Abstract
Ascertaining function-specific orchestration of NFkB in response to radiation may reveal a molecular blueprint that dictates induced relapse and metastasis of the neuroblastoma. We recently demonstrated that sustained activation of NFkB caused by ionizing radiation (IR)-initiated TNFα–NFkB feedback signaling leads to radioresistance and recurrence of neuroblastoma. We investigated whether muting IR-triggered or TNFα-dependent second-signaling feedback–dependent NFkB nuclear import results in limiting IR-altered invasion and metastasis. Neuroblastoma cells were exposed to 2 Gy and incubated for 1 h or 24 h. The cells were then treated with an NFkB-targeting peptide blocker, SN50. Upon confirming the blockade in DNA-binding activity, transcription driven transactivation of NFkB and secretion of soluble TNFα, transcriptional alterations of 93 tumor invasion/metastasis genes were assessed by using QPCR profiling and then were selectively validated at the protein level. Exposure to 2 Gy induced 63, 42 and 71 genes in surviving SH-SY5Y, IMR-32 and SK-NMC cells, respectively. Blocking post-translational nuclear import of NFkB comprehensively inhibited both initial activation of genes (62/63, 34/42 and 65/71) triggered by IR and also TNFα-mediated second signaling–dependent sustained (59/63, 32/42 and 71/71) activation of tumor invasion and metastasis signaling molecules. Furthermore, alterations in the proteins MMP9, MMP2, PYK-2, SPA-1, Dnmt3b, Ask-1, CTGF, MMP10, MTA-2, NF-2, E-Cadherin, TIMP-2 and ADAMTS1 and the results of our scratch-wound assay validate the role of post-translational NFkB in IR-regulated invasion/metastasis. These data demonstrate that IR-induced second-phase (post-translational) NFkB activation mediates TNFα-dependent second signaling and further implies that IR induced NFkB in cells that survive after treatment regulates tumor invasion/metastasis signaling.
Keywords: Neuroblastoma, Tumor invasion/metastasis, NFkB–TNFα feedback, Second signaling, QPCR profiling
Introduction
Neuroblastoma (NB), as originally described by Virchow in 1863 [1] is an embryonal tumor of the autonomic nervous system, originating from neural-crest tissues [2]. It is one of the most frequent extra cranial solid tumors in children (aged ≤ 5 years) and the most frequently diagnosed neoplasm during infancy. While the incidence of neonatal and antenatal NB is well attributed; several neonatal cases escape detection because of spontaneous regression or maturation of benign lesions to lethal meta-static spread [1, 3, 4]. Tumor recurrence poses a major challenge to curing NB and survival rates are <43 % for local and only 10 % for systemic recurrences. Even though approximately 50 % of patients have disseminated disease at diagnosis, little is known about the biology of NB invasion and metastasis. NB also has an unexplained tendency to metastasize to bone marrow, liver and non-contiguous lymph nodes. The mortality rate for children with bone metastasis is greater than 90 %. Thus, understanding and targeting the molecular determinants of metastatic NB is a key step to improving the clinical management and treatment of this tumor.
Clinical and laboratory evidence suggests that several human cancers contain populations of rapidly proliferating clonogens that can have substantial impact on tumor control after chemoradiation or radiotherapy (RT) [5]. Tumor cell repopulation may arise from remnant cells of the original neoplasm that have escaped therapeutic intervention and later become visible at the original site and/or at distant sites. Ionizing radiation (IR)-induced neoplasms occur at the edges of the irradiated field, where the IR does not cause cell death but is sufficient to induce malignant transformation [6]. IR has been shown to activate various transcription factors (TFs) including Nuclear Factor kappa B (NFkB) [7] and studies have suggested the influential role of TFs in tumor-igenesis [8]. NFkB response elements are found within the promoter and enhancer region of a wide variety of genes involved in proliferation, apoptosis, inflammation, differentiation and cell cycle control [9, 10]. Unlike other inducible TFs, a multitude of conditions and/or agents can activate NFkB, and elevated NFkB activity has been linked with tumor resistance to chemotherapy and IR [11]. Soon after the first report that clinically relevant doses of IR induced NFkB [12, 13], innumerable studies both in vitro and in vivo demonstrates that IR specifically activates NFkB. We found that IR profoundly activates NFkB in human NB cells [14, 15], leading to induced radioprotection, and further that the forced inhibition of NFkB enhanced the IR-induced cell death. Disruption of aberrantly regulated survival signaling mediated by NFkB has recently become an important target in the therapy of several chemoresistant/radioresistant cancers [16]. Recently, our in vitro and in vivo studies demonstrated that the cells from the original neoplasm that have escaped IR insults resulted in the development of concurrent radioadaptation and survival advantage mediated by sustained activation of NFkB through TNFα-dependent second-signaling (NFkB→TNFα→NFkB) feedback [17]. To that end, using in vitro and in vivo approaches, we have shown that clinical doses of radiation activate and maintain NFkB in human NB [17] and we have further elucidated the sequence of TNFα-dependent second-signaling feedback in the sustained maintenance of NFkB activation in the surviving NB cells after a course of radiotherapy in this setting [17]. More importantly, we have shown that the TNFα-dependent maintenance of NFkB activation after radiotherapy facilitates NFkB-dependent inhibitor of apoptosis (IAPs)—mediated radioresistance and survival advantage in human NB [17]. Ascertaining the molecular signaling orchestration that underlies the NB cell survival advantage after receiving clinical doses of radiation will evidently help to understand NB progression, particularly with respect to the potential of NFkB signaling in invasion and metastasis of NB. In this regard, to precisely validate the definite role of the radiation-induced NFkB-triggered TNFα-dependent second-signaling feedback associated with sustained NFkB in the regulation of NB invasion and metastasis, we investigated the transcriptional regulation of 93 tumor invasion and metastasis signaling molecules, the translation of MMP9, MMP2, PYK-2, SPA-1, Dnmt3b, Ask-1, CTGF, MMP10, MTA-2, NF-2, E-Cadherin, TIMP-2 and ADAMTS1, and tumor progression in this setting. Our results demonstrate that blocking the post-translational nuclear import of the radiation induced NFkB (both the IR-triggered initial activation and the second signaling–dependent sustained activation) comprehensively regulates tumor invasion and metastasis in human NB.
Materials and methods
Cell culture
The human NB cells SK-N-MC, IMR-32, SH-SY5Y and MC-IXC were obtained from ATCC (Manassas, VA). Culture and maintenance of the NB SH-SY5Y, IMR-32 and SKN-MC cells were performed as described earlier [17]. MCIXC cells were maintained in DMEM medium (Mediatech Inc., Herndon, VA) supplemented with 5,000 IU/ml penicillin, 5,000 lg/ml streptomycin and 10 % FBS. For passaging and for all experiments, the cells were detached by using 0.25 % trypsin/1 %EDTA, resuspended in complete medium, counted (Countess, Invitrogen) and incubated in a 95 % air/5 % CO2 humidified incubator.
Irradiation experiments and NFkB nuclear import blocking
To determine whether IR induced sustained activation of NFkB and initiation of NFkB-TNFα feedback, cells were exposed to 2 Gy by using Gamma Cell 40 Exactor (Nor-dion International Inc, Ontario, Canada) at a dose rate of0.81 Gy/min. Mock-irradiated cells were treated identically except that the cells were not subjected to IR. QPCR profiling for irradiated cells was performed after the 24 h time point and was used to compare both the initial-phase and the second-phase blocking of NFkB. We adopted this approach to avoid the robust gene activation as a part of immediate early stress response that could possibly lead to false positives. For NFkB nuclear translocation inhibition studies, the cells that were exposed to radiation were incubated for 1 or 24 h and then treated with 50 nM of the NFkB cell permeable peptide SN50 (EMD Millipore, Billerica, MA). The cells were harvested at 1 and 3 h for RNA isolation and transcriptional profiling and at 24, 48 and 72 h for protein analysis. SN50 peptide (H-Ala-Ala-Val-Ala-Leu-Leu-Pro-Ala-Val-Leu-Leu-Ala-Leu-Leu-Ala-Pro-Val-Gln–Arg–Lys–Arg–Gln–Lys–Leu–Met–Pro-OH) contains the nuclear localization sequence (360–369) of NFkB p50 that inhibits the translocation of the NFkB active complex into the nucleus [18]. All experiments were repeated at least three times in each group. The inhibition of radiation-induced sustained NFkB activation in the initiation phase and also after TNFα-dependent second signaling feedback after muting NFkB nuclear translocation was validated by using an EMSA analysis (NFkB DNA binding activity), a luciferase reporter assay, QPCR (transcriptional activation) and ELISA (NFkB-dependent TNFα secretion) according the methods reported earlier [17].
Real-time QPCR profiling of invasion/metastasis signaling pathway molecules
Total RNA extraction and real-time QPCR profiling were performed as described in our earlier studies [19, 20]. We used human tumor invasion and metastasis signaling pathway profiler (Realtimeprimers.com, Elkins Park, PA) containing 93 genes. We started with this highly selected QPCR profiler instead of an all-encompassing gene array because the selected genes provide a well-characterized profile governing tumor cell invasion and metastasis signal transduction and transcriptional targets, hence facilitating the interpretation of data, simplifying data acquisition and analysis, and avoiding genes that were not functionally characterized. More importantly, 27 of these genes, including CD44, CCR7, CTSB,CTSL1, EREG, HGF, ID1, IL1B, KISS1, MCAM, MMP1, MMP13, MMP3, MMP9, MYC, NF2, NME4, PTEN, PTGS2, SERPINE1, SPARC, SPP1, SYK, TIMP2, TNC, TP53 and VEGFA, contain NFĸB response elements and served as a set of genes that are under the direct downstream targets of NFkB. Furthermore, QPCR profiling allows detection and quantification of gene expression in real-time. Each profiling plate also had the housekeeping genes β-Actin, GAPDH, and HPRT1. The ΔΔct values were calculated by normalizing the gene expression levels to the expression of the housekeeping genes. The normalized data were then compared between groups, and the relative expression level of each gene was expressed as a fold change. When comparing each gene’s signal intensity between groups, we used a two-fold or more (≥twofold) increase or decrease as our “stringent” criterion for upregulation or downregulation and an increase/decrease of <twofold as our “less stringent” criterion. Classifying gene regulation criteria in this manner can provide an index of reliability of the gene expression data [20].
Immunoblotting
Total protein extraction and immunoblotting were performed as described in our earlier study [17]. For this study, the protein-transferred membranes were incubated with mouse monoclonal anti-MMP2, MMP9, SPA-1, PYK2, ASK-1 or Dnmt3B antibodies (Santa Cruz Bio-technology Inc., Santa Cruz, CA) and developed with the appropriate anti-mouse second antibody. Blots were stripped and reblotted with mouse monoclonal anti-α-tubulin antibody (Santa Cruz) to determine equal loading of the samples.
ELISA
ELISA was performed as described in our earlier study [17]. In brief, 100 μl of lysates (10 μg/ml) from the NB IMR-32, SH-SY5Y or MC-IXC cells that were mock irradiated, exposed to IR, exposed to IR followed by SN50 treatment (1 or 24 h post-IR) were coated on a high-binding plate (overnight at 4 °C) and blocked for 4 h (BLOT-quick blocker, Calbiochem). Coated wells were then labeled with mouse monoclonal anti-Timp2 (Biolegend Inc., San Diego, CA), -E-Cadherin, rabbit polyclonal anti-NF-2, -Adamts1, goat polyclonal anti-Ctgf, -MMP10, or MTA-2 antibodies (Santa Cruz) for 3 h (1:500) and tagged with their corresponding secondary-IgG HRP (1:2000) conjugate (Alpha Diagnostics, San Antonio, TX).
The TMB substrate (Alpha Diagnostics) was used as a detection system (20 min), and the reaction was stopped by using 1N hydrochloric acid (EMD Biosciences, La Jolla CA). The absorbance at 450 nm was read on a Synergy II multi-detection micro plate reader (Biotek Instruments, Winooski, VT), and GraphPad PRISM (GraphPad Software, Inc., La Jolla, CA) was used to perform an ANOVA with Tukey’s post hoc correction to identify significant differences among groups. A P value of <0.05 is considered statistically significant.
Scratch-wound assay
Using 30 mm diameter dishes, the human NB SH-SY5Y cells were grown to confluence in the presence of serum. The wound was made by scraping the monolayer of cells with a 10 ll pipet tip. We changed the culture medium immediately after wounding to prevent the medium from being conditioned with cell debris and factors released from the detached cells. The cells were then mock irradiated, irradiated or exposed to radiation and treated with SN50 after 1 or 24 h. All experiments were repeated at least five times in each group.
Results
Radiation activates tumor invasion/metastasis signaling molecules in surviving human neuroblastoma cells
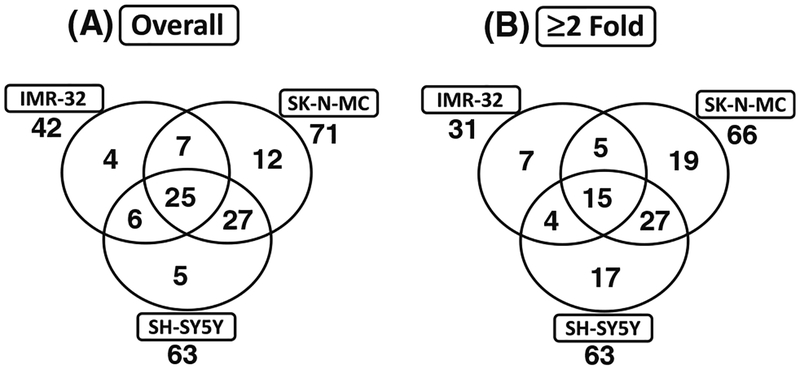
To ascertain whether radiotherapy modulates tumor invasion/metastasis signaling molecules in surviving NB cells, we utilized quantitative yet comprehensive QPCR profiling to examine the induced-alterations after the application of clinical doses of radiation. Radiation induced 42, 71 and 63 genes in IMR-32, SK-N-MC and SH-SY5Y cells, respectively (Fig. 1a). Interestingly, of these radiation induced-genes, 25 invasion/metastasis signaling molecules were upregulated commonly across all NB cell lines investigated (Fig. 1a). Application of our stringent criterion (>twofold) resulted in the identification of 31, 66 and 63 genes in the IMR-32, SK-N-MC and SH-SY5Y cells that were induced by radiation (Fig. 1b). Notably, 15 genes including Adamts1, Cdh1, Cdh11, Cdh6, Col4A2, Ctgf, Ctsl1, Hmgb1, Id1, Mmp10, Mta2, Nf2, Pax5, Serpineb5 and Timp2 were significantly upregulated after radiotherapy in all NB cell lines investigated (Fig. 1b).
Fig. 1.
Venn diagrams showing the number of tumor invasion and metastasis molecules significantly upregulated (a) without or (b) with stringent criteria (≥twofold) in human neuroblastoma (IMR-32, SKN-MC and SH-SY5Y) cells exposed to radiation (2 Gy). The numbers outside each circle represent the total number of genes upregulated in each cell-line. The numbers inside the greater part of the circles represent the genes that were selectively upregulated in that particular cell-line. The numbers in two overlapping circles represent the number of genes upregulated commonly in two cell lines. The numbers in the center represent genes upregulated in all three cell lines
Radiation-induced NFkB mediates tumor invasion/metastasis signaling molecules in surviving human neuroblastoma cells
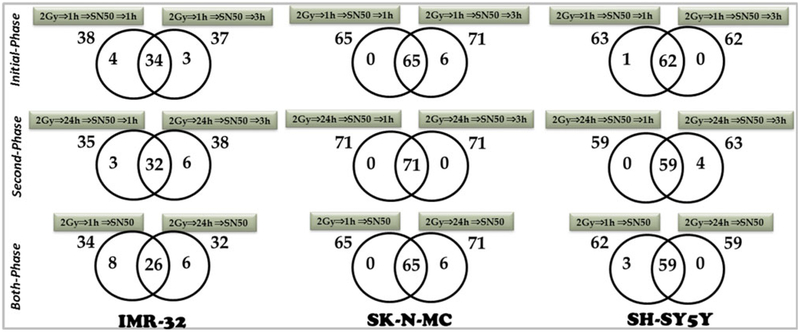
To precisely delineate whether radiation-induced NFkB mediates the activation of tumor invasion and metastasis signaling in the surviving cells, radiation-induced alterations of the invasion/metastasis signaling was investigated after muting the radiation-induced nuclear translocation of pre-existing NFkB (1 h post-irradiation) and analyzed at 1 and 3 h [IR ⇒ 1 h ⇒ SN50 ⇒ 1 h/3 h ⇒ profiling]. Multing radiation-triggered ‘initial-phase’ NFkB within 1 h post-IR suppressed 38 of 42, 65 of 71 and 63 of 63 radiation-induced invasion/metastasis signaling molecules in IMR-32, SK-N-MC and SH-SY5Y cells, respectively (Fig. 2). We observed a similar suppression of invasion/metastasis signaling molecules genes (37 of 42, 71 of 71 and 62 of 63) in the IMR-32, SK-N-MC and SH-SY5Y cells when we muted the NFkB nuclear translocation within 1 h post-IR and analyzed after 3 h (Fig. 2). Evidently, comparative analysis of the gene expression patterns after muting NFkB 1 h post-IR revealed a ‘time-independent’ suppression of 34, 65 and 62 genes in human IMR-32, SK-N-MC and SH-SY5Y cells.
Fig. 2.
Radiation-induced NFkB mediates tumor invasion/metastasis molecules in surviving human neuroblastoma cells. Venn diagrams showing the numbers of invasion/metastasis genes inhibited after muting the radiation-induced nuclear translocation of constitutive (initial-phase, [IR ⇒ 1 h ⇒ SN50 ⇒ 1 h/3 h ⇒ profiling]) NFkB, and silencing the TNFα-dependent second signaling–mediated ‘second-phase’ activation and maintenance [IR ⇒ 24 h ⇒ SN50 ⇒ 1 h/3 h ⇒ profiling] of NFkB in the human neuroblastoma IMR-32, SK N-MC and SH-SY5Y cells. In either case, the expression profiles are compared to the radiation-induced gene profiles of the specific cell line. The numbers outside each circle represent the total number of genes downregulated in the specific study time point. The numbers inside the greater part of the circles represent the genes that were selectively downregulated at that particular time point. The numbers in two overlapping circles represent the time-independent downregulated genes. Both-phase Venn diagrams showing the comparative inhibitory profiles of invasion/metastasis molecules between initial-phase and second-phase NFkB nuclear translocation blocking[IR ⇒ 1 h/24 h ⇒ SN50 ⇒ 1 h/3 h ⇒ profiling] in these neuroblastoma cell lines. The numbers outside each circle represent the total number of time-independent downregulated genes in a specific phase. The numbers inside the greater part of the circles represent the time-independent downregulated genes that were selectively downregulated in that phase. Numbers in two overlapping circles represent the time- and phase-independent downregulated genes
Radiation induced NFkB-triggered TNFα-dependent maintenance of NFkB mediates the functional orchestration of tumor invasion/metastasis in the surviving human neuroblastoma cells
To validate the role of TNFα-dependent ‘second-phase’ activation and maintenance of NFkB in the regulation of invasion/metastasis, we examined the induced alterations in invasion and metastasis signaling molecules after blocking the second phase nuclear translocation of NFkB and analyzed at 1 or 3 h [IR ⇒ 24 h ⇒ SN50 ⇒ 1 h/3 h ⇒ profiling]. We blocked TNFα-dependent ‘second phase’ NFkB activation with SN50 cell-penetrating peptide and examined at 24 h post radiation resulted in the suppression of 35 of 42, 71 of 71 and 59 of 63 radiation-induced invasion/metastasis signaling molecules in the IMR-32, SK-N-MC and SH-SY5Y cells, respectively (Fig. 2). This ‘second-phase’ NFkB blocking dependent suppression (38 of 42, 71 of 71 and 63 of 63) of invasion/metastasis signaling molecules remained consistent over an extended time period of 3 h in the IMR-32, SK-N-MC and SH-SY5Y cell lines (Fig. 2). Distinctively, comparative analysis of the gene expression patterns after muting NFkB 24 post-IR revealed a ‘time-independent’ suppression of 32, 71 and 59 genes in human IMR-32, SK-N-MC and SHSY5Y cells respectively (Fig. 2).
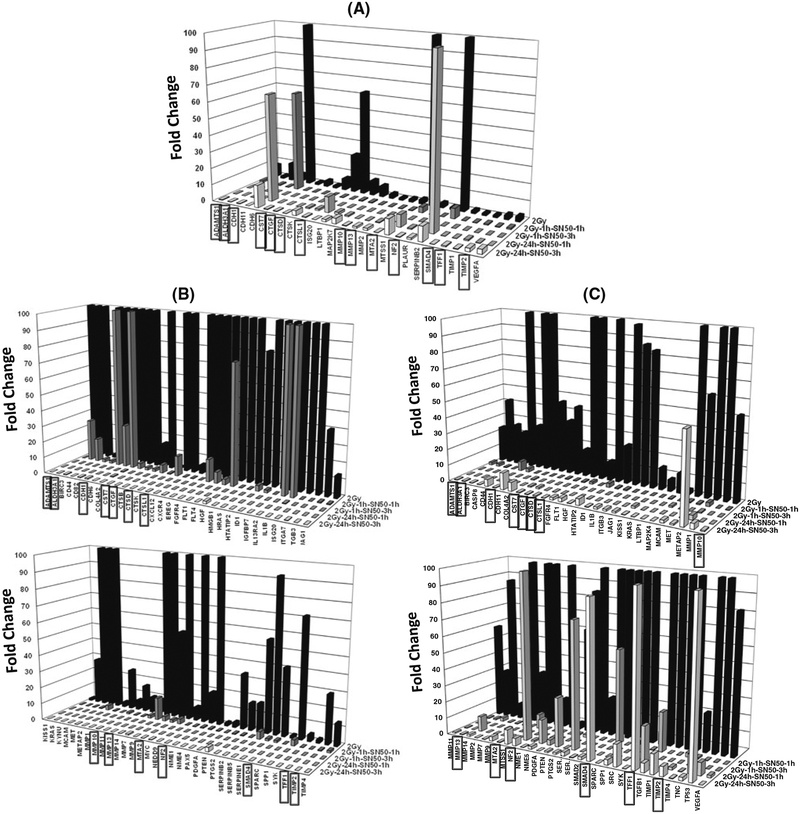
Further, gene profile comparisons revealed that radiation-induced NFkB plays a definite role in tumor invasion and metastasis at least in the surviving human NB cells (Fig. 2). To that note, of the 42 radiation-induced genes, NFkB inhibition significantly suppressed 26 invasion and metastasis signaling molecules in a ‘time-independent’ and ‘phase-independent’ manner in the human IMR-32 cells (Fig. 3a). We observed 65 out of 71 radiation-induced genes that were ‘time- and phase-independent’ suppressed in the human SK-N-MC cells (Fig. 3b). Also, 59 of 63 IR-induced genes were suppressed independent of ‘time and phase’ in the human SH-SY5Y cells (Fig. 3c). The comprehensive inhibition of invasion/metastasis signaling molecules with inhibition of radiation-induced nuclear translocation of NFkB demonstrates its ‘all-inclusive’ influence in orchestrating tumor progression in this setting. Our study identified 14 genes including Adamts1, Aldh3A1, Cdh1, Cst7, Ctgf, Ctsd, Ctsl1, Mmp10, Mmp13, Mta2, Nf2, SMAD4, Tff1 and Timp2 that were complete suppressed with NFkB inhibition in a phase- and time-independent manner in all three NB cell lines (Fig. 3a–c).
Fig. 3.
Three-dimensional histograms showing the complete suppression of radiation-induced tumor invasion/metastasis molecules in human neuroblastoma a IMR-32, b SK-N-MC and C SH-SY5Y cells after all-phase NFkB-inhibition and at all-time points investigated. A total of 26, 65 and 59 genes were completely suppressed in IMR-32, SK-N-MC and SH-SY5Y cells, respectively, after IR ⇒ 1 h/24 h ⇒ SN50 ⇒ 1 h/3 h. Fourteen genes (highlighted with the boxed labeling) were consistently inhibited after IR ⇒ 1 h/24 h ⇒ SN50 ⇒ 1 h/3 h within all three cell lines
NFkB mediates radiation induced translation and cellular localization of SPA-1, ASK1, MMP2, MMP9, PYK2, DnMT3b, CTGF, MMP10, MTA-2, NF-2, E-Cadherin, TIMP-2 and ADAMTS1 in the surviving neuroblastoma cells
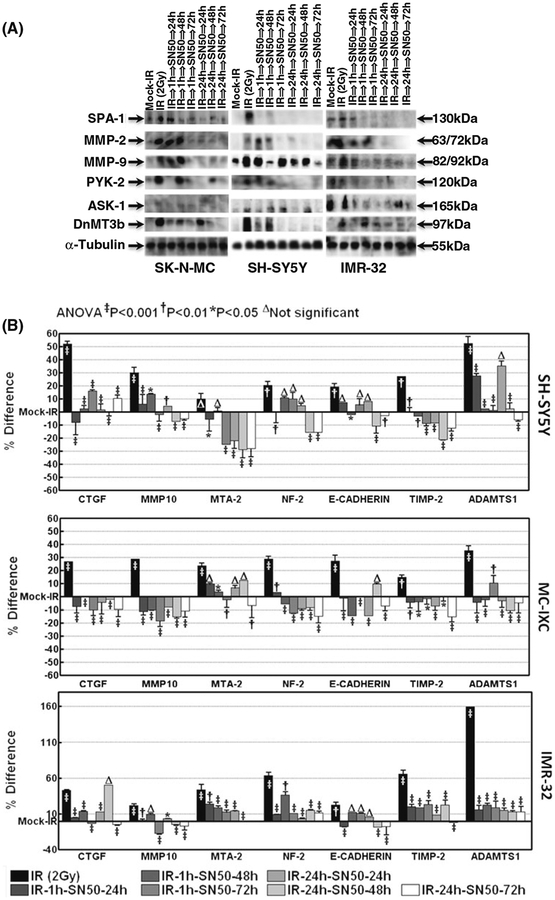
To identify whether the NFkB dependent modulation of invasion and metastasis signaling molecules after radiation promotes metastasis, we investigated the alterations in the translation and cellular localization of SPA-1, ASK1, MMP2, MMP9, PYK2, and DnMT3b after blocking the ‘initial-phase’ and ‘second-phase’ NFkB. Immunoblot analysis revealed that radiation robustly activated SPA-1, ASK1, MMP2, MMP9, PYK2, and DnMT3b in the three cell lines (Fig. 4). Conversely, muting IR-induced NFkB completely inhibited cellular localization of SPA-1, ASK1, MMP2, MMP9, PYK2, and DnMT3b in human SK-N-MC cells. We also observed a consistent inhibition of these invasion/metastasis related proteins in the IMR-32 and SHSY5Y cells (Fig. 4a). In contrast to the inhibition in the ‘initial-phase’, inhibition of ‘second-phase’ NFkB resulted in a complete suppression of SPA-1, ASK1, MMP2, MMP9, PYK2, and DnMT3b in all three cell lines. Also the immunoblot analysis revealed that muting NFkB at both initial and second phase resulted in a sustained (at least up to 3 days) inhibition of SPA-1, ASK1, MMP2, MMP9, PYK2, DnMT3b in the surviving NB IMR-32, SK-N-MC and SH-SY5Y cells (Fig. 4a).
Fig. 4.
a Representative immunoblots showing the expression levels of SPA-1, MMP2, MMP9, PYK2, ASK1, and DnMT3b in the human neuroblastoma SK-N-MC, SHSY5Y and IMR-32 cells. These cells were mock irradiated, irradiated with 2 Gy, or exposed to IR and treated with SN50 1 h or 24 h post-IR and harvested after 24, 48 and 72 h. b Histograms from ELISA analysis showing expression levels of CTGF, MMP10, MTA-2, NF-2, Adamts1, E-Cadherin and Timp2 in the human neuroblastoma SH-SY5Y, MCIXC and IMR-32 cells that were mock irradiated; irradiated with 2 Gy or exposed to IR and treated with SN50 1 h or 24 h post-IR and harvested after 24, 48 and 72 h. Group-wise comparisons were made by using ANOVA with Tukey’s post hoc correction. The radiation-alone group was compared with the mock-irradiated group. All other groups were compared to the radiation-alone group
Further, to substantiate our transcriptional data, we examined the translational modulation of CTGF, MMP10, MTA-2, NF-2, E-Cadherin, TIMP-2 and ADAMTS1, which were all significantly (>twofold) induced after radiation (Fig. 1b) and were completely suppressed after both ‘initial-phase’, and ‘second-phase’ NFkB inhibition in all three cell lines (Fig. 3a–c). Our ELISA data revealed that radiation robustly increased the translation of CTGF, MMP10, MTA-2, NF-2, E-Cadherin, TIMP-2 and ADAMTS1 in SH-SY5Y and IMR-32 cells (Fig. 4b). Conversely, muting IR-induced NFkB completely inhibited the cellular localization of CTGF, MMP10, MTA-2, NF-2, E-Cadherin, TIMP-2 and ADAMTS1 in these NB cells. To further confirm that the effect of NFkB inhibition on regulating these radiation induced proteins is a ubiquitous phenomenon in NB, we examined the regulation of these proteins in a fourth cell line, MC-IXC. We observed a consistent and significant inhibition of these proteins in the MC-IXC cells when we block the radiation-induced initial and second-phase NFkB translocation (Fig. 4b). More importantly, the results revealed that muting NFkB (during both the initial and second phases) resulted in a sustained (at least up to 3 days) inhibition of CTGF, MMP10, MTA-2, NF-2, E-Cadherin, TIMP-2 and ADAMTS1 in the surviving NB IMR-32, MC-IXC and SH-SY5Y cells (Fig. 4b).
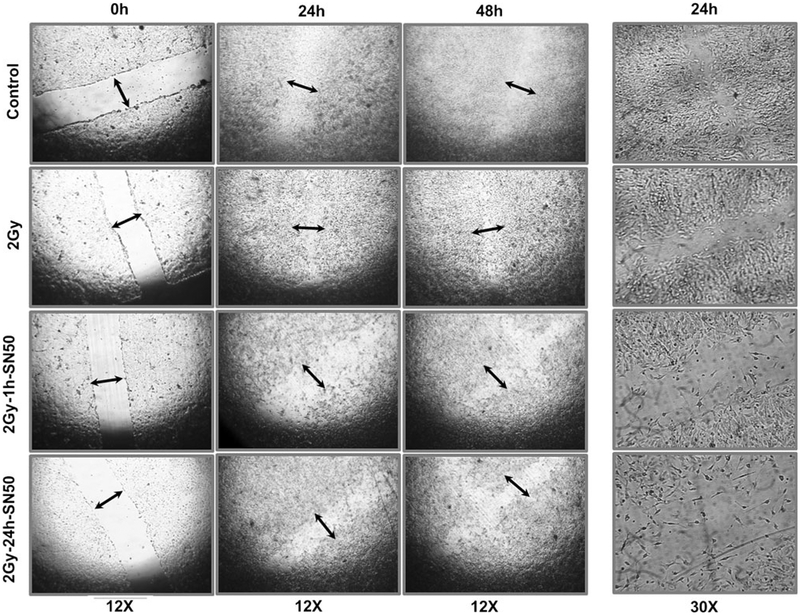
Blocking radiation-induced initial- and second-phase NFkB translocation delays wound healing in NB cells
One hour after scratching in mock-irradiated controls, the wound edge of the cell monolayer appeared irregular with cellular retractions, but the cell densities were even at the border. Cell polarization with protrusions and proliferation with higher cell densities were locally seen in the boundary cells 4 h after wounding, which spread along the border zone thereafter. At 24 h, the denuded area was almost filled in and was completely closed by 48 h (Fig. 5). Radiation exposure marginally delayed the closure of the wound. However, blocking radiation-triggered NFkB or self-orchestrated second phase NFkB significantly delayed the wound closure in these cells. To that end, we observed a measurable denuded area 48 h post-SN50 treatment (Fig. 5).
Fig. 5.
Representative photomicrographs of the scratch wound assay. The wound was made in the SH-SY5Y cells that were grown to confluence by scraping with a 10 μl pipette tip across the dish. The cells were then mock irradiated, irradiated or exposed to radiation and treated with SN50 after 1 or 24 h. All experiments were repeated at least five times in each group. Photomicrographs were captured at 24 and 48 h post-treatment
Discussion
Currently, the insight on the onset of NB metastasis, particularly after a course of therapy, and the signaling mechanisms, if any, that influence such progression are largely unknown. Our results demonstrate that radiation-induced nuclear translocation of pre-existing ‘initial-phase’ NFkB drives the activation of invasion/metastasis signaling in the surviving NB cells. Further, our data indicate that the radiation-induced nuclear translocation of TNFα-dependent ‘second-phase’ NFkB maintains the invasion/metastasis in surviving NB cells. Functional links between cellular signal transduction responses and DNA damage recognition, damage-induced repair, and cell death in response to therapy, including radiotherapy, have been well recognized. Conversely, surviving tumor cells, in particular NB cells [21] respond to the effects of IR oppositely by inducing pro-survival signal transduction pathways. We have reported elevated levels of constitutive NFkB in NB cells and an enhanced NFkB DNA-binding activity as an IR response [14, 15, 22]. More importantly, studies have causally linked the induced NFkB activity to the responsiveness to therapy and survival of NB cells [23–25]. Recently, we demonstrated that the orchestration of functional molecular signaling in response to radiotherapy maintains NFkB, which in-turn mediates the survival advantage leading to NB relapse [17]. Herein, we provide insight into a direct and definite role for radiation-maintained NFkB in surviving tumor cells in the onset and regulation of tumor invasion and metastasis. To our knowledge, this study provides the first evidence that the clinically relevant dose of IR preserves the invasion/metastasis signal transduction in NB cells through persistent (~3 days) NFkB activation.
Metastasis is a highly complex and organized process that comprises multiple but interrelated steps, during which tumor cells leave the primary tumor, gain access to the circulatory system, are carried to a distant site, eventually exit the circulation and grow in that different microenvironment. The first step of metastasis is local invasion, which requires the malignant cells retract their cell-to-cell adhesion properties and become motile, a process that enables them to invade the surrounding tissues. During the second step called intravasation, tumor cells penetrate the endothelium of blood or lymphatic vessels to enter the vascular or lymphatic circulation. Few circulating tumor cells however, are able to survive and form tumor aggregates or emboli with circulating leukocytes and platelets and get them arrested at distant sites. When arrested, some tumor cells will be able to extravasate blood and lymphatic vessels and colonize at the metastatic site. Under the influence of a complex number of environmental factors, tumor cells will undergo additional genetic and epigenetic changes that will allow them to proliferate and generate an angiogenic response necessary for the formation of a large metastatic tumor. Several genes that regulate angiogenesis, invasion, proliferation and metastasis have been shown to contain kB-binding sites. Earlier studies have shown that various adhesion molecules [26], iNOS [27], and chemokine receptors [28] are closely linked with the metastatic ability of the tumor, which are, in turn, regulated by NFkB. Further studies have shown that inhibiting constitutive NFkB activity by expressing mutant IkBα (IkBαM) suppressed tumor metastasis [29]. These results clearly implicate NFkB in playing roles in cell migration and organ-specific homing of metastatic breast cancer cells. However, our study provides the first evidence that radio-therapy-associated increase in NFkB nuclear translocation activates invasion and metastasis signal transduction in surviving NB cells. In contrast to the earlier studies discussed above, our results convincingly demonstrate the comprehensive activation of the invasion/metastasis signaling as a consequence of a radiation response. Further, our results indicate that radiation-induced NFkB has a definite influence in the regulation of a large number of these radiation-induced metastasis signal transduction molecules. More importantly, the results suggest that this signal transduction response is clearly not a transient phenomenon after clinical radiation, but rather is a more structured on-setting and programmed signal transduction that is consistent with the persistent activation of NFkB through its own signaling orchestration.
To investigate the hypothesis that the radiation-orchestrated NFkB not only initiates the onset of metastasis signaling but is required for the functional response in NB, we used a unique approach to block the selective phases of radiation-induced NFkB activation. In addition, our results not only reveal that the radiation-induced persistent maintenance of NFkB plays a key role in endorsing the initiation and orchestration of metastasis, but also indicate that metastasis-related molecular targets might play key roles in this is pathway. We have identified 65, 59 and 26 metastasis-related molecules that are actuated unfailingly by NFkB which is independent of the radiation-triggered initial-phase or TNFα-dependent self-orchestrated second-phase in SK N-MC, SH-SY5Y and IMR-32 cells (Fig. 3a–c). Remarkably, these NFkB regulated genes, Adamts1, Aldh3A1, Cdh1, Cst7, Ctgf, Ctsd, Ctsl1, Mmp10, Mmp13, Mta2, Nf2, SMAD4, Tff1 and Timp2 were both phase and time independent in all the NB cell-lines investigated. Interestingly, we observed a constrained inhibition of select genes, including METAP2, SMAD4, TFF1 and TP53, after the ‘second-phase’ NFkB inhibition (IR ⇒ 24 h ⇒ SN50 ⇒ 3 h) in the SH-SY5Y cells. The inhibition profile differences at the later time point, in this particular cell line is bit perplexing, and we speculate that it may be attributable to that cell type. SH-SY5Y cells are N-type NB cells that have a neuronal morphology, and are less substrate adherent and highly invasive [30].
Further, the protein analysis revealed a robust increase in the levels of SPA-1, ASK1, MMP2, MMP9, PYK2, DnMT3b, CTGF, MMP10, MTA-2, NF-2, E-Cadherin, TIMP-2 and ADAMTS1 after both radiation-triggered and second signaling–maintained NFkB in all three cell lines (Fig. 4a, b). More importantly, our results indicate that radiation-triggered and maintained NFkB drives the expression of these molecules in the surviving NB cells. ‘Early-phase’ inhibition of radiation-induced NFkB showed no regulation of radiation-induced MMP9 in the SK-N-MC cells at least after 24 and 48 h. Other studies have shown that the TNFα-induced MMP-9 expression and secretion maintain a lag period with maximal levels detected after 18–24 h [31]. The unaltered MMP9 levels that we observed after 24–48 h could be a cause-effect of initial radiation response, and this lag may take its time to reflect ‘initial-phase’ NFkB blocking–dependent inhibition of MMP9. We also observed complete inhibition of radiation-induced MMP9 at 72 h in all three cell lines. Earlier studies reported a plethora of evidence that NFkB interacts with those above mentioned metastasis-related molecules in a wide variety of tumors. However, the knowledge on the metastasis signaling and the influence of NFkB on those signaling is rare and fragmentary for NB [32, 33]. Our study provides the first insights on the molecular blue print of the radiation-induced metastasis signaling.
In conclusion, our results clearly indicate that (i) clinical doses of radiation induce invasion/metastasis signaling molecules, (ii) radiation-triggered NFkB plays a key role in the onset of metastasis signaling, and (iii) radiation-induced NFkB triggers second signaling through a feedback-dependent self-orchestration and thereby maintains its activation and promotes progression of NB metastasis in the surviving NB cells. Further we identified a unique comprehensive collection of NB cell type–specific NFkB-dependent activation of metastasis signaling molecules. More importantly, we identified 20 NFkB-dependent metastasis signaling molecules (by transcriptional profiling and/or translational levels) in tumor progression after a course of radiotherapy. The definite role for each of these molecules and their unique downstream molecular signaling in the preferment of metastasis as well as the translation of these in vitro findings in more relevant pre-clinical models remain to be explored and are currently in the research pipeline of our laboratory.
Acknowledgments
The authors were supported by National Institutes of Health (COBRE-1P20GM103639–01), American Cancer Society (Grant ACS-IRG-05-066-01), research development funds from the Department of Radiation Oncology at University of Oklahoma Health Sciences Center to N. Aravindan and the National Aeronautics and Space Administration (NASA) Ground-Based Studies in Space Radiobiology, Grant NNX12-AC32G to M. Natarajan.
Contributor Information
Sheeja Aravindan, Radiation Biology Research Laboratory, Department of Radiation Oncology, University of Oklahoma Health Sciences Center, BMSB 737, 940 Stanton L. Young Boulevard, Oklahoma City, OK, USA.
Mohan Natarajan, Department of Pathology, School of Medicine, University of Texas Health Sciences Center at San Antonio, San Antonio, TX, USA.
Terence S. Herman, Radiation Biology Research Laboratory, Department of Radiation Oncology, University of Oklahoma Health Sciences Center, BMSB 737, 940 Stanton L. Young Boulevard, Oklahoma City, OK, USA
Natarajan Aravindan, Email: naravind@ouhsc.edu, Radiation Biology Research Laboratory, Department of Radiation Oncology, University of Oklahoma Health Sciences Center, BMSB 737, 940 Stanton L. Young Boulevard, Oklahoma City, OK, USA; Department of Pathology, University of Oklahoma Health Sciences Center, Oklahoma City, OK, USA; Department of Anesthesiology, University of Oklahoma Health Sciences Center, Oklahoma City, OK 73104, USA.
References
- 1.Burgos-Tiburcio A et al. (2011) Development of targeted therapy for squamous cell carcinomas of the head and neck. Expert Rev Anticancer Ther 11(3):373–386 [DOI] [PubMed] [Google Scholar]
- 2.He H et al. (2011) The novel protein TSR2 inhibits the transcriptional activity of nuclear factor-kappaB and induces apoptosis. Mol Biol (Mosk) 45(3):496–502 [PubMed] [Google Scholar]
- 3.Valera FC et al. (2010) NF-kappaB expression predicts clinical outcome for nasal polyposis. Rhinology 48(4):408–441 [DOI] [PubMed] [Google Scholar]
- 4.Macha MA et al. (2011) Guggulsterone (GS) inhibits smokeless tobacco and nicotine-induced NF-kappaB and STAT3 pathways in head and neck cancer cells. Carcinogenesis 32(3): 368–380 [DOI] [PubMed] [Google Scholar]
- 5.McGinn CJ, Kinsella TJ (1992) The experimental and clinical rationale for the use of S-phase-specific radiosensitizers to overcome tumor cell repopulation. Semin Oncol 19(4 Suppl 11): 21–28 [PubMed] [Google Scholar]
- 6.Parisi MT et al. (1999) Complications of cancer therapy in children: a radiologist’s guide. Radiographics 19(2):283–297 [DOI] [PubMed] [Google Scholar]
- 7.Aggarwal BB (2004) Nuclear factor-kappaB: the enemy within. Cancer Cell 6(3):203–208 [DOI] [PubMed] [Google Scholar]
- 8.Abal M et al. (2006) Molecular pathology of endometrial carcinoma: transcriptional signature in endometrioid tumors. Histol Histopathol 21(2):197–204 [DOI] [PubMed] [Google Scholar]
- 9.Baeuerle PA, Baltimore D (1991) Hormonal control regulation of gene transcription In: Cohen P, Foulkes JG (eds) Molecular aspects of cellular regulation. Elsevier/North Holland Biomedical Press, Amsterdam, pp 409–432 [Google Scholar]
- 10.Lenardo MJ, Baltimore D (1989) NF-kappa B: a pleiotropic mediator of inducible and tissue-specific gene control. Cell 58(2): 227–229 [DOI] [PubMed] [Google Scholar]
- 11.Orlowski RZ, Baldwin AS Jr (2002) NF-kappaB as a therapeutic target in cancer. Trends Mol Med 8(8):385–389 [DOI] [PubMed] [Google Scholar]
- 12.Prasad AV et al. (1994) Activation of nuclear factor kappa B in human lymphoblastoid cells by low-dose ionizing radiation. Radiat Res 138(3):367–372 [PubMed] [Google Scholar]
- 13.Mohan N, Meltz ML (1994) Induction of nuclear factor kappa B after low-dose ionizing radiation involves a reactive oxygen intermediate signaling pathway. Radiat Res 140(1):97–104 [PubMed] [Google Scholar]
- 14.Aravindan N et al. (2008) Curcumin inhibits NFkappaB mediated radioprotection and modulate apoptosis related genes in human neuroblastoma cells. Cancer Biol Ther 7(4):569–576 [DOI] [PubMed] [Google Scholar]
- 15.Aravindan N et al. (2008) Alteration of apoptotic signaling molecules as a function of time after radiation in human neuroblastoma cells. Mol Cell Biochem 310(1–2):167–179 [DOI] [PubMed] [Google Scholar]
- 16.Piva R, Belardo G, Santoro MG (2006) NF-kappaB: a stress-regulated switch for cell survival. Antioxid Redox Signal 8(3–4):478–486 [DOI] [PubMed] [Google Scholar]
- 17.Veeraraghavan J et al. (2011) Radiation-triggered tumor necrosis factor (TNF) alpha-NFkappaB cross-signaling favors survival advantage in human neuroblastoma cells. J biol chem 286(24): 21588–21600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin YZ et al. (1995) Inhibition of nuclear translocation of transcription factor NF-kappa B by a synthetic peptide containing a cell membrane-permeable motif and nuclear localization sequence. J Biol Chem 270(24):14255–14258 [DOI] [PubMed] [Google Scholar]
- 19.Aravindan N et al. (2011) Irreversible EGFR Inhibitor EKB-569 targets low-LET gamma-radiation-triggered rel orchestration and potentiates cell death in squamous cell carcinoma. PLoS ONE 6(12):e29705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veeraraghavan J et al. (2011) Low-dose gamma-radiation-induced oxidative stress response in mouse brain and gut: regulation by NFkappaB-MnSOD cross-signaling. Mutat Res 718(1–2):44–55 [DOI] [PubMed] [Google Scholar]
- 21.Giagkousiklidis S et al. (2005) Sensitization for gamma-irradiation-induced apoptosis by second mitochondria-derived activator of caspase. Cancer Res 65(22):10502–10513 [DOI] [PubMed] [Google Scholar]
- 22.Madhusoodhanan R et al. (2009) NFkappaB Signaling Related Molecular Alterations in Human Neuroblastoma Cells after Fractionated Irradiation. J Radiat Res(Tokyo) 50(4):311–324 [DOI] [PubMed] [Google Scholar]
- 23.Armstrong MB et al. (2006) Signaling from p53 to NF-kappaB determines the chemotherapy responsiveness of neuroblastoma. Neoplasia 8(11):967–977 [DOI] [PubMed] [Google Scholar]
- 24.Bian X et al. (2001) NF-kappa B activation mediates doxorubicin-induced cell death in N-type neuroblastoma cells. J Biol Chem 276(52):48921–48929 [DOI] [PubMed] [Google Scholar]
- 25.Bian X et al. (2002) Constitutively active NFkappa B is required for the survival of S-type neuroblastoma. J Biol Chem 277(44): 42144–42150 [DOI] [PubMed] [Google Scholar]
- 26.van de Stolpe A et al. (1994) 12-O-tetradecanoylphorbol-13-acetate- and tumor necrosis factor alpha-mediated induction of intercellular adhesion molecule-1 is inhibited by dexamethasone. Functional analysis of the human intercellular adhesion molecular-1 promoter. J biol chem 269(8):6185–6192 [PubMed] [Google Scholar]
- 27.Thomsen LL, Miles DW (1998) Role of nitric oxide in tumour progression: lessons from human tumours. Cancer metastasis rev 17(1):107–118 [DOI] [PubMed] [Google Scholar]
- 28.Helbig G et al. (2003) NF-kappaB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4. J biol chem 278(24):21631–21638 [DOI] [PubMed] [Google Scholar]
- 29.Fujioka S et al. (2003) Function of nuclear factor kappaB in pancreatic cancer metastasis. Clin Cancer Res 9(1):346–354 [PubMed] [Google Scholar]
- 30.Corey JM et al. (2010) Patterning N-type and S-type neuroblastoma cells with Pluronic F108 and ECM proteins. J Biomed Mater Res Part A 93(2):673–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okamoto T et al. (2002) S−nitrosothiols inhibit cytokine-mediated induction of matrix metalloproteinase-9 in airway epithelial cells. Am J Respir Cell Mol Biol 27(4):463–473 [DOI] [PubMed] [Google Scholar]
- 32.Chayka O et al. (2009) Clusterin, a haploinsufficient tumor suppressor gene in neuroblastomas. J Natl Cancer Inst 101(9): 663–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Granchi D et al. (2004) In vitro blockade of receptor activator of nuclear factor-kappaB ligand prevents osteoclastogenesis induced by neuroblastoma cells. Int J Cancer 111(6):829–838 [DOI] [PubMed] [Google Scholar]