAIDS. Miles to Go. Global AIDS Update. Geneva: Joint United Nations Programme on HIV/AIDS, 2018.
- 7.Lippman SA, El Ayadi AM, Grignon JS, Puren A, Liegler T, Venter WDF, et al. Improvements in the South African HIV care cascade: findings on 90-90-90 targets from successive population-representative surveys in North West Province. J Int AIDS Soc. 2019;22(6):e25295–e. 10.1002/jia2.25295 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.SANAC. Let Our Actions Count: South Africa’s National Strategic Plan for HIV, TB and STIs 2017–2022. Pretoria: South African National AIDS Council, 2017.
- 9.NDOH. National HIV testing services: Policy 2016. Pretoria: National Department of Health, 2016.
- 10.Houle B, Clark SJ, Gomez-Olive FX, Kahn K, Tollman SM. The unfolding counter-transition in rural South Africa: mortality and cause of death, 1994–2009. PLoS One. 2014;9(6):e100420 Epub 2014/06/25. 10.1371/journal.pone.0100420 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kabudula CW, Houle B, Collinson MA, Kahn K, Gomez-Olive FX, Clark SJ, et al. Progression of the epidemiological transition in a rural South African setting: findings from population surveillance in Agincourt, 1993–2013. BMC Public Health. 2017;17(1):424 Epub 2017/05/11. 10.1186/s12889-017-4312-x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shisana O, Rehle T, Simbayi LC, Parker R, Zuma K, Bhana A, et al. South African national HIV prevalence, HIV incidence, behaviour and communication survey. Cape Town: HSRC Press, 2005. [Google Scholar]
- 13.Shisana O, Rehle T, Simbayi LC, Zuma K, Jooste S, Pillay-van-Wyk V, et al. South African national HIV prevalence, incidence, behaviour and communication survey 2008: a turning tide among teenagers. Cape Town: HSRC Press, 2009. [Google Scholar]
- 14.Shisana O, Rehle T, Simbayi LC, Zuma K, Jooste S, Zungu N, et al. South African national HIV prevalence, incidence and behaviour survey, 2012. Cape Town: HSRC Press, 2014. [DOI] [PubMed] [Google Scholar]
- 15.Simbayi LC, Zuma K, Zungu N, Moyo S, Marinda E, Jooste S, et al. South African national HIV prevalence, incidence, behaviour and communication survey, 2017. Cape Town: HSRC Press, 2019. [Google Scholar]
- 16.StatsSA. Census in brief. Pretoria: Statistics South Africa, 2001.
- 17.StatsSA. Spatial Metadata. Pretoria: Statistics South Africa, 2011.
- 18.StatsSA. Mid-year population estimates 2017. Pretoria: Statistics South Africa, 2017.
- 19.Saunders JB, Aasland OG, Babor TF, De La Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption-II. Addiction. 1993;88(6):791–804. 10.1111/j.1360-0443.1993.tb02093.x [DOI] [PubMed] [Google Scholar]
- 20.NDOH. Health sector HIV prevention strategy. Pretoria: National Department of Health, 2017.
- 21.Asaolu IO, Gunn JK, Center KE, Koss MP, Iwelunmor JI, Ehiri JE. Predictors of HIV Testing among Youth in Sub-Saharan Africa: A Cross-Sectional Study. PloS one. 2016;11(10):e0164052–e. 10.1371/journal.pone.0164052 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mokgatle MM, Madiba S. High Acceptability of HIV Self-Testing among Technical Vocational Education and Training College Students in Gauteng and North West Province: What Are the Implications for the Scale Up in South Africa? PLOS ONE. 2017;12(1):e0169765 10.1371/journal.pone.0169765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller CL, Nkala B, Closson K, Chia J, Cui Z, Palmer A, et al. The Botsha Bophelo Adolescent Health Study: A profile of adolescents in Soweto, South Africa. 2017. 2017;18(1). Epub 2017-01-31. 10.4102/sajhivmed.v18i1.731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Denison JA, Pettifor A, Mofenson LM, Kasedde S, Marcus R, Konayuma KJ, et al. Youth engagement in developing an implementation science research agenda on adolescent HIV testing and care linkages in sub-Saharan Africa. AIDS (London, England). 2017;31 Suppl 3(Suppl 3):S195–S201. Epub 2017/07/01. 10.1097/QAD.0000000000001509 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaede B, Versteeg M. The state of the right to health in rural South Africa In: Padarath A, English R, editors. South African health review. Durban: Health Systems Trust; 2011. p. 99–106. [Google Scholar]
- 26.McLaren ZM, Ardington C, Leibbrandt M. Distance decay and persistent health care disparities in South Africa. BMC Health Services Research. 2014;14(1):541 10.1186/s12913-014-0541-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iwuji CC, Orne-Gliemann J, Larmarange J, Okesola N, Tanser F, Thiebaut R, et al. Uptake of Home-Based HIV Testing, Linkage to Care, and Community Attitudes about ART in Rural KwaZulu-Natal, South Africa: Descriptive Results from the First Phase of the ANRS 12249 TasP Cluster-Randomised Trial. PLOS Medicine. 2016;13(8):e1002107 10.1371/journal.pmed.1002107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Comulada WS, Wynn A, van Rooyen H, Barnabas RV, Eashwari R, van Heerden A. Using mHealth to Deliver a Home-Based Testing and Counseling Program to Improve Linkage to Care and ART Adherence in Rural South Africa. Prevention Science. 2018. 10.1007/s11121-018-0950-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geoffroy E, Schell E, Jere J, Khozomba N. Going door-to-door to reach men and young people with HIV testing services to achieve the 90-90-90 treatment targets. Public health action. 2017;7(2):95–9. Epub 2017/06/21. 10.5588/pha.16.0121 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maughan-Brown B, Lloyd N, Bor J, Venkataramani AS. Changes in self-reported HIV testing during South Africa’s 2010/2011 national testing campaign: gains and shortfalls. Journal of the International AIDS Society. 2016;19(1):20658 10.7448/IAS.19.1.20658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cremin I, Cauchemez S, Garnett GP, Gregson S. Patterns of uptake of HIV testing in sub-Saharan Africa in the pre-treatment era. Tropical Medicine & International Health. 2012;17(8):e26–e37. 10.1111/j.1365-3156.2011.02937.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Treves-Kagan S, El Ayadi AM, Pettifor A, MacPhail C, Twine R, Maman S, et al. Gender, HIV Testing and Stigma: The Association of HIV Testing Behaviors and Community-Level and Individual-Level Stigma in Rural South Africa Differ for Men and Women. AIDS and behavior. 2017;21(9):2579–88. 10.1007/s10461-016-1671-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matovu JKB, Denison J, Wanyenze RK, Ssekasanvu J, Makumbi F, Ovuga E, et al. Trends in HIV counseling and testing uptake among married individuals in Rakai, Uganda. BMC public health. 2013;13:618-. 10.1186/1471-2458-13-618 . [DOI] [PMC free article] [PubMed] [Google Scholar]



