Abstract
Background
The reported national case fatality rates (CFRs) for coronavirus disease 2019 (COVID-19) shows a sex bias with males > females. The relative lengths of the index (2D) and ring (4D) fingers (digit ratio; 2D:4D) is a sexually dimorphic (males < females) proxy of fetal sex steroids (low 2D:4D indicates high prenatal testosterone/low prenatal estrogen).
Aim
To examine sex-specific relationships of 2D:4D per nation with national values of COVID-19 CFRs. Study design: COVID-19 CFRs and the percent of male deaths were related to mean national (self-reported) 2D:4D by sex and hand from a large online survey (the BBC Internet Study).
Subjects
103,482 men and 83,366 women.
Outcome measures
Relationships of mean national 2D:4D with CFRs from 41 countries and with national male death rates from 16 countries.
Results
Male right and left hand 2D:4D showed positive relationships with CFR. These relationships remained significant after removing the influence of female 2D:4D. A positive association of male right and left 2D:4D was detected with the percentage of male deaths.
Conclusions
At the national level, high mean 2D:4D (indicating low prenatal testosterone/high prenatal estrogen) is associated with high CFRs and percent male mortality. At the individual level, high 2D:4D may be a risk factor for severity of COVID-19 in males. We speculate that male 2D:4D is a negative correlate for expression of the SARS-CoV2 receptor (ACE2).
Keywords: COVID-19, Coronavirus, Digit ratio, Case fatality rate, Testosterone, ACE2
Highlights
-
•
Digit ratio (2D:4D) is a sexually dimorphic correlate of prenatal sex steroids.
-
•
High 2D:4D indicates low prenatal testosterone/high prenatal estrogen.
-
•
Relationships of national mean 2D:4D with SARS-CoV2 (COVID-19) case fatality rates were investigated.
-
•
Male 2D:4D showed positive relationships with case fatality rates and mortality.
-
•
Low prenatal testosterone may be a risk factor for COVID-19 in males.
1. Introduction
Research has documented an excess of male relative to female deaths in the SARS-CoV2 (COVID-19) pandemic [1,2]. This sex-dependent pattern has been observed also for other pathogenic coronaviruses (CoVs), including the severe acute respiratory syndrome (SARS)-CoV and the Middle East respiratory syndrome (MERS)-CoV [3]. The excess of severe male cases may provide clues as to the pathogenic effects of COVID-19 and the immunological responses to these effects. Males tend to generate less robust immune responses than females and are more susceptible to a variety of infectious agents including RNA viruses [[4], [5], [6]]. This has led to the suggestion that testosterone (among other sex-steroids) may have a negative effect on the human immune system [7].
Here we consider the relationship between COVID-19 case fatality rates (CFR; a measure of the national severity of the disease) and digit ratio (2D:4D), a proxy for prenatal sex-steroid levels that correlates negatively with prenatal testosterone and positively with prenatal estrogen [8,9]. National CFRs from COVID-19 vary considerably as do the percent male deaths per country [10,11]. We examined the relationship between CFRs and percent male deaths from cases of COVID-19 and mean national values of 2D:4D in males and females.
A straightforward hypothesis would be that high prenatal testosterone (low 2D:4D) is linked to immune suppression and high CFRs. However, strong immune responses in females may also lead to immunopathology, resulting in fatal outcomes [4]. Another possibility is that testosterone facilitates cell entry by SARS-CoV2 and is one of the driving factors of the epidemic (“androgen-driven COVID-19 pandemic theory”) [12,13]. Infectivity of COVID-19 depends on priming of the spike proteins by transmembrane protease, serine 2 (TMPRSS2) [14,15], and TMPRSS2 may cleave angiotensin converting enzyme 2 (ACE2) for augmented viral entry [16]. Importantly, androgen receptor activity is a requirement for the transcription of the TMPRSS2 gene, suggesting that testosterone facilitates SARS-CoV2 cell entry [12,13]. The androgen-driven COVID-19 pandemic theory concerns circulating levels of testosterone and has some support from the relationship between age-related COVID-19 mortality rates. For example, it may explain why children are more resistant to infections before adrenarche and puberty [12,13]. Thus, high mortality from SARS-CoV2 in men should be related to hypergonadism and to low (masculinized) 2D:4D.
The androgen-driven theory would predict that low 2D:4D (high prenatal testosterone) correlates with high severity of COVID-19 cases. However, there are some theory-inconsistent anomalies with the relationship between age, testosterone, and COVID-19 in males. If high testosterone is associated with mortality, one should expect a mini-peak during the activation of the hypothalamic-pituitary-gonadal axis in the 6-8th week after delivery of male neonates. In this period, the levels of sex steroids are similar to early-middle pubertal levels [17]. There is no support for elevated frequencies of COVID-19 at this age. Moreover, in men COVID-19 mortality rates increase with age [1] but testosterone levels decrease [18]. The expression of ACE2 is sex-dependent with a higher expression in females compared to males, and in the latter, levels of ACE2 decline with age [19]. Paradoxically, ACE2 is necessary for SARS-CoV2 cell entry, but its expression correlates negatively with mortality from the virus. Thus, there is the possibility that the severity of COVID-19 relates to male hypogonadism and not hypergonadism and that high (feminized) 2D:4D in men relates to increased severity of cases.
We examined the association between mean national values of 2D:4D and the CFRs, and percent of male mortality across 41 nations, thus assessing the two competing views of the relationship between testosterone and prognosis in cases of COVID-19. A negative association would support the androgen-driven COVID-19 pandemic theory, whereas a positive association would indicate that male hypogonadism relates to high severity of COVID-19 cases.
2. Material and methods
The BBC Internet Study was a multi-national and multiethnic online survey that included around 200 questions concerning sex-dependent aspects of demographics and behavior, along with self-measurement of the lengths of index finger (2D) and the ring finger (4D). Reimers [19] reports the details of recruitment and ethical issues in the Study. A sample of 255,116 participants from >100 countries completed all sections of the Study. The most commonly represented nationalities were the United Kingdom (46.9%), United States (27.7%), Canada (5.2%), and Australia (3.6%) with 11 other nations represented by >1000 participants. The predominant ethnicity was “White”, which was reported by 84.1% of participants. However, there were substantial numbers of other groups. Participants self-measured 2D and 4D of right and left hands. A diagram of the hand illustrated the measurements, taken from on the ventral side of the digit with a conventional ruler and reported to the nearest millimeter. The right and left hand 2D:4D's were calculated by dividing the 2D by 4D digit lengths, respectively. Manning et al. [20] have examined the effects of sex and ethnicity on 2D:4D in the Study. Means of 2D:4D from the Study showed the expected sex difference (males < females) and this extended across all ethnic groups. Previous research including the Study data (e.g., [20,21]) restricted the age of participants to ≥18 years and the 2D:4D range of 0.80 to 1.20. This had the effect of reducing SD's to about 0.05. In the present study, we adopted these restrictions, thus leaving for analysis 2D:4D of 103,482 men and 83,366 women.
The COVID-19 CFR statistics were obtained on April 21st from the World Health Organization (WHO) reports [10] and gender-segregated data from Global Health 50/50 [11]. The WHO reports CFRs by country but without ethnic details. Therefore, we included all ethnic groups in the national means for 2D:4D.
3. Results
3.1. Descriptive statistics
There were 41 nations in the sample (Table 1 ). Mean and standard deviation (M ± SD) of CFR was 3.89 ± 3.51 and the distribution showed a rightward skew (skew = 1.371, kurtosis = 1.043; Shapiro-Wilk W = 0.833, p < .0001). We log-transformed CFR (log CFR), which resulted in a mean of 0.400 (0.457) (skew = −0.724, kurtosis = 0.898, Shapiro-Wilk W = 0.959, p = .151). The percent of male deaths per nation was available from 16 countries (M = 60.94 ± 5.46%).
Table 1.
COVID 19 number of cases, numbers of deaths, and case fatality rate in 41 nations.
| Nation | Number cases | Number deaths | Case fatality rate | Log case fatality rate | Percent male cases | Percent male deaths |
|---|---|---|---|---|---|---|
| Argentina | 1628 | 53 | 3.256 | 0.513 | . | |
| Australia | 5956 | 45 | 0.756 | −0.121 | 48 | 60 |
| Austria | 12,640 | 243 | 1.922 | 0.284 | 50 | . |
| Belgium | 22,194 | 2035 | 9.169 | 0.962 | 45 | 50 |
| Brazil | 12,056 | 553 | 4.587 | 0.662 | . | |
| Bulgaria | 577 | 23 | 3.986 | 0.601 | . | |
| Canada | 17,049 | 349 | 2.047 | 0.311 | 48 | 56 |
| China | 83,157 | 3342 | 4.019 | 0.604 | 51 | 64 |
| Croatia | 1282 | 18 | 1.404 | 0.147 | . | |
| Czech | 5017 | 88 | 1.754 | 0.244 | 49 | . |
| Denmark | 5071 | 203 | 4.003 | 0.602 | 46 | 64 |
| Finland | 2308 | 34 | 1.473 | 0.168 | 50 | . |
| France | 77,226 | 10,313 | 13.354 | 1.126 | 61 | |
| Germany | 103,228 | 1861 | 1.803 | 0.256 | 50 | 63 |
| Greece | 1832 | 81 | 4.421 | 0.646 | 55 | 72 |
| Hungary | 895 | 58 | 6.480 | 0.812 | . | |
| Iceland | 1586 | 6 | 0.378 | −0.423 | . | |
| India | 5194 | 149 | 2.869 | 0.458 | . | |
| Ireland | 5709 | 210 | 3.678 | 0.566 | 45 | 63 |
| Israel | 9404 | 71 | 0.755 | −0.122 | . | |
| Italy | 135,586 | 17,129 | 12.633 | 1.102 | 53 | 68 |
| Japan | 4257 | 81 | 1.903 | 0.279 | 60 | . |
| Malaysia | 3963 | 63 | 1.590 | 0.201 | . | |
| Mexico | 2439 | 125 | 5.125 | 0.710 | . | |
| N Zealand | 969 | 1 | 0.103 | −0.987 | . | |
| Netherlands | 19,580 | 2101 | 10.730 | 1.031 | 47 | 61 |
| Norway | 5863 | 69 | 1.177 | 0.071 | 50 | 54 |
| Pakistan | 4072 | 58 | 1.424 | 0.154 | 72 | . |
| Philippines | 3764 | 177 | 4.702 | 0.672 | 60 | . |
| Poland | 4848 | 129 | 2.661 | 0.425 | . | |
| Portugal | 12,442 | 345 | 2.773 | 0.443 | 43 | 54 |
| Romania | 4417 | 182 | 4.120 | 0.615 | 41 | . |
| Russia | 7497 | 58 | 0.774 | −0.111 | . | |
| Singapore | 1481 | 6 | 0.405 | −0.393 | . | |
| Spain | 140,510 | 13,798 | 9.820 | 0.992 | 49 | 63 |
| Sweden | 7693 | 591 | 7.682 | 0.885 | 50 | 60 |
| Switzerland | 22,164 | 641 | 2.892 | 0.461 | 47 | 62 |
| Turkey | 34,109 | 725 | 2.126 | 0.328 | . | |
| UK | 55,246 | 6159 | 11.148 | 1.047 | . | |
| UAE | 2359 | 12 | 0.509 | −0.293 | . | |
| USA | 363,321 | 10,845 | 2.985 | 0.475 | . |
3.2. Statistical analyses
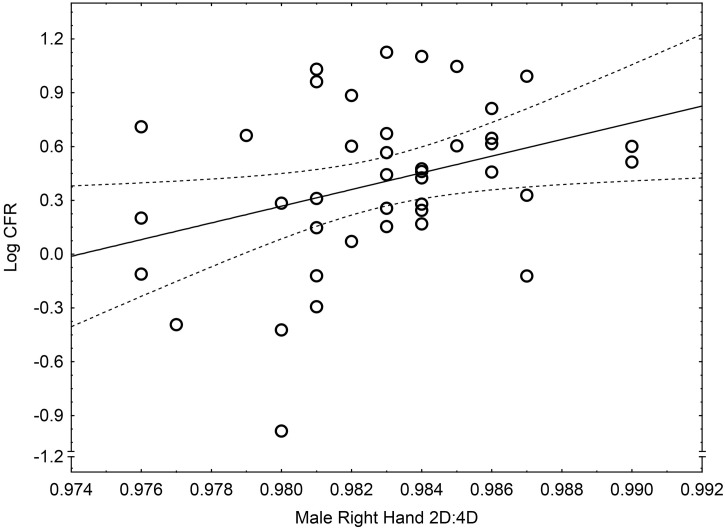
There were significant sex differences in right and left hand 2D:4D, with males < females (males: right hand M = 0.983 ± 0.003, left hand M = 0.940 ± 0.003; females: right hand M = 0.993 ± 0.004, left hand M = 0.992 ± 0.005; right hand t = 14.88, p < .0001, left hand t = 11.22, p < .0001) (Table 2, Table 3 ). Male (but not female) 2D:4D correlated positively with log CFR (males: right hand r = 0.34, p < .05 [Fig. 1 ], left hand r = 0.29, p = .07; females: right hand r = 0.03, p = .83, left hand r = 0.03, p = .83). Male 2D:4D correlated positively with female 2D:4D (right hand r = 0.37, p < .05; left hand r = 0.49, p < .001). We performing multiple regressions with the dependent variable log CFR and the independent variables male and female right 2D:4D or male and female left 2D:4D. Male right hand 2D:4D remained positively related to log CFR and female 2D:4D showed no relationship (males: right hand 2D:4D B = 51.84, SE B = 22.25, beta = 0.38, p < .05; females: right 2D:4D B = −11.26, SE B = 17.32, beta = −0.11, p = .52). Male left hand 2D:4D also remained positively related to log CFR and female 2D:4D showed no relationship (males: left 2D:4D B = 48.53, SE B = 23.81, beta = 0.36, p < .05; females: left: 2D:4D B = −13.63, SE B = 17.09, beta = −0.14, p = .43).
Table 2.
Means, SD and SEMs for right and left hand male 2D:4D in 41 nations.
| n | Males right hand |
Males left hand |
|||||
|---|---|---|---|---|---|---|---|
| Mean 2D:4D |
SD | SE | Mean 2D:4D |
SD | SE | ||
| Argentina | 125 | 0.990 | 0.048 | 0.004 | 0.988 | 0.041 | 0.004 |
| Australia | 4103 | 0.981 | 0.046 | 0.001 | 0.982 | 0.046 | 0.001 |
| Austria | 213 | 0.980 | 0.042 | 0.003 | 0.986 | 0.043 | 0.003 |
| Belgium | 764 | 0.981 | 0.045 | 0.002 | 0.984 | 0.043 | 0.002 |
| Brazil | 170 | 0.979 | 0.047 | 0.004 | 0.980 | 0.044 | 0.003 |
| Bulgaria | 172 | 0.990 | 0.047 | 0.004 | 0.989 | 0.046 | 0.004 |
| Canada | 5723 | 0.981 | 0.048 | 0.001 | 0.981 | 0.047 | 0.001 |
| China | 169 | 0.985 | 0.043 | 0.003 | 0.985 | 0.039 | 0.003 |
| Croatia | 95 | 0.981 | 0.038 | 0.004 | 0.984 | 0.038 | 0.004 |
| Czech | 146 | 0.984 | 0.04 | 0.003 | 0.986 | 0.042 | 0.003 |
| Denmark | 380 | 0.982 | 0.044 | 0.002 | 0.988 | 0.046 | 0.002 |
| Finland | 875 | 0.984 | 0.046 | 0.002 | 0.985 | 0.045 | 0.002 |
| France | 535 | 0.983 | 0.044 | 0.002 | 0.987 | 0.044 | 0.002 |
| Germany | 866 | 0.983 | 0.044 | 0.001 | 0.985 | 0.042 | 0.001 |
| Greece | 420 | 0.986 | 0.049 | 0.002 | 0.987 | 0.045 | 0.002 |
| Hungary | 97 | 0.986 | 0.04 | 0.004 | 0.988 | 0.039 | 0.004 |
| Iceland | 85 | 0.980 | 0.053 | 0.006 | 0.984 | 0.047 | 0.005 |
| India | 2274 | 0.986 | 0.056 | 0.001 | 0.986 | 0.056 | 0.001 |
| Ireland | 2307 | 0.983 | 0.049 | 0.001 | 0.983 | 0.047 | 0.001 |
| Israel | 195 | 0.987 | 0.044 | 0.003 | 0.987 | 0.042 | 0.003 |
| Italy | 248 | 0.984 | 0.041 | 0.003 | 0.986 | 0.044 | 0.003 |
| Japan | 289 | 0.984 | 0.044 | 0.003 | 0.982 | 0.043 | 0.003 |
| Malaysia | 418 | 0.976 | 0.044 | 0.002 | 0.976 | 0.041 | 0.002 |
| Mexico | 208 | 0.976 | 0.051 | 0.004 | 0.977 | 0.047 | 0.003 |
| N Zealand | 970 | 0.980 | 0.046 | 0.001 | 0.982 | 0.045 | 0.001 |
| Netherland | 1172 | 0.981 | 0.047 | 0.001 | 0.985 | 0.046 | 0.001 |
| Norway | 305 | 0.982 | 0.042 | 0.002 | 0.984 | 0.042 | 0.002 |
| Pakistan | 245 | 0.983 | 0.05 | 0.003 | 0.984 | 0.051 | 0.003 |
| Philippines | 190 | 0.983 | 0.054 | 0.004 | 0.980 | 0.051 | 0.004 |
| Poland | 197 | 0.984 | 0.05 | 0.004 | 0.989 | 0.046 | 0.003 |
| Portugal | 187 | 0.983 | 0.051 | 0.004 | 0.983 | 0.049 | 0.004 |
| Romania | 166 | 0.986 | 0.05 | 0.004 | 0.985 | 0.046 | 0.004 |
| Russia | 88 | 0.976 | 0.047 | 0.005 | 0.986 | 0.043 | 0.005 |
| Singapore | 817 | 0.977 | 0.043 | 0.002 | 0.974 | 0.042 | 0.001 |
| Spain | 468 | 0.987 | 0.052 | 0.002 | 0.988 | 0.045 | 0.002 |
| Sweden | 760 | 0.982 | 0.049 | 0.002 | 0.981 | 0.046 | 0.002 |
| Switzerland | 323 | 0.984 | 0.041 | 0.002 | 0.983 | 0.041 | 0.002 |
| Turkey | 653 | 0.987 | 0.048 | 0.002 | 0.987 | 0.048 | 0.002 |
| UK | 51,324 | 0.985 | 0.048 | 2.000E−4 | 0.986 | 0.046 | 2.000E−4 |
| UAE | 169 | 0.981 | 0.044 | 0.003 | 0.983 | 0.048 | 0.001 |
| USA | 24,571 | 0.984 | 0.053 | 3.000E−4 | 0.984 | 0.051 | 3.000E−4 |
Table 3.
Means, SD and SEMs for right and left hand female 2D:4D in 41 nations.
| n | Females right hand |
Females left hand |
|||||
|---|---|---|---|---|---|---|---|
| Mean 2D:4D |
SD | SE | Mean 2D:4D |
SD | SE | ||
| Argentina | 78 | 0.992 | 0.050 | 0.006 | 0.992 | 0.057 | 0.006 |
| Australia | 3690 | 0.990 | 0.046 | 0.001 | 0.988 | 0.044 | 0.001 |
| Austria | 170 | 0.990 | 0.046 | 0.004 | 0.994 | 0.042 | 0.003 |
| Belgium | 499 | 0.989 | 0.047 | 0.002 | 0.989 | 0.044 | 0.002 |
| Brazil | 109 | 0.994 | 0.054 | 0.005 | 0.991 | 0.051 | 0.005 |
| Bulgaria | 186 | 0.997 | 0.049 | 0.004 | 0.998 | 0.048 | 0.004 |
| Canada | 5279 | 0.994 | 0.051 | 0.001 | 0.992 | 0.050 | 0.001 |
| China | 124 | 0.989 | 0.044 | 0.004 | 0.985 | 0.050 | 0.004 |
| Croatia | 102 | 0.998 | 0.038 | 0.004 | 0.996 | 0.037 | 0.004 |
| Czech | 94 | 1.000 | 0.047 | 0.005 | 0.999 | 0.043 | 0.004 |
| Denmark | 347 | 0.987 | 0.046 | 0.002 | 0.990 | 0.048 | 0.003 |
| Finland | 675 | 0.991 | 0.044 | 0.002 | 0.990 | 0.042 | 0.002 |
| France | 376 | 0.990 | 0.045 | 0.002 | 0.986 | 0.043 | 0.002 |
| Germany | 546 | 0.993 | 0.046 | 0.002 | 0.991 | 0.044 | 0.002 |
| Greece | 392 | 0.997 | 0.054 | 0.003 | 0.998 | 0.050 | 0.003 |
| Hungary | 103 | 0.999 | 0.050 | 0.005 | 0.995 | 0.047 | 0.005 |
| Iceland | 88 | 0.986 | 0.051 | 0.005 | 0.987 | 0.050 | 0.005 |
| India | 577 | 0.997 | 0.057 | 0.002 | 0.992 | 0.059 | 0.002 |
| Ireland | 2177 | 0.991 | 0.050 | 0.001 | 0.991 | 0.049 | 0.001 |
| Israel | 131 | 1.001 | 0.052 | 0.005 | 0.996 | 0.050 | 0.004 |
| Italy | 166 | 0.994 | 0.048 | 0.004 | 0.989 | 0.047 | 0.004 |
| Japan | 163 | 0.985 | 0.046 | 0.004 | 0.982 | 0.043 | 0.003 |
| Malaysia | 350 | 0.992 | 0.049 | 0.003 | 0.991 | 0.049 | 0.003 |
| Mexico | 131 | 0.989 | 0.050 | 0.004 | 0.984 | 0.049 | 0.004 |
| N Zealand | 954 | 0.990 | 0.047 | 0.002 | 0.987 | 0.044 | 0.001 |
| Netherlands | 798 | 0.990 | 0.049 | 0.002 | 0.992 | 0.047 | 0.002 |
| Norway | 213 | 0.990 | 0.050 | 0.003 | 0.989 | 0.048 | 0.003 |
| Pakistan | 72 | 0.988 | 0.049 | 0.006 | 0.990 | 0.050 | 0.006 |
| Philippines | 201 | 0.992 | 0.057 | 0.004 | 0.991 | 0.057 | 0.004 |
| Poland | 225 | 0.999 | 0.046 | 0.003 | 0.997 | 0.044 | 0.003 |
| Portugal | 139 | 0.988 | 0.049 | 0.004 | 0.986 | 0.038 | 0.003 |
| Romania | 173 | 0.998 | 0.048 | 0.004 | 1.001 | 0.048 | 0.004 |
| Russia | 96 | 0.996 | 0.050 | 0.005 | 1.002 | 0.045 | 0.005 |
| Singapore | 944 | 0.989 | 0.046 | 0.002 | 0.986 | 0.043 | 0.001 |
| Spain | 289 | 0.995 | 0.044 | 0.003 | 0.992 | 0.049 | 0.003 |
| Sweden | 386 | 0.994 | 0.051 | 0.003 | 0.992 | 0.048 | 0.002 |
| Switzerland | 206 | 0.991 | 0.046 | 0.003 | 0.987 | 0.040 | 0.003 |
| Turkey | 595 | 0.999 | 0.050 | 0.002 | 1.000 | 0.048 | 0.002 |
| UK | 40,207 | 0.993 | 0.050 | 3.000E−4 | 0.992 | 0.047 | 3.000E−4 |
| UAE | 117 | 1.001 | 0.062 | 0.006 | 0.993 | 0.048 | 0.004 |
| USA | 21,198 | 0.996 | 0.055 | 4.000E−4 | 0.993 | 0.052 | 4.000E−4 |
Fig. 1.
The relationship between male mean right digit ratio per nation and log-transformed national case fatality rate (log CFR) in 41 nations. Note: y = −45.33 + 46.53 ∗ x; r2 = 0.12.
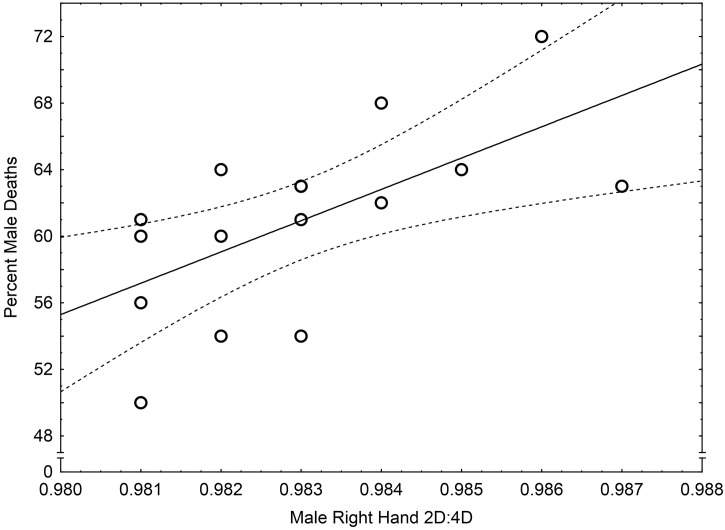
The sample size for examining 2D:4D relationships with percent of male deaths (n = 16) was small. Nevertheless, there were positive correlations for males with both right (r = 0.63, p < .01, Fig. 2 ) and left 2D:4D (r = 0.52, p < .05), and a weak association for female right hands (r = 0.50, p = .049) but no correlation for female left hands (r = 0.41, p = .12). Multiple regression analysis showed male right hand 2D:4D (B = 1513.03, SE B = 705.07, beta = 0.51, p = .050) remained significantly related to percent of male deaths independent of female right 2D:4D (B = 495.91, SE B = 459.45, beta = 0.26, p = .30). For male left hand 2D:4D there was a marginally significant relationship with percent of male deaths (B = 1122.12, SE B = 527.77, beta = 0.47, p = .053) and no relationship for female left hand 2D:4D (B = 579.00, SE B = 375.07, beta = 0.34, p = .15).
Fig. 2.
The relationship between mean male right 2D:4D per nation and the national percentage of male deaths in 16 countries. Note: y = −1787.10 + 1880 ∗ x; r2 = 0.40.
4. Discussion
We detected a positive association between mean male right hand 2D:4D per nation and log CFR (n = 41 nations), and a positive (but non-significant) association for mean male left hand 2D:4D. There were no correlations between mean female 2D:4D per nation and log CFRs. Male and female 2D:4Ds correlated positively. Male right and left hand 2D:4D correlated positively with log CFR when the influence of female 2D:4D was removed. Similar patterns of positive relationships pertained between male mean 2D:4D and percent of male deaths per nation. Here the sample size was lower (n = 16 nations), but the focus on male deaths rather than overall mortality would be expected to increase the effect size. This is what we found as the effect size for the correlation between nations increased from an r 2 value of about 10% to >30%.
Our findings support a link between high 2D:4D (low prenatal testosterone) and high severity of COVID-19 in men. However, we are considering comparative data across nations and this introduces some limitations to our analysis. Testing regimes vary across nations with some testing widely in the population and others focusing mainly on hospital admissions for COVID-19. This affects comparative values of CFRs. Therefore, we strongly advise that future investigations of associations of 2D:4D and COVID-19 severity consider patients. If there is a strong positive correlation with disease severity in men, the measurement of 2D:4D (particularly right hand 2D:4D) may be of prognostic use for the severity of COVID-19.
Advocates of the androgen-driven theory have suggested treatment with androgen blockers such as spironolactone [12,13]. This seems reasonable given the substantial excess in male mortality associated with the disease [22]. However, there is support for the counter-theory that treatment of COVID-19 should involve an increase the amount of ACE2 in the lungs [23]. ACE2 protects from lung injury, but ACE2 is also the critical SARS-CoV receptor. The severity of COVID-19 could be explained by SARS-CoV spike protein binding to ACE2, which leads to endocytosis of the virus but the loss of ACE2 from the surface of the cell. This establishes a circle of viral infection and local loss of lung protection. In support of this model, Monteil et al. [24] showed that soluble human ACE2 molecule could significantly inhibit SARS-CoV-2 infections and reduce viral load by a factor of 1000–5000.
Our findings support an association between low prenatal testosterone (high 2D:4D) and high severity of COVID-19, and high mortality in males. They are in accord with the knowledge of the variation in expression and regulation of the SARS-CoV2 receptor ACE2 gene. The ACE2 gene is more strongly expressed in females compared to males, in young males compared to old males and in healthy men compared to those with several co-morbidities including type 2 diabetes [23]. These associations suggest a negative correlation between ACE2 expression and CovID19 fatality at both population and molecular levels, which will be instrumental when designing potential prevention and treatment strategies for ACE2 binding coronaviruses in general [23]. The down-regulation of ACE2 may therefore be associated with poor prognosis from COVID-19. The finding that soluble ACE2 reduces viral load substantially supports this position [24]. Hence, ACE2 [24] and alternatives to ACE2 such as spironolactone [22] may constitute treatments for COVID-19.
ACE2 is also expressed in the Leydig cells of the testes, which indicates a regulatory function in spermatogenesis [[25], [26], [27]]. Research suggests that testosterone in men and estrogen in women up-regulates ACE2 [23]. A comparison of testicular function in COVID-19 patients and healthy controls showed similar testosterone levels in both groups but higher LH in the former, suggesting that the patients' pituitary has to work harder than that of the controls to maintain testosterone levels. This may be evidence that COVID-19 damages testicular function, however the same results would be obtained if the patients had compromised gonadal function before infection [28]. It may be relevant that men who have few or no sperm and low testosterone have high 2D:4D [29,30]. Moreover, the severity of COVID-19 is associated with cardiovascular disease and high 2D:4D correlates with early heart attacks in men [31]. Such correlations support an association between high 2D:4D and high CFRs.
In conclusion, we have found that mean male 2D:4D per nation correlates positively with CFR and percent of male deaths due to COVID-19. The effect was independent of mean female 2D:4D. Thus, high prenatal testosterone (low 2D:4D) in men may be protective of the serious effects of COVID-19. Our findings suggest that an excess of male deaths resulting from COVID-19 is not the product of high prenatal testosterone but rather a marker for prenatal hypogonadism. SARS-CoV2 enters cells via the receptor molecule ACE2. Paradoxically, the up-regulation of ACE2 relates to protective effects from COVID-19 infection, possibly because it opposes the loss of ACE2 from cell surfaces. We speculate that in men the up-regulation of ACE2 relates to high testosterone and low 2D:4D. A strong positive association between male 2D:4D and mortality may provide a biomarker for male COVID-19 susceptibility and identify those for whom it would be advisable to exercise social distancing.
CRediT authorship contribution statement
John T. Manning: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing - original draft; Writing - review & editing. Bernhard Fink: Conceptualization, Formal analysis, Investigation, Methodology, Visualization, Writing - original draft; Writing - review & editing
References
- 1.Shi Y., Yu X., Zhao H., Wang H., Zhao R., Sheng J. Host susceptibility to severe COVID-19 and establishment of a host risk score: findings of 487 cases outside Wuhan. Crit. Care. 2020;24:108. doi: 10.1186/s13054-020-2833-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan W.-J., Ni Z.-Y., Hu Y., Liang W., Ou C.-Q., He J.-X. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Channappanavar R., Fett C., Mack M., Ten Eyck P.P., Meyerholz D.K., Perlman S. Sex-based differences in susceptibility to coronavirus infection severe acute respiratory syndrome. J. Immunol. 2017;198:4046–4053. doi: 10.4049/jimmunol.1601896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein S.L., Flanagan K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016;16:626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 5.Rettew J.A., Huet-Hudson Y.M., Marriott I. Testosterone reduces macrophage expression in the mouse of toll-like receptor 4, a trigger for inflammation and innate immunity. Biol. Reprod. 2008;78:432–437. doi: 10.1095/biolreprod.107.063545. [DOI] [PubMed] [Google Scholar]
- 6.Roberts C.W., Walker W., Alexander J. Sex-associated hormones and immunity to protozoan parasites. Clin. Microbiol. Rev. 2001;14(3):476–488. doi: 10.1128/CMR.14.3.476-488.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouman A., Heineman M.J., Faas M.M. Sex hormones and the immune response in humans. Hum. Reprod. Update. 2005;11:411–442. doi: 10.1093/humupd/dmi008. [DOI] [PubMed] [Google Scholar]
- 8.Zheng Z., Cohn M.J. Developmental basis of sexually dimorphic digit ratios. Proc. Natl. Acad. Sci. U. S. A. 2011;108(39):16289–16294. doi: 10.1073/pnas.1108312108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manning J.T. Resolving the role of prenatal sex steroids in the development of digit ratio. Proc. Natl. Acad. Sci. U. S. A. 2011;108(39):16143–16144. doi: 10.1073/pnas.1113312108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization Coronavirus disease (COVID-19) situation reports – 92 (21 April 2020) https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (Accessed on April 21, 2020)
- 11.Global Health 5050 Covid-19 Sex-disaggregated Data Tracker. https://globalhealth5050.org/
- 12.Wambier C.G., Goren A., Ossimetha A., Nau G., Qureshi A.A. 2020. Androgen-driven COVID-19 Pandemic Theory. Preprint, April. [DOI] [Google Scholar]
- 13.Wambier C.G., Goren A. SARS-COV-2 infection is likely to be androgen mediated. J. Am. Acad. Dermatol. 2020 doi: 10.1016/j.jaad.2020.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heurich A., Hofmann-Winkler H., Gierer S., Liepold T., Jahn O., Pohlmann S. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J. Virol. 2014;88(2):1293–1307. doi: 10.1128/JVI.02202-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lucas J.M., Heinlein C., Kim T., Hernandez S.A., Malik M.S., True L.D. The androgen-regulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microenvironment and promotes prostate cancer metastasis. Cancer Discov. 2014;4(11):1310–1325. doi: 10.1158/2159-8290.CD-13-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurtoğlu S., Baştuğ O. Mini puberty and its interpretation. Türk. Ped. Arş. 2014;49:186–191. doi: 10.5152/tpa.2014.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maggi M., Basaria S., Ble A., Lauretani F., Bandinelli S., Ceda G.P. Correlation between testosterone and the inflammatory marker soluble interleukin-6 receptor in older men. J. Clin. Endocrinol. Metab. 2004;91(1):345–347. doi: 10.1210/jc.2005-1097. [DOI] [PubMed] [Google Scholar]
- 19.Reimers S. The BBC internet study: general methodology. Arch. Sex. Behav. 2007;36(2):147–161. doi: 10.1007/s10508-006-9143-2. [DOI] [PubMed] [Google Scholar]
- 20.Manning J.T., Churchill A.J., Peters M. The effects of sex, ethnicity, and sexual orientation on self-measured digit ratio (2D:4D) Arch. Sex. Behav. 2007;36(2):223–233. doi: 10.1007/s10508-007-9171-6. [DOI] [PubMed] [Google Scholar]
- 21.Manning J.T., Fink B. Digit ratio, nicotine and alcohol intake and national rates of smoking and alcohol consumption. Pers. Individ. Differ. 2011;50(3):344–348. doi: 10.1016/j.paid.2010.10.016. [DOI] [Google Scholar]
- 22.Cadegiani F.A. Can spironolactone be used to prevent COVID-19-induced acute respiratory distress syndrome in patients with hypertension? Am. J. Physiol. Endocrinol. Metab. 2020;318(5):E587–E588. doi: 10.1152/ajpendo.00136.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J., Jiang Q., Xia X., Liu K., Yu Z., Tao W. 2020. Individual Variation of the SARS-CoV2 Receptor ACE2 Gene Expression and Regulation; p. 2020030191. Preprints. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monteil V., Kwon H., Prado P., Hagelkrüys A., Wimmer R.A., Stahl M. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020 doi: 10.1016/j.cell.2020.04.004. April 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Douglas G.C., O’Bryan M.K., Hedger M.P., Lee D.K., Yarski M.A., Smith A.I. The novel angiotensin-converting enzyme (ACE) homolog, ACE2, is selectively expressed by adult Leydig cells of the testis. Endocrinology. 2004;145(10):4703–4711. doi: 10.1210/en.2004-0443. [DOI] [PubMed] [Google Scholar]
- 26.Langford K.G., Zhou Y., Russell L.D., Wilcox J.N., Bernstein K.E. Regulated expression of testis angiotensin-converting enzyme during spermatogenesis in mice. Biol. Reprod. 1993;48(6):1210–1218. doi: 10.1095/biolreprod48.6.1210. [DOI] [PubMed] [Google Scholar]
- 27.Leung O.S., Sernia C. The renin-angiotensin system and male reproduction: new functions for old hormones. J. Mol. Endocrinol. 2003;30(3):26370. doi: 10.1677/jme.0.0300263. [DOI] [PubMed] [Google Scholar]
- 28.Ma L., Xie W., Li D., Shi L., Mao Y., Xiong Y. Effect of SARS-CoV-2 infection upon male gonadal function: a single center-based study. medRxiv. 2020 doi: 10.1101/2020.03.21.20037267. 03.21.20037267. [DOI] [Google Scholar]
- 29.Manning J.T., Scutt D., Wilson J., Lewis-Jones D.I. The ratio of 2nd to 4th digit length: a predictor of sperm numbers and concentrations of testosterone, luteinizing hormone and oestrogen. Hum. Reprod. 1998;13(11):3000–3004. doi: 10.1093/humrep/13.11.3000. [DOI] [PubMed] [Google Scholar]
- 30.Manning J.T., Kilduff L.P., Trivers R. Digit ratio (2D:4D) in Klinefelter’s syndrome. Andrology. 2013;1(1):94–99. doi: 10.1111/j.2047-2927.2012.00013.x. [DOI] [PubMed] [Google Scholar]
- 31.Manning J.T., Bundred P.E., Kasielska-Trojan A., Smith-Straney T., Mason L. Digit ratio (2D:4D), myocardial infarction and fibrinogen in men. Early Hum. Dev. 2019;133:18–22. doi: 10.1016/j.earlhumdev.2019.04.008. [DOI] [PubMed] [Google Scholar]