To the Editor:
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the coronavirus responsible for the current pandemic of coronavirus disease 2019 (COVID-19), whose very broad clinical spectrum ranges from minor signs and symptoms such as cough and mild fever to severe pneumonia with dyspnea, tachypnea, and impaired gas exchange, leading to severe and life-threatening manifestations in approximately 15% of infected patients.1 Increased levels of proinflammatory cytokines and coagulation activation markers2 indicate that a sustained inflammatory response to viral infection and the related prothrombotic state are involved in the development of these clinical manifestations. Such a picture is reminiscent of comparable manifestations in various autoimmune/inflammatory disorders characterized by a prothrombotic state and endothelial perturbation triggered by systemic inflammation or macrophage activation2 and microangiopathies that complicate solid-organ and bone marrow transplantations.3
The complement system is a key mediator of the innate immune response that protects against infectious agents such as viruses4 but, in addition to being an important part of the immuno-defense system, it plays a critical role in promoting the inflammatory process that leads to tissue injury. Furthermore, it has long been recognized that a crosstalk between complement and the coagulation system exists.3 The activation peptide of complement component 5 (C5a) and the membrane attack complex (MAC/C5b-9) drive neutrophil activation and the inflammation that eventually leads to endothelial damage.3 However, although the role of complement in the acute respiratory distress syndrome caused by influenza, respiratory syncytial, and the previous SARS-CoVs is well established,4 its contribution to COVID-19 is still unclear. Diao et al5 have found that acute renal failure associated with tubular necrosis and abundant complement deposition develops in a significant percentage of patients with severe COVID-19, which suggests that complement plays a pathogenic role, and Gao et al6 have reported in a preprint increased serum levels of C5a in a small series of patients with severe COVID-19.
We investigated the plasma levels of sC5b-9 and C5a as markers of complement activation in 31 patients with COVID-19: 21 men and 10 women with a median age of 59 years (range, 31-85 years). The study was approved by the Ethics Committee of Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico in Milan (no. 360_2020) and was carried out in conformity with the 2013 revision of the Declaration of Helsinki.
Seventeen of these patients were admitted to our Respiratory Unit for intermediate care with continuous positive airway pressure and were considered as having moderate COVID-19; the remaining 14 were admitted to our intensive care unit for mechanical ventilation and were considered as having severe disease.2 All the patients received subcutaneous low-molecular-weight heparin at a twice-daily dose of 100 IU/kg and oral hydroxychloquine at a twice-daily dose of 200 mg. EDTA plasma samples were obtained from a single venipuncture performed 1 to 6 days after the admission, immediately frozen, and stored at −80C° before testing. The levels of soluble C5b-9 (sC5b-9) were measured by means of solid-phase assays (MicroVue Complement SC5b-9 Plus EIA kit, Quidel Corporation, San Diego, Calif), with intra- and interassay coefficients of variation of respectively 6.8% and 13.1%, and plasma C5a levels were measured using an immunoenzymatic method (MicroVue Complement C5a EIA, Quidel Corporation) with intra- and interassay coefficients of variation of less than 12%.
The controls for the complement assays were 27 healthy subjects: 19 men and 8 women with a median age of 55 years (range, 34-78 years).
The following laboratory parameters were collected from the patients’ clinical records: fibrin fragment D-dimer, C-reactive protein, IL-6, ferritin, white blood cells, neutrophils, lymphocytes, platelets, prothrombin time, activated partial thromboplastin time, and fibrinogen. The data are given as median values and ranges (minimum-maximum). The between-group differences were analyzed using the Mann-Whitney test for independent samples, and the correlations between the different parameters were evaluated using Spearman test. A P value of less than .05 was considered significant.
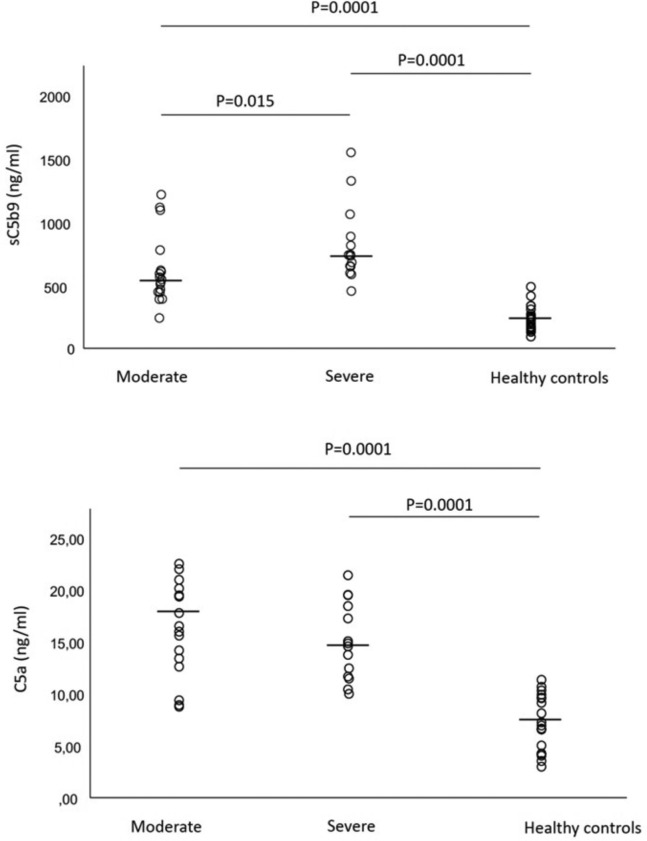
Fig 1 shows the levels of sC5b-9 and C5a in the 2 groups of patients and the normal controls. The plasma levels of sC5b-9 (upper panel) were significantly higher in the patients with moderate disease (median, 556 ng/mL; range, 253-1223 ng/mL) and those with severe disease (746 ng/mL; range, 465-1555 ng/mL) than in the healthy controls (217 ng/mL; range, 106-499 ng/mL) (P = .0001 for both), and significantly higher in the patients with severe disease than in those with moderate disease (P = .015). The plasma levels of C5a (lower panel) were higher in the patients with moderate disease (16.99 ng/mL; range, 9.24-22.99 ng/mL) and those with severe disease (15.55 ng/mL; range, 10.9-21.89 ng/mL) than in the healthy controls (7.28 ng/mL; range, 3.47-11.83 ng/mL) (P = .0001 for both), with no statistically significant difference between the 2 patient groups.
Fig 1.
Plasma levels of complement terminal complex sC5b9 (upper panel) and activated C5a (lower panel) in 17 patients with COVID-19 requiring continuous positive airway pressure (moderate) and 14 patients with COVID-19 requiring mechanical ventilation (severe). The horizontal lines represent median values.
It is worth noting that the levels of sC5b-9 were surprisingly high in a few patients whose C5a levels fell within the normal range. This can be explained by the fact that C5a is cleared more rapidly than sC5b-9 and suggests that sC5b-9 may be a more reliable marker of in vivo complement activation.
Table I presents the analyzed coagulation and inflammation parameters in the 2 patient groups. In line with recent findings,1 , 2 our cohort of patients with COVID-19 had increased levels of acute-phase proteins and coagulation system abnormalities. Although there is a slight correlation between the plasma levels of sC5b-9 and C5a and C-reactive protein levels (r = 0.439, P = .013 and r = 0.449, P = .011), the activation products of the complement cascade seem to behave as independent variables, thus raising the question as to whether measuring complement activation products is more sensitive and predictive of disease outcome. Future prospective studies also including patients with mild COVID-19 should be carried out to investigate this hypothesis. We are now conducting such a study to assess the trend of complement activation markers from the admission through hospitalization to complete remission. The increased C5a levels observed in our patients with COVID-19 are consistent with the well-established role of C5a in promoting the lung sequestration of leukocytes and pulmonary dysfunction, and it has also been shown that sC5b-9 has similar effects by causing transendothelial leukocyte migration and vascular leakage.4 Overall, these findings suggest that complement activation may contribute to the development of lung and endothelial damage in patients with COVID-19. However, consideration should also be given to the possibility that the coronavirus may directly cause damage to endothelial cells. Indeed, in a postmortem study performed in 3 patients, electron microscopy revealed viral inclusion structures in endothelial cells and histological analyses showed an accumulation of inflammatory cells associated with endothelium.7 A number of research laboratories are making a major effort to develop therapeutic strategies for controlling COVID-19. In addition to looking for drugs that prevent viral entry into target cells and virus replication, they are seeking satisfactory treatments for the serious clinical manifestations often associated with the disease, including the severe interstitial pneumonia, sepsis, heart failure, and excessive blood clotting that can lead to a fatal outcome. One of the aims of the currently investigated therapeutic strategies is to induce a marked reduction in the inflammatory response to viral infection documented by increased levels of proinflammatory cytokines. Our data show that complement activation is frequent in patients with COVID-19 and probably involved in the pathophysiology of its clinical complications. Complement may act in combination with and possibly upstream of other mediators, thus suggesting the possibility that complement-blocking drugs may be a beneficial addition to the therapeutic armamentarium against COVID-19. In particular, the blocking of complement may be obtained by specific drugs targeting C5 or the mannan-binding lectin-associated serine protease-2 by the humanized mAb eculizumab or the human mAb narsoplimab, respectively.8 Moreover, intravenous immunoglobulins may hamper complement cascade amplification, decreasing C5 activation and deposition of the membrane attack complex.9
Table I.
Coagulation and inflammation parameters expressed as median values and ranges in 17 patients with COVID-19 requiring continuous positive airway pressure (moderate) and 14 patients with COVID-19 requiring mechanical ventilation (severe)
| Severity level | D-dimer (μg/L) | CRP (mg/dL) | Ferritin (μg/L) | IL-6 (ng/L) | White cells (n/μL) | Neutrophils (n/μL) | Lymphocytes (n/μL) | Platelets n (× 103/μL) | PT ratio | aPTT ratio |
Fibrinogen (mg/dL) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Moderate | 1,275 | 3.41 | 1,122 | 20.1 | 8,060 | 6,600 | 1,000 | 342 | 1.17 | 0.91 | 472 |
| 290-21,639 | 0.55-18.24 | 69-8,633 | 1.5-268.0 | 2,830-17,910 | 1,560-12,510 | 380-3,330 | 70-799 | 0.96-5.43 | 0.71-1.21 | 229-819 | |
| Severe | 1,565 | 9.00 | 1,269 | 33.3 | 9,190 | 8,005 | 650 | 322 | 1.11 | 0.92 | 499 |
| 471-19,548 | 1.61-34.15 | 216-5,064 | 3.8-300.0 | 2,310-51,410 | 1,300-49,680 | 200-1,650 | 72-608 | 1.02-1.35 | 0.81-1.15 | 228-1,035 | |
| Normal ranges | <500 | 0.00-0.05 | 30-400 | <10 | 4,800-10,800 | 1,500-6,500 | 1,200-3,400 | 130-430 | 0.84-1.20 | 0.86-1.20 | 165-350 |
aPTT, Activated partial thromboplastin time; CRP, C-reactive protein; PT, prothrombin time.
Footnotes
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
References
- 1.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H. Clinical and immunologic features in severe and moderate Coronavirus Disease 2019. J Clin Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riedl M., Fakhouri F., Le Quintrec M., Noone D.G., Jungraithmayr T.C., Fremeaux-Bacchi V. Spectrum of complement-mediated thrombotic microangiopathies: pathogenetic insights identifying novel treatment approaches. Semin Thromb Hemost. 2014;40:444–464. doi: 10.1055/s-0034-1376153. [DOI] [PubMed] [Google Scholar]
- 4.Gralinski L.E., Sheahan T.P., Morrison T.E., Menachery V.D., Jensen K., Leist S.R. Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. mBio. 2018;9 doi: 10.1128/mBio.01753-18. e01753-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diao B, Wang C, Wang R, Feng Z, Tan Y, Wang H, et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection [published online ahead of print April 10, 2020]. medRxiv. 10.1101/2020.03.04.20031120. [DOI]
- 6.Gao T, Hu M, Zhang X, Li H, Zhu L, Liu H, et al. Highly pathogenic coronavirus N protein aggravates lung injury by MASP-2-mediated complement over-activation [published online ahead of print April 7, 2020]. medRxiv. 10.1101/2020.03.29.20041962. [DOI]
- 7.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patriquin C.J., Kuo K.H.M. Eculizumab and beyond: the past, present, and future of complement therapeutics. Transfusion Med Rev. 2019;33:256–265. doi: 10.1016/j.tmrv.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Basta M., Dalakas M.C. High-dose intravenous immunoglobulin exerts its beneficial effect in patients with dermatomyositis by blocking endomysial deposition of activated complement fragments. J Clin Invest. 1994;94:1729–1735. doi: 10.1172/JCI117520. [DOI] [PMC free article] [PubMed] [Google Scholar]