Abstract
Background
Allergen immunotherapy (AIT) is safe and effective but is typically administered under strict clinic observation to mitigate the risk of a systemic reaction to immunotherapy (SRIT). However, in the setting of the global coronavirus disease 2019 pandemic, alternative care models should be explored.
Objective
To evaluate the cost-effectiveness of home immunotherapy self-administration (HITSA) in a highly idealized circumstance for provision of maintenance AIT in a shelter-in-place or other scenarios of unforeseen reduction in nonessential medical services.
Methods
Markov modeling was used to compare in-office clinic AIT in selected patients using cohort analysis and microsimulation from the societal and health care perspectives.
Results
Assuming similar SRIT rates, HITSA was found to be a cost-effective option with an incremental cost-effectiveness ratio of $44,554/quality-adjusted life-year when considering both incremental epinephrine autoinjector costs and coronavirus disease 2019 risks. Excluding epinephrine autoinjector costs, HISTA dominated other options. However, outside of pandemic considerations, HITSA was not cost-effective (incremental cost-effectiveness ratio, $198,877,286) at annual epinephrine autoinjector costs above $287. As the incremental HITSA SRIT rate increased above 15%, clinic AIT was the most cost-effective strategy. Excluding both pandemic risks and risk of motor vehicle accident fatality from round-trip clinic transit, clinic AIT dominated other strategies. Clinic AIT was the more cost-effective option at very high fatality relative risk for HITSA or at very low annual risk of contracting coronavirus disease 2019.
Conclusions
Under idealized assumptions HITSA can be a safe and cost-effective option during a global pandemic in appropriately selected patients provided home rates of SRIT remain stable.
Key words: SARS-CoV-2, COVID-19, Allergy, Allergic rhinitis, Allergy immunotherapy, Venom immunotherapy, Systemic reaction to immunotherapy, Quality-adjusted life-years, Simulation, Economic outcomes, Epinephrine, Epinephrine autoinjectors, Anaphylaxis, Cost-effectiveness analysis, Fatality
Abbreviations used: AIT, Allergen immunotherapy; AR, Allergic rhinitis; COVID-19, Coronavirus disease 2019; EAI, Epinephrine autoinjector; ED, Emergency department; HITSA, Home allergen immunotherapy self-administration; HOMVIT, Home venom immunotherapy; ICER, Incremental cost-effectiveness ratio; QALY, Quality-adjusted life-year; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; SCIT, Subcutaneous immunotherapy; SRIT, Systemic reactions to immunotherapy; VIT, Venom immunotherapy; WTP, Willingness to pay
What is already known on this topic: Allergy/immunology clinical practices are not immune to natural disasters or global pandemics, which may force service reduction or abrupt changes in practice.
What does this article add to our knowledge: Home allergen immunotherapy can be cost-effective in highly selected patients under pandemic shelter-in-place conditions provided home systemic reactions to immunotherapy rates remain stable; however, careful patient selection is critical.
How does this study impact current management guidelines: This cost-effectiveness analysis demonstrates potential feasibility of allergen immunotherapy for appropriately screened patients. This may help inform decision making regarding how to provide this valuable allergy service in a current or future natural disaster or pandemic.
Introduction
Because of the global coronavirus disease 2019 (COVID-19) pandemic, we must take drastic and unprecedented steps for acute service reduction to execute life-saving social distancing strategies.1 Given rapid community spread from both symptomatic and asymptomatic patients,2 , 3 the World Health Organization declared COVID-19, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a global pandemic on March 11, 2020, and the president of the United States declared a state of national emergency on March 13, 2020.4 , 5 Provincial, but not national, emergencies have been declared in Canada. A worldwide strategy of social distancing and/or quarantine has been implemented to attempt to limit the spread of SARS-CoV-2.6 , 7 This strategy includes closure of nonessential businesses and services, including postponement of nonessential medical care and procedures.3 , 8, 9, 10 Recently, the American Academy of Allergy, Asthma & Immunology, the American College of Allergy, Asthma, and Immunology, and the Canadian Society of Allergy and Clinical Immunology jointly endorsed recommendations for such considerations for allergy/immunology practices.1 Recommendations included limiting initiation of immunotherapy for inhalants, and strongly considering schedule modification or outright suspension of current treatment for established patients and restarting treatment at a future date. For venom immunotherapy (VIT), no service disruption, if at all possible, was recommended given the life-threatening nature of Hymenoptera allergy.1
Allergen immunotherapy (AIT) is a safe and effective disease-modifying treatment for allergic rhinoconjunctivitis and asthma.11 Because of the potential risk of inducing life-threatening anaphylaxis, it is recommended that immunotherapy injections should be administered under medical supervision with a 30-minute postinjection observation period.11 However, under exceptional circumstances, alternative care models should be considered. We evaluated a model in which patients could be considered for home immunotherapy self-administration (HITSA) if specific criteria are met. These specific criteria include patients who are advised of the risks and benefits and provide informed consent, do not have a history of previous systemic reactions to immunotherapy (SRIT), lack comorbidities complicating anaphylaxis severity and/or treatment, have high health literacy, and are appropriately educated on storage/handling/administration of AIT (and felt to be able to handle these responsibilities in the opinion of the prescribing clinician).1 , 11 In a landscape that requires ongoing contingency planning for potential long-term service adjustments (and disruption) to providing standard of care, there are limited data to better inform the range of health and economic consequences of offering HITSA to select patients during the COVID-19 pandemic. The cost-effectiveness of HITSA has not been previously explored. This analysis was undertaken to further characterize this approach to providing a valuable core allergy/immunology service when access to in-person encounters may not be feasible or possible, as well as a supplemental model to assess the feasibility of this approach for VIT.
Methods
Model structure
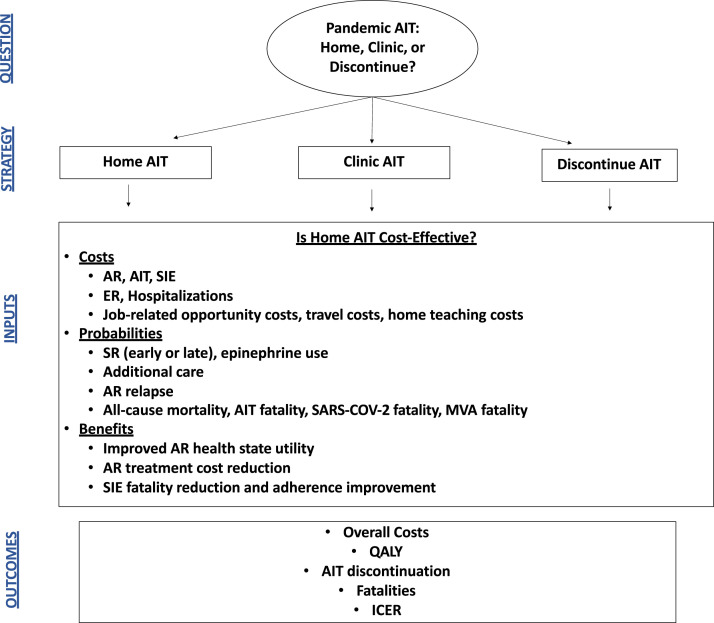
TreeAge Pro (Williamstown, Mass) was used to build a decision tree incorporating costs and probabilities that patients would experience with home immunotherapy during the 2020 pandemic. Markov cohort evaluations and microsimulations were used, because this model structure allows evaluation of health state transitions reflective of real-world outcomes. The base-case scenario was represented by a 30-year-old patient with allergic rhinitis (AR) who lives 5 miles from clinic and is transitioned to HITSA for a duration of 4 years of treatment. Additional base-case assumptions were that this patient has been receiving maintenance AIT in the office setting, has a negative history of previous SRIT, and demonstrates excellent adherence, strong contextual knowledge, and high health literacy. Cohorts of patients were randomized to 3 strategies: (1) clinic-administered AIT with 30-minute observation; (2) HITSA; and (3) provider-directed AIT discontinuation (Figure 1 ). Patients received 12 injections per year in the base case, with sensitivity exploring a higher number of injections allowing for variation in maintenance dosing. Because universal prescription of self-injectable epinephrine to all AIT patients has recently been shown to be a cost-ineffective strategy for in-clinic administration,12 patients receiving clinic-administered immunotherapy were not prescribed an epinephrine autoinjector (EAI). However, in this model, patients receiving HITSA were required to maintain 2 EAIs. All patients experiencing an SRIT were then subsequently transitioned to a required EAI strategy with clinic-administered AIT. A 50-year time horizon evaluated longer-term AIT benefits following course completion, with age-adjusted all-cause mortality evaluated in each strategy.13 Costs, probabilities, and utilities associated with AIT were derived from the literature, and strategies were evaluated from both the health care perspective and the societal perspective, which incorporated job-related and travel-related opportunity costs of observed AIT (Table I ).30 The societal perspective incorporated travel costs and patient time costs but did not include costs due to unrelated medical conditions, secondary caregiver time costs (if any), and non–health care sector costs. Incremental risks associated with compromising social distancing and contracting COVID-19 during immunotherapy injections were included in the model, and in the base case it was assumed that the COVID-19 risk would persist for the next 2 years. Cost-effectiveness was evaluated at a willingness-to-pay (WTP) threshold of $100,000/quality-adjusted life-year (QALY). Tracker modifications were used within microsimulations to evaluate episodes of anaphylaxis and immunotherapy fatalities in each strategy. The microsimulation (n = 10,000) discontinued AIT in patients with more than 2 systemic reactions, and patients receiving HITSA had a 25% reduction in patient-preference AIT discontinuation rates. Because patient-level data are not available for inputs, both the base-case cohort and microsimulation used point estimates for inputs described in Table I. The analysis followed the Consolidated Health Economic Evaluation Reporting Standards guidelines.31 This study does not involve human subjects and as such is not eligible for review per the Colorado Multiple Institutional Review Board.
Figure 1.
Model decision diagram. The base-case scenario was represented by a 30-year-old patient with AR who lives 5 miles from clinic, receiving maintenance AIT, with no history of previous systemic reaction, excellent adherence, contextual knowledge, and high health literacy transitioned to home AIT for 4 years. Cohorts of patients were randomized to 3 strategies: (1) clinic-administered AIT with 30-minute observation, (2) HITSA, and (3) AIT discontinuation. ER, Emergency room; MVA, motor vehicle accident; SIE, self-injectable epinephrine; SR, systemic reaction.
Table I.
Model inputs
| Costs (2020 dollars) | Value | Range | Reference |
|---|---|---|---|
| AR (annual cost) | $723 | $200-$2,500 | Allen-Ramey et al,14 2017 |
| AIT (annual cost) | $524 | $200-$1,200 | Hankin et al,15 2013 |
| Annual health care cost reduction from AIT | 37.6% | 10%-50% | Hankin et al,15 2013 |
| Self-injectable epinephrine twinpack | $340 | $100-$700 | GoodRx,16 2020 |
| Emergency room visit | $1,554 | $1,000-$5,000 | Clark et al,17 2014 |
| Ambulance transport | $854 | $500-$3,000 | Shaker et al,18 2020 |
| Hospitalization (per episode) | $7,593 | $5,000-$20,000 | Clark et al,17 2014 |
| Average hourly wage | $28.60 | $0-$100 | US Department of Labor, Bureau of Labor Statistics,19 2019 |
| Automobile fuel price and economy | $2.75 per gallon, regular 23 miles per gallon |
$1.20-$3.50 per gallon (18-39 mpg) | Irving Gas20 Best and worst gas mileage 201821 |
| Travel distance (round-trip) | 10 miles | 1-50 miles | Model assumption |
| Home teaching and additional home costs | $524 | $0-$1,500 | Model assumption |
| Probabilities | Value | Range | Reference |
|---|---|---|---|
| Additional care inclusive of ED care for SRIT in clinic | 58% | 5%-60% | Phillips et al,22 2011 |
| Additional care inclusive of ED care for SRIT at home | 100% | — | Model assumption |
| Hospitalization | 22% | 2%-30% | Clark et al,17 2014 |
| SRIT | 4% | 0.6%-34% | Epstein et al,23 2019 Cox et al,11 2011 Phillips et al,22 2011 |
| Proportion of early SRIT | 85% | 50%-90% | Epstein et al,23 2019 Cox et al,11 2011 |
| AIT fatality per injection | 1.3 × 10−7 | 1.3 × 10−8 to 1.3 × 10−6 | Epstein et al,23 2019 |
| Automobile fatality rate | 1.18 per 100 million vehicle miles traveled | 0.118-1.18 per 100 million vehicle miles traveled | Traffic safety facts 2016 data24 |
| AIT discontinuation (cumulative) | 45% | 6%-84% | Cox et al,25 2014 Hsu and Reisacher,26 2012 |
| Injection visits per year | 12 | 12-52 | Model assumption |
| Travel, clinic check-in, administration, and observation time | 60 min | 30-90 min | Model assumption |
| Epinephrine for SR in the first 30 min | 85% | 71%-94% | Epstein et al,23 2019 |
| Epinephrine for SR after 30 min | 15% | 8%-100% | Epstein et al,23 2019 |
| Relapse of AR after AIT | 23.4% | 0%-55% | Lee et al,27 2018 Cox et al,11 2011 |
| Probability of SARS-CoV-2 infection from allergy clinic AIT administration (annual) | 5% | 0%-90% | Model assumption |
| COVID-19 hospitalization | 12% | 1%-30% | CDC COVID-19 Response Team28 |
| COVID-19 fatality | 0.5% | 0.1%-10% | CDC COVID-19 Response Team28 |
| Utilities, Mortality, and Other Assumptions | Value | Range | Reference |
|---|---|---|---|
| Utility AR on AIT | 0.880 | 0.870-1 | Retzler et al,29 2018 |
| Utility AR | 0.864 | 0.635-0.865 | Retzler et al,29 2018 |
| US mortality table | Age-specific | — | Arias and Xu,13 2018 |
| Home AIT fatality risk increase | 10-fold increase | 5-fold to 1,000-fold increase | Estimate |
| Immediate epinephrine risk reduction | 10-fold decrease | 5-fold to 1,000-fold decrease | Estimate |
| Start age | 30 y | 10-60 y | Model assumption |
| Time horizon | 50 y | 5-70 y | Model assumption |
| Home adherence benefit | 0 | 0%-25% reduction in discontinuation | Model assumption |
SR, systemic reaction.
Costs
Costs were reported in 2020 dollars using the Consumer Price Index Inflation Calculator through the Bureau of Labor Statistics, with a 3% annual discount rate applied.19 Annual AIT costs were estimated at $524 on the basis of a retrospective analysis of Florida Medicaid claims by Hankin et al,15 which demonstrated that AIT reduced total health care costs for AR by 37.6%. Annual AR costs were based on a retrospective analysis of medical and pharmacy claims data by Allen-Ramey et al.14 Cost of home epinephrine was applied using the lowest available US retail price at www.GoodRx.com for a single package of 2 EAI devices at the 0.3-mg dose ($340).16
Clark et al17 reported health care utilization for patients evaluated in the emergency department (ED) or hospitalized for all-cause anaphylaxis using the Thomson Reuters MarketScan Commercial and Medicare Supplemental Database. In this study, 22% of ED patients were admitted and the authors used an ED anaphylaxis cost of $1157 ($1554 in 2020 dollars) and hospitalization cost of $5653 ($7593 in 2020 dollars). Costs of ambulance transport were based on Shaker et al,18 estimated at $854.
The societal perspective analysis included travel costs based on average automobile fuel price and economy,20 , 21 and job-related opportunity costs reflected by average hourly wage, estimated clinic round-trip transit time, time spent receiving injections, and the 30-minute postinjection observation time.19 Because teaching and adherence are crucial to a successful HITSA program, the base case set costs of these activities equivalent to costs of AIT, with sensitivity analyses exploring the impact of broader cost assumptions of this variable.
Costs of COVID-19 hospitalization were assumed to approximate costs of hospitalization for anaphylaxis; however, because these costs may be much greater (and these data are new, emerging, and not readily available), sensitivity analyses explored ranges 20-fold above this value ($151,860 per COVID-19 hospitalization). Additional costs of testing and treatment were not individually modeled but assumed not to exceed the $151,860 cost evaluated in the sensitivity analysis.
Probabilities and events
All patients experiencing an SRIT received epinephrine, with all HITSA patients transported by ambulance for ED evaluation and management. Patients with a grade 2 to 4 SRIT in clinic received ambulance transport and ED evaluation. Phillips et al22 reported that 4% of 773 patients receiving subcutaneous immunotherapy (SCIT) experienced an SRIT, with 58% of reactions assessed as grade 2 to 4 severity. Anaphylaxis hospitalization rates from Clark et al17 were applied to patients evaluated in the ED for SRIT.
In a survey of practicing allergists through the American Academy of Allergy, Asthma & Immunology/American College of Allergy, Asthma and Immunology, Epstein et al23 reported that 85% of SRITs occurred within 30 minutes postinjection. The model assumed that all SRITs would be promptly treated with EAI either at home or in clinic; however, sensitivity analyses explored reduced rates of home EAI administration. Epstein et al23 reported 6 to 7 fatalities from 2008 to 2016 among 54.4 million injections (modeled AIT fatality rate per injection, 1.3 × 10−7). Automobile fatality rates were incorporated on the basis of a rate of 1.18 fatalities per 100 million vehicle miles traveled.24
Estimating AIT adherence is a challenge.32 A review of SCIT adherence by Cox et al25 reported that rates may range from 6% to 84%. In a retrospective evaluation of SCIT adherence, Hsu et al26 reported a 45% attrition rate over a 4-year period. As the base case assumed maintenance immunotherapy, 12 injections were received annually but sensitivity analyses evaluated a broader range of injections in exploratory analyses. Given the 30-minute observation period in addition to parking, clinic check-in, and injection administration, the base case assumed 20 minutes of travel time with 40 minutes of clinic time and a sensitivity analysis incorporated a range from 40 to 90 minutes (travel and clinic time).
Despite the safety and efficacy of SCIT, patients may experience persistence of symptoms after a course is completed. Symptom relapse rates were extrapolated from patients failing to achieve remission, based on a retrospective review of patients who completed house-dust mite AIT by Lee et al,27 who reported persistent symptoms in 23.4% of patients after a 5-year AIT course.
The model also incorporated the current reality of the COVID-19 pandemic causing widespread SARS-CoV-2 infection, applied for the first 2 years of the simulation to patients receiving AIT or emergency care.3 Although the total duration of the COVID-19 pandemic is unknown, a 24-month time frame was chosen to represent a plausible estimate that a safe and effective vaccine will likely not be available for at least 12 to 18 months.33 Population estimates of SARS-CoV-2 infection vary, and rates may exceed 50% to 60% in some regions and countries34 , 35; furthermore, the annual probability of contracting SARS-CoV-2 attributable to visiting an allergy clinic for AIT is unknown. Given this uncertainty, the base case assumed an annual 5% COVID-19 risk attributable to repeated immunotherapy visits during the pandemic, with a wide sensitivity ranging from 0% to 90%. Rates of COVID-19 hospitalization and fatality were based on morbidity and mortality statistics reported by the Centers for Disease Control and Prevention for the United States between February 12 and March 16, 2020, where fatality risk was greatest in persons 85 years or older (10%-27%), followed by persons 65 to 84 years old (3%-11%), and with fatality rates of 1% to 3% in individuals aged 55 to 64 years and less than 1% in persons 20 to 54 years old.28 The model assumed a 0.5% hospitalization fatality rate of COVID-19 in the base case. In this same report, 12% of individuals testing positive for COVID-19 (508 of 4226) were hospitalized.28
Health state utilities
In the base case, patients began the simulation with moderate AR, which improved to mild AR with the use of AIT (see Figure E1 in this article's Online Repository at www.jaci-inpractice.org). The baseline health state utilities of moderate and mild AR were 0.864 and 0.880, respectively, based on a study by Retzler et al,29 who used health state descriptions and the axiomatically robust standard gamble approach for adults to investigate the impact of AR. Health state utilities were multiplied by life-years in each health state to derive QALYs.36 QALY can be translated to represent a perfect year of health relative to the condition of interest.
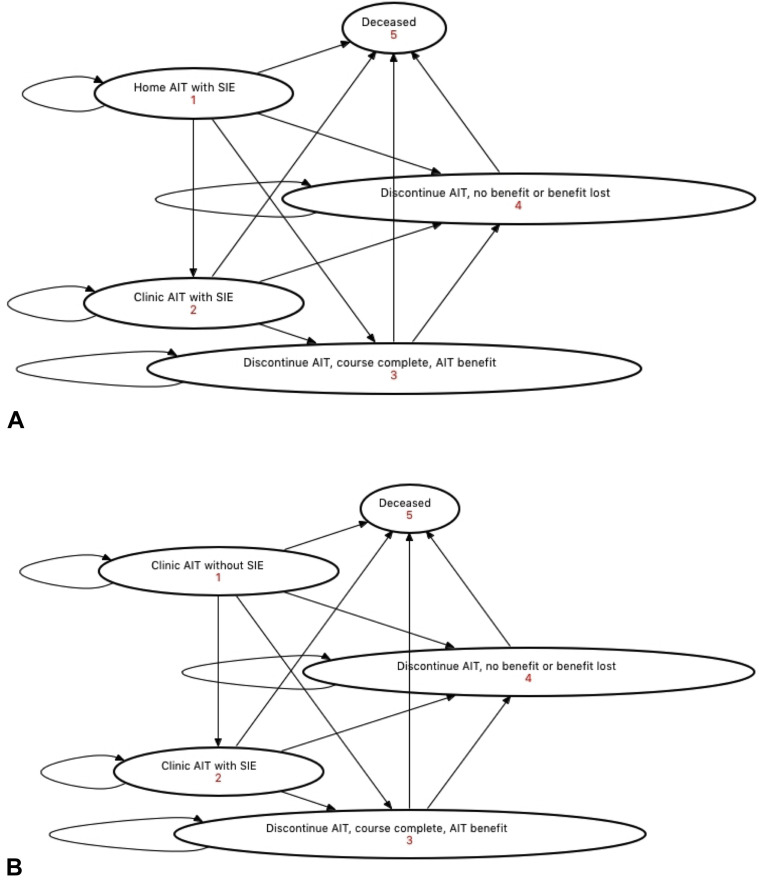
Figure E1.
Health state transition diagrams. Transitions within the HITSA cohort are shown for (A) HITSA and (B) clinic AIT. SIE, Self-injectable epinephrine.
Sensitivity analyses
Practice variation exists within AIT administration, and as such models evaluated ranges in AIT discontinuation.25 , 26 Patients in cohort evaluations continued AIT regardless of the number of previous SRITs; however, patients in the microsimulations discontinued AIT if they experienced 2 SRITs. Because the face-to-face requirement may variably create a barrier to AIT, the cohort simulation did not assume in-clinic administration had any impact on adherence or discontinuation rate, but the microsimulation accounted for less attrition in patients receiving HITSA by assuming a 25% reduction in patient-preference AIT discontinuation rates. Sensitivity analyses evaluated a wide range of epinephrine costs and fatality risk reduction associated with clinic-observed AIT with deterministic sensitivity analyses performed on all variables. Clinic-administered fatality reduction was based on previous cost-effectiveness analyses using similar methodology and included an upper limit assumption of 1000× fatality reduction associated with clinic-observed administration.37, 38, 39, 40, 41 An additional sensitivity analysis explored a 2-fold differential rate of HITSA SRIT due to dosing errors. Probability sensitivity analyses were performed incorporating stochastic effects of variable selection across sensitivity ranges using triangular modal distributions around the base-case value.
Additional probability sensitivity analysis was performed using alternate number seeding and alternative gamma distribution parameters for costs, with beta distributions for probabilities of hospitalization and SRIT. For alternative modeling because patient-level data were not available, gamma distributions assumed SD equivalent to one-half mean values and probability of hospitalization modeled by setting alpha equivalent to the number of events (r) and beta corresponding to the population at risk not experiencing hospitalization (n − r). The probability of SRIT was modeled using a mean annual event rate of 4% with SD set to 0.5%.
Supplemental model
A supplemental model was evaluated for home VIT (HOMVIT) versus clinic VIT during the pandemic. For this analysis, immunotherapy costs were assumed to be equivalent for home and clinic administration and the base-case patient receiving HOMVIT was assumed to be in the first year of a maintenance program of immunotherapy (12 injections per year in the base case with sensitivity analysis to 4 injections per year) adherent to a 5-year VIT course providing persistent benefit. Ongoing VIT was assumed to be completely protective from sting anaphylaxis, and the risk for sting anaphylaxis from discontinued immunotherapy assumed to be 50%. Anaphylaxis fatality risk (modeled at 0.29%) was based on a population-based epidemiologic study of 3 national databases published by Ma et al,42 who reported mortality rates of 0.25% to 0.33% for patients hospitalized for anaphylaxis or evaluated in the ED for this diagnosis. Annual sting risk was assumed to be 5%.43 Health state utility of venom allergy (untreated because of discontinuation of VIT) was assumed to be 0.91 on the basis of standard gamble assessment of severe allergic reaction utility values from Finnell et al.44 No differential epinephrine cost was assumed because all patients maintained EAIs.
Results
Cohort analysis, societal perspective
Compared with a baseline strategy of clinic AIT, HITSA was cost-effective in the base case ($44,554/QALY) with both incremental EAI costs and COVID-19 risks included. The cost of HITSA was $16,464, clinic AIT was $16,394, and AIT discontinuation was $18,332, with corresponding effectiveness of each strategy being 22.1077, 22.1061, and 21.9077 (Table II ). Excluding EAI costs, HISTA dominated (eg, lowest cost, highest degree of benefit) other options, costing $15,210 compared with clinic costs of $16,283.
Table II.
Cost-effectiveness of pandemic home AIT from a societal perspective
| Strategy | Cost ($) | Effectiveness (QALY) | CE | ICER ($/QALY) | NMB ($) |
|---|---|---|---|---|---|
| Clinic AIT | $16,394 | 22.1061 | $742 | — | $2,194,214 |
| Home AIT | $16,464 | 22.1077 | $745 | $44,554 | $2,194,302 |
| Discontinue AIT | $18,332 | 21.9077 | $837 | Dominated | $2,172,434 |
| Microsimulation∗ | Cost ($)† | Effectiveness (QALY)† | CE | ICER ($/QALY) | NMB ($)† | Fatality | AIT Early discontinuation† |
|---|---|---|---|---|---|---|---|
| Clinic AIT | $16,380 ± $4,909 | 22.0913 ± 3.8201 | $741 | — | $2,192,752 ± $379,798 | 0 | 47.0% ± 50.0% |
| Home AIT | $15,934 ± $4,915 | 22.1503 ± 3.8101 | $719 | Dominant | $2,199,093 ± $379,043 | 0 | 38.6% ± 48.7% |
| Discontinue AIT | $18,354 ± $3,165 | 21.9335 ± 3.7821 | $837 | Dominated | $2,174,996 ± $375,048 |
CE, Cost-effectiveness; NMB, net monetary benefit.
The microsimulation (n = 10,000) discontinued AIT in patients with more than 2 systemic reactions, and patients receiving home AIT had a 25% reduction in patient-preference AIT discontinuation rates.
Values presented as mean ± SD.
Excluding pandemic considerations, when differential EAI costs were considered, HITSA was not cost-effective (incremental cost-effectiveness ratio [ICER], $198,877,286) unless annual EAI costs fell below $287. At base-case EAI costs without pandemic risks, HITSA cost $16,455 and clinic AIT $16,277, producing 22.107785 and 22.107784 QALYs, respectively. Excluding both pandemic risks and risk of motor vehicle accident fatality from round-trip clinic transit, clinic AIT dominated other strategies.
Cohort analysis, health care perspective
When job-related costs were excluded, the ICER of HITSA was $774,125, with HITSA costing $16,352 and clinic AIT costing $15,128. HITSA was not cost-effective from this perspective unless annual EAI cost fell below $21. Excluding pandemic risks, the ICER of HITSA was $1,486,751,997 when job-related costs were excluded, and without job-related costs or pandemic risks considered, HITSA was not cost-effective when EAI costs were excluded (ICER $210,889,379).
Microsimulation, societal perspective
HITSA was the dominant strategy in microsimulation (n = 10,000) from the societal perspective, in which HITSA was modeled with an adherence benefit over clinic AIT, with HITSA costs of $15,934 ± $4,915, clinic AIT costs of $16,380 ± $4,909, and costs of discontinuation of $18,354 ± $3,165. Associated effectiveness of each strategy was 22.1503 ± 3.8101 QALY for HITSA, 22.0913 ± 3.8201 QALY for clinic, and 21.9335 ± 3.7821 QALY for discontinuation. Although no fatalities occurred in the societal microsimulation, 38.6% ± 48.7% of HITSA patients discontinued AIT compared with 47.0% ± 50.0% of clinic AIT patients.
Microsimulation, health care perspective
In the health care perspective microsimulation, HITSA was cost-effective (ICER, $10,358). HITSA cost $15,863 ± $4,855, clinic AIT cost $15,085 ± $5,182, and discontinuation cost $18,332 ± $3,174. Associated effectiveness of each strategy was 22.1407 ± 3.8118 QALY (HITSA), 22.0657 ± 3.8563 QALY (clinic AIT), and 21.9074 ± 3.7935 QALY (AIT discontinuation).
Deterministic sensitivity analyses
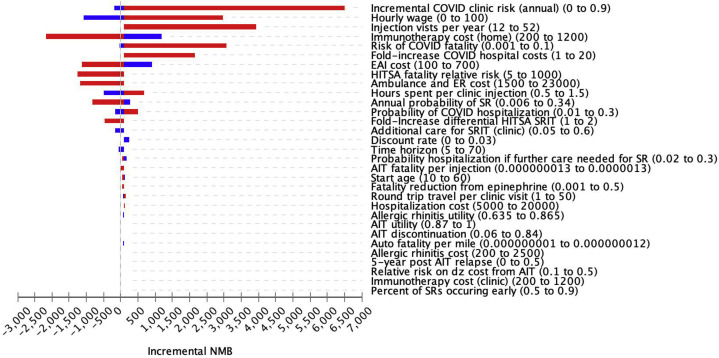
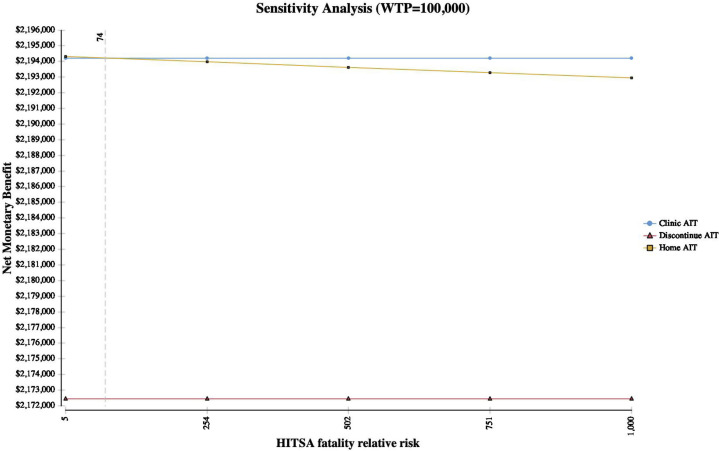
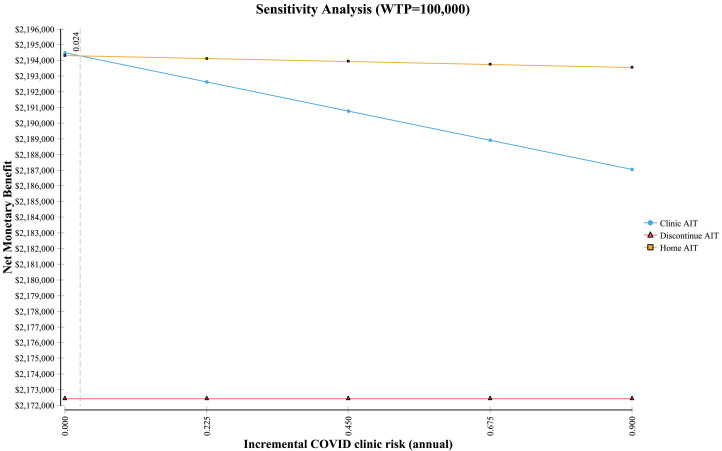
HITSA was a cost-effective consideration across multiple parameters in the sensitivity analysis, with greatest incremental net monetary benefit (a composite measure representing the value of a QALY at the WTP threshold of $100,000/QALY) within sensitivity ranges evaluated for multiple variables including annual COVID-19 risk, hourly wage, HITSA costs, EAI costs, home fatality relative risks, ambulance and ED costs, time spent receiving injections, risk of SRIT and additional care probability, and time horizon (Figure 2 , Tornado plot). As COVID-19–associated costs increased, HITSA became more preferred. However, at a 15% increased HITSA SRIT rate, clinic AIT was the most cost-effective strategy. In addition, at equivalent SRIT rates for HITSA and clinic AIT, when the HITSA fatality relative risk was increased 74-fold, clinic AIT was associated with the greatest net monetary benefit (WTP, $100,000/QALY) (Figure 3 ). Clinic AIT was also the preferred strategy when the annual risk of contracting COVID-19 in the allergy clinic fell below 2.4% (see Figure E2 in this article's Online Repository at www.jaci-inpractice.org).
Figure 2.
Tornado diagram of 1-way sensitivity analyses comparing strategies of HITSA vs clinic AIT during the COVID-19 pandemic. Clinic AIT is the reference scenario. Blue bars represent values below and red bars those above base-case assumptions. Values in parentheses represent range of changes explored. Changes associated with positive gain in NMB are cost-effective at a WTP of $100,000/QALY. Dz, Disease; ER, emergency room; NMB, net monetary benefit; SR, systemic reaction.
Figure 3.
Sensitivity analysis of HITSA fatality risk increase during the COVID-19 pandemic. Dotted line represents the threshold at which the net monetary benefit of clinic AIT exceeds HITSA. Note: Axes do not start at 0.
Figure E2.
Sensitivity analysis of incremental risk of COVID-19 from visiting allergy clinic for immunotherapy. Dotted line represents the threshold at which the net monetary benefit of HITSA exceed clinic AIT. Note: Axes do not start at 0.
Probabilistic sensitivity analyses
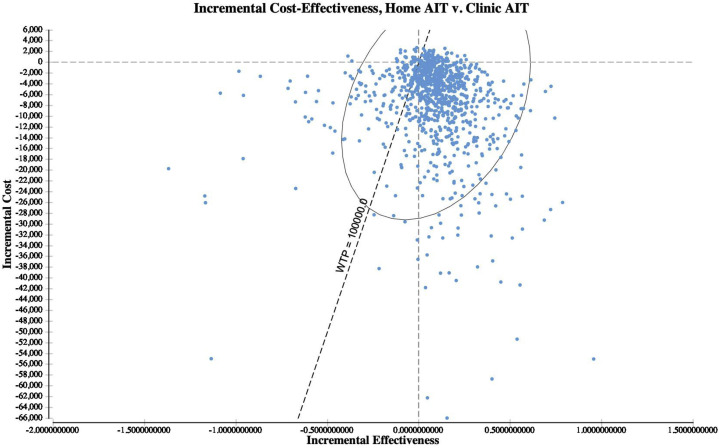
From a societal perspective, HITSA was the most cost-effective strategy (WTP $100,000) in 83.7% of probabilistic simulations across variable distributions (n = 10,000) (Figure 4 ; see Figure E3 in this article's Online Repository at www.jaci-inpractice.org). Holding HITSA and clinic AIT SRIT rates constant, HITSA was the optimal strategy in 90.2% of simulations. Considering the health care perspective, HITSA was the preferred option in 74.3% of simulations. Using alternative distributions, HITSA was preferred in 84.7% (societal) and 81.3% (health care perspective) of simulations. In analyses of alternate random number seeding, HITSA was the preferred strategy in 84.1% of simulations from the societal perspective and 80.6% of simulations from the health care perspective.
Figure 4.
Incremental cost-effectiveness scatter plot (probability sensitivity analysis). From a societal perspective, HITSA was the most cost-effective strategy (WTP, $100,000) in 83.7% of probabilistic simulations across variable distributions (n = 10,000).
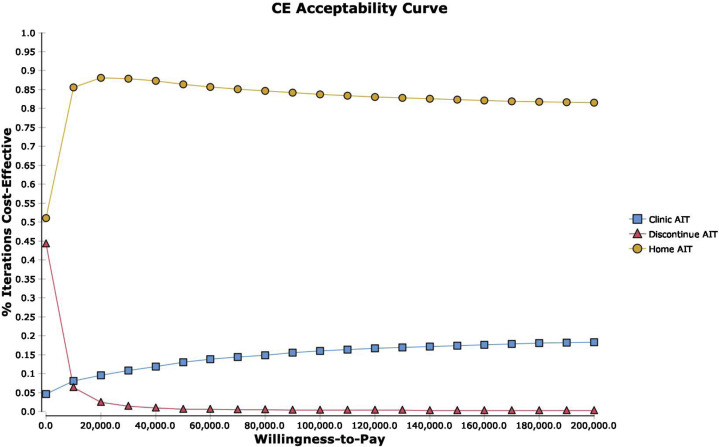
Figure E3.
CE curve. Strategies are compared across WTP thresholds to evaluate the percent of probability sensitivity analysis iterations that are most cost-effective. CE, Cost-effectiveness.
Supplemental VIT model
In a VIT model, HOMVIT was also cost-effective. In the cohort analysis from a societal perspective, both clinic VIT and HOMVIT were more effective than VIT discontinuation (HOMVIT 25.3557, clinic VIT 25.3538, VIT discontinuation 23.0417). The incremental cost of clinic VIT was $1485, and HOMVIT remained cost-effective unless the differential rate of SRIT was quadrupled. HOMVIT remained preferred unless HOMVIT-related fatality risk exceeded 800-fold, differing from the AIT analysis because EAI costs were held equal between groups in the supplemental VIT analysis. As with HITSA, as the differential rate of HOMVIT SRIT increased over clinic rates, probability sensitivity analysis simulations demonstrated a less pronounced distinction between location of VIT.
Discussion
AIT is a defining feature of an allergy/immunology practice, but in the setting of a pandemic this therapy is likely subject to service disruptions and potential reprioritization of health care service utilization. To help preserve ongoing consistency with AIT and VIT services for allergy/immunology patients under such unusual and unprecedented circumstances, present models of care must be reexamined to test assumptions about safety and efficacy of certain core services, aiming to continue delivery of care, creatively. HITSA and HOMVIT for highly selected patients in maintenance therapy are 2 such measures to consider.
Under COVID-19 pandemic conditions, in the carefully selected patient, HITSA and HOMVIT can be cost-effective options. Both HITSA and HOMVIT could be considered as a preference-sensitive decision for highly selected patients meeting the criteria laid forth in the base case, provided clinic infrastructure is established to support this method of care delivery. However, there are practical issues that may limit immediate implementation relating to patient education, adherence, and infrastructure. In addition, patients would need close monitoring and if home SRIT rates increase HITSA would need to be reconsidered. In the decision model evaluated, patients experiencing HITSA SRIT were transitioned back to a clinic AIT strategy. This analysis may provide clarity in considering these options as a way to continue immunotherapy during this pandemic; however, many clinics may not have necessary protocols in place to support a home administration option urgently.
Although HITSA was not cost-effective when analyzed from the contextually specific health care perspective using a cohort approach except at very low EAI device price, this perspective is narrow and excludes other common societal costs not directly related to the medical service. Alternative modeling in this context using microsimulation did denote possible cost-effectiveness, with the microsimulation accounting for the potential of improved adherence with HITSA. HOMVIT was robustly cost-effective, given that a key difference was no additional EAI unit cost in the model versus in-clinic administration.
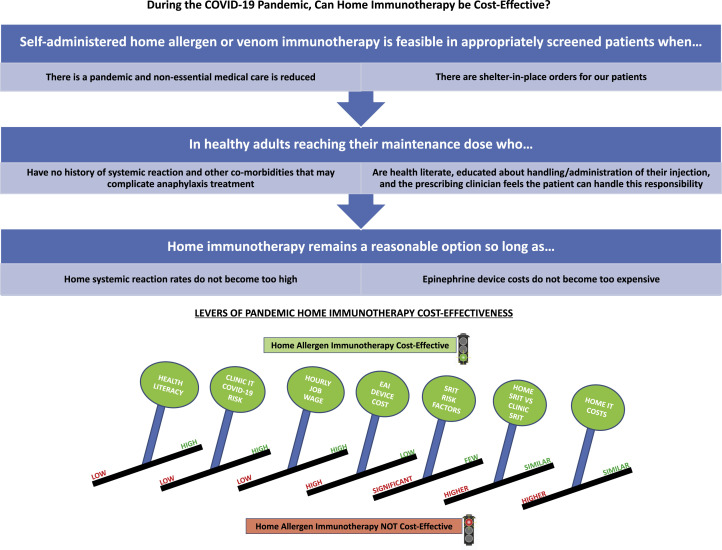
The HITSA cost-effectiveness is dependent on a very narrow set of assumptions and is sensitive to multiple levers (Figure 5 ). Although the risk of contracting a pandemic infection is a significant lever, it is of note that HITSA would also be cost-effective outside of a pandemic as long as EAI device price is below $287. HITSA is highly sensitive to higher wage earners, where home administration mitigates the opportunity cost of time away from their job to receive AIT, and to keeping the cost to administer HITSA low, given a threshold at which in-office administration is favored. There are also a defined range of safety-related concerns contributing to HITSA's cost-effectiveness. HITSA remains cost-effective versus in-office administration as long as SRIT fatality risks do not exceed 74-fold greater than the in-clinic risk; however, at a 15% increased HITSA SRIT rate, clinic AIT is the most cost-effective option. A key lever is EAI device price, which is unrelated to AIT itself. In the nonpandemic societal perspective, the pandemic health care perspective models, and in 1-way sensitivity analysis, the viability of HITSA cost-effectiveness hinges on EAI device acquisition price being far below current market value (which was previously shown to have a value-based ceiling price of $24).41 Herein lies a key difference between administration settings—although for routine office AIT, universal EAI prescription in someone without a history of SRIT is not cost-effective,12 in HITSA universal EAI prescription would be necessary. This reinforces continued negative effects from exorbitant EAI device pricing,45 and is unequivocally a lever that could be changed through advocacy or payer pressure on device manufacturers, which may potentially lower health care costs and increase access to a service. The HOMVIT model is not affected by device price given all VIT patients have an EAI.
Figure 5.
Summary overview of key findings. Summary diagram of the key assumptions and constraints in analyzing the cost-effectiveness of home AIT and VIT.
HITSA and HOMVIT may raise the question of “just because you can, doesn't mean you should.” During a pandemic, HITSA and HOMVIT (even for highly selected patients) would both be a fundamental evolution, which may alter current AIT revenue streams and pose a significant barrier to implementation. Revenue issues, sustainability and penetration of service, and physician acceptability may all require some adjustment. There are also concerns regarding safety of administration and how to identify the optimal patient for HITSA/HOMVIT. Immunotherapy would still be mixed and administered by the office staff in the first year, and mixed but just not clinic-administered thereafter. This could be countered by an increase in patients who may opt for AIT with a HITSA option available. HOMVIT may help increase VIT adherence though the rational for starting VIT is distinctly different from that for starting AIT. This safety issue must also be addressed. HITSA and HOMVIT are modeled only as maintenance therapy, in an adherent and well-screened population without a history of SRIT, and with good contextual knowledge, high health literacy, and ability/willingness to rapidly self-administer EAI (1 or 2 doses) if an SRIT occurs. There would have to be a screening tool developed to help identify patients who are ready for this transition, a patient decision-support tool for considering this option, and some increase in nursing education for patients during the buildup phase to improve familiarity with the injection process, thereby reducing dosing and administration errors. Visiting and telemedicine nursing services could be considered, though this would have to stay within a certain price for the therapies to remain cost-effective and would depend on local social distancing strategies in place.
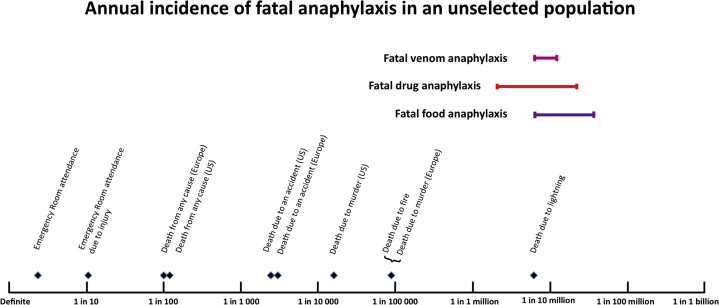
AIT and VIT are safe therapies with overall low rates of SRIT. All patients on the HITSA and HOMVIT therapies would have an EAI device and training in their use. Models could also be explored where the maintenance dose is drawn up at the office and mailed to a patient, to reduce dosing errors. However, we can use examples of several models of homecare where medications of similar potency and potential for significant adverse events are routinely given at home, some daily. A notable example of this is insulin, which is given multiple times a day, and has life-threatening potential of harm similar to any form of AIT if given incorrectly. Patients with diabetes are given intensive education to support safe and proper administration at home as routine practice. Provided education is appropriate, there is every reason to think that 12 maintenance injections could be handled as such in a pandemic setting, in select patients. To contextualize risk, the estimated rate of AIT fatality per injection, 1.3 × 10−7, is on par with the estimated risks of fatal penicillin anaphylaxis (8.0 × 10−8),46 per-patient omalizumab fatality (5.8 × 10−6),42 , 47 , 48 and the general population risk of home-peanut introduction in US infants (0.3-3 × 10−7)49 (see Figure E4 in this article's Online Repository at www.jaci-inpractice.org). Penicillin is administered without mandated routine medical observation in the general population, omalizumab is approved for preference-sensitive home administration after the first 4 uneventful doses in Europe at provider discretion for patients with appropriate indication, and young infants routinely have peanut introduced at home.46 , 50, 51, 52
Figure E4.
Estimated rates of fatal venom, drug, and food anaphylaxis in the general population compared with other risks. Reproduced from Turner et al.E1
Most importantly, this model is formulated as a direct response to the COVID-19 pandemic that is causing service disruption and forced changes in health care service utilization and provides a promising approach to preserve as much normal service as possible in select patients. It is an option to consider, and not formally endorsed by any professional societies or national guidelines at this time. Although these cost-effectiveness models for HITSA and HOMVIT are not designed to provide a practical “how to” guideline on how to implement this scenario, the discussion herein can all be considered if this model is adopted. Regardless of the acceptability of this approach by allergists and patients, the standard manner of delivering AIT to patients in the setting of global pandemic must be significantly altered. At the very least, patients receiving AIT or VIT should be contacted to discuss the ongoing infectious risks associated with continued in-office AIT/VIT visits, and office steps to mitigate such risks. Allergists should also initiate discussion regarding interruption in AIT dosing schedules, including the potential for symptoms to worsen as well as alterations to the AIT schedule if/when injections are resumed, which may necessitate more frequent visits to build back up to the dosing at the time of interruption and additional indirect costs associated with time lost from work, co-payments, and so forth. At the time of this writing, several states and municipalities have mandated interruptions in outpatient office visits, which are expected to increase across North America as the COVID-19 pandemic broadens in scope. Whether allergists agree with or are prepared for these realities, they may be forced into a situation in which face-to-face office visits must cease, at least temporarily. Preparation and consideration of these elements can assist planning should drastic measures be necessary.
The analysis has some limitations. Foremost, the model is deliberately very narrow as to the type of patient entered into the simulation—younger and no history of SRIT. Asthma severity and other comorbidities were deliberately excluded, and we created a fairly ideal patient type for parsimony in this proof-of-concept model. This limits generalizability to a number of other scenarios, but this model is meant to introduce the feasibility and cost-effectiveness of the concept, and additional models are required to broaden its application. Second, the model is dependent on having an adherent, health-literate, and contextually aware patient. There will need to be a significant upgrade to nursing support to educate patients, as well as creation of decision-support and other tools to help identify ideal patients (including screening for health literacy), but this can be achieved. However, although our model cannot precisely quantify the importance and potential difficulty in selecting such patients, this is critical. Before adopting HITSA/HOMVIT, it is crucial to develop home protocols to ensure proper administration, appropriate observation, and clear understanding of necessary activity restrictions—a situation not dissimilar to that for oral immunotherapy. Moreover, patients would need to have access to emergency medical services in the event of an SRIT, and an understanding of when these services should be activated. This may pose limitation in some more rural settings. Third, as the potential for a higher rate of incremental discontinuation in the HITSA model increases, the strategy becomes less cost-effective (and it is possible adherence may decrease with home AIT, though this will have to be monitored closely to better understand how this affects adherence). Fourth, this model does not account for clinician or patient acceptability. Fifth, the most appropriate scenario for deployment of these models (if systems are in place to support it) is during a pandemic, such as COVID-19, where shelter-in-place orders are in effect, social distancing/quarantining is mandated, and nonessential face-to-face health care services have been reduced (eg, “red zone conditions”). That said, with EAI device costs less than $287, HITSA could be cost-effective outside such conditions, and the models are exceptionally sensitive to EAI price and potential supply chain disruptions, a sixth key limitation. Seventh, this model only pertains to maintenance AIT and VIT, but the assumptions and ranges of sensitivity are broad enough and simulated out to weekly injections that could potentially serve as a model for this in certain phases of buildup, though that may require additional modeling. Eighth, the base assumptions are sensitive to current (and low) rates of SRIT that are equivalent between home and clinic administration, but HITSA remains the more cost-effective option when fatality associated from SRIT does not exceed 74-fold that of the in-office rate, and HOMVIT until this exceeds 800-fold. Ninth, we did not evaluate downstream consequences of clinic AIT during the pandemic including increased risk of secondary and tertiary community spread of SARS-CoV-2 from asymptomatic carriers receiving clinic AIT; however, this aspect of compromised social distancing would only serve to accentuate the cost-effectiveness of HITSA.
In the normal setting, HITSA and HOMVIT models may not be preferred practice. However, in the midst of an unimaginable global pandemic, where major service disruptions are occurring and likely to continue, rethinking how health care can be delivered provides the opportunity to preserve ongoing patient care. HITSA and HOMVIT, under the right assumptions and used in the right patients, can be done safely, providing a valuable service option for our patients. In the setting of the current pandemic, this is an avenue that some clinicians may choose to discuss with selected patients, and this analysis serves as a catalyst for possible shared decision making in the appropriate context. Although additional modeling is required to more robustly explore the sensitivity and generalizability of other patient groups and scenarios, creative thinking and a willingness to adapt may prove to be a most valuable resource that the field can mobilize to persevere under less than ideal circumstances. Clear, concise, and evidence-informed risk communication is essential, especially in the time of a global pandemic. This study provides a framework through which physicians can consider relative risks and benefits contextually of a novel approach to preserve patient care while maintaining safety in unprecedented times.
Footnotes
M.G. is supported by the Agency for Healthcare Research and Quality (grant no. 5K08HS024599-02).
Conflicts of interest: M. S. Shaker is a member of the Joint Taskforce on Allergy Practice Parameters; has a family member who is CEO of Altrix Medical; and serves on the Editorial Board of Journal of Food Allergy and Annals of Allergy, Asthma, and Immunology. G. Mosnaim received research grant support from AstraZeneca (AZ) and GlaxoSmithKline (GSK) and currently receives research grant support from Propeller Health; owned stock in Electrocore; and served as a consultant and/or member of a scientific advisory board for GSK, Sanofi-Regeneron, Teva, Novartis, AZ, Boehringer Ingelheim, and Propeller Health. J. Oppenheimer has been involved in research/adjudication for AZ, GSK, Sanofi, and Novartis; has been a consultant for GSK, AZ, and Sanofi; is associate editor of Annals of Allergy, Asthma, and Immunology, AllergyWatch; section editor of Current Opinion of Allergy; has received royalties from Up to Date; is board liaison American Board of Internal Medicine for American Board of Allergy and Immunology; and is a member of the Joint Taskforce on Allergy Practice Parameters. D. Stukus is a consultant for DBV Therapeutics, Before Brands, and Abbott Nutrition. E. M. Abrams is on the National Advisory Board for Food Allergy Canada and is on the National Food Allergy Action Plan Action Steering Team for Food Allergy Canada. M. Greenhawt is supported by the Agency for Healthcare Research and Quality (grant no. 5K08HS024599-02); is an expert panel and coordinating committee member of the National Institute of Allergy and Infectious Diseases–sponsored Guidelines for Peanut Allergy Prevention; has served as a consultant for the Canadian Transportation Agency, Thermo Fisher, Intrommune, and Aimmune Therapeutics; is a member of physician/medical advisory boards for Aimmune Therapeutics, DBV Technologies, Sanofi/Genzyme, Genentech, Nutricia, Kaleo Pharmaceutical, Nestle, Acquestive, Allergy Therapeutics, Allergenis, Aravax, and Monsanto; is a member of the Scientific Advisory Council for the National Peanut Board; has received honorarium for lectures from Thermo Fisher, Aimmune Therapeutics, DBV Technologies, Before Brands, multiple state allergy societies, the American College of Allergy, Asthma, & Immunology, and the European Academy of Allergy and Clinical Immunology; is an associate editor for Annals of Allergy, Asthma, and Immunology; and is a member of the Joint Taskforce on Allergy Practice Parameters.
Online Repository.
References
- 1.Shaker M.S., Oppenheimer J., Grayson M., Stukus D., Hartog N., Hsieh E.W.Y., et al. COVID-19: pandemic contingency planning for the allergy and immunology clinic. J Allergy Clin Immunol Pract. 2020;8:1477–1488.e5. doi: 10.1016/j.jaip.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elsevier Novel Coronavirus Information Center. https://www.elsevier.com/connect/coronavirus-information-center Available from: Accessed March 25, 2020.
- 3.Johns Hopkins University Coronavirus Resource Center. https://coronavirus.jhu.edu Available from: Accessed March 25, 2020.
- 4.World Health Organization WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 Available from: Accessed March 25, 2020.
- 5.The White House Proclamation on declaring a national emergency concerning the novel coronavirus disease (COVID-19) outbreak. https://www.whitehouse.gov/presidential-actions/proclamation-declaring-national-emergency-concerning-novel-coronavirus-disease-covid-19-outbreak/ Available from: Accessed March 25, 2020.
- 6.Remuzzi A., Remuzzi G. COVID-19 and Italy: what next? Lancet. 2020;395:1225–1228. doi: 10.1016/S0140-6736(20)30627-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koo J., Cook A., Park M., Sun Y., Sun H., Lim J. Interventions to mitigte early spread of SARS-CoV-2 in Singapore: a modelling study. Lancet Infect Dis. 2020;20:678–688. doi: 10.1016/S1473-3099(20)30162-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 9.Knowles H., Natanson H., Berger M., Hawkins D., Mettler K. Spain, France take drastic measures to fight coronavirus; Georgia delays presidential primary. The Washington Post. March 14, 2020. https://www.washingtonpost.com/world/2020/03/14/coronavirus-latest-news/ Accessed March 25, 2020.
- 10.Waldrop T. Self-isolation, quarantine and California’s stay-at-home order: what the terms mean and how they differ. CNN Health. March 24, 2020. https://www.cnn.com/2020/03/19/health/shelter-in-place-isolation-quarantine-definition/index.html Available from: Accessed March 25, 2020.
- 11.Cox L., Nelson H., Lockey R., Calabria C., Chacko T., Finegold I., et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011;127:S1–S55. doi: 10.1016/j.jaci.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 12.Sun D., Cafone J., Shaker M., Greenhawt M. The cost-effectiveness of requiring universal vs contextual self-injectable epinephrine autoinjector for allergen immunotherapy. Ann Allergy Asthma Immunol. 2019;123:582–589. doi: 10.1016/j.anai.2019.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Arias E., Xu J. United States Life Tables, 2015. Natl Vital Stat Rep. 2018;67:1–63. [PubMed] [Google Scholar]
- 14.Allen-Ramey F., Mao J., Blauer-Peterson C., Rock M., Nathan R., Halpern R. Healthcare costs for allergic rhinitis patients on allergy immunotherapy: a retrospective observational study. Curr Med Res Opin. 2017;33:2039–2047. doi: 10.1080/03007995.2017.1359517. [DOI] [PubMed] [Google Scholar]
- 15.Hankin C.S., Cox L., Bronstone A., Wang Z. Allergy immunotherapy: reduced health care costs in adults and children with allergic rhinitis. J Allergy Clin Immunol. 2013;131:1084–1091. doi: 10.1016/j.jaci.2012.12.662. [DOI] [PubMed] [Google Scholar]
- 16.GoodRx, Inc Epinephrine (Epipen) prices, cost, coupons, & savings tips. https://www.goodrx.com/epinephrine-epipen Available from: Accessed March 25, 2020.
- 17.Clark S., Wei W., Rudders S.A., Camargo C.A., Jr. Risk factors for severe anaphylaxis in patients receiving anaphylaxis treatment in US emergency departments and hospitals. J Allergy Clin Immunol. 2014;134:1125–1130. doi: 10.1016/j.jaci.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 18.Shaker M., Chalil J.M., Tran O., Vlahiotis A., Shah H., King T., et al. Commercial claims costs related to health care resource use associated with a diagnosis of peanut allergy. Ann Allergy Asthma Immunol. 2020;124:357–365.e1. doi: 10.1016/j.anai.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 19.US Department of Labor, Bureau of Labor Statistics. CPI inflation calculator. March 22, 2020. https://data.bls.gov/cgi-bin/cpicalc.pl Available from: Accessed March 25, 2020.
- 20.Irving Oil. On the road. www.theirving.com Available from: Accessed March 25, 2020.
- 21.Cars.com Editors. Best and worst gas mileage 2018. https://www.cars.com/articles/best-and-worst-gas-mileage-2018-1420698621218/ Available from: Accessed March 25, 2020.
- 22.Phillips J.F., Lockey R.F., Fox R.W., Ledford D.K., Glaum M.C. Systemic reactions to subcutaneous allergen immunotherapy and the response to epinephrine. Allergy Asthma Proc. 2011;32:288–294. doi: 10.2500/aap.2011.32.3446. [DOI] [PubMed] [Google Scholar]
- 23.Epstein T.G., Liss G.M., Berendts K.M., Bernstein D.I. AAAAI/ACAAI Subcutaneous Immunotherapy Surveillance Study (2013-2017): fatalities, infections, delayed reactions, and use of epinephrine autoinjectors. J Allergy Clin Immunol Pract. 2019;7:1996–2003.e1. doi: 10.1016/j.jaip.2019.01.058. [DOI] [PubMed] [Google Scholar]
- 24.National Highway Traffic Safety Administration Traffic safety facts 2016 data. https://crashstats.nhtsa.dot.gov/Api/Public/Publication/812554 Available from: Accessed March 25, 2020.
- 25.Cox L.S., Hankin C., Lockey R. Allergy immunotherapy adherence and delivery route: location does not matter. J Allergy Clin Immunol Pract. 2014;2:156–160. doi: 10.1016/j.jaip.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 26.Hsu N.M., Reisacher W.R. A comparison of attrition rates in patients undergoing sublingual immunotherapy vs subcutaneous immunotherapy. Int Forum Allergy Rhinol. 2012;2:280–284. doi: 10.1002/alr.21037. [DOI] [PubMed] [Google Scholar]
- 27.Lee J.H., Kim S.C., Choi H., Jung C.G., Ban G.Y., Shin Y.S., et al. A retrospective study of clinical response predictors in subcutaneous allergen immunotherapy with house dust mites for allergic rhinitis. Allergy Asthma Immunol Res. 2018;10:18–24. doi: 10.4168/aair.2018.10.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.CDC COVID-19 Response Team Severe outcomes among patients with coronavirus disease 2019 (COVID-19) — United States, February 12–March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:343–346. doi: 10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Retzler J., Grand T.S., Domdey A., Smith A., Romano Rodriguez M. Utility elicitation in adults and children for allergic rhinoconjunctivitis and associated health states. Qual Life Res. 2018;27:2383–2391. doi: 10.1007/s11136-018-1910-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanders G.D., Neumann P.J., Basu A., Brock D.W., Feeny D., Krahn M., et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316:1093–1103. doi: 10.1001/jama.2016.12195. [DOI] [PubMed] [Google Scholar]
- 31.Husereau D., Drummond M., Petrou S., Carswell C., Moher D., Greenberg D., et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ. 2013;346:f1049. doi: 10.1136/bmj.f1049. [DOI] [PubMed] [Google Scholar]
- 32.Senna G., Ridolo E., Calderon M., Lombardi C., Canonica G.W., Passalacqua G. Evidence of adherence to allergen-specific immunotherapy. Curr Opin Allergy Clin Immunol. 2009;9:544–548. doi: 10.1097/ACI.0b013e328332b8df. [DOI] [PubMed] [Google Scholar]
- 33.Collins F. To beat COVID-19, social distancing is a must. NIH Director’s Blog. March 19, 2020. https://directorsblog.nih.gov/2020/03/19/to-beat-covid-19-social-distancing-is-a-must/ Available from: Accessed March 25, 2020.
- 34.KCRA Staff Newsom: more than half of California projected to develop COVID-19. KCRA Channel 3. March 20, 2020. https://www.kcra.com/article/newsom-more-than-half-of-california-projected-to-contract-covid-19/31791343# Available from: Accessed March 25, 2020.
- 35.Achenbach J., Wan W., Sun L.H. Coronovirus forecasts are grim: “it’s going to get worse”. The Washington Post. March 11, 2020. https://www.washingtonpost.com/health/coronavirus-forecasts-are-grim-its-going-to-get-worse/2020/03/11/2a177e0a-63b4-11ea-acca-80c22bbee96f_story.html Available from: Accessed March 25, 2020.
- 36.Neumann P.J., Cohen J.T. QALYs in 2018—advantages and concerns. JAMA. 2018;319:2473–2474. doi: 10.1001/jama.2018.6072. [DOI] [PubMed] [Google Scholar]
- 37.Shaker M., Kanaoka T., Feenan L., Greenhawt M. An economic evaluation of immediate vs non-immediate activation of emergency medical services after epinephrine use for peanut-induced anaphylaxis. Ann Allergy Asthma Immunol. 2019;122:79–85. doi: 10.1016/j.anai.2018.06.035. [DOI] [PubMed] [Google Scholar]
- 38.Shaker M., Greenhawt M. Providing cost-effective care for food allergy. Ann Allergy Asthma Immunol. 2019;123:240–248.e1. doi: 10.1016/j.anai.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 39.Shaker M.S., Greenhawt M.J. Analysis of value-based costs of undesignated school stock epinephrine policies for peanut anaphylaxis. JAMA Pediatr. 2019;173:169–175. doi: 10.1001/jamapediatrics.2018.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shaker M., Greenhawt M. Cost-effectiveness of stock epinephrine autoinjectors on commercial aircraft. J Allergy Clin Immunol Pract. 2019;7:2270–2276. doi: 10.1016/j.jaip.2019.04.029. [DOI] [PubMed] [Google Scholar]
- 41.Shaker M., Greenhawt M. Association of fatality risk with value-based drug pricing of epinephrine autoinjectors for children with peanut allergy: a cost-effectiveness analysis. JAMA Netw Open. 2018;1:e184728. doi: 10.1001/jamanetworkopen.2018.4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma L., Danoff T.M., Borish L. Case fatality and population mortality associated with anaphylaxis in the United States. J Allergy Clin Immunol. 2014;133:1075–1083. doi: 10.1016/j.jaci.2013.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Golden D.B., Demain J., Freeman T., Graft D., Tankersley M., Tracy J., et al. Stinging insect hypersensitivity: a practice parameter update 2016. Ann Allergy Asthma Immunol. 2017;118:28–54. doi: 10.1016/j.anai.2016.10.031. [DOI] [PubMed] [Google Scholar]
- 44.Finnell S.M., Carroll A.E., Downs S.M. The utility assessment method order influences measurement of parents’ risk attitude. Value Health. 2012;15:926–932. doi: 10.1016/j.jval.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 45.Shaker M., Bean K., Verdi M. Economic evaluation of epinephrine auto-injectors for peanut allergy. Ann Allergy Asthma Immunol. 2017;119:160–163. doi: 10.1016/j.anai.2017.05.020. [DOI] [PubMed] [Google Scholar]
- 46.Macy E., Vyles D. Who needs penicillin allergy testing? Ann Allergy Asthma Immunol. 2018;121:523–529. doi: 10.1016/j.anai.2018.07.041. [DOI] [PubMed] [Google Scholar]
- 47.Genentech. Xolair (omalizumab) highlights of prescribing information. San Fransisco, CA: Genentech; 2018. Available from: https://www.gene.com/download/pdf/xolair_prescribing.pdf. Accessed March 20, 2020.
- 48.Shaker M., Briggs A., Dbouk A., Dutille E., Oppenheimer J., Greenhawt M. Estimation of health and economic benefits of clinic versus home administration of omalizumab and mepolizumab. J Allergy Clin Immunol Pract. 2020;8:565–572. doi: 10.1016/j.jaip.2019.09.037. [DOI] [PubMed] [Google Scholar]
- 49.Turner P.J., Jerschow E., Umasunthar T., Lin R., Campbell D.E., Boyle R.J. Fatal anaphylaxis: mortality rate and risk factors. J Allergy Clin Immunol Pract. 2017;5:1169–1178. doi: 10.1016/j.jaip.2017.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Novartis. Novartis receives European Commission approval for self-administration of Xolair across all indications. 2018. https://www.novartis.com/news/media-releases/novartis-receives-european-commission-approval-self-administration-xolair-across-all-indications Available from: Accessed March 20, 2020.
- 51.Togias A., Cooper S.F., Acebal M.L., Assa’ad A., Baker J.R., Jr., Beck L.A., et al. Addendum guidelines for the prevention of peanut allergy in the United States: report of the National Institute of Allergy and Infectious Diseases-sponsored expert panel. J Allergy Clin Immunol. 2017;139:29–44. doi: 10.1016/j.jaci.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Netting M.J., Campbell D.E., Koplin J.J., Beck K.M., McWilliam V., Dharmage S.C., et al. An Australian Consensus on infant feeding guidelines to prevent food allergy: outcomes from the Australian Infant Feeding Summit. J Allergy Clin Immunol Pract. 2017;5:1617–1624. doi: 10.1016/j.jaip.2017.03.013. [DOI] [PubMed] [Google Scholar]
Reference
- Turner P.J., Jerschow E., Umasunthar T., Lin R., Campbell D.E., Boyle R.J. Fatal anaphylaxis: mortality rate and risk factors. J Allergy Clin Immunol Pract. 2017;5:1169–1178. doi: 10.1016/j.jaip.2017.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]