Highlights
-
•
SARS-CoV-2 (novel coronavirus) and SARS-2003 both have a similar mechanism of infection, i.e, binding the spike protein on the viral surface to the ACE2 receptors on the host cell surface.
-
•
The devastating cytokine explosion attributed to the SARS-CoV-2 infection leads to severe shock, oedema and multiple organ failure.
-
•
Administering the COVID-19 patients with an infusion of multipotent MSCs can help to combat the COVID-19 as these cells will inhibit the exaggerated immune response and encourage endogenous repair of the lung epithelial cells.
-
•
The mesenchymal stem cell therapy does not have any adverse side effects on the patient.
-
•
In this review we have highlighted all the implications associated with MSC therapy application in case of COVID-19 and strongly place our argument in support of this.
Keywords: COVID-19, Mesenchymal stem cells, SARS-CoV-2, Immunomodulatory, Cytokines
Abstract
The COVID-19 disease is caused by a positive stranded RNA virus called SARS-CoV-2. The virus mainly targets the pulmonary epithelial cells as it’s initial site of infection by letting its surface spike protein interact and bind to the host ACE2 receptor. The internalization and gradual replication of the virus results in an exaggerated immune response triggering release of many pro-inflammatory cytokines and chemokines. This immune storm is responsible for multiple health hazards in the host ultimately leading to multiple organ failure. Mesenchymal stem cell therapy offers a promising approach towards mitigating the delirious effects of the infection in the COVID-19 patients. This therapy has shown to reduce the expression of pro-inflammatory cytokines as well as repair of damaged tissues in COVID-19 patients. This review has been organized to put forward the positive aruments and implications in support of mesenchymal stem cell therapy as a necessary approach for treating COVID-19 patients.
1. Introduction
The end of the year 2019 marked the beginning of a new challenge for humanity when several cases of severe respiratory ailments were reported in the city of Wuhan, Hubei province, China. The cases were earlier confused with regular flu and thought to be caused by the normal seasonal influenza virus. The accurate prognosis of the illness was very difficult to make in the beginning but was simulataneously identified to be a virus borne disesase. Due to the increasing severity in the following days, on January 1, the virus was declared novel. Upon the complete phylogenetic analysis of the viral genome consisting of 29,903 nucleotides, it was found that this novel coronavirus had 89.1 % similar nucleotides to a class of Severe Acute Respiratory Syndrome (SARS) – like coronavirus. This novel virus belonged to the genus Betacoronavirus having the subgenus Sarbecovirus [1]. The novel coronavirus was earlier known to be found in Chinese bats [2]. WHO has assigned a brief name to the virus, SARS-CoV-2 and COVID-19 is the name assigned to the SARS-CoV-2 associated disease.
Till date no dedicated therapeutic or vaccination strategies have been implemented or confirmed to prevent COVID-19. Accessory therapeutic manueveurs including corticosteroid mediated inflammation reduction, convalescent plasma therapy, antibiotics for treatment of secondary bacterial sepsis and non-specific antivirals, etc., do not show much effectivity in severe cases of COVID-19. The main reason behind the failure of these therapies is the cytokine storm in the lungs generated by the virus. In the computed tomography scans, these cytokine storms (an augmented immune response in the body towards any external stimulus) appear as inflammatory lesions with ground-glass opacity [3,4].
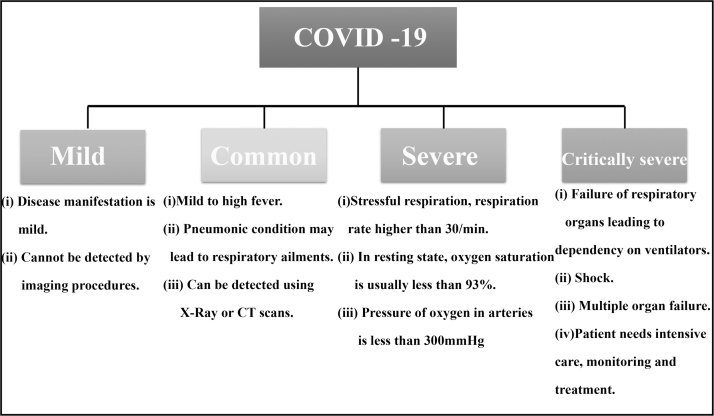
Most of the gravely sick and deceased patients do not develop any severe clinical manifestations in the earlier stages of COVID-19. The commonly reported symptoms are cough, throat pain, mild/high fever, muscle soreness, or body ache. A sudden deterioration in the health condition of the patients are seen in the later stages of diseases progression. Rapid failure of multiple organs and ARDS (Acute Respiratory Distress Syndrome) results in death within a very short period of time. Cytokine storm has been indicated as the presumable causal factor for ARDS and multiple organ failure [5,6] (Fig. 1).
Fig. 1.
Classification of COVID-19 on clinical basis issued by the National Health Commission of China.
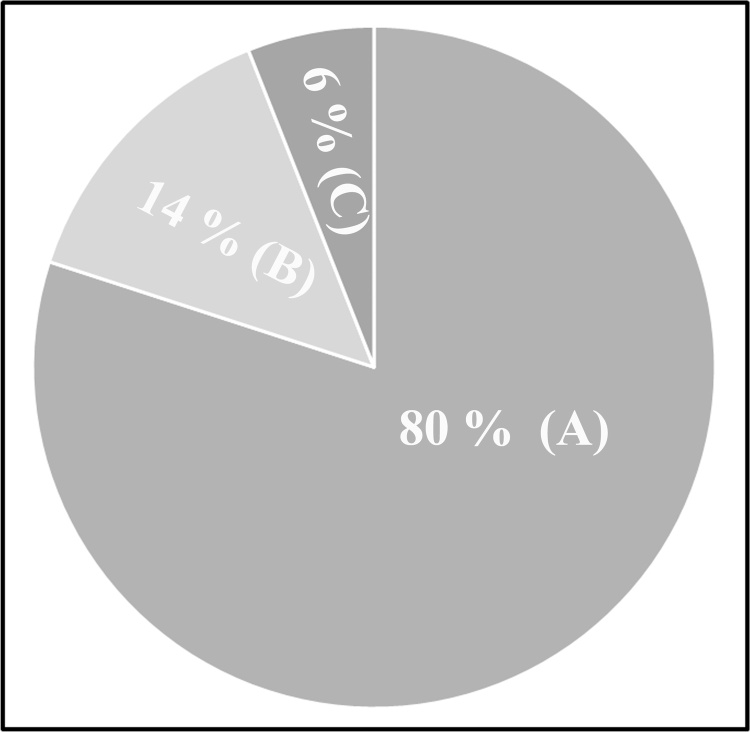
WHO has approximated the case fatality rate (CFR) of COVID-19 as ranging from 0.3 to 1%, higher than that of influenza A which has a CFR of 0.1 %. Various epidemiological studies conducted in countries implementing COVID-19 mitigation strategies has reported that around 80 % of patients suffering from COVID-19 had symptomless or mild disease. 14 % of the patients had severe ailments, and 6% of them were in critical condition [7] (Fig. 2).
Fig. 2.
Data obtained from epidemiological studies conducted in various countries reporting the incidence of COVID-19 suggested that (A). 80 % of the patients suffering from COVID-19 were asymptomatic. (B). 14 % of them had severe symptoms, and (C). 6% of the reported patients were critically ill.
1.1. Pathogenesis of SARS-CoV-2
SARS-CoV-2 recognizes the angiotensin I converting enzyme 2 (ACE2) receptor by its spike glycoprotein (SARS-CoV-2S), which is a class I fusion protein. The virus is consecutively fused with the host cells. S1 subunit, via its receptor-binding domain, mediates the attachment of the spike protein. The protein changes its conformation after binding with the receptor. S2 domain of the spike protein is responsible for membrane fusion and internalization of the virus [8]. The host cell entry and spread are facilitated by priming of the spike protein of the virus by cellular transmembrane protease, serine 2 (TMPRSS2) [[9], [10], [11]].
In the lungs, the ACE2 receptor and TMPRSS2 is commonly expressed in alveolar type II cells along with capillary endothelial cells. Therefore the characteristic symptom of SARS-CoV-2 infection is the severe respiratory ailment. This viral infection within the lungs results in a cytokine storm, thus elevating the level of many pro-inflammatory cytokines. The increased level of these cytokines leads to oedema, dysfunction in exchange of air, acute respiratory distress, and various other secondary infections, which may eventually cause death [[9], [10], [11], [12]].
1.2. Why mesenchymal stem cells?
Presently, cell-based therapy, and above all, stem cell therapy has proven itself to be one of the most promising therapeutic approaches that provide opportunities to treat several diseases that were considered incurable earlier [13]. MSC therapy is preferred over other therapeutic strategies because they are free of ethical and social issues, they have a high proliferation rate and a low invasive nature. Mesenchymal cells can be obtained from various sources, including adipose tissues, dental pulp, bone marrow, umbilical cord, menstrual blood, fetal liver, and Bichat's fat pad.
MSCs can also be isolated from various adult tissues such as the infrapatellar fat pad, abdominal fat pad, and tissues associated with neonates such as placenta, Wharton's (gelatinous substance providing insulation and protection to the umbilical cord) jelly, cord blood, and amniotic fluid. These stem cells are multipotent (i.e., having several fates). Storage of mesenchymal cells can be done so that they can be repetitively used for therapeutic purposes as they expand to volume in a suitable and short period of time. So far, the clinical trials of mesenchymal stem cells have not shown any unfavorable reaction towards the allogeneic MSCs. The efficacy and safety of the MSCs have been documented in many clinical trials very well [14].
1.3. Therapeutic achievements
Remarkable reversal of severe COVID-19 symptoms even in critical condition were reported in two clinical studies conducted in China. These clinical studies discovered a novel therapeutic strategy as well as existing natural mechanism resisting acute inflammatory pneumonia.
A total of ten patients were taken as subjects, and the study was conducted on seven SARS-CoV-2 positive patients, out of which four of them showed severe symptoms, two showing common types of the syndrome, and one of them was critically ill. The other three patients with severe symptoms were enrolled for placebo control [9]. Clinical-grade human MSCs were intravenously administered to each of the seven patients. The patients were treated with 1 × 106 MSCs per kilogram body weight while their condition was worsening severely. They were observed for a total period of 14 days. Before the infusion of MSCs, all the patients had high fever (body temperatures ranging from 38.5 °C to 39 °C), reduced oxygen saturation, dyspnea (shortness of breath) and pneumonia. The study showed that almost all the symptoms displayed by the patients before infusion, subsided under 2–4 days after they received the infusion. The oxygen saturation, with or without oxygen uptake (approximately 5 L/minute), rose to ≥ 95 % at rest. The profile of the immune system constitution was investigated during MSC transplantation. CyTOF (mass cytometric) analysis of peripheral blood of the patient was done, revealing the fact that The peripheral lymphocytes increased after the treatment, with a shift towards the regulatory phenotype for both dendritic cells and CD4+T cells. In case of the two patients having common symptoms, no significant increase in dendritic cells (DC,CXCR3−) or CXCR3- (regulatory T cells) were reported. Whereas in the case of the patients with severe symptoms, both dendritic cells as well as regulatory T cells were found to have increased following the treatment. Also, in the 3 severe control patients enrolled for placebo treatment, no significant increase in CXCR3− dendritic cell were observed. The percentages of CXCR3+CD+T cells, CXCR3+CD4+T cells and CXCR3+ natural killer cells in the peripheral blood mononuclear cells were remarkably high compared to the healthy control, before the MSC infusion. This triggered the cytokine storm as a result of inflammation. But within 6 days, it was found that the overactivation of natural killer cells, as well as T cells, nearly subsided, and the subpopulation of the cells reverted to the normal levels. The MSC infusion didn't show any side effects. CT scans of chest revealed that pneumonia infiltration was remarkably reduced. Most of the patients were reported negative for the SARS-CoV-2 nucleic acid test within 7–14 days after the infusion. The most exciting and extraordinary finding of the study was the overall improvement of an elderly patient who was critically ill previously.
Another study involved a critically ill ventilator ridden COVID-19 patient who was administered with human umbilical cord MSC (hUCMSC). This patient was treated with 3 infusions of 5 × 107 hUCMSC at the interval of 3 days, and the patient was able to walk within 4 days of her second cell infusion. The essential parameters like T-cell counts were restored back to normal levels. The patient displayed no observable side effects [15]. Till date about 17 completed clinical studies along with 70 trials are registered on https://clinicaltrials.gov reported positive results in treating respiratory disorders using MSCs. The study entitled “A study to evaluate the potential role of mesenchymal stem cells in the treatment of idiopathic pulmonary fibrosis” (clinical trial number: NCT01385644) was mainly aimed to provide evidence of safe infusion of placental MSCs from unrelated or related Human leukocyte antigen (HLA) mismatched or HLA identical donors in the Idiopathic Pulmonary Fibrosis (IPF) treatment. Results of the study provided evidence of stabilized and improved lung function. The other study entitled human mesenchymal stem cells for acute respiratory distress syndrome (ARDS) was aimed to infuse allogenic bone marrow derived human MSCs intravenously in patients having ARDS and assess the safety of the intravenous infusion (clinical trial number: NCT01775774). The study was conducted in two phases, with the first phase involving the intravenous infusion of stromal MSCs in 9 patients with ARDS. It was revealed that the infusion of the MSCs was safe and no treatment-related adverse events or infusion-linked events were reported [16]. The second phase of the study suggested that the severity of acute lung injury was reduced in a sheep model having bacterial pneumonia on treatment with hMSCs [17].
Out of the 70 registered trials, one registered trial (clinical trial number: NCT04313322) at https://clinicaltrials.gov involved the intravenous administration of Wharton’s Jelly MSCs (3 doses consisting of 1 × 106/kg, 3 days apart) for treatment of COVID-19 diagnosed patients. Another registered trial (clinical trial number: NCT04315987) suggested the use of NestCell®, which is an MSC therapy produced by Cellavita (Germany based company) and has proved safe in various clinical trials. One registered trial was estimated to enroll 24 subjects diagnosed with COVID-19 (between 18–75 years of age) for treatment of SARS-CoV-2 induced pneumonia using MSCs obtained from dental pulp (clinical trial number: NCT04302519). Several other trials included the use of umbilical cord MSCs for treating COVID-19 triggered pneumonia. Also, Chinese clinical trial registry site, http://www.chictr.org.cn, has reported registration of 20 clinical trials in this regard.
2. MSC therapy: analysis of outcomes
COVID-19 triggers an exaggerated immune reaction in the body by producing large amounts of various inflammatory factors including several cytokines, chemokines and immune reactive cells. It can be hypothesized that the MSC therapy might prevent the triggering of cytokine storm by the activated immune system, and the reparative properties of the stem cells might promote endogenous repair [18]. Mesenchymal stem cells when intravenously injected will lead to some part of the population getting entrapped in the lungs. While trapped within the lungs, a wide variety of soluble mediators, including antimicrobial peptides, anti-inflammatory cytokines, extracellular vesicles, and angiogenic growth factors are released by the MSCs [[19], [20], [21]]. The pattern of anti-inflammatory mediators released is specific for the inflammatory lung environment encountered. The pattern of these released mediators are regulated through differential activation of the damage and pathogen-associated molecular pathogen receptors expressed on the cell surfaces of MSCs [22], including TLRs (toll-like receptors) that, in case of COVID-19, are activated by viral unmethylated CpF-DNA (TLR9) and viral RNA (TLR3), leading to sub sequential cellular signalling pathways and thereafter activation of MSCs [23]. Keratinocyte Growth Factor (KGF) and angiopoietin-1 (Ang-1) secreted by MSCs promote the restoration of disrupted alveolar-capillary barriers due to the pathogenesis of ARDS [24]. Extracellular vesicles containing specific miRNA ans inhibitory mRNAs are also known to regulate the protective effects of MSCs in pre-clinical models of non-infectious acute lung inhuries or bacterial sepsis in lungs [25]. The pulmonary microenvironment could be recovered with the help of these MSCs, thus protecting the alveolar epithelial cells. Hence, pulmonary fibrosis of the lungs could be prevented, which may lead to curing COVID-19 caused pneumonia. In the multiple disease condition, the immunomodulatory effects will be responsible for improved function after MSC infusion. A variety of paracrine factors are secreted by these cells. These paracrine factors interact with the immune cells, eventually leading to immunomodulation. The vigorous anti-inflammatory activities of MSCs will actually be responsible for improvements after their infusion in COVID-19 patients.
It has been found that after infusion of MSCs in COVID-19 patients, the number of peripheral lymphocytes increased while the levels of C-reactive protein (CRP) decreased. The overactivated cytokine-secreting immune cells dwindled within 6 days in the circulating blood. Furthermore, after the MSC treatment, the population of regulatory dendritic cells (CD14+CD11bmid) increased. A major pro-inflammatory cytokine, TNF-α (Tumour Necrosis Factor), exhibited a decline in its levels in the MSC treated COVID-19 patients, with an elevation in the concentration of IL-10 (Interleukin-10) compared to the patients treated with conventional therapy [9]. Table 1 depicts the various immunomodulatory interventions by the MSCs.
Table 1.
Mesenchymal Stem Cells deprived of ACE2 receptors benefit the patients having SARS-CoV-2 infection via immunomodulatory functions [9].
| Pathological Complications | MSC therapy intervention (Immunoregulatory functions) |
|---|---|
|
|
|
|
|
|
|
|
For further elucidation of the underlying mechanisms of MSC-dependent treatment of COVID-19 patients, 10 x RNA-sequencing was done [9], which implicated that MSCs were free from infection with SARS-CoV-2. The fact that MSCs are resistant to viral infections compared to their differentiated progenies, reflects the significant role played by the intrinsic ISGs (interferon-stimulated genes) in these cells. The expression of ISGs forestalls viral infection [26]. MSCs express several ISGs including Interferon Induced Transmembrane Family (IFITM), SAT1, IFI6, PMAIP1, ISG15, CCL2, p21/CDKN1a etc., many of which are known to show typical anti-viral responses. The member proteins of the Interferon Induced Transmembrane Family are peculiar as they pre-empt infection before the virus can cross the lipid bilayer of cell.
This activity attributed to the IFITM proteins prevented infection of cultured cells by several viruses like Ebola virus, influenza A virus, dengue virus and SARS coronavirus [27]. Thus the two unique antiviral mechanisms provided by MSCs, in the context of respiratory viral infection like COVID-19, are constitutive elevation of levels of MSC-specific ISGs to act as regulators of antiviral protection and secondary response to IFN, which induces ISG and hence offers broad viral resistance.
3. Conclusion
SARS-CoV-2 (novel coronavirus) and SARS-2003, both have a similar mechanism of infection, i.e, binding the spike protein on the viral surface to the ACE2 receptors on the host cell surface. Thus all the tissues and organs expressing the ACE2 receptor are susceptible to the SARS-CoV-2 infection. Since the alveolar epithelial cells have a high propensity of ACE2 receptors, they are the most adversely affected during SARS-CoV-2 infection. The devastating cytokine explosion attributed to the SARS-CoV-2 infection leads to severe shock, oedema and multiple organ failure. Administering the COVID-19 patients with an infusion of multipotent MSCs can help to combat the COVID-19 as these cells will inhibit the exaggerated immune response and encourage endogenous repair of the lung epithelial cells by improving the microenvironment. The mesenchymal stem cell therapy has not tey shown any adverse side effects on the patient. In this review we have highlighted all the implications associated with MSC therapy application in case of COVID-19 and strongly place our argument in support of this.
Declaration of Competing Interest
The authors report no conflicts of interest.
Acknowledgements
KR and SR thanks IIEST Shibpur and Mahatma Gandhi Central University Motihari, Bihar, respectively.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.btre.2020.e00467.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G., Hu Y., Tao Z.W., Tian J.H., Pei Y.Y., Yuan M.L., Zhang Y.L., Dai F.H., Liu Y., Wang Q.M., Zheng J.J., Xu L., Holmes E.C., Zhang Y.Z. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu D., Zhu C., Ai L., He T., Wang Y., Ye F., Yang L., Ding C., Zhu X., Lv R., Zhu J., Hassan B., Feng Y., Tan W., Wang C. Genomic characterization and infectivity of a novel SARS-like coronavirus in Chinese bats. Emerg. Microbes Infect. 2018;7:154. doi: 10.1038/s41426-018-0155-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Metcalfe S.M. Mesenchymal stem cells and management of COVID-19 pneumonia. Med. Drug Discov. 2020;5 doi: 10.1016/j.medidd.2020.100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ye Q., Wang B., Mao J. The pathogenesis and treatment of the `Cytokine Storm’ in COVID-19. J. Infect. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.An update on the epidemiological characteristics of novel coronavirus pneumoniaCOVID-19. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:139–144. doi: 10.3760/cma.j.issn.0254-6450.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Anderson R.M., H. H, Klinkenberg D., Hollingsworth T.D. How will country-based mitigation measures influence the course of the COVID-19 epidemic. Lancet. 2020:931–934. doi: 10.1016/S0140-6736(20)30567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jitendra Subhash R., Aroni C., Abhijeet K., Shashikant R. 2020. Targeting SARS-CoV-2 Spike Protein of COVID-19 With Naturally Occurring Phytochemicals: an in Silco Study for Drug Development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leng Z., Zhu R., Hou W., Feng Y., Yang Y., Han Q., Shan G., Meng F., Du D., Wang S., Fan J., Wang W., Deng L., Shi H., Li H., Hu Z., Zhang F., Gao J., Liu H., Li X., Zhao Y., Yin K., He X., Gao Z., Wang Y., Yang B., Jin R., Stambler I., Lim L.W., Su H., Moskalev A., Cano A., Chakrabarti S., Min K.J., Ellison-Hughes G., Caruso C., Jin K., Zhao R.C. Transplantation of ACE2(-) mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11:216–228. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W.J., Wang D., Xu W., Holmes E.C., Gao G.F., Wu G., Chen W., Shi W., Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffmann M., Kleine-Weber H., Krüger N., Müller M., Drosten C., Pöhlmann S. The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. bioRxiv. 2020 2020.01.31.929042. [Google Scholar]
- 13.Golchin A., Farahany T.Z. Biological products: cellular therapy and FDA approved products. Stem Cell Rev. Rep. 2019;15:166–175. doi: 10.1007/s12015-018-9866-1. [DOI] [PubMed] [Google Scholar]
- 14.Golchin A., Farahany T.Z., Khojasteh A., Soleimanifar F., Ardeshirylajimi A. The clinical trials of mesenchymal stem cell therapy in skin diseases: an update and concise review. Curr. Stem Cell Res. Ther. 2019;14:22–33. doi: 10.2174/1574888X13666180913123424. [DOI] [PubMed] [Google Scholar]
- 15.Bing Liang J.C., Li Tao, Haiying Wu, Yang Wenjie, Li Yanjiao, Jianchun Li, Fangang Nie C.Y., Ma Zhaoxia, Yang Mingxi, Nie Panrong, Gao Yanfeng, Chuanyun Qian M.H. 2020. Clinical Remission of a Critically Ill COVID-19 Patient Treated Byhuman Umbilical Cord Mesenchymal Stem Cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson J.G., Liu K.D., Zhuo H., Caballero L., McMillan M., Fang X., Cosgrove K., Vojnik R., Calfee C.S., Lee J.W., Rogers A.J., Levitt J., Wiener-Kronish J., Bajwa E.K., Leavitt A., McKenna D., Thompson B.T., Matthay M.A. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir. Med. 2015;3:24–32. doi: 10.1016/S2213-2600(14)70291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asmussen S., Ito H., Traber D.L., Lee J.W., Cox R.A., Hawkins H.K., McAuley D.F., McKenna D.H., Traber L.D., Zhuo H., Wilson J., Herndon D.N., Prough D.S., Liu K.D., Matthay M.A., Enkhbaatar P. Human mesenchymal stem cells reduce the severity of acute lung injury in a sheep model of bacterial pneumonia. Thorax. 2014;69:819–825. doi: 10.1136/thoraxjnl-2013-204980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glenn J.D., Whartenby K.A. Mesenchymal stem cells: emerging mechanisms of immunomodulation and therapy. World J. Stem Cells. 2014;6:526–539. doi: 10.4252/wjsc.v6.i5.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee R.H., Pulin A.A., Seo M.J., Kota D.J., Ylostalo J., Larson B.L., Semprun-Prieto L., Delafontaine P., Prockop D.J. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krasnodembskaya A., Song Y., Fang X., Gupta N., Serikov V., Lee J.W., Matthay M.A. Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells. 2010;28:2229–2238. doi: 10.1002/stem.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu S., Park J., Liu A., Lee J., Zhang X., Hao Q., Lee J.W. Mesenchymal stem cell microvesicles restore protein permeability across primary cultures of injured human lung microvascular endothelial cells. Stem Cells Transl. Med. 2018;7:615–624. doi: 10.1002/sctm.17-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liotta F., Angeli R., Cosmi L., Fili L., Manuelli C., Frosali F., Mazzinghi B., Maggi L., Pasini A., Lisi V., Santarlasci V., Consoloni L., Angelotti M.L., Romagnani P., Parronchi P., Krampera M., Maggi E., Romagnani S., Annunziato F. Toll-like receptors 3 and 4 are expressed by human bone marrow-derived mesenchymal stem cells and can inhibit their T-cell modulatory activity by impairing Notch signaling. Stem Cells. 2008;26:279–289. doi: 10.1634/stemcells.2007-0454. [DOI] [PubMed] [Google Scholar]
- 23.Waterman R.S., Tomchuck S.L., Henkle S.L., Betancourt A.M. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS One. 2010;5 doi: 10.1371/journal.pone.0010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee J.W., Krasnodembskaya A., McKenna D.H., Song Y., Abbott J., Matthay M.A. Therapeutic effects of human mesenchymal stem cells in ex vivo human lungs injured with live bacteria. Am. J. Respir. Crit. Care Med. 2013;187:751–760. doi: 10.1164/rccm.201206-0990OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monsel A., Zhu Y.G., Gennai S., Hao Q., Hu S., Rouby J.J., Rosenzwajg M., Matthay M.A., Lee J.W. Therapeutic effects of human mesenchymal stem cell-derived microvesicles in severe pneumonia in mice. Am. J. Respir. Crit. Care Med. 2015;192:324–336. doi: 10.1164/rccm.201410-1765OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu X., Dao Thi V.L., Huang Y., Billerbeck E., Saha D., Hoffmann H.H., Wang Y., Silva L.A.V., Sarbanes S., Sun T., Andrus L., Yu Y., Quirk C., Li M., MacDonald M.R., Schneider W.M., An X., Rosenberg B.R., Rice C.M. Intrinsic immunity shapes viral resistance of stem cells. Cell. 2018;172(423-438):e25. doi: 10.1016/j.cell.2017.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bailey C.C., Zhong G., Huang I.C., Farzan M. IFITM-family proteins: the cell’s first line of antiviral defense. Annu. Rev. Virol. 2014;1:261–283. doi: 10.1146/annurev-virology-031413-085537. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.