Abstract
Objectives:
Accurate estimates of ASD-associated medical costs are essential for predicting future care needs, allocating resources, identifying best practices, and modelling cost-effectiveness. Most existing studies either employed subjective cost data and/or ascertained ASD using self-reported or ICD-coded diagnoses. Such ascertainment is especially problematic for identifying milder ASD among older individuals never diagnosed with ASD.
Methods:
This 1976–2000 population-based birth-cohort study was set in Olmsted, County, MN. ASD cases and age- sex-matched unaffected controls were identified by applying uniform operational-research criteria for ASD (using DSM-IV-TR guidelines) following rigorous review of provider-linked medical and public/private/home-school records available for all members from birth to maximum age 21 years. Medical-cost estimates for the 901 case-control pairs used line-item provider-linked billing data (including all payers) from 2003–2014 (ages 3–38 years). Out-patient pharmaceutical costs were unavailable. Temporal changes in diagnostic criteria/clinical practice/public awareness/access were addressed by separating analyses into 5-year age-group/4-year calendar-period cells. Unadjusted and adjusted (age and age plus co-occurring conditions) cost estimates were provided for cases, controls, and case-control differences. Additional factors (co-occurring conditions/percent hospitalized/intellectual disability) were investigated using unadjusted descriptive analyses.
Results:
Cell sample sizes ranged 93–402 for age-groups 3–19 years and 45–395 for age-groups 20–38 years. Unadjusted, age-adjusted, and fully-adjusted medical-costs were significantly higher for cases versus controls in 100% of cells for age-groups 3–19 years; and in 50% (unadjusted), 38% (age-adjusted), and 12% (fully-adjusted) of cells for age-groups 20–38 years.
Conclusions:
These unique estimates can help inform construction of cost-effectiveness models, decisions by payers/providers/policy-makers, and predictions of lifetime costs.
Précis:
Uniform operational-research criteria for identifying ASD during childhood plus objective medical-costs provide reliable overall and incremental medical-cost estimates for ages 3 through 38 years.
INTRODUCTION
Autism Spectrum Disorder (ASD) affects a broad range of individuals across multiple domains (personal/educational/societal/etc.),1,2 each with considerable economic consequences.1,3–18 While non-healthcare costs contribute most of the economic burden,1,3,5,16,18,19 numerous cost-of-illness investigations reveal autism is also associated with substantially higher direct-medical costs;3,6,7–14 published estimates vary widely, with cases 2- to 10-fold higher than controls.7–11,14 Differences also exist regarding contributions of age and co-occurring conditions.3–5,12,14,15,20 Between-study variability reflects disparate questions/rationales/audiences. Patient and cost-data sources differ depending on the perspective (society, government-insurer/private-insurer/provider/employer/individual). The choice between prevalence versus incidence data depends on whether short-term or lifetime estimates are desired. Consideration of controls and/or adjustment for co-occurring conditions differs depending on whether overall- or incremental-costs are preferred.21,22
Between-study differences can also reflect data limitations. Few investigations contained both objective cost estimates and reliable case-ascertainment. Cost estimates derived from self/parental report of service type and frequency are subject to participation and recall bias.24 While more objective cost estimates were afforded using administrative data, the vast majority of such studies relied solely on International Classification of Diseases (ICD) discharge diagnosis codes25 for ASD case-ascertainment.
Use of ICD-diagnosis codes for identifying autism may be problematic, especially for adults.26–38 While autism is largely considered a condition of childhood,39 autism-associated health issues, disability, and service needs can continue throughout adulthood.3,36,37 Despite increasing awareness of the need for cost data on older individuals,36,40–45 very few studies on ASD-associated medical costs include primary data collected on adults. The shortage reflects that individuals now in their mid-30s were age 3-years >3 decades ago, before explicit diagnostic criteria were broadly applied, awareness was heightened, and service-availability increased. The Diagnostic and Statistical Manual (DSM) is the primary source of explicit criteria for assigning clinical diagnoses of mental conditions. First mention of autism as a distinct DSM diagnosis was in 1980.46 Before this date, children presenting with autistic characteristics were often assigned diagnoses such as childhood schizophrenia or mental retardation (now termed intellectual disability). DSM criteria for pervasive developmental disorder, not otherwise specified (PDDNOS) and Asperger’s disorder were not introduced until 1987 and 1994 respectively.47,48
Diagnostic substitution, expanded criteria, and greater awareness increase the likelihood that adult individuals who exhibited signs and symptoms that met criteria for ASD as children may have never received an ICD-code specific to ASD.26 This potential for detection bias also affects case-ascertainment based on, “Have you/your child ever received a diagnosis of autism?” The present study used resources and approaches described below, in an effort to provide objective, essentially complete ASD-associated direct medical-cost estimates with minimal potential for referral, detection, recall, or participation bias.
METHODS
Study Design/Setting
This retrospective population-based birth-cohort study was set in Olmsted County, MN, (2010 census 144,248) and was approved by Mayo Clinic and Olmsted Medical Center (OMC) Institutional Review Boards (IRB) and the Rochester Epidemiology Project (REP) Cost and Utilization Committee.
Resources
REP:
Rochester, MN, (the Olmsted County seat) is approximately 80 miles from the nearest major metropolitan area and home to Mayo Clinic, a large tertiary-care referral center. Thus, essentially all medical care received by local residents is provided by either Mayo Clinic or OMC, a second group practice, and their affiliated hospitals.49 A unique identifier is assigned each patient seen at Mayo and other medical providers, including OMC and most private practitioners in the area. REP affords vital status and residency status for all County residents for all dates 1965-present.50 REP provider-linked medical records contain line-item detail from every contact for each individual.51,52
REP Cost Data Warehouse (CDW):
Through a data-sharing agreement between Mayo Clinic and OMC, patient-level administrative data on all billed services are shared, archived, and available for use in approved research studies. Data include date, type, frequency, and billed-charge for every item or service provided each individual, regardless of age or payer type (including the uninsured). Using widely-accepted valuation techniques for administrative claims data developed by the Patient Outocmes Research Team (PORT),53 an algorithm was previously developed to generate standardized inflation-adjusted cost estimates, with a nationally-representative dollar cost assigned each line item.54,55 Costs for this study were adjusted to 2014 dollars. Costs for over-the-counter medications, outpatient prescription medications, home- and long-term care, and behavioral therapy provided by the very few private-practice psychologists/sociologist not affiliated with Mayo Clinic or OMC are not included. A detailed description of the costing methodology is provided elsewhere.56
Independent School District (ISD) #535 School Records:
A contractual agreement between ISD #535 school board and Mayo Clinic IRB affords access for approved research studies to the cumulative grade- and high-school records of all birth cohort children registered at any of the 43 public, parochial, and private schools, including home-schooled students and students who subsequently left due to relocation, graduation, or death.57 Conditions/procedures for using school records follow Minnesota Law concerning data privacy [M.S.13.32,subd.3(f)] and Federal Law concerning disclosure of personally-identifiable information from educational records without prior consent [(34CFR99.31(a)(6)(i)(c)]. Records include results of cognitive and achievement tests (both those administered by a clinician and those administered within the classroom setting); Individualized Education Program reports and Educational Disability Classification (EDC) codes; and school outcomes (e.g. dropout, expulsion, absenteeism, graduation status). Records also include notes related to any type of learning difficulty or performance/behavior concern documented by teachers/parents/school psychologists/physicians/social workers/school nurses and counselors, or in correspondence with private tutorial/therapeutic facilities.57
Olmsted County Birth Cohort:
The Olmsted County Birth Cohort includes all 43,215 live births from 1/1/1976–12/31/2000 born to mothers who were Olmsted County residents at time of delivery.57 In accordance with Minnesota state privacy law--statute 144.335, the 3,325 individuals (7.7%) who subsequently denied authorization for use of medical records in research58 were excluded from review, leaving 39,890 children. The target population for ASD identification was limited to 31,220 individuals (78%) remaining in Olmsted County on or after age 3 years, when social, communication, and behavioral ASD-like problems can be recognized with more certainty.39 All 31,220 individuals were followed retrospectively in REP medical and ISD #535 school records from birth until earliest of relocation outside Olmsted County, death, or age 21 years to identify those who met ASD research criteria (ASD-R).
ASD-R case identification
As described in detail elsewhere,35 ASD-R case identification followed criteria outlined in DSM Fourth Edition, Text Revision (DSM-IV-TR)59 and encompassed autistic disorder, Asperger’s disorder, and PDDNOS. The rigorous epidemiologic approach to identifying all ASD-R incident cases among the 31,220 birth-cohort members involved multiple sources of information within REP medical records and ISD #535 school records. The effort was led by a team of experts including a senior child psychologist, two developmental pediatricians, a speech/language pathologist, and an MD epidemiologist with 20-years expertise researching childhood developmental and behavioral disorders. The effort consisted of four main steps. Step one involved electronically obtaining all ICD-codes from medical records and EDC codes from school records assigned each individual from birth to age 21 years that were relevant to neurodevelopmental/psychiatric disorders (NPD) for which signs/symptoms overlapped with core social, communicative, and behavioral features of ASD and/or commonly coexisted with ASD. The codes were categorized into NPD clusters (e.g., childhood psychosis, developmental/cognitive, speech/language, non-psychosis childhood psychiatric clusters) (Supplement, Figure 1).35 Individuals with ≥1 ICD- or EDC-code within one or more clusters were defined as an NPD case (N=4,301). Based on various combinations of ICD- and EDC-codes, the NPD cluster profile, and a priori relative likelihood of harboring true ASD cases, 1,766 of the 4,301 individuals were further identified in Step two as meeting criteria for “potential ASD” (PASD). In Step three, PASD cases’ medical/school records were manually reviewed by trained abstractors under close supervision by team members. Abstractors carefully read all documentation within pertinent sections from birth to age 21 years. Using a systematic, multi-staged process, detailed protocols, and an extensive Data Dictionary (developed by the research team), abstractors identified descriptive phrases within the records that mapped to any of the 58 ASD signs/symptoms contributing to the 12 DSM-IV-TR criteria.59 In Step four, research-team members reviewed the collected information and applied clinical expertise and judgement to exclude initially identified cases whose problems/symptoms likely resulted from other conditions, e.g. psychosis, bipolar disorder, major depression. In total, 1,056 individuals were ultimately determined to have met ASD-R criteria.35
Study Sample
Due to relatively recent changes in costing methodologies within the REP Cost Data Warehouse applied retrospectively from 2003 forward,56 this study of ASD-associated medical costs was limited to 901 ASD-R cases who, as of 1/1/2003, remained in Olmsted County and had not refused research authorization. For each case, one non-ASD control was randomly selected from all 1976–2000 birth-cohort members who 1) was of same sex, birth year, and birth date (± 60 days), 2) was an Olmsted Count resident as of 1/1/2003, 3) was last seen at a REP-provider within ±2 years of the case’s last visit date before 12/31/2014, 4) had not refused research authorization,58 and 5) had not met criteria for ASD-R. Analyses were limited to these 901 matched-pairs.
Data Collection
Each member of the 901 case-control pairs was followed in the Cost Data Warehouse for all REP-provider encounters from 1/1/2003 until earliest of relocation outside Olmsted County, death, date of last encounter, or study end (12/31/14). To ensure similar lengths of observation for both members of a pair, follow-up was truncated at earliest end of follow-up for either member. To reduce confounding by temporal changes in ASD criteria, awareness, practice style, and/or costing methodologies, analyses were divided into three calendar periods (2003–2006, 2007–2010, and 2011–2014) and seven age groups (0–4, 5–9, 10–14, 15–19, 20–24, 25–29, and 30–38), totaling 21 unique cells. Data were analyzed separately for each age-group/calendar-period cell. Individuals were partitioned into each cell based on birth date, age at start of each calendar-period, and date of last follow-up. Supplement, Figure 2 provides examples of partitioning for three hypothetical individuals. Given the matching process, length of observations within each cell were essentially the same for both members of each matched-pair.
To adjust analyses of medical costs for non-ASD medical/psychiatric conditions, we used Johns Hopkins Adjusted Clinical Groups (ACG) System® software60 to assign Resource Utilization Band (RUB) summary measures of overall-morbidity.61 A RUB value was assigned each individual for each age-group/calendar-period cell, using clinical diagnoses assigned within 12-months before cell entry. To avoid over-adjustment, ASD-specific codes and codes associated with (or substituting for) ASD were excluded from the RUB calculation (see Figure Legend).
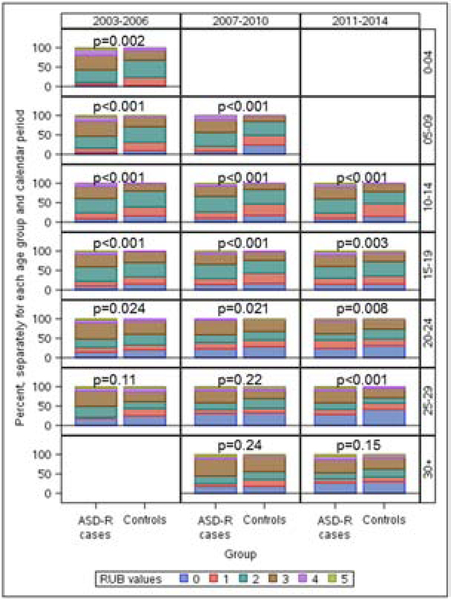
Figure.
Distribution of the RUB summary measure of overall-morbidity for research-identified ASD ASD-R) cases and age- sex-matched controls in each age-group/calendar-period cell
Bar graph comparing ASD-R cases with non-ASD controls within each age-group/calendar-period cell for the distribution of Resource Utilization Band (RUB) summary measures of overall-morbidity within 12-months before cell entry. Given that individuals were born 1/1/1976 through 12/31/2000 and followed from 1/1/2003 through 12/31/2014, no individuals met age-group criteria for four cells, and no individuals were <3 years of age in the age group 0–4 years for calendar period 2003–2006. RUB values were assigned using Johns Hopkins Adjusted Clinical Groups (ACG) System® software.60 ACG software first categorizes an individual’s ICD-9-CM-coded diagnoses based on persistence, severity, and etiology of the condition; diagnostic certainty; and need for specialty care. RUB values are then assigned based on aggregations of ACGs with similar expected resource use; 0 – Non-users; 1 – Healthy Users; 2 – Low Morbidity Users; 3 – Moderate Morbidity Users; 4 – High Morbidity Users; 5 – Very High Morbidity Users.61 To avoid over-adjustment, ASD-specific diagnosis codes (i.e., autistic disorder, early infantile autism, infantile autism, PDDNOS, autism spectrum disorders, Asperger’s disorder) as well as codes associated with (or substituting for) ASD, (e.g., childhood psychosis; developmental cognitive disabilities and developmental delay; ASD-associated speech and language impairments; childhood psychiatric, non-psychoses) were excluded from the RUB calculation (i.e., were allowed to contribute to ASD-incremental costs).
Separate descriptive analyses were conducted to analyze direct-medical costs as a function of IQ among ASD-R cases. Information on IQ was abstracted from medical/school records as part of a previous investigation of IQ that was limited to ASD-R cases.35 Analyses in the present study were limited to 760 of the 901 ASD-R cases for whom either documented IQ scores or an ICD code for intellectual disability were available. Cases were categorized as low IQ (≤85) versus average/higher IQ (≥86), based on their last full-scale IQ score obtained between ages 3 and 21 years; persons without IQ scores but with an ICD-code for intellectual disability were categorized as low IQ.
Statistical Methods
Analyses were performed using SAS version 9.4 software package (SAS Institute, Inc; Cary, NC). Unadjusted costs were analyzed within each cell for cases and controls separately and for cost-differences between each control and his/her matched case. Statistical significance of unadjusted case-control differences for costs and RUB values were assessed using the two-sided Wilcoxon signed rank test given the skewed nature of these outcome distributions. Unadjusted case-control comparisons for percent with ≥1 inpatient hospitalization in each cell used the exact two-sided McNemar’s test. Unadjusted comparisons between ASD-R cases with IQ ≤85 versus ≥86 for medical costs within each cell used a two-sided Wilcoxon rank-sum test.
To estimate incremental costs, we used generalized linear multivariate modeling adjusted for age at cell entry in one model and, in a second model, additionally adjusted for the RUB overall-morbidity measure, using diagnoses 12-months before cell entry. These adjusted approaches employed two-part models to account for zero costs when appropriate, 53,62 and incorporated a generalized linear model with family distribution based on the modified Park test recommended by Manning and Mullahy.63 These approaches account for the skewed cost distribution while enabling coefficients to be directly back-transformed into the original dollar scale.64,65 We analyzed cost differences between ASD-R cases and controls using the method of recycled predictions, setting all individuals as ASD-R cases or as controls, while keeping all other individual characteristics constant.66,67 Bootstrapped 95% confidence intervals of the mean differences were calculated based on 1,000 bootstrap samples.
Tests of statistical significance were limited to within-cell comparisons, i.e., those with independent observations. As shown in Supplementary Figure 2, individuals transitioned across cells with advancing age and calendar year; thus, independent observations were lost for across-cell comparisons; tests of significance were not conducted. Statements regarding across-cell differences were based on visual comparisons of point-estimate trends and are referred to as ‘visually-observed’ throughout the text.
RESULTS
The 901 case-control pairs from the 1976–2000 birth cohort included in this study (74% male) were followed for outcomes from 1/1/2003 through 12/31/2014 (ages 3 through 38 years). Table 1 provides frequency counts and % male after partitioning each ASD-R case into age-group/calendar-period cells. No individuals met age-group/calendar-periods criteria for four of the 21 cells, leaving 17 cells for analysis. Table 1 values for age- and sex-matched non-ASD controls were the same as for cases. For the nine cells with age-groups 3–19 and eight cells with age-groups 20–38, the number of matched-pairs within each cell ranged from 93–402 and 45–395 respectively. Case-control comparisons for RUB measures of overall-morbidity (Figure) revealed RUB values were significantly higher for ASD-R cases than controls in 100% of cells for age-groups 3–19 and 50% of cells for age-groups 20–38. Table 2 compares ASD-R cases with controls within each cell for percent with ≥1 inpatient hospitalization. The proportion hospitalized was significantly greater for cases versus controls in seven of nine cells (78%) for age-groups 3–19 and one of eight cells (12%) for age-groups 20–38. Table 3 provides unadjusted direct-medical costs for ASD-R cases and controls; unadjusted costs were significantly higher for cases versus controls in 100% of cells for age-groups 3–19 and 50% of cells for age-groups 20–38. Table 4 provides predicted-mean costs for ASD-R cases and controls and predicted-mean-cost differences (cases minus controls). When adjusted for age alone, predicted-mean-cost differences reached statistical significance (i.e., 95% CI values excluded zero) in 100% of cells for age-groups 3–19 and 3 of 8 cells (38%) for age-groups 20–38. When additionally adjusted for RUB overall-morbidity, point-estimates for predicted-mean-cost differences each appeared visually lower than point-estimates adjusted for age alone, but cost-differences remained significantly higher for cases versus controls in 100% of cells for age-groups 3–19 and one of eight cells (12%) for age-groups 20–38. It is cautioned that between-age-group differences in significance levels (Figure, Tables 2–4) could reflect generally smaller sample sizes for older versus younger age groups. Table 5 compares medical costs for ASD-R cases with low versus average/higher IQ. While visually-observed comparisons of point-estimates for predicted-mean costs appeared greater for low versus average/higher IQ in 15 of 17 cells (88%), between-category differences reached statistical significance in only eight of 17 cells (47%).
Table 1.
Of the 901 research-identified ASD (ASD-R) cases (74% male), the total number of cases and % male after partitioning into age-group/calendar-period cells.† The total number and % male in each cell were the same for the 901 age- and sex-matched non-ASD controls.
| Age group (years) | Calendar period | ||
|---|---|---|---|
| 2003–2006 | 2007–2010 | 2011–2014 | |
| 3–4 | 93 (73.1%)†,* | --** | --** |
| 5–9 | 303 (74.6%) | 135 (71.9%) | --** |
| 10–14 | 384 (76.6%) | 357 (74.5%) | 180 (71.7%) |
| 15–19 | 375 (74.4%) | 402 (76.1%) | 355 (74.4%) |
| 20–24 | 186 (71.5%) | 336 (72.9%) | 395 (74.9%) |
| 25–29 | 57 (64.9%) | 149 (70.5%) | 259 (70.3%) |
| 30–38 | --** | 45 (64.4%) | 114 (64.9%) |
Abbreviations: ASD, Autism Spectrum Disorder.
For each individual, date of cell entry and exit was based on their birth date, age specific to the calendar period, and final follow-up date.
Given that the birth cohort ended as of 12/31/2000, no individuals were <3 years of age within the age group 0–4 years for calendar-period 2003–2006.
Given that individuals were born 1/1/1976 through 12/31/2000 and followed from 1/1/2003 through 12/31/2014, no individuals met age-group criteria for these cells.
Table 2.
The number (proportion) of research-identified ASD (ASD-R) cases and matched controls with at least one inpatient hospitalization in each age-group/calendar-period cell†
| Calendar period / Age group (years) | No. of matched pairs | No. (%) with at least one
inpatient hospitalization |
p-value‡ | |
|---|---|---|---|---|
| ASD-R cases | Matched controls | |||
| 2003–2006 | ||||
| 3–4* | 93 | 7 (7.5%) | 1 (1.1%) | 0.070 |
| 5–9 | 303 | 33 (10.9%) | 10 (3.3%) | <0.001 |
| 10–14 | 384 | 44 (11.5%) | 11 (2.9%) | <0.001 |
| 15–19 | 375 | 59 (15.7%) | 34 (9.1%) | 0.007 |
| 20–24 | 186 | 33 (17.7%) | 25 (13.4%) | 0.29 |
| 25–29 | 57 | 10 (17.5%) | 15 (26.3%) | 0.30 |
| 30–38** | -- | -- | -- | -- |
| 2007–2010 | ||||
| 3–4** | -- | -- | -- | -- |
| 5–9 | 135 | 12 (8.9%) | 4 (3.0%) | 0.077 |
| 10–14 | 357 | 26 (7.3%) | 11 (3.1%) | 0.017 |
| 15–19 | 402 | 46 (11.4%) | 27 (6.7%) | 0.030 |
| 20–24 | 336 | 38 (11.3%) | 35 (10.4%) | 0.79 |
| 25–29 | 149 | 23 (15.4%) | 11 (7.4%) | 0.029 |
| 30–38 | 45 | 7 (15.6%) | 12 (26.7%) | 0.30 |
| 2011–2014 | ||||
| 3–4** | -- | -- | -- | -- |
| 5–9** | -- | -- | -- | -- |
| 10–14 | 180 | 32 (17.8%) | 16 (8.9%) | 0.020 |
| 15–19 | 355 | 51 (14.4%) | 15 (4.2%) | <0.001 |
| 20–24 | 395 | 44 (11.1%) | 28 (7.1%) | 0.056 |
| 25–29 | 259 | 35 (13.5%) | 31 (12.0%) | 0.68 |
| 30–38 | 114 | 14 (12.3%) | 17 (14.9%) | 0.69 |
Abbreviations: ASD, Autism Spectrum Disorder
For each individual, date of cell entry and exit was based on their birth date, age specific to the calendar period, and final follow-up date.
Obtained using exact McNemar’s test for comparing correlated proportions.
Given that the birth cohort ended as of 12/31/2000, no individuals were <3 years of age within the age group 0–4 years for calendar-period 2003–2006.
Given that individuals were born 1/1/1976 through 12/31/2000 and followed 1/1/2003 through 12/31/2014, no individuals met age-group criteria for these cells
Table 3.
Unadjusted medical costs among research-identified ASD (ASD-R) cases and matched controls in each age-group/calendar-period cell†
| Calendar period / Age group (years) | No. of matched pairs | ASD-R cases | Matched controls | Cost Differenc (case minus control) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (SD),$ | Median (IQR), $ | % With zero costs | Mean (SD), $ | Median (IQR), $ | % with zero costs | Median (IQR), $ | p-value‡ | ||
| 2003–2006 | |||||||||
| 3–4* | 93 | 4158 (5358) | 2214 (677, 6080) | 3.2 | 1215 (2171) | 597 (272, 973) | 1.1 | 1166 (43, 4637) | <0.001 |
| 5–9 | 303 | 8932 (30826) | 2769 (1061, 6513) | 1.3 | 1670 (2645) | 748 (341, 1829) | 5.9 | 1618 (12, 4874) | <0.001 |
| 10–14 | 384 | 5761 (12530) | 2241 (801, 5470) | 2.3 | 1958 (3556) | 920 (330, 2159) | 5.5 | 946 (−183, 3905) | <0.001 |
| 15–19 | 375 | 8366 (32536) | 2020 (741, 6022) | 3.7 | 3331 (10132) | 1130 (406, 2730) | 6.9 | 587 (−597, 3639) | <0.001 |
| 20–24 | 186 | 8602 (24540) | 1448 (287, 3820) | 10.2 | 3786 (7557) | 903 (219, 3551) | 14.0 | 54 (−1106, 2268) | 0.09 |
| 25–29 | 57 | 6637 (9184) | 2848 (427, 9623) | 7.0 | 7508 (13750) | 1383 (195, 9917) | 12.3 | 91 (−2276, 3397) | 0.76 |
| 30–38** | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 2007–2010 | |||||||||
| 3–4** | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 5–9 | 135 | 4787 (12003) | 1471 (367, 4107) | 5.2 | 1390 (3338) | 418 (132, 1249) | 13.3 | 563 (−114, 2992) | <0.001 |
| 10–14 | 357 | 6081 (20056) | 1504 (514, 3365) | 5.3 | 1992 (4569) | 696 (282, 1758) | 9.5 | 507 (−347, 2138) | <0.001 |
| 15–19 | 402 | 5676 (14429) | 1954 (617, 5252) | 5.0 | 3417 (7723) | 1329 (366, 3038) | 8.7 | 393 (−1071, 3035) | <0.001 |
| 20–24 | 336 | 4844 (13367) | 925 (209 3665) | 18.2 | 3665 (10615) | 695 (152, 2187) | 15.8 | 164 (−792, 2011) | 0.003 |
| 25–29 | 149 | 6109 (15695) | 1153 (203, 4384) | 16.8 | 4048 (12469) | 514 (89, 1894) | 20.8 | 395 (−473, 2927) | 0.002 |
| 30–38 | 45 | 8302 (17391) | 2268 (132, 7923) | 17.8 | 10380 (15802) | 5950 (486, 15264) | 8.9 | −1172 (−7646, 2268) | 0.14 |
| 2011–2014 | |||||||||
| 3–4** | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 5–9** | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 10–14 | 180 | 8286 (20281) | 2583 (921, 6067) | 3.9 | 2737 (4939) | 988 (332, 2629) | 5.6 | 1011 (−144, 4638) | <0.001 |
| 15–19 | 355 | 9483 (30040) | 1810 (450, 6982) | 7.0 | 3445 (9888) | 1228 (371, 3000) | 9.3 | 394 (−791, 4418) | <0.001 |
| 20–24 | 395 | 5539 (14222) | 1233 (349, 4351) | 10.4 | 3683 (10381) | 663 (167, 2240) | 12.7 | 326 (−710, 2403) | <0.001 |
| 25–29 | 259 | 8241 (26489) | 1429 (319, 4712) | 11.6 | 4223 (8740) | 793 (180, 3659) | 12.7 | 110 (−1263, 2996) | 0.04 |
| 30–38 | 114 | 10749 (21709) | 2457 (392, 10343) | 14.0 | 7408 (15889) | 1319 (320, 8936) | 12.3 | 341 (−2533, 6092) | 0.12 |
Abbreviations: ASD, Autism Spectrum Disorder; IQR, interquartile range; SD, standard deviation.
For each individual, date of cell entry and exit was based on their birth date, age specific to the calendar period, and final follow-up date.
Within each age-group/calendar-period cell, costs were compared between ASD-R cases and their matched controls using a two-sided Wilcoxon signed rank test given the paired nature of the data and skewed distributions.
Given that the birth cohort ended as of 12/31/2000, no individuals were <3 years of age within the age group 0–4 years for calendar-period 2003–2006.
Given that individuals were born 1/1/1976 through 12/31/2000 and followed from 1/1/2000 through 12/31/2014, no individuals met age-group criteria for these cells.
Table 4.
Predicted mean direct medical costs for research-identified ASD (ASD-R) cases and matched controls in each age-group/calendar-period cell†,‡,
| Calendar period / Age group (years) | No. of matched pairs | A. Adjusted for age* |
B. Adjusted for age and RUB
values* |
||||
|---|---|---|---|---|---|---|---|
| Predicted mean costs,
$ |
Predicted mean cost difference, $ (95% CI)** | Predicted mean costs,
$ |
Predicted mean cost difference, $ (95% CI)** | ||||
| ASD-R cases | Matched controls | ASD-R cases | Matched controls | ||||
| 2003–2006 | |||||||
| 3–4*** | 93 | 4245 | 1223 | 3022 (2033, 4138) | 4048 | 1276 | 2772 (1718, 3963) |
| 5–9 | 303 | 9038 | 1742 | 7296 (4340, 10914) | 7593 | 2105 | 5488 (3169, 8878) |
| 10–14 | 384 | 5898 | 2070 | 3828 (2643, 5166) | 5437 | 2235 | 3202 (2103, 4494) |
| 15–19 | 375 | 8142 | 3423 | 4719 (1914, 9496) | 7200 | 3861 | 3339 (1132, 5773) |
| 20–24 | 186 | 8420 | 3870 | 4550 (1387, 8322) | 7799 | 4191 | 3607 (881, 6684) |
| 25–29 | 57 | 6448 | 7734 | −1286 (–5538, 2530) | 6258 | 8058 | –1801 (–5369, 1886) |
| 30–38**** | -- | -- | -- | -- | -- | -- | -- |
| 2007–2010 | |||||||
| 3–4**** | -- | -- | -- | -- | -- | -- | -- |
| 5–9 | 135 | 4788 | 1352 | 3436 (1398, 5453) | 3279 | 2008 | 1272 (158, 2449) |
| 10–14 | 357 | 5484 | 2281 | 3203 (1734, 5119) | 4651 | 2673 | 1978 (659, 3333) |
| 15–19 | 402 | 5594 | 3471 | 2122 (626, 3771) | 5371 | 3628 | 1743 (296, 3423) |
| 20–24 | 336 | 4916 | 3613 | 1303 (−452, 3120) | 4624 | 3873 | 752 (−972, 2529) |
| 25–29 | 149 | 5965 | 4148 | 1816 (−1153, 4882) | 5734 | 4380 | 1353 (−1553, 4187) |
| 30–38 | 45 | 8747 | 9852 | −1105 (−7653, 5965) | 7716 | 11119 | −3403 (−8368, 1067) |
| 2011–2014 | |||||||
| 3–4**** | -- | -- | -- | -- | -- | -- | -- |
| 5–9**** | -- | -- | -- | -- | -- | -- | -- |
| 10–14 | 180 | 8797 | 2776 | 6021 (3171, 9466) | 6512 | 3820 | 2692 (787, 4849) |
| 15–19 | 355 | 9535 | 3589 | 5946 (2812, 9504) | 8598 | 3877 | 4721 (2356, 7521) |
| 20–24 | 395 | 5498 | 3715 | 1783 (136, 3324) | 5284 | 3878 | 1406 (−46, 2835) |
| 25–29 | 259 | 8187 | 4251 | 3937 (1159, 7798) | 7178 | 4958 | 2220 (−96, 5277) |
| 30–38 | 114 | 10861 | 7338 | 3523 (−896, 8501) | 9879 | 8278 | 1602 (−2512,5526) |
Abbreviations: ASD, Autism Spectrum Disorder; CI, Confidence Intervals; RUB, Resource Utilization Bands.
For each individual, date of cell entry and exit was based on their birth date, age specific to the calendar period, and final follow-up date.
Two-part modelling was employed to estimate costs in each age-group/calendar-period cell for which the ratio of the number of cases plus controls with zero costs divided by the total number of cases plus controls was ≥5%, i.e., ages 15–19, 20–24, and 25–29 years in 2003–2006; each age in 2007–2010; and ages 15–19, 20–24, 25–29, and 30–38 years in 2011–2014.
Predicted costs were adjusted for each individual’s age at cell entry and their RUB value for diagnoses within the 12 months before cell entry.
Cost differences were obtained using the method of recycled predications, 95% confidence intervals (CI) were calculated based on 1,000 bootstrap samples; bolded text indicates 95% CI values that do not include zero (i.e., statistically significant differences between cases and controls).
Given that the birth cohort ended as of 12/31/2000, no individuals were <3 years of age within the age group 0–4 years for calendar-period 2003–2006.
Given that individuals were born 1/1/1976 through 12/31/2000 and followed 1/1/2003 through 12/31/2014, no individuals met age-group criteria for these cells.
Table 5.
Unadjusted medical costs among research-identified ASD (ASD-R) cases with IQ values less than average (≤85) versus average or higher (≥86) in each age-group/calendar-period cell.
| Calendar period / Age group (years) | ASD-R cases with IQ
≤85 |
ASD-R cases with IQ
≥85 |
Cost difference |
||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Mean (SD), $ | Median (IQR), $ | % with zero costs | No. | Mean (SD), $ | Median (IQR), $ | % with zero costs | p-value* | |
| 2003–2006 | |||||||||
| 3–4** | 34 | 6309 (7469) |
4407 (703,7296) |
2.9 | 2.9 | 2842 (2588) |
1681 (677,5527) |
6.9 | 0.07 |
| 5–9 | 101 | 9404 (16797) |
4105 (1612,8296) |
0 | 111 | 5953 (13666) |
2704 (933,5941) |
1.8 | 0.015 |
| 10–14 | 120 | 7671 (17969) |
1963 (724,6091) |
3.3 | 204 | 5254 (9611) |
2616 (944,5762) |
1.0 | 0.60 |
| 15–19 | 123 | 10231 (48530) |
1965 (559,7663) |
4.9 | 234 | 7846 (21455) |
2321 (982,5632) |
3.4 | 0.41 |
| 20–24 | 75 | 12067 (32771) |
1469 (391,6453) |
8 | 95 | 5702 (14284) |
1057 (226,3219) |
12.6 | 0.19 |
| 25–29 | 25 | 5966 (8091) |
3221 (531,8219) |
0 | 26 | 8199 (10797) |
2671 (427,16924) |
7.7 | 0.99 |
| 30–38*** | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 2007–2010 | |||||||||
| 3–4*** | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 5–9 | 46 | 8175 (16768) |
2228 (454,5811) |
6.5 | 48 | 2566 (3342) |
1325 (373,3729) |
4.2 | 0.23 |
| 10–14 | 119 | 9082 (28192) |
1949 (909,5039) |
3.4 | 141 | 4582 (13825) |
1319 (488,3425) |
5.0 | 0.008 |
| 15–19 | 127 | 6670 (12909) |
2226 (832,6667) |
1.6 | 228 | 4527 (8299) |
1909 (459,4173) |
7.5 | 0.024 |
| 20–24 | 122 | 6722 (19460) |
1180 (282,5958) |
13.9 | 198 | 3474 (7562) |
697 (100,2682) |
21.2 | 0.029 |
| 25–29 | 61 | 7283 (18416) |
2155 (437,6407) |
11.5 | 73 | 4501 (12694) |
857 (111,2248) |
19.2 | 0.015 |
| 30–38 | 21 | 9409 (18879) |
3768 (830,7923) |
14.3 | 20 | 8799 (17581) |
2571 (811,9513) |
10.0 | 0.91 |
| 2011–2014 | |||||||||
| 3–4** | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 5–9*** | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 10–14 | 58 | 12486 (19120) |
4158 (1221,13527) |
3.4 | 67 | 4433 (6642) |
2177 (973,5136) |
4.5 | 0.036 |
| 15–19 | 115 | 13934 (39681) |
2474 (714,8593) |
4.3 | 155 | 6209 (10692) |
1530 (431,6845) |
6.5 | 0.08 |
| 20–24 | 122 | 5347 (12810) |
1190 (331,4742) |
9.0 | 236 | 5546 (15241) |
1242 (336,3976) |
11.9 | 0.58 |
| 25–29 | 97 | 11296 (37978) |
2092 (708,5265) |
11.3 | 151 | 5952 (14613) |
972 (250,3966) |
11.9 | 0.013 |
| 30–38 | 50 | 9333 (14964) |
3397 (451,10775) |
12.0 | 54 | 11384 (26409) |
1203 (290,6785) |
16.7 | 0.16 |
Abbreviations: ASD, Autism Spectrum Disorder; IQ, intelligence quotient; IQR, interquartile range; SD, standard deviation.
For each individual, date of cell entry and exit was based on their birth date, age specific to the calendar period, and final follow-up date.
Of 901 ASD-R incidence cases, 760 (84.4%) had either documented IQ scores or clinical diagnosis codes for intellectual disability (ID); cases were dichotomized into two groups based on their last full scale IQ score obtained between ages 3 and 21 years (mean [SD], 11.2 [3.8] years) or clinical diagnoses of ID for those without IQ scores available.
Within each age-group/calendar-period cell, cost distributions were compared between the ASD-R cases with low IQ (i.e., ≤85 or a diagnosis code for intellectual disability) versus average or higher IQ (≥86) using a two-sided Wilcoxon rank sum test.
Given that the birth cohort ended as of 12/31/2000, no individuals were <3 years of age within the age group 0–4 years for calendar-period2003–2006.
Given that individuals were born 1/1/1976 through 12/31/2000 and followed 1/1/2003 through 12/31/2014, no ASD-R cases met age-group criteria for these cells.
DISCUSSION
This population-based longitudinal study benefited from both objective cost estimates and uniform application of rigorous research criteria for identifying ASD-R cases and age- sex-matched non-ASD controls. To help address temporal changes in diagnostic criteria/practice styles/public awareness, etc., analyses for each outcome were divided into 17 age-group/calendar-period cells. Analyses of direct-medical costs within the nine cells for age-groups 3–19, revealed case-control mean-cost ratios (mean-costs for cases divided by mean-costs for controls) within each cell ranged from 1.7–5.3, 1.6–3.5, and 1.4–3.6 for unadjusted, age-adjusted, and adjusted for age plus co-occurring conditions respectively; case-control cost differences reached significance in 100% of cells for each analysis type. Analyses of direct-medical costs within the eight cells for age-groups 20–38 revealed case-control mean-cost ratios ranged from 0.8–2.3, 0.9–1.9, and 0.7–1.8 for unadjusted, age-adjusted, and adjusted for age plus co-occurring conditions respectively; the percentages of cells with significant case-control differences were 50% (unadjusted), 38% (age-adjusted), and 12% (adjusted for age plus co-ccurring conditions). Visual comparisons between age-groups and between unadjusted and adjusted findings suggest that ASD incremental-medical costs decline with adulthood, perhaps reflecting transition from ASD-specific treatment among youth toward treatment of co-occurring conditions among adults.
Results of ASD-associated direct-medical costs for age-groups 3–19 were generally consistent with published findings for children/adolescents.7,9–11,13,15 Relatively fewer reports of ASD-associated medical-costs exist for adults. ASD-associated lifetime medical costs were calculated by Ganz15 and Buescher et al.3 using synthetic-cohort designs. Ganz reported ASD incremental-medical costs decreased with each year of age from $44,446 at age 3 to $835 at age 66.15 Buescher et al. reported medical costs attributable to ASD were much higher for adults than for children;3 adult medical costs were calculated using published medical-cost estimates for psychiatric encounters among ASD cases age 3–20 years4 and applying the assumption that medical costs for ages ≥18 were 1.5 times those for ages 6–17.3
Very few studies of ASD-associated medical costs collected primary data on adults. Shimabukuro et al.’s study of privately-insured individuals ages 1–21 years found incremental-medical expenditures for ASD were highest for ages 1–4 and lowest for ages 11–17.11 Vohra et al.12 reported Medicaid enrollees age 22–64 with ASD had higher medical costs compared to propensity-matched controls. Zerbo et al.14 found medical costs for Kaiser Permanente members age ≥18 with ASD were nearly double those for age- sex-matched controls. While differences between our and previous findings for adult medical costs may reflect our relatively smaller sample sizes, each study of ASD-associated medical costs among adults cited above relied on either self-reported or ICD-coded diagnoses for identifying autism. As noted in Introduction, such case-ascertainment is potentially biased toward more severe, sicker cases;26–38 the potential may be greater for adults.26,28,29
Strong associations between ASD and several selected medical/psychiatric conditions were reported previously for both youth and adults.10,12,20,37,69–73 Previous findings for children/adolescents are consistent with our finding that RUB overall-morbidity was significantly greater for cases versus controls in 100% of cells for ages 3–19. Our finding that case-control differences reached significance in only 50% of cells for ages 20–38 differs somewhat from previous adult findings. Differences between age-groups in our study and differences between our and previous adult findings may both reflect our study’s relatively smaller sample sizes for older individuals. Between-study differences may also reflect differences in sample selection, case-ascertainment, and aims. The perspective for some previous adult-studies was the economic burden for Medicaid or hospitalized populations.12,30,37 Almost all adult studies cited above relied on self-reported or ICD-coded diagnoses for ASD case-ascertainment; the potential diagnostic bias toward more severe, sicker cases may be greater for Medicaid or hospitalized populations.68 Most previous studies of co-occurring conditions among persons with ASD intentionally focused on certain select medical/psychiatric conditions, with an aim toward identifying ASD associations that might inform treatment and/or reveal common pathways.37,69–73 Because our aims included estimating ASD-incremental costs, we followed Valderas et al.’s suggestion that summary measures consisting of multiple conditions and empirically-derived weights may have greater utility for case-mix adjustment in cost-of-illness studies,74 and we used RUB overall-morbidity.
Some,7,9,10,75,76 but not all,5,8,12,77 studies of ASD-associated hospitalizations found higher hospitalization rates for persons with versus without autism. Of cited studies, higher rates forASD cases were found for five7,9,10,75,76 of seven5,7,8,9,10,75,76 that included children, and one75 of three12,75,77 that included adults. Similar77 or lower12 hospitalization rates for adults with ASD versus controls were found by Nicolaidis et al.77 and Vohra et al.12 Among persons with ASD, Lokhandwala et al.75 found hospitalization rates were lower for ages >21 versus ages 10–20. We found significantly higher hospitalization rates for cases versus controls in 78% of cells age 3–19 and 12% of cells age 20–38. Visually-observed age-associated trends in hospitalizations appeared steady and marked for controls and more irregular and smaller for cases. While greater age-associated increases in hospitalization rates for controls versus cases could contribute to lower case-control differences for adults versus youth found by us and others;75 smaller sample sizes for older versus younger individuals may have also contributed to present-study findings. Further investigations are needed to understand factors responsible for ASD-associated hospitalizations and the contribution of hospitalizations to ASD-associated medical costs for children and adults.78
The prevalence of intellectual disability is greater for persons with versus without ASD.18 Buescher et al.3 reported mean annual medical costs among U.S. youth with ASD were ~2-fold higher for those with versus without intellectual disability. Interpretation of our visual observation that point-estimates of mean-medical costs in Table 5 appeared much greater for ASD cases with low versus average/higher IQ in 14 of 17 cells (82%), but only seven cells (41%) reached statistical significance is limited in part by small sample sizes and loss of within-cell matching between cases for sex and length-of-follow-up that resulted from division of cases into two groups. Further studies of intellectual disability and ASD-associated medical costs are needed, especially for adults.
Study strengths/limitations
The present study had access to a large population-based birth cohort and detailed information contained in essentially complete medical and school records for each cohort member.57 ASD-ascertainment was based on DSM-IV-TR59 criteria using a multi-staged process and labor-intensive systematic record review.35 Because we attempted to apply DSM-IV-TR59 case criteria uniformly from birth to age 21 years for each member of the 1976 through 2000 birth cohort, and costs were estimated from 2003 (minimum age = 3) through 2014 (maximum age = 38), findings address the serious shortage of medical-cost estimates among adults who met operational research criteria for autism as children. Both overall and incremental estimates of direct-medical costs were provided; incremental estimates were conducted using matching and regression approaches. National average dollar costs were assigned for essentially all direct-medical services, including all payers/providers and the uninsured. Autism signs/symptoms are typically clinically-detectable around 3-years of age; for this study, we considered all individuals who met ASD-R criteria as an ASD case as of age 3; individuals were followed longitudinally, with censoring, from minimum age 3-years to maximum age 38-years; ASD-associated medical costs over that age range could (with more effort) be calculated.
The present study has several limitations. No estimates were provided for informal/indirect medical care or non-health-care use, each recognized as contributing substantially to the ASD economic burden.3,5,18 Medical costs excluded over-the-counter medications, and outpatient-prescription medications. Previous studies reveal markedly higher prescription-medication costs for persons with versus without ASD.7,12 Costs excluded home-health- and long-term-care services and behavioral therapy administered by the very few psychologists/sociologists not affiliated with Mayo or OMC. No estimates were afforded for middle-aged or elderly individuals.
Estimates were for a single population (86% white in 2010). Compared with Minnesota and all other upper mid-west states, Olmsted County residents exhibit similar age-, sex-, and racial-distributions, and similar mortality and chronic-disease rates, but higher income and education.79 Low racial diversity and higher income/education levels, and the fact that medical care is delivered by few providers, could compromise generalizability of findings to different racial and socioeconomic groups and different health-care environments. 5,75,80,81
ASD Identification was based on DSM-IV-TR59 rather than more recent DSM-582 criteria. Baio et al.80 used the Autism and Developmental Disabilities Monitoring (ADDM) Network surveillance system.81 to compare DSM-5 and DSM-IV-TR criteria for ASD prevalence and characteristics. ADDM applies DSM-IV-TR criteria using an approach for ASD case-ascertainment similar to that used in the present study. Baio et al. concluded the number and characteristics of children meeting DSM-IV-TR ASD criteria were similar to those for children meeting DSM-5 criteria, overall and when stratified by sex, race/ethnicity, diagnostic subtype, and level of intellectual ability.80
Conclusions/Implications
The importance of understanding the economic burden of autism among adults is increasingly recognized.15,36,40–45 The uniform application of rigorous research criteria for identifying ASD and the objective estimates of ASD-associated medical costs from ages 3 through 38 years provided here can help refine predictions of future care needs and inform decisions by individuals/providers/payers/policymakers regarding resource allocation, treatment options, and various proposals for health care delivery and/or reimbursement.
Several important questions remain. The present study provides descriptive estimates of ASD-associated medical costs for ages 3–38 years. Cost-effectiveness studies and economic modeling await further investigation to determine if costs are appropriate, too low, or too high for the benefit gained. If higher medical costs for younger ASD cases versus controls observed here and in other studies are unnecessarily high, where might efforts toward cost-reduction be focused? Do the relatively smaller case-control differences in medical costs observed here for older age groups reflect reductions in care-seeking behavior, changes in the type of care needed, reduced access, or unmet needs.16,77,83 Future studies are needed, both to confirm or reject findings presented here, and that also include estimates of medical-costs collected on middle-aged/elderly individuals who meet research criteria for ASD. Improving the quality-of-life for persons of all ages with autism and their care-givers will require additional data on informal/indirect costs, unmet care needs, and patient satisfaction.
Supplementary Material
HIGHLIGHTS.
Reliable accurate estimates of both overall and incremental ASD-associated direct-medical costs are needed to predict future care needs, allocate resources, identify best practices, and model cost-effectiveness. Few ASD cost-of-illness studies had simultaneous access to both objective cost data and ASD case-ascertainment beyond self-reported or ICD-coded diagnoses. Most available estimates are focused on children and/or adolescents; few studies obtained primary data on adults.
This population-based 1976–2000 birth-cohort study identified ASD cases and age- sex-matched non-ASD controls by applying uniform operational-research criteria (based on DSM-IV-TR guidelines) following review of provider-linked medical and public/private/home-school records from birth to maximum age 21 years. Provider-linked administrative data, including all payers, afforded objective medical-cost estimates (ages 3 to maximum 38 years) for 901 case-control pairs. Analyses of case-control medical-cost differences included unadjusted, age-adjusted, and adjusted for age plus co-occurring conditions. Temporal changes were addressed by separating analyses into approximately 5-year age-group/4-year calendar-period cells.
Consistent with published findings for children/adolescents, incremental-medical costs were significantly higher for cases versus controls in 100% of cells for age-groups 3–19 years. However, differing from suggestions that case-control medical-cost differences increased with adulthood, we observed significant case-control differences in incremental costs for only 12% of cells for age-groups 20–38 years. This study provided objective overall and incremental-medical cost estimates for persons age 3–38 years who met standardized case-criteria during childhood, included unaffected controls and relevant measures of co-occurring conditions, and considered temporal changes in diagnostic criteria/practice styles/public awareness.
Acknowledgments:
The authors wish to acknowledge Dr. Leonard T. Kurland (deceased) for his vision in initiating the Rochester Epidemiology Project and Dr. Robert C. Colligan (deceased) for his insight, enthusiasm, and collegiality across his 47 years of research in developmental disabilities at the Mayo Clinic. We thank Dr. Ruth Stoeckel for her expert input with establishing ASD research criteria and study coordinators Ms. Candice Klein, Mr. Thomas Bitz, and other members of the team, for their outstanding contributions to data collection. We are most appreciative of the guidance provided by Dr. Sue Visscher and members of the Rochester Epidemiology Project Cost and Utilization Committee. We are thankful to Independent School District No. 535 for their longstanding cooperation and collaboration.
Funding/Support: This study was funded by a National Institutes of Health, Public Health Service research grant (Award Number MH093522) and was made possible by the Rochester Epidemiology Project (National Institutes of Health, National Institute on Aging, Award Number RO1 AG034676).
Role of the Sponsors: The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. In support of the manuscript, the NIH had no role in design and conduct of the study; data collection, analysis, and interpretation; or preparation and review of the manuscript. Publication of study results was not contingent on sponsors’ approval or censorship of the manuscript.
Financial Disclosure: Beyond grant funding from the National Institutes of Health, the authors have no direct or indirect financial disclosures to report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Amendah D, Grosse SD, Peacock G, Mandell DS. The Economic Costs of Autism: A Review In: Amaral D, Geschwind D, Dawson G eds, Autism Spectrum Disorders Oxford, England: Oxford University Press, 2011. [Google Scholar]
- 2.Picardi A, Gigantesco A, Tarolla E, et al. Parental burden and its correlates in families of children with autism spectrum disorder: A multicentre study with two comparison groups. Clin Pract Epidemiol Ment Health 2018;14:143–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buescher AV, Cidav Z, Knapp M, Mandell DS. Costs of autism spectrum disorders in the United Kingdom and the United States. JAMA Pediatr 2014;168(8):721–8. [DOI] [PubMed] [Google Scholar]
- 4.Cidav Z, Lawer L, Marcus SC, Mandell DS. Age-related variation in health service use and associated expenditures among children with autism. J Autism Dev Disord 2013;43(4):924–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lavelle TA, Weinstein MC, Newhouse JP, et al. Economic burden of childhood autism spectrum disorders. Pediatrics 2014;133(3):e520–e529 https://pediatrics.aappublications.org/content/pediatrics/133/3/e520.full.pdf. [Accessed June 21, 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leslie DL, Martin A. Health care expenditures associated with autism spectrum disorders. Arch Pediatr Adolesc Med 2007;161(4):350–5. [DOI] [PubMed] [Google Scholar]
- 7.Croen LA, Najjar DV, Ray GT, Lotspeich L, Bernal P. Comparison of health care utilization and costs of children with and without autism spectrum disorders in a large group-model health plan. Pediatrics 2006;118(4):e1203–11. https://pediatrics.aappublications.org/content/pediatrics/118/4/e1203.full.pdf. [Accessed June 21, 2019]. [DOI] [PubMed] [Google Scholar]
- 8.Liptak GS, Stuart T, Auinger P. Health care utilization and expenditures for children with autism: Data from U.S. national samples. J Autism Dev Disord 2006;36(7):871–9. [DOI] [PubMed] [Google Scholar]
- 9.Mandell DS, Cao J, Ittenbach R, Pinto-Martin J. Medicaid expenditures for children with autistic spectrum disorders: 1994 to 1999. J Autism Dev Disord 2006;36(4):475–85. [DOI] [PubMed] [Google Scholar]
- 10.Peacock G, Amendah D, Ouyang L, Grosse SD. Autism spectrum disorders and health care expenditures: the effects of co-occurring conditions. J Dev Behav Pediatr 2012;33(1):2–8. [DOI] [PubMed] [Google Scholar]
- 11.Shimabukuro TT, Grosse SD, Rice C. Medical expenditures for children with an autism spectrum disorder in a privately insured population. J Autism Dev Disord 2008;38(3):546–52. [DOI] [PubMed] [Google Scholar]
- 12.Vohra R, Madhavan Suresh, Sambamoorthi Usha. Comorbidity prevalence, healthcare utilization, and expenditures of Medicaid enrolled adults with autism spectrum disorders. Autism 2017;21(8):995–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L, Leslie DL. Health care expenditures for children with autism spectrum disorders in Medicaid. J Am Acad Child Adolesc Psychiatry 2010;49(11):1165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zerbo O, Qian Y, Ray T, et al. Healthcare service utilization and cost among adults with autism spectrum disorders in a U.S. integrated healthcare system. Autism in Adulthood 2019;1(1):27–36. 10.1089/aut.2018.0004. [Accessed June 21, 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ganz ML. The lifetime distribution of the incremental costs of autism. Arch Pediatr Adolesc Med 2007;161(4):343–9. [DOI] [PubMed] [Google Scholar]
- 16.Leigh JP, Grosse SD, Cassady D, Melnikow J, Hertz-Picciotto. Spending by California’s Department of Developmental Services for persons with autism across demographic and expenditure categories. PLoS One 2016. 25;11(3):e0151970. https://journals.plos.org/plosone/article/file?id=10.1371/journal.pone.0151970&type=printable. [Accessed June 19, 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shattuck PT, Narendorf SC, Cooper B, et al. Postsecondary education and employment among youth with an autism spectrum disorder. Pediatrics 2012;129(6):1042–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Autism Speaks. Autism and Health: A Special Report by Autism Speaks. 2017. https://www.autismspeaks.org/sites/default/files/2018-09/autism-and-health-report.pdf [Accessed June 21, 2019].
- 19.Rogge N and Janssen J. The economic costs of autism spectrum disorder: A literature review. J Autism Dev Disord 2019;49(7):2873–2900. [DOI] [PubMed] [Google Scholar]
- 20.Gurney JG, McPheeters ML, Davis MM. Parental report of health conditions and health care use among children with and without autism: National survey of children’s health. Arch Pediatr Adolesc Med 2006;160(8):825–30. [DOI] [PubMed] [Google Scholar]
- 21.Clabaugh G, Ward MM. Cost-of-illness studies in the United States: A systematic review of methodologies used for direct cost. Value Health 2008;11(1):13–21. [DOI] [PubMed] [Google Scholar]
- 22.Kim DD, Basu A. Estimating the medical care costs of obesity in the United States: Systematic review, meta-analysis, and empirical analysis. Value Health 2016;19(5):602–613. [DOI] [PubMed] [Google Scholar]
- 23.Brusco NK, Watts JJ. Empirical evidence of recall bias for primary health care visits. BMC Health Services Research 2015;15(381). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4572632/pdf/12913_2015_Article_1039.pdf. [Accessed November 19, 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhandari A, Wagner T. Self-reported utilization of health care services: Improving measurement and accuracy. Med Care Res Rev 2006;63(2):217–35. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. International Classification of Diseases, 9th Revision, Clinical (ICD-9-CM). Salt Lake City, Utah: Medicode, 1996. [Google Scholar]
- 26.Nicolaidis C, Kripke CC, Raymaker D. Primary care for adults on the autism spectrum. Med Clin North Am 2014;98(5):1169–91. 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brugha TS, McManus S, Bankart J, et al. Epidemiology of autism spectrum disorders in adults in the community in England. Arch Gen Psychiatry 2011;68(5):459–65. [DOI] [PubMed] [Google Scholar]
- 28.Dodds L, Spencer A, Shea S, et al. Validity of autism diagnoses using administrative health data. Chronic Dis Can 2009;29(3):102–7. [PMC free article] [PubMed] [Google Scholar]
- 29.Hollocks MJ, Lerh JW, Magiati I, Meiser-Stedman R, Brugha TS. Anxiety and depression in adults with autism spectrum disorder: A systematic review and meta-analysis. Psychol Med 2019;49(4):559–72. [DOI] [PubMed] [Google Scholar]
- 30.Houghton R, Ong RC, Bolognani F. Psychiatric comorbidities and use of psychotropic medications in people with autism spectrum disorder in the United States. Autism Res 2017;10(12):2037–47. [DOI] [PubMed] [Google Scholar]
- 31.Idring S, Lundberg M, Sturm H, et al. Changes in prevalence of autism spectrum disorders in 2001–2011: Findings from the Stockholm youth cohort. J Autism Dev Disord 2015;45(6):1766–73. [DOI] [PubMed] [Google Scholar]
- 32.Miller JS, Farley M, Coon H, et al. Autism spectrum disorder reclassified: A second look at the 1980s Utah/UCLA Autism Epidemiologic Study. J Autism Dev Disord 2013; 43(1): 200–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pinborough-Zimmerman J, Bilder D, Satterfield R, Hossain S, McMahon W. The impact of surveillance method and record source on autism prevalence: Collaboration with Utah Maternal and Child Health programs. Matern Child Health J 2010;14(3):392–400. [DOI] [PubMed] [Google Scholar]
- 34.Thomas P, Zahorodny W, Peng B, et al. The association of autism diagnosis with socioeconomic status. Autism 2012;16 (2):201–13. [DOI] [PubMed] [Google Scholar]
- 35.Myers SM. Voigt RG, Colligan RC, et al. Autism spectrum disorder: Incidence and time trends over two decades in a population-based birth cohort. J Autism Dev Disord 2019;49(4):1455–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fombonne E. Autism in adult life. Can J Psychiatr 2012;57(5):273–4. [DOI] [PubMed] [Google Scholar]
- 37.Kohane IS, McMurry A, Weber G, et al. The co-morbidity burden of children and young adults with autism spectrum disorders. PLoS ONE 2012;7(4):e33224. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0033224. [Accessed June 21, 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Close HA, Lee LC, Kaufmann CN, Zimmerman AW. Co-occurring conditions and change in diagnosis in autism spectrum disorders. Pediatrics 2012;129(2):e305–16. https://pediatrics.aappublications.org/content/pediatrics/129/2/e305.full.pdf [Accessed June, 21, 2019]. [DOI] [PubMed] [Google Scholar]
- 39.Zablotsky B, Colpe LJ, Pringle BA, et al. Age of parental concern, diagnosis, and service initiation among children with autism spectrum disorder. Am J Intellect Dev Disabil 2017;122(1):49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Interagency Autism Coordinating Committee. Interagency Autism Coordinating Committee Strategic Plan for Autism Spectrum Disorder Research. 2011; https://iacc.hhs.gov/publications/strategic-plan/2011/strategic_plan_2011.pdf. [Accessed June 21, 2019].
- 41.Mandell DS. Adults with autism: A new minority. J Gen Intern Med 2013;28(6):751–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murphy C, Wilson CE, Robertson DM, et al. Autism spectrum disorder in adults: Diagnosis, management, and health services development. Neuropsychiatr Dis Treat 2016;12:1669–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perkins EA, Berkman KA. Into the unknown: Aging with autism spectrum disorders. Am J Intellect Dev Disabil 2012;117(6):478–96. [DOI] [PubMed] [Google Scholar]
- 44.Piven J, Rabins P, on behalf of the Autism-in-Older Adults Working Group. Autism spectrum disorders in older adults: Toward defining a research agenda. J Am Geriatr Soc 2011;59(11):2151–55. [DOI] [PubMed] [Google Scholar]
- 45.Wright SD. Autism Spectrum Disorder in Middle and Later Life. Philadelphia, PA: Jessica Kingsley Publisher, 2016. [Google Scholar]
- 46.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (3rd ed.). Washington, DC: American Publishing, Inc., 1980. [Google Scholar]
- 47.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (3rd ed., Text Revision). Washington, DC: American Publishing, Inc., 1987. [Google Scholar]
- 48.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (4th ed.). Arlington, VA: American Psychiatric Publishing, Inc, 1994. [Google Scholar]
- 49.The Dartmouth Institute for Health Policy & Clinical Practice. Dartmouth Atlas of Healthcare. http://archive.dartmouthatlas.org/. [Accessed June 21, 2019]. [PubMed]
- 50.St Sauver JL, Grossardt BR, Yawn BP, Melton JL 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: The Rochester Epidemiology Project. Am J Epidemiol 2011;173(9):1059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Melton LJ, 3rd. History of the Rochester Epidemiology Project. Mayo Clin Proc 1996;71(3):266–74. [DOI] [PubMed] [Google Scholar]
- 52.St Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: The Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol 2012a;41(6):1614–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lave JR, Pashos CL, Anderson GF, et al. Costing medical care: Using Medicare administrative data. Med Care 1994;32(7 Suppl):JS77–89. [PubMed] [Google Scholar]
- 54.Leibson CL, Katusic SK, Barbaresi WJ, Ransom J, O’Brien PC. Use and costs of medical care for children and adolescents with and without attention-deficit/hyperactivity disorder. JAMA 2001; 285(1):60–6. [DOI] [PubMed] [Google Scholar]
- 55.Long KH, Rubio-Tapia A, Wagie AE, et al. The economics of coeliac disease: A population-based study. Aliment Pharmacol Ther 2010;32(2):261–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Visscher SL, Naessens JM, Yawn BP, Reinalda MS, et al. Developing a standardized healthcare cost data warehouse. BMC Health Serv Res 2017;17(1):396 https://bmchealthservres.biomedcentral.com/track/pdf/10.1186/s12913-017-2327-8?site=bmchealthservres.biomedcentral.com. [Accessed June 21, 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Katusic SK, Colligan RC, Myers SM, et al. What can large population-based birth cohort study ask about past, present and future of children with disorders of development, learning and behaviour? J Epidemiol Community Health 2017;71(4):410–16.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Melton LJ 3rd,. The threat to medical-records research. N Engl J Med 1997;337(20):1466–70. [DOI] [PubMed] [Google Scholar]
- 59.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (4th ed., Text Revision). Washington, DC: American Psychiatric Publishing, Inc., 2000. [Google Scholar]
- 60.Johns Hopkins Bloomberg School of Public Health. The Johns Hopkins ACG® System. http://www.acg.jhsph.org/. [Accessed June 21, 2019].
- 61.Johns Hopkins Bloomberg School of Public Health; The Johns Hopkins ACG® System: Excerpt from Technical Reference Guide, Version 11.1, December 2016. https://www.hopkinsacg.org/document/acg-system-version-11-1-technical-reference-guide/. [Accessed June 21, 2019].
- 62.Buntin MB, Zaslavsky AM. Too much ado about two-part models and transformation? Comparing methods of modeling Medicare expenditures. J Health Econ 2004. May;23(3):525–42. [DOI] [PubMed] [Google Scholar]
- 63.Manning WG, Mullahy J. Estimating log models: To transform or not to transform? J Health Econ 2001;20(4):461–94. [DOI] [PubMed] [Google Scholar]
- 64.Mullahy J Much ado about two: Reconsidering retransformation and the two-part model in health econometrics. J Health Econ 1998;17(3):247–81. [DOI] [PubMed] [Google Scholar]
- 65.Birnbaum HG, Ben-Hamadi R, Greenberg PE, et al. Determinants of direct cost differences among US employees with major depressive disorders using antidepressants. Pharmacoeconomics 2009;27(6):507–17. [DOI] [PubMed] [Google Scholar]
- 66.Basu A, Arondekar BV, Rathouz PJ. Scale of interest versus scale of estimation: comparing alternative estimators for the incremental costs of a comorbidity. Health Econ 2006;15(10):1091–107. [DOI] [PubMed] [Google Scholar]
- 67.Esposito D, Bagchi AD, Verdier JM, Bencio DS, Kim MS. Medicaid beneficiaries with congestive heart failure: Association of medication adherence with healthcare use and costs. Am J Manag Care 2009;15(7):437–45. [PubMed] [Google Scholar]
- 68.Wang L, Mandell DS, Lawer L, Cidav Z, Leslie DL. Healthcare service use and costs for autism spectrum disorder: A comparison between Medicaid and private insurance. J Autism Dev Disord 2012;43(5):1057–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Davignon MN, Qian Y, Massolo M, Croen LA. Psychiatric and medical conditions in transition-aged individuals with ASD. Pediatrics 2018;141(Suppl 4):S335–45. [DOI] [PubMed] [Google Scholar]
- 70.Tye C, Runicles AK, Whitehouse AJO, Alvares GA. Characterizing the interplay between autism spectrum disorder and comorbid medical conditions: An integrative review. Front Psychiatry 2019;9:751 10.3389/fpsyt.2018.00751. [Accessed May 20, 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Muskens JB, Velders FP, Staal WG. Medical comorbidities in children and adolescents with autism spectrum disorders and attention deficit hyperactivity disorders: A systematic review. Eur Child Adolesc Psychiatry 2017;26(9):1093–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rydzewska E, Hughes-McCormack LA, Gillberg C, et al. Prevalence of long-term health conditions in adults with autism: Observational study of a whole country population. BMJ Open 2018;8(8):e023945. https://journals.sagepub.com/doi/pdf/10.1177/1362361318791279. [Accessed June 21, 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Croen LA, Zerbo O, Qian Y, et al. The health status of adults on the autism spectrum. Autism 2015;19(7):814–23. [DOI] [PubMed] [Google Scholar]
- 74.Valderas JM, Starfield B, Sibbald B, Salisbury C, Roland M. Defining comorbidity: implications for understanding health and health services. Ann Fam Med 2009;7(4):357–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lokhandwala T, Khanna R, West-Strum D. Hospitalization burden among individuals with autism. J Autism Dev Disord 2012;42(1):95–104. [DOI] [PubMed] [Google Scholar]
- 76.Cummings JR, Lynch FL, Rust KC, et al. Health services utilization among children with and without autism spectrum disorders. J Autism Dev Disord 2016;46(3):910–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nicolaidis C, Raymaker D, McDonald K, et al. Comparison of healthcare experiences in autistic and non-autistic adults: A cross-sectional online survey facilitated by an academic– community partnership. J Gen Intern Med 2013;28(6):761–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nathenson RA, Zablotsky B. The transition to the adult health care system among youths with autism spectrum disorder. Psychiatr Serv 2017;68(7):735–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.St Sauver JL, Grossardt BR, Leibson CL, et al. Generalizability of epidemiological findings and public health decisions: An illustration from the Rochester Epidemiology Project. Mayo Clin Proc 2012b;87(2):151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Baio J, Wiggins L, Christensen DL, et al. Prevalence of autism spectrum disorder among children aged 8 years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. MMWR Surveill Summ 2018;;67(6):1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liptak GS, Benzoni LB, Mruzek DW, et al. Disparities in diagnosis and access to health services for children with autism: Data from the National Survey of Children’s Health. J Dev Behav Pediatr 2008;29(3):152–60. [DOI] [PubMed] [Google Scholar]
- 82.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (5th ed.). Washington, DC: American Psychiatric Publishing, Inc, 2013. [Google Scholar]
- 83.Turcotte P, Matthew M, Shea LL, Brusilovskiy E, Nonnemacher SL. Service needs across the lifespan for individuals with autism. J Autism Dev Disord 2016;46(7):2480–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.