Abstract
Diagnostic platforms providing biomarkers that are highly predictive for diagnosing, monitoring, and stratifying cancer patients are key instruments in the development of personalized medicine. We demonstrate that tumor cells transfer (mutant) RNA into blood platelets in vitro and in vivo, and show that blood platelets isolated from glioma and prostate cancer patients contain the cancer-associated RNA biomarkers EGFRvIII and PCA3, respectively. In addition, gene-expression profiling revealed a distinct RNA signature in platelets from glioma patients compared with normal control subjects. Because platelets are easily accessible and isolated, they may form an attractive platform for the companion diagnostics of cancer.
Introduction
Targeted tumor therapy and personalized medicine are critically depending on disease profiling and the development of companion diagnostics.1–5 Mutations in tumor-derived nucleic acids can be highly predictive for the response to targeted treatment of cancer (eg, KRAS mutations in colorectal cancer, BRAF mutations in melanoma, and EGFR mutations in lung carcinoma).5 However, obtaining easily accessible high-quality nucleic acids remains a significant developmental hurdle.1–3 Blood generally contains 150 000 to 350 000 platelets (thrombocytes) per microliter,6 which exert their diverse functions7 and provide a highly available biomarker source for research and clinical use.8 Moreover, blood platelet isolation is relatively simple and is a standard procedure in blood bank/hematology laboratories. Because platelets do not contain a nucleus, their RNA transcripts, needed for functional maintenance, are derived from megakaryocytes during platelet origination.6,8,9 Platelet RNA can be readily isolated and subjected to gene-expression analysis.8–10 Here we show that blood platelets take up tumor-derived secreted membrane vesicles that can contain tumor-associated RNA and that platelets can serve as a potential biomarker source for cancer diagnostics.
Methods
Platelet isolation and tissue resection
Tumor tissue resection and whole blood harvesting from glioma and prostate cancer patients were performed at the VU University Medical Center (Amsterdam, The Netherlands) and at Norrlands Universitets Sjukhus (Umeå, Sweden), all after informed consent and following ethical guidelines in accordance with the Declaration of Helsinki. Platelets were isolated from whole blood collected in purple-cap BD Vacutainers containing EDTA anticoagulant by standard centrifugation. The cells were removed by centrifugation at room temperature for 20 minutes at 120g, which was repeated for 5 minutes. The platelets were isolated from the supernatant by centrifugation at room temperature for 20 minutes at 360g, after which the platelet pellet was washed twice in PBS, 0.8% EDTA and collected in 100 μL PBS. Platelet quality (activation and aggregation) as well as purity were assessed by microscopic analysis. Next, isolated platelet pellets were snap-frozen for further use.
Microvesicle isolation, labeling, and transfer
Microvesicles were isolated from U87/U87-EGFRvIII glioma and 22Rv1 prostate cancer cells and labeled as described previously.11 After U87-EGFRVIII microvesicle incubation, the platelets were washed and treated with RNAse enzymes to ensure that the EGFRvIII RNA was delivered into the platelets and therefore protected from RNAse-mediated degradation. The microvesicle uptake was analyzed using the LSM-710 confocal microscope system with the ZEN 2010 software (Carl Zeiss) and a 63× oil immersion objective (Carl Zeiss). Platelets were stained with Texas red-conjugated wheat germ agglutinin (Invitrogen) to indicate platelet structure and analyzed for microvesicle uptake by the presence of green PKH67 (Sigma-Aldrich). Electron microscopy was performed as described elsewhere.12
RT-PCR
RT-PCR for EGFRvIII, PCA3, and GAPDH was performed as described previously,11,12 using the following primer sets:
Nested EGFRvIII primers: PCR1, forward, 5′-CCAGTATTGAT- CGGGAGAGC-3′; and reverse, 5′-TGTGGATCCAGAGGAGGAGT-3′; PCR2, forward, 5′-GAGCTCTTCGGGGAGCAG-3′; and reverse, 5′-GCCCTTCGCACTTCTTACAC-3′.
Nested PCA3 primers (exons 2-3): PCR1, forward, 5′-AGTCCGCTGTGAGTCT-3′; and reverse, 5′-CCATTTCAGCAGATGTGTGG-3′; PCR2, forward, 5′-ATCGACGGCACTTTCTGAGT-3′; and reverse, 5′-TGTGTGGC- CTCAGATGGTAA-3′.
GAPDH primers: forward, 5′-GAAGGTGAAGGTCGGAGTC-3′; and reverse, 5′-TCAGAAGATGGTGATGGGATTTC-3′.
Results
Cancer cells secrete membrane vesicles that transfer mutant RNA into blood platelets
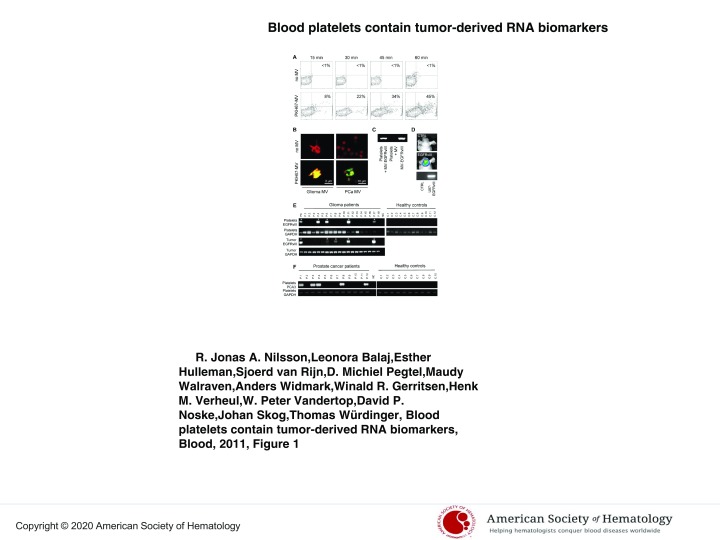
In Figure 1, we show that platelets isolated from healthy human control subjects have the ability to take up secreted RNA-containing membrane vesicles derived from human cancer cells. We isolated platelets form healthy donor subjects, as well as secreted membrane vesicles from glioma and prostate cancer cells11,12 (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Next, we labeled the isolated microvesicles with green fluorescent dye PKH67 and subsequently incubated them with the blood platelets. We determined uptake and accumulation of labeled tumor-derived microvesicles in the blood platelets by FACS analysis (Figure 1A). To confirm that the microvesicles were internalized by the platelets, we used confocal microscopy, demonstrating significant uptake of PKH67-labeled microvesicles derived from glioma and prostate cancer cells (Figure 1B). The secreted membrane vesicles from the glioma cells analyzed here contained tumor-associated RNA, including mutant EGFRvIII.11 To establish that tumor-derived RNA can be transferred from cancer cells to blood platelets, we demonstrate microvesicle-mediated transfer of mutant EGFRvIII RNA into platelets from healthy control subjects by RT-PCR (Figure 1C). We detected EGFRvIII RNA by RT-PCR in platelets from healthy donor subjects that were incubated with membrane vesicles isolated from EGFRvIII-positive glioma cells, and not in platelets incubated with microvesicles from EGFRvIII-negative glioma cells. Next, we implanted U87-EGFRvIII glioma cells expressing firefly luciferase in the mouse brain13; and after 2 weeks, the mice were imaged using firefly luciferase-mediated bioluminescence imaging showing significant tumor growth (Figure 1D), after which 500 μL of blood was withdrawn from the heart. Platelets were isolated, and RT-PCR was used to demonstrate the presence of EGFRvIII mRNA in platelets in vivo (Figure 1D).
Figure 1.
Uptake of tumor-derived RNA in platelets. (A) U87 glioma-derived microvesicles were labeled with PKH67 green fluorescent dye and incubated with isolated platelets. After 15, 30, 45, and 60 minutes of incubation in the presence and absence of microvesicles, the platelets were washed and subjected to FACS analysis of PKH67 fluorescence. (B) Platelets were incubated with PKH67-labeled microvesicles (MV) from glioma or prostate cancer (PCa) patients, stained with Texas-red wheat germ agglutinin, and analyzed by confocal microscopy for PKH67-labeled microvesicle uptake. Merged images and size bars are shown. (C) RNA was isolated from RNAse-treated platelets after incubation with U87 glioma-derived microvesicles under different conditions. RT-PCR was performed to detect EGFRvIII RNA. MV/MV-EGFRvIII indicates microvesicles isolated from U87/U87-EGFRvIII cells. (D) Mice were implanted with U87-Fluc-EGFRvIII cells or no cells and imaged after 2 weeks. Shown are representative bioluminescence images and corresponding EGFRvIII RT-PCR on mouse platelets. (E) RNA was isolated from platelets from 12 healthy control subjects and 26 glioma patients (only 18 patients shown here) and subjected to RT-PCR analysis. Corresponding glioma tissue biopsies served as control. Platelet activation and heterogeneous EGFRvIII tissue distribution may have caused the possible false-negative signals. PC indicates U87-EGFRvIII RNA; NC, H2O; and nd, not determined. *Positive signal. (F) RNA was isolated from platelets from healthy control subjects (n = 10) and prostate cancer patients (n = 12) and subjected to PCA3 and GAPDH RT-PCR analysis.
Blood platelets from cancer patients contain tumor-derived mutant RNA
To determine whether circulating blood platelets isolated from glioma patients contain the RNA biomarker EGFRvIII, we compared blood platelets from healthy donor subjects to blood platelets from glioma patients (Figure 1E; summarized in supplemental Table 1). In addition to the RNA isolated from blood platelets, we isolated RNA from the corresponding glioma tissues. As proof of concept, we used RT-PCR to determine whether mutant EGFRvIII RNA was detectable in resected glioma tissues and in platelets from the same patient (n = 26), as well as in platelets from healthy control subjects (n = 12). The samples were coded, and RT-PCR was performed in a blinded fashion. A total of 21% of the glioma tissue samples contained the EGFRvIII transcript (supplemental Table 1), similar as observed earlier.11 Notably, EGFRvIII was amplified from platelets in 80% of the EGFRvIII-positive patients, and from none of the platelets of the healthy donors (n = 12), whereas GAPDH RNA was detected in all platelet samples. To demonstrate that the presence of tumor-associated messages is not unique to platelets from glioma patients, we report the presence of RNA coding for the prostate cancer marker PCA3 in platelets from prostate cancer patients (n = 12) and their absence in platelets from healthy control subjects (n = 10; Figure 1F).
Gene-expression profiling identifies a glioma signature in blood platelets
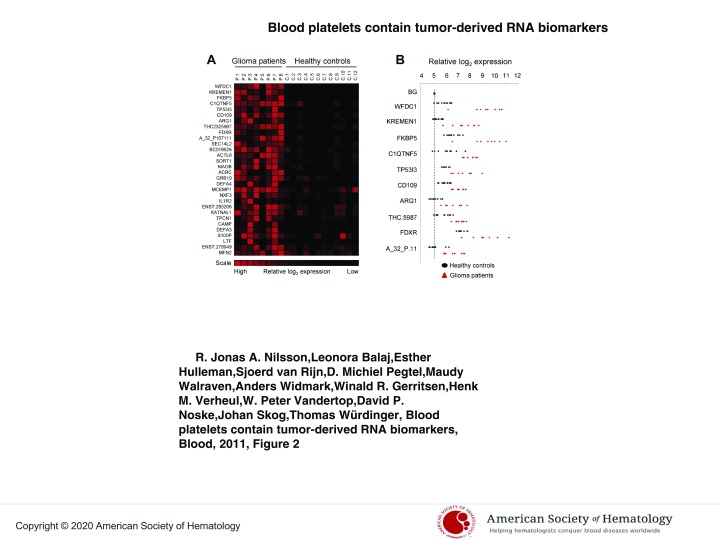
Finally, using gene-expression arrays, we determined the RNA profiles of platelets isolated from healthy control subjects (n = 12) and glioma patients (n = 8; supplemental Methods). SAM analysis was performed to determine that we obtained distinct RNA expression profiles and allowed us to compile a glioma-associated gene-expression signature (Figure 2A). Interestingly, several of the potential biomarkers were hardly detectable in control samples, whereas in the blood platelets from the glioma patients they were highly expressed (Figure 2B).
Figure 2.
Glioma-associated gene expression signature. (A) Platelet RNA from glioma patients and healthy control subjects was subjected to gene-expression arrays. SAM analysis was used to determine significantly expressed genes. A heatmap of the top-30 up-regulated RNAs is shown. (B) Individual expression levels for the top-10 RNAs. Dashed line indicates background (BG).
Discussion
Here we demonstrate that membrane vesicles secreted by cancer cells are vehicles capable of transferring tumor-derived (mutant) RNA into platelets, as shown by confocal microscopy and RT-PCR. It is established that tumor cells can release RNA into the circulation via a variety of microvesicle types.11,12,14–16 However, additional mechanisms are emerging; for instance, circulating microRNAs (miRNAs) have been detected in conjunction with argonaute 2 protein,16 as well as in complex with high density lipoprotein.17 Therefore, tumor-derived RNA molecules in platelets may also transfer via microvesicle-independent mechanisms. Interest in platelets and their ability to interact with intravascular components have enticed several groups to pursue the use of platelets as a protein source for cancer biomarkers and investigating their function in disease.18–22 Several platelet proteins were identified as potential cancer biomarkers, including PF423 and thrombospondin-1.24 Interestingly, thrombospondin-1 was shown to be a negative regulator of angiogenesis and affects platelet-mediated recruitment of bone marrow–derived cells to sites of tumor angiogenesis.25 In addition, platelet-derived lysophosphatidic acid was shown to support breast cancer metastasis and was identified as a potential therapeutic target by interfering with the recruitment of bone marrow–derived cells to angiogenic sites.26 It was recently shown that megakaryocytes selectively transport mRNAs into platelets, allowing only a subset of RNAs to be transferred into platelets,27 and Calverly et al identified a subset of megakaryocyte/platelet-derived mRNAs that are differentially expressed in lung cancer metastasis.28 However, any role of tumor-derived RNA in platelet biology and cancer remains to be investigated, and it remains unclear to what extent the transferred tumor-derived RNA can be functionally translated into proteins, thereby manipulating platelet function, as previously shown to occur in endothelial cells after uptake of tumor-derived microvesicles.12 A recent study demonstrated miR-28 to be deregulated in platelets of cancer patients.29 Because platelets have a functional miRNA machinery,10,30 it would also be of interest to determine whether tumor-derived miRNAs can functionally repress translation in platelets, as was demonstrated to take place in monocytes on microvesicle-mediated delivery of tumor-derived miRNAs.31 Besides the microvesicle uptake shown here, it was reported that platelets can efficiently release protumoral microvesicles themselves,18,32,33 thereby possibly allowing for a blood-based distribution network of (tumor-derived) RNA. Thus, the spread of RNA by tumor cells via platelets provides a strategic opening for cancer surveillance. The results presented here demonstrate that platelets contain tumor-associated RNA and therefore may possibly serve as an attractive platform for the companion diagnostics of cancer in the context of personalized medicine.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
The authors thank Prof Dr Xandra O. Breakefield for continuous support, Jantine Posthuma de Boer for assistance with blood collection, and Nitesh Mistry for help with the electron microscope.
This work was supported by Lion's Cancer Research Foundation, Umeå University (Sweden); Stichting Translational Research CCA/VU University Medical Center, Swedish Research Council (R.J.A.N.); and NWO-VIDI (T.W.).
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: R.J.A.N., J.S., and T.W generated and analyzed data and were involved in experimental strategy and design, writing, and editing of the manuscript; L.B. and S.v.R. generated PCR data and edited the manuscript; E.H. and D.M.P. generated microvesicle uptake data and edited the manuscript; and M.W., A.W., W.R.G., H.M.V., W.P.V., and D.P.N. provided patient material and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Thomas Würdinger, Neuro-oncology Research Group, Cancer Center Amsterdam, VU University Medical Center, De Boelelaan 1117, 1081 HV, Amsterdam, The Netherlands; e-mail: t.wurdinger@vumc.nl.
References
- 1.Bernards R. It's diagnostics, stupid. Cell. 2010;141(1):13–17. doi: 10.1016/j.cell.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 2.Khleif FN, Doroshow JH, Hait WN, et al. AACR-FDA-NCI Cancer Biomarkers Collaborative consensus report: advancing the use of biomarkers in cancer drug development. Clin Cancer Res. 2010;16(13):3299–3318. doi: 10.1158/1078-0432.CCR-10-0880. [DOI] [PubMed] [Google Scholar]
- 3.Vlassov VV, Laktionov PP, Rykova EY. Circulating nucleic acids as a potential source for cancer biomarkers. Curr Mol Med. 2010;10(2):142–165. doi: 10.2174/156652410790963295. [DOI] [PubMed] [Google Scholar]
- 4.Papadopoulos N, Kinzler KW, Vogelstein B. The role of companion diagnostics in the development and use of mutation-targeted cancer therapies. Nat Biotechnol. 2006;24(8):985–995. doi: 10.1038/nbt1234. [DOI] [PubMed] [Google Scholar]
- 5.Sharma SV, Bell DW, Settleman J, et al. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7(3):169–181. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 6.George JN. Platelets. Lancet. 2000;355(9214):1531–1539. doi: 10.1016/S0140-6736(00)02175-9. [DOI] [PubMed] [Google Scholar]
- 7.Leslie M. Cell biology: beyond clotting. The powers of platelets. Science. 2010;328(5978):562–564. doi: 10.1126/science.328.5978.562. [DOI] [PubMed] [Google Scholar]
- 8.Gnatenko DV, Dunn JJ, McCorkle SR, et al. Transcript profiling of human platelets using microarray and serial analysis of gene expression. Blood. 2003;101(6):2285–2293. doi: 10.1182/blood-2002-09-2797. [DOI] [PubMed] [Google Scholar]
- 9.Goodall AH, Burns P, Salles I, et al. Transcription profiling in human platelets reveals LRRFIP1 as a novel protein regulating platelet function. Blood. 2010;116(22):4646–4656. doi: 10.1182/blood-2010-04-280925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagalla S, Shaw C, Kong X, et al. Platelet microRNA-mRNA coexpression profiles correlate with platelet reactivity. Blood. 2011;117(19):5189–5197. doi: 10.1182/blood-2010-09-299719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skog J, Würdinger T, van Rijn S, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10(12):1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nilsson J, Skog J, Nordstrand A, et al. Prostate cancer-derived urine exosomes: a novel approach to biomarkers for prostate cancer. Br J Cancer. 2009;100(1):1603–1607. doi: 10.1038/sj.bjc.6605058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wurdinger T, Badr C, Pike L, et al. A secreted luciferase for ex vivo monitoring of in vivo processes. Nat Methods. 2008;5(2):171–173. doi: 10.1038/nmeth.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zomer A, Vendrig T, Hopmans ES, et al. Exosomes: fit to deliver small RNA. Commun Integr Biol. 2010;3(5):447–450. doi: 10.4161/cib.3.5.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rak J. Microparticles in cancer. Semin Thromb Hemost. 2010;36(8):888–906. doi: 10.1055/s-0030-1267043. [DOI] [PubMed] [Google Scholar]
- 16.Arroyo JD, Chevillet JR, Kroh EM, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A. 2011;108(12):5003–5008. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vickers KC, Palmisano BT, Shoucri BM, et al. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011;13(4):423–433. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11(2):123–134. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pinedo HM, Verheul HM, D'Amato RJ, Folkman J. Involvement of platelets in tumour angiogenesis? Lancet. 1998;352(9142):1775–1777. doi: 10.1016/s0140-6736(98)05095-8. [DOI] [PubMed] [Google Scholar]
- 20.Klement GL, Yip T-T, Cassiola F, et al. Platelets actively sequester angiogenesis regulators. Blood. 2009;113(12):2835–2842. doi: 10.1182/blood-2008-06-159541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kerr BA, Miocinovic R, Smith AK, et al. Comparison of tumor and microenvironment secretomes in plasma and in platelets during prostate cancer growth in a xenograft model. Neoplasia. 2010;12(5):388–396. doi: 10.1593/neo.10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzalez FJ, Rueda A, Sevilla I, et al. Shift in the balance between circulating thrombospondin-1 and vascular endothelial growth factor in cancer patients: relationship to platelet alpha-granule content and primary activation. Int J Biol Markers. 2004;19(3):221–228. doi: 10.5301/JBM.2008.1959. [DOI] [PubMed] [Google Scholar]
- 23.Cervi D, Yip TT, Bhattacharya N, et al. Platelet-associated PF4 as a biomarker of early tumor growth. Blood. 2008;111(3):1201–1207. doi: 10.1182/blood-2007-04-084798. [DOI] [PubMed] [Google Scholar]
- 24.Zaslavsky A, Baek KH, Lynch RC, et al. Platelet-derived thrombospondin-1 is a critical negative regulator and potential biomarker of angiogenesis. Blood. 2010;115(22):4605–4613. doi: 10.1182/blood-2009-09-242065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng W, Madajka M, Kerr BA, et al. A novel role for platelet secretion in angiogenesis: mediating bone marrow-derived cell mobilization and homing. Blood. 2011;117(14):3893–3902. doi: 10.1182/blood-2010-08-304808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boucharaba A, Serre CM, Grès S, et al. Platelet-derived lysophosphatidic acid supports the progression of osteolytic bone metastases in breast cancer. J Clin Invest. 2004;114(12):1714–1725. doi: 10.1172/JCI22123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cecchetti L, Tolley ND, Michetti N, et al. Megakaryocytes differentially sort mRNAs for matrix metalloproteinases and their inhibitors into platelets: a mechanism for regulating synthetic events. Blood. 2011;118(7):1903–1911. doi: 10.1182/blood-2010-12-324517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calverley DC, Phang TL, Choudhury QG, et al. Significant downregulation of platelet gene expression in metastatic lung cancer. Clin Transl Sci. 2010;3(5):227–232. doi: 10.1111/j.1752-8062.2010.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Girardot M, Pecquet C, Boukour S, et al. miR-28 is a thrombopoietin receptor targeting microRNA detected in a fraction of myeloproliferative neoplasm patient platelets. Blood. 2010;116(3):437–445. doi: 10.1182/blood-2008-06-165985. [DOI] [PubMed] [Google Scholar]
- 30.Landry P, Plante I, Ouellet DL, et al. Existence of a microRNA pathway in anucleate platelets. Nat Struct Mol Biol. 2009;16(9):961–966. doi: 10.1038/nsmb.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, et al. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci U S A. 2010;107(14):6328–6333. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janowska-Wieczorek A, Wysoczynski M, Kijowski J, et al. Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int J Cancer. 2005;113(5):752–760. doi: 10.1002/ijc.20657. [DOI] [PubMed] [Google Scholar]
- 33.Heijnen HF, Schiel AE, Fijnheer R, et al. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999;94(11):3791–3799. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.