Dear Editor,
While daily repetitive transcranial magnetic stimulation (rTMS) is FDA-approved to treat depression and obsessive-compulsive disorder, we are still unclear about its therapeutic mechanism of action. Better understanding of the fundamental mechanisms of rTMS-induced changes would likely refine and improve this therapy.
Animal studies suggest that 10 Hz rTMS may work through long-term potentiation (LTP), a form of synaptic plasticity [1]. In humans, several studies have demonstrated that n-methyl-D-aspartate (NMDA) receptor activity, a critical step in the LTP pathway, is necessary [2,3], but not sufficient [4,5] for 0.1 Hz rTMS paired with ischemic nerve block (INB)- and theta burst stimulation (TBS)-induced motor plasticity. Surprisingly, there are no pharmacologic studies testing whether 10 Hz rTMS, as used clinically, depends on synaptic plasticity. We hypothesize that 10 Hz rTMS enhances motor excitability through NMDA receptor-dependent LTP of glutamatergic synapses, and that partial agonism of this pathway by d-cycloserine (DCS) is sufficient to further potentiate motor excitability in healthy humans. Consistent with our hypothesis, we observed that participants in this small crossover study appeared to have more cumulative potentiation after 10 Hz rTMS with DCS relative to placebo.
We recruited ten healthy adults (6 men, 26–37 years old) into a randomized, double-blind, crossover study approved by the Medical University of South Carolina Institutional Review Board. All participants provided informed consent prior to any research procedures. We included healthy participants without TMS contraindications between the ages of 18 and 50.
We randomly assigned participants to begin the crossover study with either 100 mg d-cycloserine (DCS, an NMDA receptor partial agonist) or identical microcrystalline cellulose capsules (Tidewater pharmacy, Mt. Pleasant, SC) in a blinded manner on separate visits. All 10 participants received both medications, half received active drug first. We administered the drug approximately 2 hours before rTMS as done previously [6].
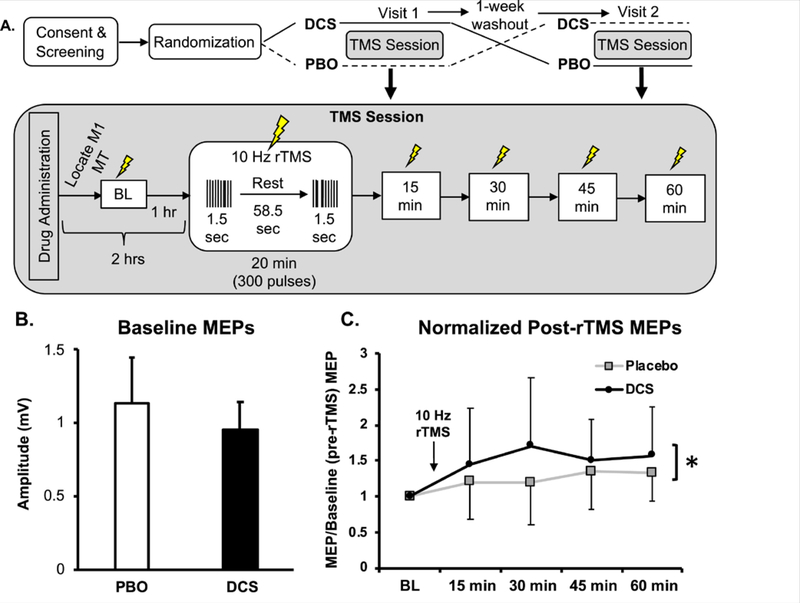
We obtained baseline measurements of motor excitability after drug was administered in order to differentiate whether the drug affected basal excitability (baseline) as opposed to rTMS-induced plasticity (15–60 minutes after rTMS, Fig. 1A). Following baseline measurements, we administered 20 minutes of 10 Hz rTMS. We then remeasured motor excitability at 15 minute increments for 60 minutes.
Fig. 1. NMDA receptor partial agonist d-cycloserine (DCS) enhances 10 Hz rTMS-induced motor-evoked potentials (MEPs).
(A) Experimental crossover design. Top: Overview of full experiment with grayed insets representing TMS session (below). DCS: d-cycloserine, PBO: placebo, MT: motor threshold, M1: motor cortex (“hotspot”). (B) Averaged raw MEPs and standard deviations (error bars) single-pulse MEPs before rTMS: placebo 1.129±0.32 mV; DCS 0.956±0.19 mV); p = 0.173. (C) Averaged normalized (to baseline) MEP values and standard deviations (error bars) for each individual time point after 10 Hz rTMS conclusion: 15 minutes: placebo (1.20±0.52), DCS (1.45±0.78); 30 minutes: placebo (1.19±0.58), DCS (1.70±0.95), effect size: 0.387, power: 0.360; 45 minutes, placebo (1.35±0.53), DCS (1.50±0.57); 60 minutes, placebo (1.33±0.41), DCS (1.57±0.68). *p = 0.038 overall (average across time points) treatment effect between placebo and DCS, independent of time.
We ensured all TMS and rTMS pulses were given within 2 mm of the target motor “hotspot” (over the left motor cortex (M1)) using Brainsight neuronavigation (Brainsight, Rogue Research, Quebec, Canada). We obtained resting motor thresholds (rMT), defined as the lowest stimulator intensity to elicit ≥ 5/10 MEPs, using a modified Mills-Nithey approach; whereby we started above the rMT, and descended in intensity until ≥ 6/10 response failures. Because we used different TMS machines for generating single pulse MEPs and for delivering rTMS, we obtained an independent rMT for single-pulse TMS (>1 mV, Magstim) and for rTMS (>50 μV, MagVenture).
We administered single TMS pulses (jittered 4–7 sec apart) with the Magstim 2002 capacitor (Magstim, UK) while recording motor evoked potentials (MEPs) from surface electromyography (EMG) electrodes (Natus, USA) placed over the right abductor pollicis brevis (APB) analyzed with Spike2 software. The raw signal was amplified and filtered by CED 1902 and 1401 microprocessor or DAQ (Cambridge Electronic Devices, UK). We collected two bins of twenty pulses and averaged these together for each time point: before rTMS (baseline) and 15, 30, 45, and 60 minutes after rTMS. We administered 10 Hz rTMS over the same motor location with a figure- 8 MagPro R30 with B65 cooled coil (MagVenture, Denmark) for 20 minutes (1.5/58.5 second duty cycle) for a total of 300 pulses at 80% rMT in order to potentiate MEPs, as done previously [7]. Following at least a 1-week washout period, we repeated the experiment with the other drug.
At the conclusion of each session we asked participants which pill they believed they had received and their confidence. They guessed correctly 13/20 times (p = 0.445 two-tailed McNemar matched Chi Square test), with a mean confidence rating of 2.8/10 for correct and 5.0/10 for incorrect guessers. 1/10 reported a side effect from DCS (“felt minimally funny”) and 1/10 from placebo (“drowsy”). Visual analog scale TMS pain ratings were 2.35 on 1–10 scale. All participants tolerated and completed the study.
We normalized time-course MEPs to the participant’s baseline MEP average, and consequently did not include baseline data in our statistical model. We then analyzed the time course data with a general linear mixed model for continuous outcomes with a random effect for repeated measures using PROC MIXED in SAS software (Cary, NC, USA). We fit a series of models testing the effects of drug, time, and drug-time interaction, and controlled for order in the crossover design. We set a priori level of significance at p < 0.05.
Medication did not significantly affect baseline motor excitability (obtained ~1 hour after drug administration, and prior to rTMS; student’s two-tailed paired t-test, p = 0.173 (Fig. 1B)).
Treatment order was a significant predictor of outcome (F = 5.19, p = 0.026): MEPs were higher if DCS was received in the first visit, and we accounted for this in our statistical model of medication effect. We also controlled for time (F = 0.19, p = 0.91) and condition by time interaction (F = 0.33; p = 0.80) and found these were not significant predictors of outcome.
When participants received d-cycloserine, we observed an overall increase in least squares means from 1.268687 to 1.554 (difference of 0.28787, standard error 0.14 [F = 4.48, df = 62; p = 0.038; data in Fig. 1C]). While these results are with a small sample size, and should be interpreted cautiously, they appear to be consistent with our general hypothesis that the therapeutic effects of 10 Hz rTMS may be accomplished through NMDA receptor-mediated LTP of relevant synapses. It is our opinion that understanding how TMS changes brain synapses and circuits will likely help this tool reach its full potential. Moreover, these results suggest that 10 Hz rTMS clinical efficacy could conceivably be enhanced by mechanistic synergy through pharmacologic augmentation.
Acknowledgements
The authors would like to thank Lisa McTeague, PhD, and Colleen Hanlon, PhD for helpful suggestions and for Amy Wahlquist, MS, Steven Lauzon, MS and Viswanathan Ramakrishnan, PhD for statistical support.
Funding source: This work was supported by the DART Training Grant at MUSC, National Institutes of Health, Grant number: R25DA020537.
Contributor Information
Joshua C. Brown, Department of Psychiatry and Behavioral Sciences, Medical University of South Carolina, Charleston, SC, USA; Department of Neurology, Medical University of South Carolina, Charleston, SC, USA.
William H. DeVries, Department of Psychiatry and Behavioral Sciences, Medical University of South Carolina, Charleston, SC, USA
Jeffrey E. Korte, Department of Public Health Sciences, Medical University of South Carolina, Charleston, SC, USA
Gregory L. Sahlem, Department of Psychiatry and Behavioral Sciences, Medical University of South Carolina, Charleston, SC, USA
Leonardo Bonilha, Department of Neurology, Medical University of South Carolina, Charleston, SC, USA.
E. Baron Short, Department of Psychiatry and Behavioral Sciences, Medical University of South Carolina, Charleston, SC, USA.
Mark S. George, Department of Psychiatry and Behavioral Sciences, Medical University of South Carolina, Charleston, SC, USA
Ralph H. Johnson, Veterans Administration Medical Center, Charleston, SC, USA
References
- [1].Vlachos A, Muller-Dahlhaus F, Rosskopp J, Lenz M, Ziemann U, Deller T. Repetitive magnetic stimulation induces functional and structural plasticity of excitatory postsynapses in mouse organotypic hippocampal slice cultures. J Neurosci 2012;32(48):17514–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ziemann U, Hallett M, Cohen LG. Mechanisms of deafferentation-induced plasticity in human motor cortex. J Neurosci 1998;18(17):7000–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Huang YZ, Chen RS, Rothwell JC, Wen HY. The after-effect of human theta burst stimulation is NMDA receptor dependent. Clin Neurophysiol 2007;118(5): 1028–32. [DOI] [PubMed] [Google Scholar]
- [4].Teo JT, Swayne OB, Rothwell JC. Further evidence for NMDA-dependence of the after-effects of human theta burst stimulation. Clin Neurophysiol 2007;118(7): 1649–51. [DOI] [PubMed] [Google Scholar]
- [5].Selby B, MacMaster FP, Kirton A, McGirr A. d-cycloserine blunts motor cortex facilitation after intermittent theta burst transcranial magnetic stimulation: a double-blind randomized placebo-controlled crossover study. Brain Stimul 2019;12(4):1063–5. [DOI] [PubMed] [Google Scholar]
- [6].Nitsche MA, Jaussi W, Liebetanz D, Lang N, Tergau F, Paulus W. Consolidation of human motor cortical neuroplasticity by D-cycloserine. Neuropsychopharmacology 2004;29(8):1573–8. [DOI] [PubMed] [Google Scholar]
- [7].Jung SH, Shin JE, Jeong YS, Shin HI. Changes in motor cortical excitability induced by high-frequency repetitive transcranial magnetic stimulation of different stimulation durations. Clin Neurophysiol 2008;119(1):71–9. [DOI] [PubMed] [Google Scholar]