Abstract
Objective
Preoperative inflammatory parameters are associated with outcome in renal cell carcinoma; however, their predictive value in tumors with sarcomatoid dedifferentiation (sRCC) is uncertain. We aimed to evaluate the association between preoperative and postoperative inflammatory parameters and the outcome of patients with locoregional and metastatic sRCC who underwent nephrectomy.
Methods and Materials
After obtaining IRB approval, we identified 230 patients with sRCC treated between 1994 – 2018 with a complete blood count drawn ≤1 month before nephrectomy. Preoperative neutrophil-lymphocyte ratio (NLR), lymphocyte-monocyte ratio and platelet-lymphocyte ratio were evaluated as continuous variables. Postoperative NLR, 1–8 weeks after surgery, and % change in NLR were calculated. Cox regression models were used to identify predictors of outcome.
Results
The study cohort included 105 metastatic patients and 112 patients with locoregional disease. Patients with metastatic disease had significantly higher preoperative NLR (4.31 vs. 3.29) and PLR (248 vs. 194), and lower preoperative LMR (2.6 vs. 3.23). Median follow-up for patients with locoregional and metastatic disease was 36 months and 20 months, respectively, and estimated 5-year cancer specific survival (CSS) rates were 56% and 15%, respectively. Preoperative NLR was a significant predictor of CSS for both metastatic (HR=1.23, 95% CI 1.1–1.37, p<0.001) and locoregional (HR=1.09, 95% CI 1–1.2, p=0.049) patients. For metastatic patients, postoperative NLR was significantly associated with CSS on univariate analysis; however, change in NLR was not associated with outcome.
Conclusions
Preoperative NLR is associated with CSS in locoregional and metastatic sRCC. NLR should be considered when establishing future predictive models for sRCC.
Keywords: kidney, sarcomatoid renal cell carcinoma, neutrophils, lymphocytes, prognosis
Introduction
Sarcomatoid dedifferentiation occurs in 10%−20% of patients with advanced renal cell carcinoma (RCC) and is the most aggressive phenotype of RCC.[1, 2] Renal cell carcinoma with sarcomatoid dedifferentiation (sRCC) is resistant to most treatments with a median overall survival ranging from 10 months to 2 years depending on the metastatic status at diagnosis.[2] Clinical characteristics associated with outcome include tumor stage and size, regional lymph node involvement, presence of distant metastases, presence of tumor necrosis and the percentage of sarcomatoid dedifferentiation.[3–5]
Preoperative inflammatory parameters, especially neutrophil-lymphocyte ratio (NLR), have been shown to predict outcome in various solid tumors, including localized and metastatic RCC.[6–8] Moreover, a decline in NLR following systemic treatment for metastatic RCC was significantly associated with prolonged survival.[9, 10] The predictive role of preoperative inflammatory variables in patients with sRCC was previously evaluated in a series of patients who underwent nephrectomy, most of whom had localized disease. On univariable analyses preoperative NLR, platelet-lymphocyte ratio (PLR) and lymphocyte-monocyte ratio (LMR) were significantly associated with outcome, and NLR remained an independent predictor of outcome on multivariable analysis.[5] The association between preoperative NLR and outcome in patients with metastatic sRCC, and the predictive role of NLR dynamics in this group of patients are unknown.
The aim of the current study was to evaluate the predictive role of the preoperative inflammatory parameters NLR, LMR and PLR in patients with sRCC who underwent curative or cytoreductive nephrectomy. Furthermore, we aimed to evaluate whether a change in NLR after surgery was associated with treatment outcome.
Materials and Methods
After obtaining Institutional Review Board approval, we queried our prospectively maintained nephrectomy database and identified 230 patients treated for locoregional or metastatic sRCC between the years 1994–2018 who had a complete blood count drawn ≤1 month before the procedure. Thirteen patients who received systemic therapy prior to nephrectomy were excluded, leaving a total of 217 patients for further analyses.
Clinical characteristics collected at presentation included patient age, sex, body mass index (BMI) and smoking status (categorized as current, former and never smoker). Karnofsky Performance Status was collected for patients with metastatic disease. Tumor specimens were reviewed by genitourinary pathologists and tumor histology, size, TNM-stage and surgical margin status were reported. All patients had varying degrees of sarcomatoid dedifferentiation within their tumor. Patients were considered to have metastatic disease at presentation if they had evidence of metastases ≤1 month from nephrectomy.
A complete blood count was obtained for all patients within 1 month prior to the procedure. Preoperative inflammatory parameters evaluated included NLR, LMR and PLR. The postoperative NLR was calculated from complete blood counts collected within 1–8 weeks after nephrectomy. Blood counts obtained <1 week after the procedure were not included to avoid an elevation in white-blood cell count associated with the surgical procedure itself. When multiple tests were done – the median and minimum postoperative NLR values were evaluated; % change in NLR was calculated based on previous publications as [(postoperative NLR/ preoperative NLR) −1] *100 and stratified into three groups: >25% decrease, no change (<25% decrease to <25% increase) and >25% increase.[9, 10]
After the procedure, patients were followed with serial imaging. Disease recurrence was determined for patients who presented with locoregional disease as radiographic evidence of progression of previously stable lesions, appearance of new lesions, pathologic evidence of metastatic disease or initiation of systemic treatment.
Clinical and pathological characteristics were reported using descriptive statistics and compared between patients with locoregional and metastatic disease using the Fisher exact test for categorical variables and the Wilcoxon rank-sum test for continuous variables. Univariate linear regressions were performed to determine the association between baseline characteristics and NLR, PLR and LMR. Pearson correlation plots were used to correlate preoperative NLR, LMR and PLR.
The primary study endpoint was cancer related death. Secondary outcomes included overall survival (OS) for all patients and recurrence free survival (RFS) for patients with locoregional disease. Cancer specific survival (CSS) was defined from the date of surgery to death due to renal cancer or treatment related complications. The Kaplan-Meier method was used to estimate the probability of CSS; patients were censored at the date of their last follow-up or death from other causes. Time to relapse was defined as the time from surgery to the diagnosis of relapse; patients without relapse were censored at the date of their last follow-up or death. Kaplan Meier curves were plotted to compare outcomes among patients with high and low hematological parameters stratified by the median and by quartiles. Univariable Cox regression analyses were used to evaluate the association between preoperative clinicopathologic and hematologic parameters and CSS for patients treated for metastatic disease and both CSS and RFS for patients treated for locoregional disease. Variables significantly associated with the outcome on univariable analyses were included in a multivariable Cox regression model. Landmark analyses at 3 and 8 weeks were conducted to explore the predictive value of postoperative NLR and % change in NLR on CSS. All statistical analyses were two-sided. A P-value of less than 0.05 was considered statistically significant. All statistical analyses were performed using R v3.5.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
The study cohort included a total of 217 patients, 112 of whom (52%) were treated for locoregional sRCC and 105 (48%) who underwent cytoreductive nephrectomy for metastatic disease. Clinicopathological characteristics of the study cohort are reported in Table 1. Patients presenting with metastatic disease were more likely to have larger tumors (p<0.001), a higher rate of T3/T4 tumors (p=0.002), and a higher rate of nodal involvement (p=0.002).
Table 1 –
Clinical and pathological characteristics of the study cohort (n=217) stratified by the absence and presence of metastatic disease at the time of nephrectomy
| Variable* | Overall (n=217) | Locoregional disease (n=112) | Metastatic disease (n=105) | p |
|---|---|---|---|---|
| Age in years | 61 (52, 69) | 62 (53, 70) | 60 (52, 68) | 0.3 |
| Sex | 0.6 | |||
| Female | 52 (24%) | 29 (26%) | 23 (22%) | |
| Male | 165 (76%) | 83 (74%) | 82 (78%) | |
| BMI | 28.5 (24.7, 31.6) | 28.5 (25.5, 31.8) | 28.4 (24.0, 31.6) | 0.3 |
| Smoking Status (n=212) | 0.5 | |||
| Current | 20 (9%) | 13 (12%) | 7 (7%) | |
| Former | 100 (47%) | 50 (45%) | 50 (50%) | |
| Never | 92 (43%) | 48 (43%) | 44 (44%) | |
| KPS (n=94)** | NA | |||
| ≥ 80 | 81 (86%) | NA | 81 (86%) | |
| < 80 | 13 (14%) | NA | 13 (14%) | |
| Tumor Histology | 0.6 | |||
| ccRCC | 164 (76%) | 87 (78%) | 77 (73%) | |
| non-ccRCC | 53 (24%) | 25 (22%) | 28 (27%) | |
| Tumor Size in cm | 9.4 (6.5, 12) | 8.0 (5.5, 10.7) | 10.0 (7.5, 12.5) | <0.001 |
| T Stage Group (n=214) | 0.002 | |||
| T1/T2 | 45 (21%) | 33 (30%) | 12 (12%) | |
| T3/T4 | 169 (79%) | 78 (70%) | 91 (88%) | |
| N Stage | 0.002 | |||
| N0/ Nx | 169 (78%) | 97 (87%) | 72 (69%) | |
| N1/ N2 | 48 (22%) | 15 (13%) | 33 (31%) | |
| WBC count (103/μL) | 7.80 (6.50, 9.50) | 7.40 (6.10, 9.15) | 8.10 (6.80, 10.10) | 0.007 |
| RBC count (106/μL) | 4.43 (4.01, 4.74) | 4.43 (4.04, 4.72) | 4.41 (3.98, 4.75) | 0.7 |
| PLT count (n=214, 103/μL) | 311 (234, 420) | 281 (224, 407) | 343 (253, 422) | 0.004 |
| Absolute Neutrophils count (103/μL) | 5.40 (4.30, 7.00) | 5.10 (3.90, 6.43) | 5.77 (4.60, 7.60) | <0.001 |
| Absolute Lymphocytes count (103/μL) | 1.40 (1.10, 1.80) | 1.50 (1.17, 1.90) | 1.40 (1.10, 1.70) | 0.084 |
| Absolute Monocytes count (103/μL) | 0.50 (0.40, 0.70) | 0.50 (0.30, 0.60) | 0.50 (0.40, 0.70) | 0.009 |
| NLR | 3.82 (2.90, 5.40) | 3.29 (2.50, 4.44) | 4.31 (3.31, 6.08) | <0.001 |
| LMR | 2.86 (2.00, 4.00) | 3.23 (2.25, 4.21) | 2.60 (1.62, 3.50) | <0.001 |
| PLR (n=214) | 216 (161, 316) | 194 (146, 274) | 248 (175, 344) | <0.001 |
| Median post-op NLR (n=89)** | 4.4 (3.1,6.3) | NA | 4.4 (3.1,6.3) | NA |
| Minimum post-op NLR (n=89)** | 3.25 (2.33,5.27) | NA | 3.25 (2.33,5.27) | NA |
| Median post-op NLR % Change Groups (n=89)** | NA | |||
| >25% Decrease | 21 (24%) | NA | 21 (24%) | |
| >25% Increase | 27 (30%) | NA | 27 (30%) | |
| No Change | 41 (46%) | NA | 41 (46%) | |
| Minimum post-op NLR % Change Groups (N=89)** | NA | |||
| >25% Decrease | 40 (45%) | NA | 40 (45%) | |
| >25% Increase | 12 (13%) | NA | 12 (13%) | |
| No Change | 37 (42%) | NA | 37 (42%) | |
BMI = body mass index; KPS = Karnofsky performance status; ccRCC = clear cell renal cell carcinoma; WBC = white blood cell; RBC = red blood cell; PLT = platelets; NLR = neutrophil-lymphocyte ratio; LMR = lymphocyte-monocyte ratio; PLR = platelet-lymphocyte ratio; NA = not applicable.
Continuous variables are reported as median and inter-quartile range and categorical ariables are reported as number and percent.
Only included for patients with metastatic disease.
A complete blood count was obtained at a median of 9 days (IQR 6, 15) prior to surgery. The median (IQR) preoperative NLR, LMR and PLR for the whole cohort were 3.82 (2.90, 5.40), 2.86 (2.00, 4.00) and 216 (161, 316), respectively. The different ratios were significantly correlated with one another (Figure 1). Patients with metastatic disease had a higher preoperative NLR (median 4.31 vs. 3.29, p<0.001) and PLR (median 248 vs. 194, p<0.001) and a lower preoperative LMR (median 2.60 vs. 3.23, p<0.001) when compared with patients with locoregional disease. Among patients with metastatic disease, non-clear cell histology was associated with NLR (β=2.03, p<0.001), PLR (β=0.73, p=0.02) and LMR (β=−1.08, p=0.015). Among patients with locoregional disease, tumor size was associated with NLR (β=0.18, p<0.001), PLR (β=0.05, p=0.044) and LMR (β=−0.08, p=0.016), nodal involvement was associated with NLR (β=1.28, p=0.043) and LMR (β=−1.06, p=0.011), and BMI was associated with PLR (β=−0.04, p=0.023, Supplementary Table 1).
Figure 1 -.
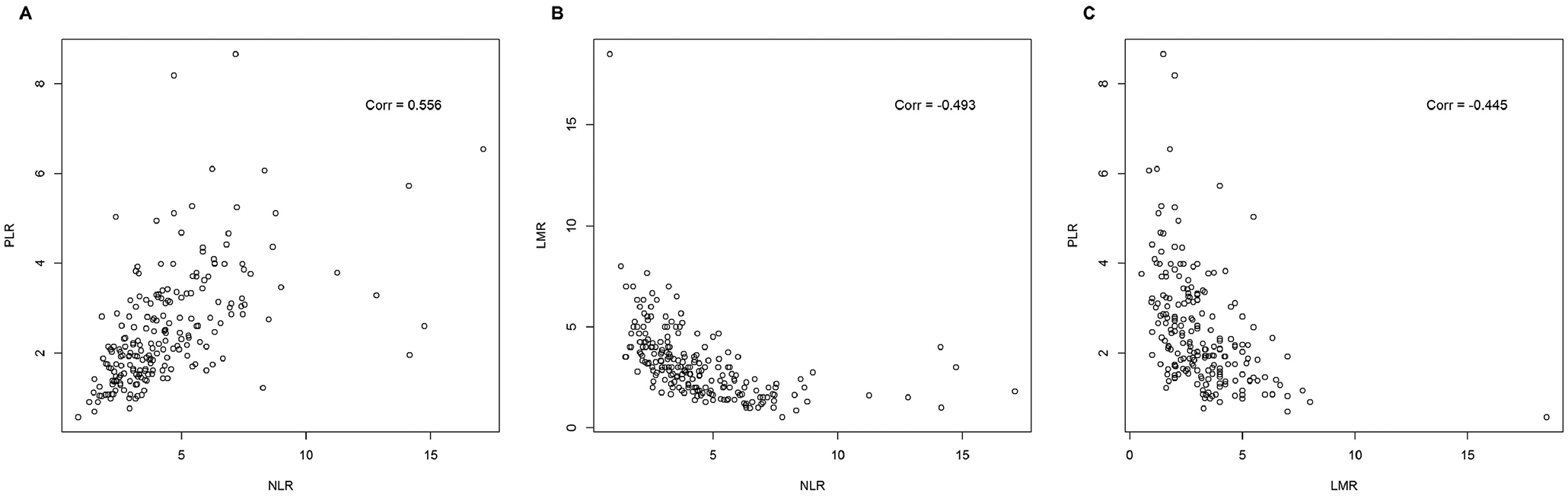
Pearson correlation plots of (A) neutrophil-lymphocyte ratio and platelet-lymphocyte ratio, (B) neutrophil-lymphocyte ratio and lymphocyte-monocyte ratio, and (C) lymphocyte-monocyte ratio and platelet-lymphocyte ratio for the whole cohort (n=214); p-value < 0.001 for all correlations
Postoperative NLR, within 1–8 weeks after the procedure, was available for 89/105 (85%) of metastatic patients and 64/112 (57%) of patients with locoregional disease. We did not include postoperative NLR in further analyses associated with patients with locoregional disease since the lack of this information for 43% of patients may create a selection bias. When selecting the minimum 1–8-week postoperative NLR value, the median (IQR) postoperative NLR was 3.25 (2.33, 5.27) and 40/89 (45%) patients had a >25% decrease in NLR. When analyzing the median value, the median postoperative NLR was 4.4 (3.10, 6.30) and 21/89 (24%) patients had a >25% decrease in NLR. The Pearson correlations between the preoperative and postoperative NLR were 0.54 (p<0.001) and 0.43 (p<0.001) when using minimal and median values, respectively.
Median follow-up for patients with locoregional and metastatic disease was 35.5 months and 20.4 months, respectively. During follow-up 50 patients treated for locoregional disease died, 46/50 due to their disease, and 78 patients treated for metastatic disease died, 77/78 due to their disease. Estimated 5-year CSS for patients with locoregional and metastatic disease were 55.6% and 14.8%, respectively. Similarly, 5-year OS were 53.8% and 14.3%, respectively. 5-year RFS for patients presenting with locoregional disease was 31.9% (Figure 2, Supplementary Figure 1). Univariate analyses of patients with metastatic disease showed that tumor N-stage, preoperative NLR and preoperative PLR were significantly associated with CSS. N-stage and preoperative NLR remained significant predictors of CSS on multivariate analysis (Table 2). On multivariate analysis of patient with locoregional disease sex, T-stage and preoperative NLR were significant predictors of CSS (Table 3). Significant predictors of RFS on multivariate analysis of patients with locoregional disease were tumor size and T-stage. Preoperative LMR was associated with RFS on univariate analysis however was not an independent predictor of RFS when adjusting for tumor size and T-stage (Table 3).
Figure 2 –
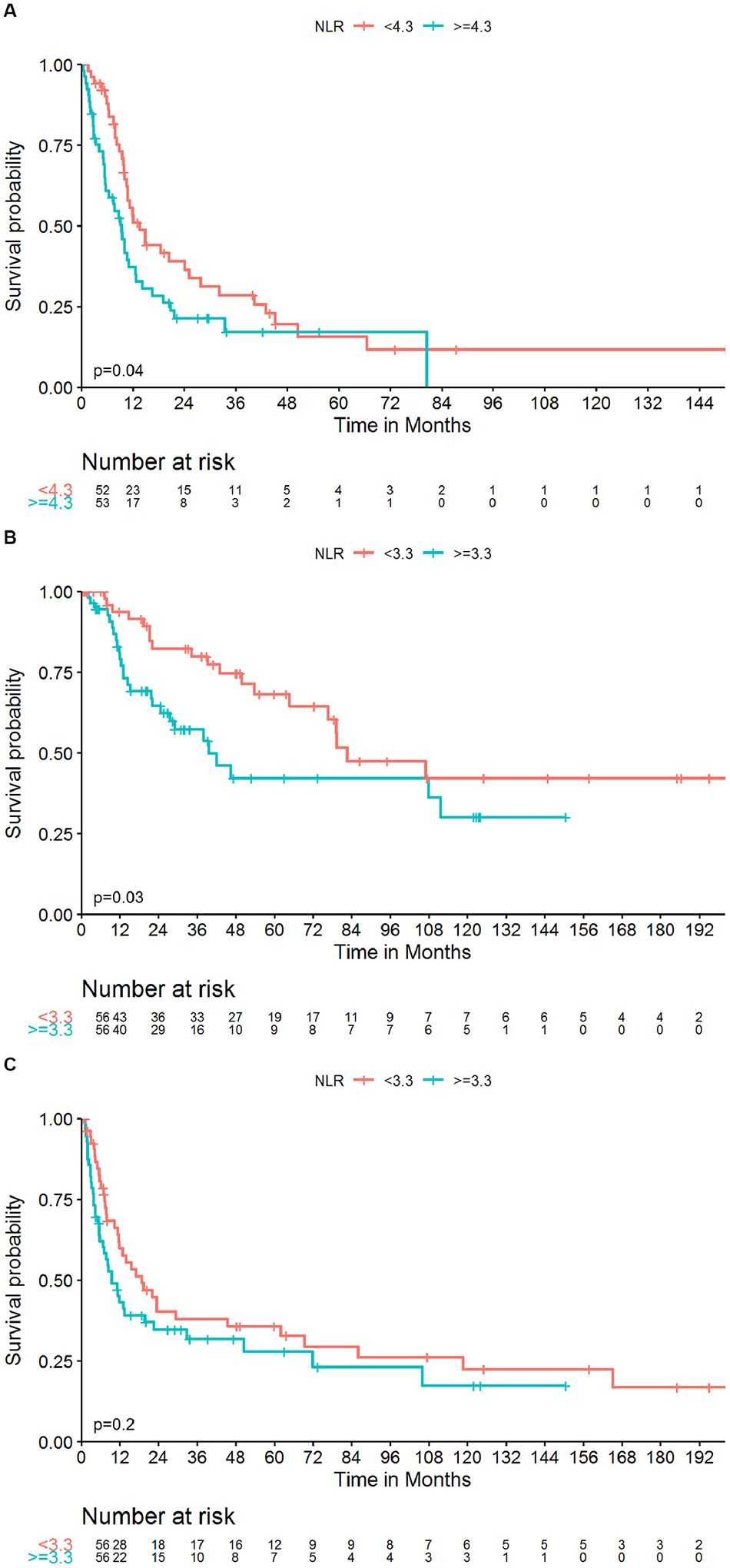
Kaplan-Meier curves of CSS in patients with metastatic sRCC (A) and CSS (B) and RFS (C) in patients with locoregional sRCC stratified by preoperative NLR above and below the median values; p-values were obtained by the log-rank test
Table 2 -.
Univariable and multivariable analyses of predictors of cancer specific survival for patients who underwent cytoreductive nephrectomy for metastatic sarcomatoid RCC (n=105)
| Variable | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Age | 1.02 | 1.00, 1.04 | 0.10 | |||
| Sex | ||||||
| Female | Ref. | |||||
| Male | 0.97 | 0.56, 1.69 | >0.9 | |||
| BMI (n=102) | 0.99 | 0.95, 1.04 | 0.8 | |||
| Smoking Status (n=101) | ||||||
| Current | Ref. | |||||
| Former | 1.83 | 0.65, 5.12 | 0.3 | |||
| Never | 1.62 | 0.57, 4.61 | 0.4 | |||
| KPS Category (n=94) | ||||||
| <80 | Ref. | |||||
| ≥80 | 0.90 | 0.45, 1.78 | 0.8 | |||
| Histology | ||||||
| ccRCC | Ref. | |||||
| non-ccRCC | 1.61 | 0.98, 2.65 | 0.058 | |||
| Tumor Size (n=103) | 1.02 | 0.96, 1.08 | 0.5 | |||
| T Stage Group (n=103) | ||||||
| T1/T2 | Ref. | |||||
| T3/T4 | 1.49 | 0.68, 3.27 | 0.3 | |||
| N Stage | ||||||
| N0/Nx | Ref. | Ref. | ||||
| N1/N2 | 2.37 | 1.48, 3.79 | <0.001 | 2.62 | 1.62, 4.23 | <0.001 |
| WBC | 1.04 | 0.98, 1.10 | 0.2 | |||
| RBC | 0.80 | 0.54, 1.19 | 0.3 | |||
| PLT/100 | 1.19 | 0.98, 1.45 | 0.072 | |||
| Neutrophils | 1.06 | 1.00, 1.13 | 0.070 | |||
| Lymphocytes | 0.79 | 0.54, 1.15 | 0.2 | |||
| Monocytes | 1.10 | 0.46, 2.60 | 0.8 | |||
| NLR | 1.20 | 1.08, 1.34 | <0.001 | 1.23 | 1.10, 1.37 | <0.001 |
| LMR | 0.94 | 0.82, 1.06 | 0.3 | |||
| PLR/100 | 1.19 | 1.02, 1.38 | 0.027 | |||
BMI = body mass index; KPS = Karnofsky performance status; ccRCC = clear cell renal cell carcinoma; WBC = white blood cells; RBC = red blood cells; PLT = platelets; NLR = neutrophil-lymphocyte ratio; LMR = lymphocyte-monocyte ratio; PLR = platelet-lymphocyte ratio; NA = not applicable; Ref. = reference.
Table 3 -.
Univariable and multivariable analyses of predictors of cancer specific survival and recurrence free survival for patients who underwent nephrectomy for locoregional sarcomatoid RCC (n=112)
| CSS Univariable | CSS Multivariable | RFS Univariable | RFS Multivariable | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p |
| Age | 1.01 | 0.98, 1.04 | 0.4 | 1.00 | 0.98, 1.02 | 0.9 | ||||||
| Sex | ||||||||||||
| Female | Ref. | Ref. | Ref. | |||||||||
| Male | 0.46 | 0.25, 0.86 | 0.015 | 0.51 | 0.27, 0.97 | 0.040 | 0.73 | 0.44, 1.21 | 0.2 | |||
| BMI (n=111) | 0.98 | 0.92, 1.05 | 0.6 | 1.00 | 0.96, 1.04 | >0.9 | ||||||
| Smoking Status (n=111) | ||||||||||||
| Current | Ref. | Ref. | ||||||||||
| Former | 0.47 | 0.17, 1.25 | 0.13 | 1.24 | 0.48, 3.19 | 0.7 | ||||||
| Never | 0.44 | 0.16, 1.18 | 0.10 | 1.33 | 0.52, 3.40 | 0.6 | ||||||
| Histology | ||||||||||||
| ccRCC | Ref. | Ref. | ||||||||||
| non-ccRCC | 1.27 | 0.67, 2.42 | 0.5 | 0.66 | 0.37, 1.16 | 0.2 | ||||||
| Tumor Size | 1.02 | 0.95, 1.09 | 0.7 | 1.08 | 1.03, 1.14 | 0.003 | 1.07 | 1.01, 1.13 | 0.017 | |||
| T Stage Group (n=111) | ||||||||||||
| T1/T2 | Ref. | Ref. | Ref. | Ref. | ||||||||
| T3/T4 | 2.65 | 1.27, 5.53 | 0.010 | 2.41 | 1.15, 5.07 | 0.020 | 2.20 | 1.28, 3.76 | 0.004 | 2.00 | 1.17, 3.43 | 0.012 |
| N Stage | ||||||||||||
| N0/Nx | Ref. | Ref. | ||||||||||
| N1/N2 | 2.13 | 1.06, 4.30 | 0.035 | 1.95 | 1.05, 3.62 | 0.036 | ||||||
| WBC | 1.09 | 1.03, 1.16 | 0.005 | 1.03 | 0.98, 1.09 | 0.3 | ||||||
| RBC | 0.69 | 0.38, 1.27 | 0.2 | 0.93 | 0.59, 1.46 | 0.8 | ||||||
| PLT/100 (n=109) | 1.06 | 0.85, 1.31 | 0.6 | 1.10 | 0.93, 1.30 | 0.3 | ||||||
| Neutrophils | 1.11 | 1.04, 1.18 | 0.002 | 1.04 | 0.98, 1.10 | 0.2 | ||||||
| Lymphocytes | 1.02 | 0.58, 1.81 | >0.9 | 0.96 | 0.62, 1.47 | 0.8 | ||||||
| Monocytes | 2.25 | 0.84, 6.07 | 0.11 | 1.63 | 0.80, 3.32 | 0.2 | ||||||
| NLR | 1.16 | 1.05, 1.28 | 0.003 | 1.10 | 1.00, 1.22 | 0.049 | 1.09 | 1.00, 1.20 | 0.059 | |||
| LMR | 0.84 | 0.68, 1.04 | 0.11 | 0.86 | 0.73, 1.00 | 0.050 | 0.91 | 0.78, 1.06 | 0.2 | |||
| PLR/100 (n=109) | 1.09 | 0.84, 1.42 | 0.5 | 1.16 | 0.93, 1.43 | 0.2 | ||||||
CSS = cancer specific survival; RFS = recurrence free survival; BMI = body mass index; ccRCC = clear cell renal cell carcinoma; WBC = white blood cells; RBC = red blood cells; PLT = platelets; NLR = neutrophil-lymphocyte ratio; LMR = lymphocyte-monocyte ratio; PLR = platelet-lymphocyte ratio; Ref. = reference.
Eighty-three patients with metastatic sRCC were included in the landmark analysis at week 8 evaluating the association between postoperative NLR and CSS. Minimum postoperative NLR (HR=1.16; 95% CI 1.02, 1.31; p=0.02) and median postoperative NLR (HR=1.11; 95% CI 1.02, 1.21; p=0.019) were associated with CSS. Similarly, in the landmark analysis at week 3 (n=46), minimum postoperative NLR was associated with CSS (HR=1.15, 95% CI 1.02, 1.29, p=0.019). However, relative change in NLR in both analyses was not a significant predictor of outcome. We did not include the postoperative NLR in a multivariable model due to the high correlation with preoperative NLR.
Discussion
Preoperative NLR is a predictor of outcome among patients with RCC. A systematic review of the literature showed an association between NLR>3 and an increased risk of recurrence in patients with localized RCC, and a decreased OS and progression free survival (PFS) in locally advanced and metastatic RCC.[7] Gu et al. reported the only series that specifically evaluated the predictive role of preoperative inflammatory parameters in patients with sRCC. In their series of 103 patients, most of whom (74%) had non-metastatic disease, NLR, PLR and LMR were significantly associated with decreased OS on univariable analyses. In a multivariable analysis including these preoperative parameters and pathologic features, NLR remained an independent predictor of OS (HR=4.1; 95% CI 1.5–10.9; P = 0.006). The study did not evaluate the role of NLR separately in the group of patients with metastatic sRCC.[5] The findings of the current study support the role of preoperative NLR in predicting CSS for patients with locoregional sRCC. Furthermore, we showed that higher preoperative NLR is significantly associated with a decrease in CSS in patients with metastatic disease, expanding its application to patients undergoing cytoreductive nephrectomy for sRCC. Interestingly, in both cohorts a higher NLR and PLR and lower LMR were associated with non-clear cell histology.[5]
The large variability in neutrophil levels between healthy individuals, and the variations in reported NLR cut-off values, complicate the use of preoperative NLR when counseling individual patients. Longitudinal evaluation of NLR may therefore be beneficial when using NLR to risk stratify patients.[11] A decrease in postoperative NLR was associated with a better outcome in patients operated for colorectal cancer, non-small cell lung cancer and bladder cancer.[12–14] Furthermore, in patients with bladder cancer who underwent cystectomy, an increase in postoperative NLR during follow-up preceded the detection of recurrence.[15] In RCC, the role of NLR dynamics has been evaluated mostly as a predictor of response to systemic treatment in the metastatic setting.[9, 10] In a large group of patients treated with targeted therapy, an increase in NLR 6 weeks after treatment was associated with reduced objective response rates, PFS and OS.[9] A similar finding was reported in patients with metastatic RCC treated with immune check-point blockade. In these patients, an NLR increase by ≥25% 6 weeks after initiation of treatment was associated with significantly worse PFS and OS, regardless of baseline NLR levels.[10] Ohno et al. evaluated the role of postoperative NLR in predicting recurrence after nephrectomy for patients with localized clear cell RCC. Consistent with previous reports they showed that increased preoperative NLR was significantly associated with lower RFS. Surprisingly, increased postoperative NLR was associated with a higher RFS rate and the group of patients who had an increased preoperative NLR and decreased postoperative NLR had the worst outcome.[16] The association between NLR dynamics and outcome in patients with sRCC has not been previously investigated. In the current study, while the level of postoperative NLR predicted outcome, a decrease in NLR after cytoreductive nephrectomy was not significantly associated with outcome. The lack of association may be the result of residual metastatic disease which still affects the NLR even after cytoreductive nephrectomy. The role of NLR change in patients with localized disease should be evaluated by future studies.
The mechanism underlying the association between NLR and outcome is not fully understood. Neutrophils may play an active role in tumor initiation, growth and metastases.[11] The role of neutrophils in tumor initiation is evident in CXCR2 deficient tumor models. The chemokine receptor CXCR2 is a key mediator of neutrophil migration and its deficiency profoundly suppressed inflammation-driven and spontaneous tumorigenesis in the skin and intestines.[17] Similarly, the use of an anti-neutrophil antibody in a K-ras mutant mouse model of lung cancer resulted in a significant decrease in the number of lung tumors.[18] Neutrophils may promote tumor progression by a direct effect on tumor cells and by stimulating angiogenesis.[18–20] In neutrophil elastase deficient mouse models of lung cancer, tumor cell proliferation and angiogenesis were significantly reduced, leading to inhibition of tumor development.[18, 19] In pancreatic islet carcinogenesis mouse models, transient depletion of neutrophils significantly suppressed VEGF:VEGF-receptor association, and markedly reduced the frequency of initial angiogenic switching, further supporting the role of neutrophils in angiogenesis.[20] The role of neutrophils in the promotion of metastatic disease is controversial, however several cancer models have supported its role especially during the early steps of the metastatic cascade.[21, 22] Finally, tumors themselves may secrete factors leading to the release of granulocytes from the bone marrow elevating their number in the blood.[11]
The central role of T-lymphocytes in cancer immunology has been highlighted recently with the rise of immunotherapy as an effective modality for treating cancer.[23–26] In a pooled analysis of studies evaluating the association between tumor-infiltrating lymphocytes and outcome, both CD3+ and CD8+ tumor infiltrating lymphocytes were significantly associated with a positive effect on survival.[23] While most of the focus is given to tumor infiltrating lymphocytes, Spitzer et al. showed that peripheral CD4+ T-cells conferred protection against new tumors and significantly expanded in patients responding to immunotherapy.[25] Additionally, B-lymphocytes have a central immunomodulatory role and can inhibit tumor progression by the development of tumor-reactive antibodies, priming CD4+ and CD8+ T-cells and activating NK-cells and macrophages to promote tumor killing and phagocytosis, respectively.[26] However, certain subsets of both T- and B-lymphocytes may act to decrease the immune response towards a tumor, emphasizing the complexity of this association.[24, 26] The NLR is a measure that combines information for both neutrophils and lymphocytes and may reflect the balance between tumor promoting inflammatory process and tumor suppressing host immunity.[9] Nevertheless, the true association between NLR values measured in peripheral blood and the immune process in the tumor microenvironment requires further study.
The limitations of the current study include its retrospective design and the possibility for selection bias. We did not control our analyses for tumor necrosis and percent sarcomatoid dedifferentiation as this information was not accurately captured in our database. Larger cohorts are required to evaluate optimal cutoff points for NLR in sRCC patients, however Kaplan-Meier curves of CSS in metastatic patients showed a separation when stratified by quartiles, suggesting a continuous scale of the marker may add predictive information. In addition, we were unable to evaluate the predictive value of NLR in patients with sRCC treated with immunotherapy. Given the potential role of check-point inhibition in sRCC, future studies should evaluate the predictive role of NLR in this setting.[27, 28]
Conclusions
The current study supports the association between preoperative NLR and CSS in patients treated for localized sRCC and demonstrates a similar association in patients treated for metastatic sRCC. Therefore, NLR should be evaluated as a possible biomarker in future risk stratifying tools for patients with sRCC.
We did not find an association between postoperative changes in NLR and outcome in patients with metastatic sRCC. Additional studies are required to evaluate the role of postoperative change in NLR in predicting outcome following nephrectomy for localized sRCC.
Supplementary Material
Highlights.
Preoperative NLR is higher in metastatic vs. locoregional sarcomatoid RCC
Preoperative NLR predicted CSS in both metastatic and locoregional disease
Changes in NLR after cytoreductive nephrectomy were not associated with survival
NLR should be considered when establishing predictive models for sarcomatoid RCC
Funding
This work was supported by The Sidney Kimmel Center for Prostate and Urologic Cancers. This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest and Disclosure Statement
All authors have nothing to disclose.
References
- [1].Shuch B, Bratslavsky G, Linehan WM, Srinivasan R. Sarcomatoid renal cell carcinoma: a comprehensive review of the biology and current treatment strategies. The oncologist. 2012;17:46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lebacle C, Pooli A, Bessede T, Irani J, Pantuck AJ, Drakaki A. Epidemiology, biology and treatment of sarcomatoid RCC: current state of the art. World journal of urology. 2018. [DOI] [PubMed] [Google Scholar]
- [3].Shuch B, Bratslavsky G, Shih J, Vourganti S, Finley D, Castor B, et al. Impact of pathological tumour characteristics in patients with sarcomatoid renal cell carcinoma. BJU international. 2012;109:1600–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zhang BY, Thompson RH, Lohse CM, Leibovich BC, Boorjian SA, Cheville JC, et al. A novel prognostic model for patients with sarcomatoid renal cell carcinoma. BJU international. 2015;115:405–11. [DOI] [PubMed] [Google Scholar]
- [5].Gu L, Ma X, Li H, Chen L, Xie Y, Zhao C, et al. Prognostic value of preoperative inflammatory response biomarkers in patients with sarcomatoid renal cell carcinoma and the establishment of a nomogram. Scientific reports. 2016;6:23846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Templeton AJ, McNamara MG, Seruga B, Vera-Badillo FE, Aneja P, Ocana A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Journal of the National Cancer Institute. 2014;106:dju124. [DOI] [PubMed] [Google Scholar]
- [7].Boissier R, Campagna J, Branger N, Karsenty G, Lechevallier E. The prognostic value of the neutrophil-lymphocyte ratio in renal oncology: A review. Urologic oncology. 2017;35:135–41. [DOI] [PubMed] [Google Scholar]
- [8].Grimes N, Hannan C, Tyson M, Thwaini A. The role of neutrophil-lymphocyte ratio as a prognostic indicator in patients undergoing nephrectomy for renal cell carcinoma. Canadian Urological Association journal = Journal de l’Association des urologues du Canada. 2018;12:E345–E8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Templeton AJ, Knox JJ, Lin X, Simantov R, Xie W, Lawrence N, et al. Change in Neutrophil-to-lymphocyte Ratio in Response to Targeted Therapy for Metastatic Renal Cell Carcinoma as a Prognosticator and Biomarker of Efficacy. European urology. 2016;70:358–64. [DOI] [PubMed] [Google Scholar]
- [10].Lalani AA, Xie W, Martini DJ, Steinharter JA, Norton CK, Krajewski KM, et al. Change in Neutrophil-to-lymphocyte ratio (NLR) in response to immune checkpoint blockade for metastatic renal cell carcinoma. Journal for immunotherapy of cancer. 2018;6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nature reviews Cancer. 2016;16:431–46. [DOI] [PubMed] [Google Scholar]
- [12].Guthrie GJ, Roxburgh CS, Farhan-Alanie OM, Horgan PG, McMillan DC. Comparison of the prognostic value of longitudinal measurements of systemic inflammation in patients undergoing curative resection of colorectal cancer. British journal of cancer. 2013;109:24–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jin F, Han A, Shi F, Kong L, Yu J. The postoperative neutrophil-to-lymphocyte ratio and changes in this ratio predict survival after the complete resection of stage I non-small cell lung cancer. OncoTargets and therapy. 2016;9:6529–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kang M, Jeong CW, Kwak C, Kim HH, Ku JH. The Prognostic Significance of the Early Postoperative Neutrophil-to-Lymphocyte Ratio in Patients with Urothelial Carcinoma of the Bladder Undergoing Radical Cystectomy. Annals of surgical oncology. 2016;23:335–42. [DOI] [PubMed] [Google Scholar]
- [15].Morizawa Y, Miyake M, Shimada K, Hori S, Tatsumi Y, Nakai Y, et al. Neutrophil-tolymphocyte ratio as a detection marker of tumor recurrence in patients with muscle-invasive bladder cancer after radical cystectomy. Urologic oncology. 2016;34:257 e11–7. [DOI] [PubMed] [Google Scholar]
- [16].Ohno Y, Nakashima J, Ohori M, Gondo T, Hatano T, Tachibana M. Followup of neutrophil-to-lymphocyte ratio and recurrence of clear cell renal cell carcinoma. The Journal of urology. 2012;187:411–7. [DOI] [PubMed] [Google Scholar]
- [17].Jamieson T, Clarke M, Steele CW, Samuel MS, Neumann J, Jung A, et al. Inhibition of CXCR2 profoundly suppresses inflammation-driven and spontaneous tumorigenesis. The Journal of clinical investigation. 2012;122:3127–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gong L, Cumpian AM, Caetano MS, Ochoa CE, De la Garza MM, Lapid DJ, et al. Promoting effect of neutrophils on lung tumorigenesis is mediated by CXCR2 and neutrophil elastase. Molecular cancer. 2013;12:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Houghton AM, Rzymkiewicz DM, Ji H, Gregory AD, Egea EE, Metz HE, et al. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nature medicine. 2010;16:219–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:12493–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Huh SJ, Liang S, Sharma A, Dong C, Robertson GP. Transiently entrapped circulating tumor cells interact with neutrophils to facilitate lung metastasis development. Cancer research. 2010;70:6071–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Spicer JD, McDonald B, Cools-Lartigue JJ, Chow SC, Giannias B, Kubes P, et al. Neutrophils promote liver metastasis via Mac-1-mediated interactions with circulating tumor cells. Cancer research. 2012;72:3919–27. [DOI] [PubMed] [Google Scholar]
- [23].Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. British journal of cancer. 2011;105:93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. [DOI] [PubMed] [Google Scholar]
- [25].Spitzer MH, Carmi Y, Reticker-Flynn NE, Kwek SS, Madhireddy D, Martins MM, et al. Systemic Immunity Is Required for Effective Cancer Immunotherapy. Cell. 2017;168:487–502 e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Yuen GJ, Demissie E, Pillai S. B lymphocytes and cancer: a love-hate relationship. Trends in cancer. 2016;2:747–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Joseph RW, Millis SZ, Carballido EM, Bryant D, Gatalica Z, Reddy S, et al. PD-1 and PD-L1 Expression in Renal Cell Carcinoma with Sarcomatoid Differentiation. Cancer immunology research. 2015;3:1303–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kawakami F, Sircar K, Rodriguez-Canales J, Fellman BM, Urbauer DL, Tamboli P, et al. Programmed cell death ligand 1 and tumor-infiltrating lymphocyte status in patients with renal cell carcinoma and sarcomatoid dedifferentiation. Cancer. 2017;123:4823–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


