Abstract
Objective
To examine the impact of flavor, device type, and health warning messages on youth preference for electronic nicotine delivery systems (ENDS), and to provide evidence and data to inform the FDA’s potential regulatory actions on ENDS.
Design:
An online discrete choice experiment (DCE) was conducted in September 2015. Each participant was given 9 choice sets and asked to choose one out of two alternative ENDS products, with varying characteristics in three attributes (flavor, device type and warning message). The impact of the attributes on the probability of choosing ENDS was analyzed using conditional and nested logit regressions, controlling for individual socio-demographic characteristics and current smoking status.
Setting and Participants
A general population sample of 515 participants (50 ever users and 465 never users of ENDS) aged 14–17 were recruited to complete the experiment using an online panel.
Results
Fruit/sweets/beverage flavors significantly increase the probability of choosing ENDS among youth (p<0.01 for never-users and <0.1 for ever-users) and flavor has the most pronounced impact among three attributes. Among never-users, menthol flavor also increases (p<0.05) the probability of choosing ENDS compared with tobacco flavor. Vaping devices that are modifiable, compared with cigarette-like e-cigarettes, increase (p<0.05) the probability of choosing ENDS among adolescent never-users. Warning messages reduce (p<0.01) the probability of choosing ENDS among never-users.
Conclusions and Relevance
Restricting fruit/sweets/beverage flavors in ENDS, regulating modifiable vaping devices, and adopting strong health warning messages may reduce the uptake of ENDS among youth.
Keywords: ENDS, public policy, priority/special populations, packaging and labelling, prevention
Introduction
Recent years have seen a striking increase in the use of electronic nicotine delivery systems (ENDS), particularly among youth and young adults.1,2 According to the National Youth Tobacco Surveys (NYTS), ENDS use among high school students increased from 1.5% in 2011 to 16.0% in 2015, rendering it the most-used tobacco product by this group. 3 ENDS use among middle school students showed a similar, but less dramatic, trend. Nevertheless, 5.3% of middle school students were currently using ENDS in 2015, which was the most-used tobacco product among this group as well.3
The 2009 Family Smoking Prevention and Tobacco Control Act (the TCA) gave the U.S. Food and Drug Administration (FDA) authority to regulate the manufacturing, distribution and marketing of tobacco products, including ENDS. The future FDA’s regulatory decisions will hinge on the net public health impact of ENDS, which is not well-studied. 1–7 On the one hand, nicotine has adverse effects on youth’s central nervous system8 and ENDS may become a gateway product for youth to transition into combustible tobacco products.9–12 As most tobacco use initiate at a young age,13,14 preventing youth from ever taking up ENDS is the key to future tobacco control and public health.2 On the other hand, from the perspective of the continuum of risks, because ENDS are less harmful than conventional combustible tobacco products, switching to ENDS may reduce the harmful health consequences of smoking.15–18 In the final deeming rule, the FDA considered these two aspects, and requested more evidence in each aspect.1
Flavors
Characterizing flavors in cigarettes other than menthol has been banned by the FDA since 2009. Flavors in ENDS, however, are a feature potentially salient to youth use and which remain unregularted.1 There is a great variety of flavors other than menthol or tobacco flavors in ENDS, including fruit, sweets, and beverage flavors.19–21 Sweet- and fruit- flavors have been shown to be very appealing to youth and young adults. 21–25 Beverage flavors in ENDS are often mixed drink flavors such as pina colada, mojito, and margarita, which are sweet and fruity as well and potentially appealing to young people.26
Flavor is a risk factor associated with ENDS initiation among youth and young adults. Compared to older adults, young people are more likely to use flavored ENDS at onset and preferred sweet flavors.21,22 For adolescents, flavors are reported as a common reason for them to experiment with or choose ENDS and the primary reason for their first try.27–29 This association between flavor and ENDS onset may be moderated by risk perceptions. Studies show that youth perceive fruit- and other flavored ENDS less harmful than tobacco flavored ones, and are more interested in trying menthol-, candy/sweet-, fruit- flavored ENDS compared with tobacco- flavored ones. 23–25 Marketing of flavored ENDS may also play a role in use onset among youth. One recent study shows that flavored ENDS ads were more appealing to youth than non-flavored ones, and that youth reported greater interest in buying and trying ENDS if they were exposed to ads of flavored ENDS.30
Nonetheless, characterizing flavors are not completely risk-free – they may contain chemicals that irritate respiratory systems.19,20 In addition, several cinnamon-flavored e-liquids contain a toxic chemical – cinnamaldehyde. 31 Menthol, coffee, and strawberry flavored aerosols were found to reduce cell viability and metabolic activity, with strawberry flavored products being the most toxic among all tested flavors. 32
Flavored ENDS use was also associated with susceptibility of cigarette smoking among youth non-smokers,33 suggesting the role of characterizing flavors in the possible “gateway effect” of ENDS. However, some evidence suggests that flavors may be an incentive for smokers to switch to ENDS, and thus may help them quit smoking combustible cigarettes.1,34,35 In light of insufficient evidence on this issue, the FDA specifically calls for additional data and research to address the effect of flavors on youth initiation, use, and dual use of ENDS and tobacco products.1
Device Types
Unlike other tobacco products, ENDS encompass a variety of devices that differ in shapes, sizes, and names. According to a systematic review by Glasser et al. (2017),36 ENDS devices can be broadly classified into subtypes ‘cigarette-like e-cigarettes’ type (including rechargeable and disposable) and the ‘modifiable vaping products’ type that is rechargeable, including e-go style (vape pens, vape pipes, e-cigars, e-hookahs, e-pipes) and ‘open tank’ style.37–39 Some e-go and open-tank style ENDS allow for personalization of devices and modifications of nicotine levels, as well as mixing of e-juices or flavors.
Studies further show that various device types may have differential appeals to different users. Modifiable or advance types were perceived by current ENDS users to be more effective in helping smoking abstinence and more satisfying.40 Established ENDS users were also more likely to use advance types than to use cigarette-like e-cigarettes.41 In contrast, ever users of ENDS were more likely to use “cigarette-like” devices than to use advance devices.41 It was also reported that ENDS users commonly initiated with “cigarette-like” devices and later transitioned into using more advanced types.42
Among US adolescents in Connecticut, rechargeable devices were shown to be more prevalent than disposable devices for both first use and current use of ENDS.43 However, it is unclear whether youth initiated ENDS by using “cigarette-like” e-cigarettes or advance types such as e-go and open-tank styles. In addition, very little is understood about how device types influence ENDS uptake and choices, in relation to other features such as flavors.
Warning Messages
Health warning messages are another important attribute that may influence youth choice of ENDS through impacting their belief and perceptions related to tobacco. 44,45 A review of the effectiveness of health warning messages concluded that comprehensive warning messages may prevent youth from smoking initiation.46 Recent experiments found similar evidence that Canadian youth and US young adults are less likely to choose ENDS if a health warning message is present.29,47
While the deeming rule requires a nicotine warning statement in ENDS ads and on their packages, the health warning message specified in the deeming rule is limited only to the addictive nature of nicotine.1 There is an additional health warning statement on the FDA Center for Tobacco Products (CTP) website that describes the potential risks and benefits of ENDS as unknown. Moreover, some ENDS products carry voluntary warnings that are deemed stronger than the one the FDA requires.48–50 For example, MarkTen, an ENDS brand owned by Altria Group, has a warning message that contains more risk information about ENDS use, such as “very toxic by inhalation” and “increase your heart rate and blood pressure”. 48–50 The effects of these warning messages, including the FDA required one and alternative warning messages, on youth uptake and choices of ENDS, remain unknown.51
This study aimed to better understand how different attributes influence youth’s decisions to choose ENDS by simultaneously analyzing the impacts of flavors, health warning messages and device types on ENDS choices, using an online Discrete Choice Experiment (DCE). DCE is a stated-preference technique that has been increasingly used in tobacco research in recent years.29,47,52–54 This method also allows us to compare the relative importance of flavors, health warning messages and device types, and thus identify the most salient attribute in youth ENDS choices for regulatory purposes. Finally, given that the existing DCE studies in ENDS use either come from Canada28 or solely focus on the US adult population,29,53,54 this study fills an important research gap by providing the first DCE study on the impact of product attributes on ENDS choices among US adolescents.
Participants
A general population sample of 515 adolescents, aged 14–17, were recruited through the KnowledgePanel, which used both probability-based and random-digit-dialing sampling to recruit panelists and their youth household members. In September 2015, adolescents were recruited through parents who were panelists and provided consents. Post-stratification weights were constructed to adjust for sample design and survey non-responses. Among the 515 adolescents, 50 were ever-users of ENDS and 465 were never-users. The incidence of ever-users is very close to that found in the Population Assessment of Tobacco and Health (PATH) Wave 1 survey, which was 11%. 55
Experiment Design
The design (Table 1) has one two-level attribute for device type (cigalike e-cigarettes, e-go/Mods/APVs), one three-level attribute for flavors (tobacco, menthol, and fruit/sweets/beverage), and one four-level attribute for warning messages (None, FDA proposed warning message, FDA CTP warning message, and MarkTen warning message from Altria Group). The levels of each attribute were chosen as they are distinctive from each other and may inform different regulatory actions1,26, 36,48–50, and together lead to 24 (2×3×4) possible hypothetical products.
Table 1,
Product attributes and corresponding levels.
| Attributes | Levels |
|---|---|
| Device type | 1. Cigarette-like e-cigarettes |
| 2. Vaping products i.e.: EGO style, Mods,& APVs which can be modified | |
| Flavor | 1. Tobacco |
| 2. Menthol | |
| 3. Fruit/Sweets/Beverage | |
| Warning | 1. None |
| 2. “WARNING A: This product contains nicotine derived from tobacco. Nicotine is an addictive chemical.” | |
| 3. “WARNING B: This product contains nicotine derived from tobacco. Nicotine is an addictive chemical. The FDA cautions that electronic vaping products have not been fully studied, so we currently don’t know the potential risks of e-cigarettes when used as intended, how much nicotine or other potentially harmful chemicals are being inhaled during use, or whether there are any benefits associated with using these products.” | |
| 4. “WARNING C: This product is not a smoking cessation product and has not been tested as such. This product is intended for use by persons of legal age or older, and not by children, women who are pregnant or breastfeeding, or persons with or at risk of heart disease, high blood pressure, diabetes, or taking medicine for depression or asthma. Nicotine is addictive and habit-forming, and is very toxic by inhalation, in contact with the skin, or if swallowed.” | |
Note: Warning A is the FDA-proposed warning which is slightly different from the one final rules require.
Warning B is the FDA CTP statement in 2014, which can be found at http://dracutps.org/sites/dracutsd/files/file/file/fda_public_health_focus_electronic_cigarettes.pdf. Warning C is the MarkTen warning.
In the next step, Sawtooth software and the Balanced Overlap method was used to select two hypothetical products to form the choice set or pair. For all participants regardless of their ENDS use history, these choice sets contain an opt-out option of not using any hypothetical ENDS. In addition, ever-users were given an additional opt-out option to choose their most-used ENDS product, and thereby chose among two hypothetical products and two opt-out options. An algorithm was used to guarantee that neither of the two hypothetical ENDS products are identical to their-most used ENDS product in those three attributes, while ensuring all ten versions of choice sets were asked to ever-user participants to preserve the integrity of the design. Examples of these choice sets can be found Figures 1 and 2.
Figure 1.
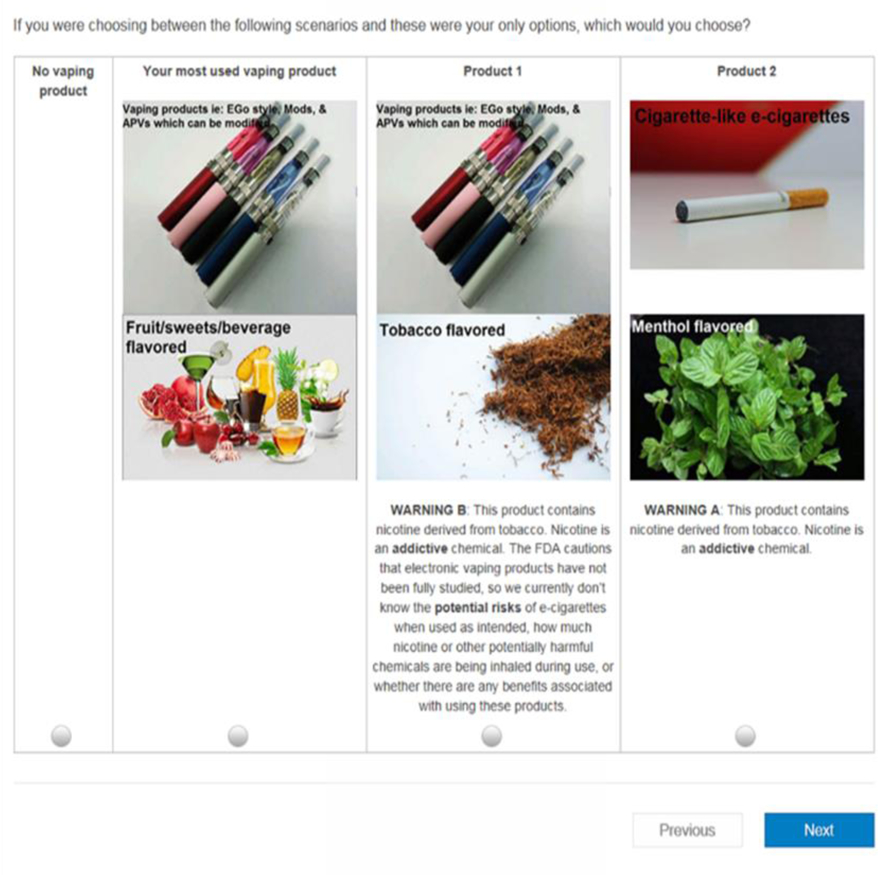
Example of a DCE choice set for ever-users of ENDS
Figure 2.
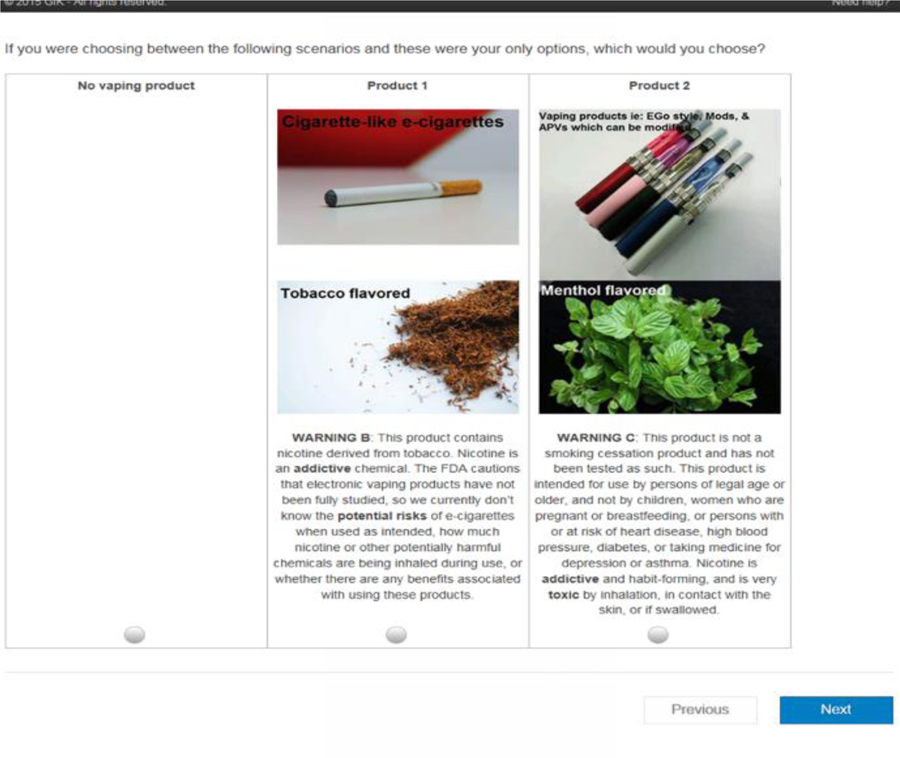
Example of a DCE choice set for never-users of ENDS
Finally, the design resulted in 90 unique choice sets with a D-efficiency of 0.98, very close to a fully balanced and orthogonal design that has a D-efficiency of 1. To avoid exhaustion and errors from making too many choices, 56 these choice sets were then divided into 10 versions, each containing 9 choice sets, and respondents were randomly assigned to answering one out of these 10 versions.
Methods and Analyses
Following previous studies, 29,47,52–54 conditional logit regressions were used to analyze the effects of flavors, warning messages and device types on the choice of using e-cigarettes. In addition, according to the power analysis described in de Bekker-Grob et al. (2015),57 both the sample size of ENDS never users and that of ever users exceed the sample size requirement to detect an effect size of 0.1 with a statistical power of 0.8 and a confidence level at 95%. Therefore, we analyzed ENDS never-user and ever-user samples separately. Furthermore, since we have a large sample of never-users, nested logit regressions were also used to analyze these attributes for this group.
Compared with conditional logit, nested logit makes the assumption that decision-making takes two steps: participants first choose between the “opt-out” option and hypothetical products, and then, conditional on choosing hypothetical products instead of opting out, choosing between the two hypothetical products. In other words, this method models decision trees with two branches, with one branch leading to opting out and the other leading to the use or uptake of a hypothetical product. (See Figures 1 and 2)52 Because never-users have not initiated ENDS use, their decision-making is more likely to follow this format, which is the rationale for estimating a nested logit model for never-users.
Two alternative specifications or models were employed to analyze the effects of these attributes on the probability of choosing ENDS. In the first model (Model A), the flavor attribute was constructed as an ordinal variable (1=tobacco, 2=menthol, 3=fruit/sweets/beverages), whereas in the second model (Model B), it was constructed as a dichotomous variable (0=tobacco and 1=menthol and fruit/sweets/beverage). In addition, in Model A, health warning messages were constructed as an ordinal variable (1=no warning messages, 2= FDA Deeming warning message, 3=FDA CTP warning message, and 4=MarkTen warning message); in Model B, health warning messages were constructed as a dichotomous variable (0=no warning message or FDA Deeming messages, and 1=FDA CTP and MarkTen warning messages). In both models, device type was constructed as a dichotomous variable with the value of 1 indicating e-Go/Mods/APVs style ENDS and the value of 0 indicating cigarette-like e-cigarettes.
In addition to these attributes, individual characteristics such as gender, age, race/ethnicity, family income, household size, parent’s education and current smoking status were controlled for in all analyses. Standard errors were clustered at the individual level to take account of the correlation among choices made by the same participant. It is also worth noting that both the conditional and nested logit models intrinsically assume independence of irrelevant alternatives (IIA) that the relative probability of choosing A over B is independent of an additional item C.52 We conducted Hausman and McFadden and likelihood-ratio tests to test this assumption. All analyses were conducted using Stata 14.
Results
Table 2 provides separate descriptive statistics for key variables by ENDS use status (never-users and ever-users), with detailed definitions of the variables presented in online supplemental table. For both groups, about half of the sample was male. On average, the participants were a little over 15 years old, living in a household with 4 people. The two most frequent parental education level were “<=12th grade or no diploma” (28%) and “high school graduate or diploma” (21%) for ever-users, and “high school graduate or diploma” (29%) and “bachelor’s degree” (25%) for never-users, respectively. The two most frequent family income levels among never-users of ENDS were $85,000–$124,999 (25%) and <$40,000 (24%), whereas among ever-users, they were <$40,000 (52%), and $60,000–$84,999 (19%). The composition of race/ethnicity was similar between the two groups: 50–56% of both samples were White, Non-Hispanic; 21–28% were Hispanic; 13–14% were Black, Non-Hispanic; and the rest were Non-Hispanics of other or multiple races. The only significant difference between the two samples was the prevalence of currently smoking cigarettes, which was 42% among ever-users, whereas among never-users, it was only 4%.
Table 2,
Summary Statistics (Total N=515)
| Mean[SD] or Proportion | Never-users (N=465) | Ever-users (N=50) |
|---|---|---|
| Male | 0.50 | 0.54 |
| Age | 15.54 [1.14] | 15.45 [0.95] |
| Family income | 12.69 [4.11] | 10.42 [4.54] |
| <$40,000 | 0.24 | 0.52 |
| $40,000–$59,999 | 0.16 | 0.11 |
| $60,000–$84,999 | 0.20 | 0.19 |
| $85,000–$124,999 | 0.25 | 0.11 |
| $125,000–$174,999 | 0.10 | 0.04 |
| ≥$175,000 | 0.05 | 0.04 |
| Parent’s Education | 10.37 [2.08] | 9.60 [2.34] |
| <=12th grade no diploma | 0.09 | 0.28 |
| high school graduate/diploma | 0.29 | 0.21 |
| some college/no degree | 0.16 | 0.17 |
| associate degree | 0.08 | 0.09 |
| bachelor’s degree | 0.25 | 0.15 |
| master degree | 0.10 | 0.06 |
| professional/doctorate degree | 0.04 | 0.04 |
| Household size | 4.47 [1.52] | 4.20 [1.43] |
| Currently Smoking | 0.04 | 0.42 |
| White, Non-Hispanic | 0.56 | 0.50 |
| Black, Non-Hispanic | 0.13 | 0.14 |
| Other, Non-Hispanic | 0.05 | 0.02 |
| Hispanic | 0.21 | 0.28 |
| 2+ races, Non-Hispanic | 0.04 | 0.06 |
Note: Samples were weighted to be representative of a general youth population. Family income was measured using an ordinal variable with 19 levels). Parent’s education (highest degree received) was measured using an ordinal variable with 12 levels
Table 3 shows the results of the analyses for ENDS ever-users for both of the two specifications discussed in the Method section. The sample contains 1,800 observations, generating from 50 respondents choosing among 4 options for 9 times (50×9×4). Both models suggest that flavors marginally (p<0.1) increase the likelihood of choosing ENDS. Results from Model B further suggest that, compared with tobacco flavor, the fruit/sweets/beverage flavor marginally significantly increases (p<0.1) the probability of choosing an ENDS product, whereas menthol flavor does not. In addition, device types and warning messages do not significantly influence ever-users’ choice of an ENDS product. The IIA test did not reject the null hypothesis that the IIA assumption holds.
Table 3,
the Effects of Attributes on the Probability of Choosing ENDS – Ever ENDS users, conditional logit regressions.
| Attributes | Model A (1) | Attributes | Model B (2) |
|---|---|---|---|
| Flavor | Flavor | ||
| Flavor (ordinal) |
0.682* (0.376) | Tobacco (omitted) | -- |
| -- | Menthol | 0.065 (0.680) |
|
| -- | Fruit/Sweets/Beverage | 1.277* (0.699) |
|
| Device types | Device types | ||
| Cig-like e-cigs (omitted) | -- | Cig-like e-cigs (omitted) | -- |
| EGO/Mods/APVs | 0.188 (0.426) |
EGO/Mods/APVs | 0.182 (0.427) |
| Warning | Warning | ||
| Warning (ordinal) | No warning or FDA messages (omitted) | -- | |
| 0.354 (0.328) |
FDA/CTP Statement or MarkTen | 0.530 (0.731) |
|
| N | 1,800 | N | 1,800 |
Note:
p<0.1. Samples were weighted to be representative of a general youth population. Clustered S.E. are in parentheses. For the sake of convergence, Race/Ethnicity was controlled for using a dichotomous indicator for White instead of a group of dummy variables. Model A: Hausman-McFadden Chi2=5.76, p=0.93. Model B: Hausman-McFadden Chi2=11.78, p=0.55.
Results of the analyses for ENDS never-users are presented in Table 4. Both conditional and nested logit were analyzed in two models. Since each of 465 respondents chose among 3 options (two hypothetical ENDS products and the opt-out option (not using ENDS)) for 9 times, the original sample size was 12,555 (465×9×3). After dropping the non-responses and skipped choices, the final analytical sample contains 12,525 observations. About 84% of youth never-users always chose not to use any product, whereas only 34% of youth ever-users always chose not to use any product. The IIA test did not reject the null hypothesis that the IIA assumption holds at the 5% level.
Table 4,
the Effects of Attributes on the Probability of Choosing ENDS – Never-users of ENDS
| Attributes | Model A |
Attributes | Model B |
||
|---|---|---|---|---|---|
| CL (1) | NL (2) | CL(3) | NL (4) | ||
| Flavor | Flavor | ||||
| Flavor (ordinal) | 0.497*** (0.112) |
0.280** (0.133) |
Tobacco (omitted) | -- | -- |
| -- | -- | Menthol | 0.443** (0.209) |
0.270** (0.136) |
|
| -- | -- | Fruit/Sweets/Beverage | 0.980*** (0.232) |
0.601** (0.250) |
|
| Device type | Device type | ||||
| Cig-like e-cigs (omitted) | -- | -- | Cig-like e-cigs (omitted) | -- | -- |
| EGO/Mods/APVs | 0.232* (0.131) |
0.168** (0.083) |
EGO/Mods/APVs | 0.221* (0.132) |
0.171** (0.087) |
| Warning | Warning | -- | -- | ||
| Warning (ordinal) | No warning or FDA messages (omitted) | ||||
| −0.143* | −0.072* | FDA/CTP Statement or MarkTen | −0.328*** | −0.178* | |
| (0.078) | (0.044) | (0.127) | (0.100) | ||
| N | 12,525 | 12,525 | N | 12,525 | 12,525 |
Note: Samples were weighted to be representative of a general youth population.
p<0.01,
p<0.05,
p<0.1. Clustered S.E. are in parentheses. Race/Ethnicity was controlled for using a group of dummy variables with White, Non-Hispanic as omitted category. Model A: CL Hausman-McFadden chi2=7.78, p=1.00; NL chi2=4.55, p=0.10. Model B: CL Hausman-McFadden chi2=23.40, p=0.50; NL Chi2=5.09, p=0.08.
For ENDS never-users, conditional logit regression results (Column 3) suggest that, compared with tobacco flavor, both menthol (p<0.05) and fruit/sweets/beverage (p<0.01) flavors significantly increase the probability of choosing ENDS, with the latter having a larger impact. Vaping devices that are modifiable, compared with cigarette-like e-cigarettes, increase (p<0.1 in Columns 1 and 3; p<0.05 in Columns 2 and 4) the probability of choosing ENDS among adolescent never-users. Warning messages measured in ordinal levels marginally (p<0.1 in Columns 1 and 2) reduce the probability of choosing ENDS. In addition, FDA CTP statement and MarkTen warning messages, compared to FDA-proposed warning messages or no warning message, (p<0.01; Column 3) significantly decrease the probability of choosing ENDS among never-users.
Although the coefficient estimates in these models do not provide a direct interpretation of the effect size,53 their relative magnitudes illustrate the relative impact of different attributes. A comparison indicates that among never-users, fruit/sweet/beverage flavor has the largest impact on ENDS choices, followed by menthol flavor, FDA-CTP/MarkTen warning messages, and modifiable devices.
Conclusions and Discussion
Our findings have several important policy implications. First, corroborating the previous findings on the role of characterizing flavors in increasing smoking initiation and escalation among youth and young adults,21–25,27–30 we found that flavors likely play a similar role in ENDS uptake and preference among youth. Specifically, both menthol and fruit/sweets/beverage flavors increase the probability that a youth never-user chooses ENDS. In addition, Fruit/sweets/beverage flavors may also matter to youth ENDS ever users. Therefore, regulating characterizing flavors could have the potential to reduce youth initiating ENDS.
Modifiable vaping devices, compared with cigarette-like e-cigs, also increase the probability that a youth never-user chooses ENDS. This is consistent with existing evidence that youth ENDS users tend to initiate with rechargeable ENDS.43 This finding further adds to the evidence that youth ENDS never- users show more interest in advanced or modifiable device types for their first try. Unlike adult smokers who may initiate cigarette-like e-cigarettes that mimic cigarettes,58 youth may consider vaping devices more acceptable than cigarette-like e-cigs that may be associated with smoking. Their preference for advanced devices is also independent from their preference for flavors.
Finally, youth never-users were responsive to health warning messages, suggesting that warnings may be effective in deterring youth uptake of ENDS. This finding is consistent with a Canadian study that shows youth non-smokers, compared with youth smokers and adults, are more likely to choose ENDS when there are no warning messages.29 Our results also suggest that, compared with no warnings or FDA Deeming warning messages, FDA CTP statement and MarkTen warning messages reduce the probability that a never-user would choose ENDS. This finding is consistent with a recent focus group study showing that participants considered MarkTen warnings to be stronger that a nicotine statement.49 It also corroborates recent evidence that young adult smokers, compared with older adult smokers, are less likely to choose ENDS when exposed to strong health warning messages.47 The combined evidence suggests that the warning message required by the FDA may deter youth uptake of ENDS, but stronger warnings could have a greater impact.
Our research has several limitations. DCE is a method based on hypothetical choices, and thus contains bias that deviates from behaviors in reality. About 10% participants completed the survey in less than 8 minutes which may render their answers less reliable. Nonetheless, the results and conclusions still hold after dropping these participants who finished the survey relatively quickly. The demographic group was limited to a general population sample of youth aged 14–17 and did not include young adults who are also at a high risk of initiating ENDS. We also did not include prices in the attributes to reduce the burden on youth participants. But prices can be an important attribute and should be further studied in future youth DCEs. Finally, future studies may consider conducting DCEs among subgroups stratified by gender, age, and ethnicities.
Findings from this study nonetheless fill an important research gap by providing the first evidence of simultaneous consideration of flavors, warning messages and device types on youth choices of ENDS in the U.S.53 Together with other studies, 47,53 our findings shed light on potential consequences of future FDA regulatory actions on ENDS related to flavors, warning messages, and device types. From the perspective of population health, a regulation that has a positive impact on both cessation and initiation (increasing the probability of quitting a tobacco product among current users, while simultaneously decreasing the probability of initiation among never users) is preferred. However, when a regulation imposes opposite impacts on cessation and initiation, the benefits from one outcome should be weighed against the costs from the other outcome.
In addition, how different populations value ENDS attributes differently is center to the consideration of a regulation. The net public health benefits may be achieved through regulating attributes that are more important to ENDS initiation than to adult smokers’ transition to ENDS, especially when implemented with other policies that incentivize them to quit smoking or switch to ENDS. This implies a potential opportunity to achieve positive public health benefits by regulating characterizing flavors, as they are the most important to youth among the three attributes, yet may not be as important to adult smokers in their quitting or switching behaviors.47,53 Similarly, regulating modifiable device types and strong warning messages may be effective in deterring youth never users from choosing ENDS if these policies do not deter adult smokers to switch from combustible cigarettes to ENDS. 49, 51 Future studies may focus on product standards and how to better communicate warning messages to youth through stronger or more visible warnings on ENDS packages or in advertisements.
Findings from this study may also inform ENDS regulatory policies in other counties where the products are becoming more popular. In particular, many European Union (EU) member states have requested health warnings,59 which may deter youth from initiating ENDS use. Future consideration of regulating charactering flavors may further reduce the likelihood of ENDS initiation among youth in these countries.
Supplementary Material
What this study adds.
What is already known on this subject:
Characterizing flavors, device type, and health warning messages may be associated with youth preference for electronic nicotine delivery systems (ENDS).
What important gaps in knowledge exist on this topic:
It is unclear how characterizing flavors, device type, and health warning messages simultaneously influence ENDS choices among US adolescents.
There is no evidence on the relative effects of these three attributes on youth initiation.
What this study adds:
Characterizing flavors have the most pronounced impact among three attributes.
Restricting fruit/sweets/beverage flavors in ENDS, regulating device type, and adopting strong health warning messages may reduce the uptake of ENDS among youth.
Acknowledgement:
GfK was paid for data collection. Ce Shang had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding statement:
This study was funded by the NCI U01CA154254 and 1U01CA154248–04.
Footnotes
Conflicts of interest: none.
Ethics approvals:
The experiment and survey were conducted with ethics clearance from the institutional review boards/ethics committee at the University of Illinois at Chicago.
References
- 1.Department of Health and Human Services, Food and Drug Administration, 21 CFR Parts 1100, 1140, and 1143 [Docket No. FDA–2014–N–0189], RIN 0910–AG38. Deeming Tobacco Products To Be Subject to the Federal Food, Drug, and Cosmetic Act, as Amended by the Family Smoking Prevention and Tobacco Control Act; Restrictions on the Sale and Distribution of Tobacco Products and Required Warning Statements for Tobacco Products, ACTION: Final rule. Available at: http://www.fda.gov/TobaccoProducts/Labeling/RulesRegulationsGuidance/ucm394909.htm. Accessed June 7th, 2016. [PubMed]
- 2.U.S. Department of Health and Human Services. E-Cigarette Use Among Youth and Young Adults. A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2016. [Google Scholar]
- 3.Singh T, Kennedy S, Marynak K, et al. Characteristics of Electronic Cigarette Use Among Middle and High School Students - United States, 2015. Morbidity and Mortality Weekly Report, 2016. December 30;65(5051):1425–1429. 10.15585/mmwr.mm655051a2. Available at https://www.cdc.gov/mmwr/volumes/65/wr/mm655051a2.htm [DOI] [PubMed] [Google Scholar]
- 4.The Wild West of e-cigarettes just ended with a new, sweeping federal rule. Available at: http://www.vox.com/2016/5/5/11595784/fda-rule-e-cigarettes-tobacco. Accessed June 7th, 2016.
- 5.Nutt DJ, Phillips LD, Balfour D, et al. Estimating the Harms of Nicotine-Containing Products Using the MCDA Approach. European Addiction Research, 2014; 20(5): 218–225. 10.1159/000360220. [DOI] [PubMed] [Google Scholar]
- 6.Riccardo RP. E-Cigarettes: Public Health England’s Evidence-Based Confusion. The Lancet. 2015; 386 (10000): 1237–1238. 10.1016/S0140-6736(15)00133-6. [DOI] [PubMed] [Google Scholar]
- 7.McKee M, Capewell S Evidence about Electronic Cigarettes: A Foundation Built on Rock or Sand? BMJ, 2015; 351: h4863 10.1136/bmj.h4863. [DOI] [PubMed] [Google Scholar]
- 8.U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress. A Report of the Surgeon General. 2014; Available at http://www.surgeongeneral.gov/library/reports/50-years-of-progress/full-report.pdf.
- 9.Bunnell RE, Agaku IT, Arrazola RA, et al. Intentions to Smoke Cigarettes Among Never-Smoking US Middle and High School Electronic Cigarette Users: National Youth Tobacco Survey, 2011–2013. Nicotine & Tobacco Research. 2015; 17(2):228–235. 10.1093/ntr/ntu166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leventhal AM, Strong DR, Kirkpatrick MG, et al. Association of Electronic Cigarette Use With Initiation of Combustible Tobacco Product Smoking in Early Adolescence. Journal of the American Medical Association. 2015; 314(7):700–707. 10.1001/jama.2015.8950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barrington-Trimis JL, Urman R, Berhane K et al. E-Cigarettes and Future Cigarette Use. Pediatrics. 2016: e20160379 10.1542/peds.2016-0379. [DOI] [PMC free article] [PubMed]
- 12.McCabe SE, Veliz P, McCabe VV, et al. Smoking behaviors and intentions among current e-cigarette users, cigarette smokers, and dual users: A national survey of U.S. high school seniors. Preventive Medicine 2017. June;99:228–235. 10.1016/j.ypmed.2017.02.025. Epub 2017 Mar 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eissenberg T & Balster R Initial Tobacco Use Episodes in Children and Adolescents: Current Knowledge, Future Directions. Drug and Alcohol Dependence.2000, 59, 41–60. [DOI] [PubMed] [Google Scholar]
- 14.Shang C The Effect of Smoke-Free Air Law in Bars on Smoking Initiation and Relapse among Teenagers and Young Adults. Int. J. Environ. Res. Public Health 2015, 12(1), 504–520; 10.3390/ijerph120100504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McNeill A, Brose LS, Calder R, et al. E-Cigarettes: An Evidence Update. Public Health England. 2015.
- 16.Hajek P, Etter JF, Benowitz N, et al. Electronic Cigarettes: Review of Use, Content, Safety, Effects on Smokers and Potential for Harm and Benefit. Addiction. 2014; 109(11): 1801–1810. 10.1111/add.12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention, ‘‘Electronic Cigarette Use Among Adults: United States, 2014,’’ National Center for Health Statistics (NCHS) Data Brief, 2015; No. 217.
- 18.Farsalinos KE, Romagna G, Tsiapras D, et al. Characteristics, Perceived Side Effects and Benefits of Electronic Cigarette Use: A Worldwide Survey of More than 19,000 Consumers. International Journal of Environmental Research and Public Health. 2014; 11(4):4356–4373. 10.3390/ijerph110404356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farsalinos KE, Kistler KA, Gilman G, et al. Evaluation of Electronic Cigarette Liquids and Aerosol for the Presence of Selected Inhalation Toxins. Nicotine & Tobacco Research. 2015; 17(2):168–174. 10.1093/ntr/ntu176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allen JG, Flanigan SS, LeBlanc, et al. Flavoring Chemicals in E-Cigarettes: Diacetyl, 2, 3 - Pentanedione, and Acetoin in a Sample of 51 Products, Including Fruit-, Candy-, and Cocktail-Flavored E-Cigarettes. Environ Health Perspect. 2015. 10.1289/ehp.1510185. [DOI] [PMC free article] [PubMed]
- 21.Stanton CA, Villanti AC, Watson C, et al. Flavoured tobacco products in the USA: synthesis of recent multidiscipline studies with implications for advancing tobacco regulatory science. Tobacco Control. 2016. November;25(Suppl 2):ii1–ii3. 10.1136/tobaccocontrol-2016-053486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrell MB, Weaver SR, Loukas A, et al. 2017 Flavored e-cigarette use: Characterizing youth, young adult, and adult users. Prev Med Rep. 2016. November 11;5:33–40. eCollection 2017 Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooper M, Harrell MB, Pérez A, et al. Flavorings and Perceived Harm and Addictiveness of E-cigarettes among Youth. Tobacco Regulatory Science, Volume 2, Number 3 Tobacco Regulatory Science, Volume 2, Number 3, July 2016, pp. 278–289(12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ford A, MacKintosh AM, Bauld L, et al. Adolescents’ responses to the promotion and flavouring of e-cigarettes. Int J Public Health. 2016. March;61(2):215–24. 10.1007/s00038-015-0769-5. Epub 2015 Dec 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pepper JK, Ribisl KM, Brewer NT. Adolescents’ interest in trying flavoured e-cigarettes. Tob. Control 2016;25:ii62–ii66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackler RK, VanWinkle CK, Bumanlag IM, et al. Alcohol-flavoured tobacco products. Tobacco Control Published Online First: 07 June 2017. 10.1136/tobaccocontrol-2016-053609 [DOI] [PubMed]
- 27.Ambrose BK, Day HR, Rostron B, et al. Flavored Tobacco Product Use Among U.S. Youth Aged 12–17 Years, 2013–2014. Journal of the American Medical Association. 2015; 314(17):1871–1873. 10.1001/jama.2015.13802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kong G, Morean ME, Cavallo DA, et al. Reasons for Electronic Cigarette Experimentation and Discontinuation among Adolescents and Young Adults. Nicotine & Tobacco Research. 2014; ntu257 10.1093/ntr/ntu257. [DOI] [PMC free article] [PubMed]
- 29.Czoli CD, Goniewicz M, Islam T, Kotnowski K, & Hammond D Consumer preferences for electronic cigarettes: results from a discrete choice experiment. Tobacco Control. 2015. 10.1136/tobaccocontrol-2015-052422. [DOI] [PubMed]
- 30.Vasiljevic M, Petrescu DC, Marteau TM. Impact of advertisements promoting candy-like flavoured e-cigarettes on appeal of tobacco smoking among children: an experimental study. Tobacco Control 2016;25:e107–e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Behar RZ, Davis B, Wang Y, et al. Identification of Toxicants in Cinnamon-Flavored Electronic Cigarette Refill Fluids. Toxicology In Vitro. 2014; 28(2):198–208. 10.1016/j.tiv.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 32.Leigh NJ, Lawton RI, Hershberger PA, et al. Flavourings significantly affect inhalation toxicity of aerosol generated from electronic nicotine delivery systems (ENDS). Tobacco Control 2016;25:ii81–ii87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen JC, Das B, Mead E, et al. Flavored E-cigarette Use and Cigarette Smoking Susceptibility among Youth. Tobacco Regulatory Science, Volume 3, Number 1, January 2017, pp. 68–80(13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farsalinos KE, Romagna G, Tsiapras D, et al. Impact of Flavour Variability on Electronic Cigarette Use Experience: An Internet Survey. International Journal of Environmental Research & Public Health. 2013; 10(12): 7272–7282. 10.3390/ijerph10127272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barbeau AM, Burda J, Siegel M Perceived Efficacy of E-Cigarettes Versus Nicotine Replacement Therapy Among Successful E-Cigarette Users: A Qualitative Approach. Addiction Science & Clinical Practice. 2013; 8(1): 5 10.1186/1940-0640-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glasser AM, Collins L, Pearson JL,et al. Overview of Electronic Nicotine Delivery Systems: A Systematic Review. American Journal of Preventive Medicine, Volume 52, Issue 2, February 2017, Pages e33–e66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown CJ, Cheng JM. Electronic cigarettes: product characterization and design considerations. Tob Control. 2014; 23(Suppl 2): ii4–10. 10.1136/tobaccocontrol-2013-051476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wollscheid KA, Kremzner ME. Electronic cigarettes: Safety concerns and regulatory issues. Am J Health Syst Pharm. 2009; 66(19): 1740–2. 10.2146/ajhp090127. [DOI] [PubMed] [Google Scholar]
- 39.Cheah NP, Chong NW, Tan J, Morsed FA, Yee SK. Electronic nicotine delivery systems: regulatory and safety challenges: Singapore perspective. Tob Control. 2014; 23(2):119–25. 10.1136/tobaccocontrol-2012-050483. [DOI] [PubMed] [Google Scholar]
- 40.Etter JF. Addiction, 2016 Characteristics of users and usage of different types of electronic cigarettes: findings from an online survey. [DOI] [PubMed]
- 41.Giovenco DP, Lewis MJ, Delnevo CD. Factors associated with e-cigarette use: a national population survey of current and former smokers. Am J Prev Med. 2014;47(4):476–480. 10.1016/j.amepre.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yingst JM, Veldheer S, Hrabovsky S, Nichols TT, Wilson SJ, & Foulds J (2015). Factors Associated With Electronic Cigarette Users’ Device Preferences and Transition From First Generation to Advanced Generation Devices. Nicotine & Tobacco Research, 17(10), 1242–1246. 10.1093/ntr/ntv052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krishnan-Sarin S, Morean ME, Camenga DR, et al. E-cigarette Use Among High School and Middle School Adolescents in Connecticut. Nicotine & Tobacco Research. 2015;17(7):810–818. 10.1093/ntr/ntu243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goodall C, Appiah O. Adolescents’ Perceptions of Canadian Cigarette Package Warning Labels: Investigating the Effects of Message Framing. Health Communication Vol. 23, Iss. 2,2008 [DOI] [PubMed] [Google Scholar]
- 45.White V, Webster B Wakefield M (2008), Do graphic health warning labels have an impact on adolescents’ smoking-related beliefs and behaviours?. Addiction, 103: 1562–1571. [DOI] [PubMed] [Google Scholar]
- 46.Hammond D Health warning messages on tobacco products: a review. Tob Control. 2011. September;20(5):327–37. 10.1136/tc.2010.037630. [DOI] [PubMed] [Google Scholar]
- 47.Pesko MF, Kenkel DS, Wang H, & Hughes JM The effect of potential electronic nicotine delivery system regulations on nicotine product selection. Addiction. 2016; 111(4): 734–44. 10.1111/add.13257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shang C, Chaloupka FJ The Trend of Voluntary Warnings in Electronic Nicotine Delivery System Magazine Advertisements Int. J. Environ. Res. Public Health 2017, 14(1), 62; 10.3390/ijerph14010062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wackowski OA; Hammond D; O’Connor RJ; Strasser AA; Delnevo CD Smokers’ and e-cigarette users’ perceptions about e-cigarette warning statements. Int. J. Environ. Res. Public Health 2016, 13, 655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dire Warnings by Big Tobacco on E-Smoking. Available at: http://mobile.nytimes.com/2014/09/29/business/dire-warnings-by-big-tobacco-on-e-smoking-.html?partner=rss&emc=rss&smid=tw-nytimes&_r=0&referrer=. Accessed June 7th, 2016
- 51.Wackowski OA, Hammond D, O’Connor RJ, et al. Considerations and Future Research Directions for E-Cigarette Warnings—Findings from Expert Interviews. Int. J. Environ. Res. Public Health 2017, 14(7), 781; 10.3390/ijerph14070781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marti J Assessing preferences for improved smoking cessation medications: a discrete choice experiment. European Journal of Health Economics. 2012; 13(5), 533–548. 10.1007/s10198-011-0333-z. [DOI] [PubMed] [Google Scholar]
- 53.Marti J, Buckell J, Maclean J, Sindelar J To vape or smoke? A discrete choice experiment among US adult smokers. NBER working paper. 2016; No. w22079. http://www.nber.org/papers/w22079 Accessed June 7th, 2016.
- 54.Salloum RG, Maziak W, Hammond D, et al. Eliciting preferences for waterpipe tobacco smoking using a discrete choice experiment: implications for product regulation. BMJ Open. 2015; 5(9). 10.1136/bmjopen-2015-009497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hyland A, Conway K, Borek N, et al. , on behalf of the PATH Study Team. Highlighted Findings From Wave 1 of the Population Assessment of Tobacco and Health (PATH) Study. March 2016. 2016 SRNT Plenary Chicago, Illinois. [Google Scholar]
- 56.Bridges JF, Hauber AB, Marshall D, et al. Conjoint analysis applications in health--a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value in Health. 2011; 14(4): 403–413. 10.1016/j.jval.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 57.de Bekker-Grob EW, Donkers B, Jonker MF, Stolk EA. Sample Size Requirements for Discrete-Choice Experiments in Healthcare: a Practical Guide. Patient. 2015; 8:373–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McQueen A, Tower S, Sumner W. Interviews with “vapers”: implications for future research with electronic cigarettes. Nicotine Tob Res. 2011;13(9) 860–867. 10.1093/ntr/ntr088. [DOI] [PubMed] [Google Scholar]
- 59.O’Leary R, Borland R, Stockwell T, et al. Claims in vapour device (e-cigarette) regulation: A Narrative Policy Framework analysis. International Journal of Drug Policy 2017 44 (2017) 31–40 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


