ABSTRACT
Crohn's disease (CD) is associated with an increased risk of developing colorectal cancer. In particular, cases in which long-term survival is achieved by patients with local recurrence of CD-associated rectal cancer are rare. We report a case in which curative resection was achieved for a 47-year-old man with long-standing CD and locally recurrent rectal cancer. In this case, the patient obtained a long-term survival without recurrence after surgical resection with adjuvant chemotherapy and immunosuppressive therapy. In the management of inflammatory bowel disease patients with cancer, the management of both cancer and inflammatory bowel disease treatment is important for the long-term prognosis.
INTRODUCTION
Some epidemiological studies have reported that Crohn's disease (CD) is associated with an increased risk of developing small bowel and colorectal cancer (CRC) and that the prognosis of CRC associated with Crohn's colitis is poorer than that of sporadic colorectal carcinoma.1–6 In particular, the long-term survival of locally recurrent rectal cancer associated with Crohn's colitis is extremely rare. Both cancer and CD treatment are needed for long-term survival. We report a case in which curative resection was achieved in a patient with local recurrence from CD-associated rectal cancer. The patient achieved long-term survival without cancer recurrence after surgical resection with both chemotherapy and immunosuppressive therapy.
CASE REPORT
The patient was a 47-year-old man from Japan who had been diagnosed with ileocolonic CD 30 years previously. The diagnosis of ileocolonic CD was preceded by anal fistula. His condition was maintained with 5-aminosalicylate, elemental diet, and temporary corticosteroids; however, he had multiple ileal and right-colonic longitudinal ulcers and a deep anorectal ulcer. His colorectal and distal ileal stenosis gradually progressed, and anal pain appeared and increased. Anorectal cancer was suspected, and colonoscopy under anesthesia was performed, but sufficient findings, such as endoscopic images and suggestive biopsy results, were not detected because of severe anorectal stenosis. A barium enema showed severe anorectal stenosis, deformity of the total colon, and multiple ileal longitudinal ulcers (Figure 1). Magnetic resonance imaging showed circumferential anorectal wall thickening (Figure 2). He underwent total proctocolectomy with distal ileal resection reconstructed by ileostomy at 32 years of age because of highly suspicious anorectal cancer (Figure 3). A pathological examination detected anorectal cancer (well-differentiated adenocarcinoma with T3 invasion and without lymph node metastasis).
Figure 1.
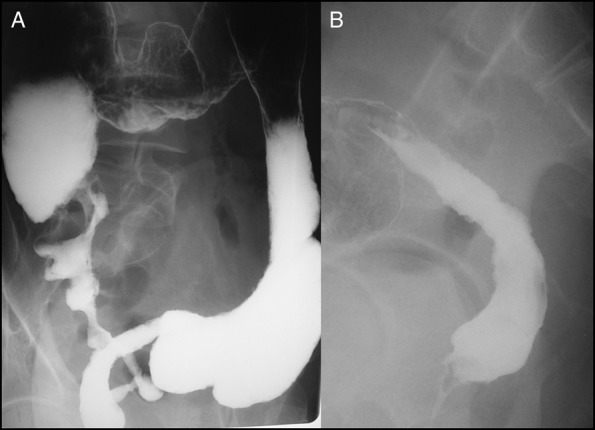
(A and B) Barium enema showing severe anorectal stenosis, deformity of the total colon, and multiple ileal longitudinal ulcers.
Figure 2.
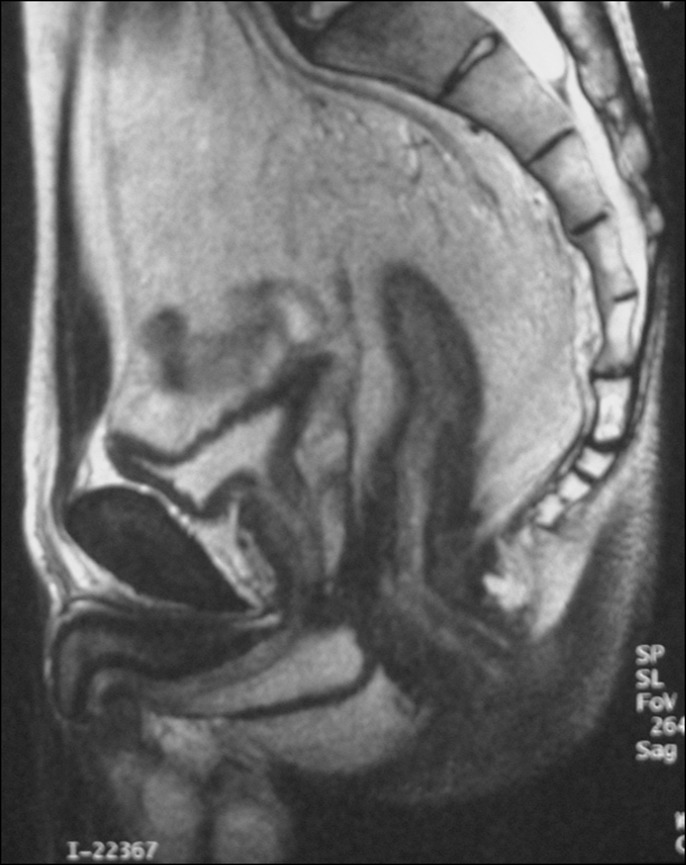
Magnetic resonance imaging showing circumferential anorectal wall thickening.
Figure 3.
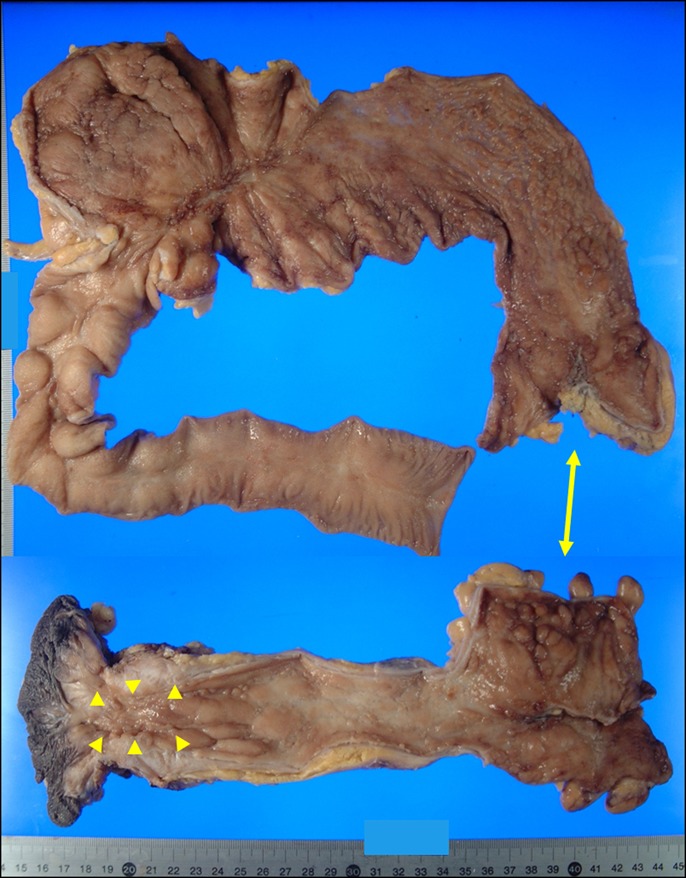
A resected specimen obtained during the first surgery, where multiple longitudinal ulcers were seen (arrow: originally connected site; arrowheads: cancer area).
Remission of ileal CD was obtained by surgical resection, and 5-FU and leucovorin therapy for adjuvant chemotherapy was introduced. Remission was maintained with nutritional therapy and mesalazine without the use of antitumor necrosis factor agents and immunomodulators, including during the chemotherapy period. Six years later, locally recurrent rectal cancer lesions appeared in the pelvic cavity, although a clinical remission of ileal CD was maintained (Figure 4). Total pelvic exenteration with a urinary ileal conduit was performed, which achieved pathologically curative resection. Postoperative chemotherapy was administered using capecitabine and oxaliplatin therapy. He developed severe watery diarrhea during chemotherapy, which did not improve with alteration of his regimen, and chemotherapy was discontinued. Fortunately, he has been cancer-free for 9 years after resection of the local recurrence. In the management of ileal CD the exacerbation of ileal CD occurred during chemotherapy and ileal ulcers were pointed out (Figure 5). Because we considered the risk of cancer recurrence to be high, the patient received nutritional therapy, mesalazine, and temporary corticosteroids without immunosuppressive therapy for 5 years after surgery. Because he experienced repeated remission and relapse of ileal CD without cancer recurrence for 5 years, immunosuppressive treatment including an antitumor necrosis factor agent and azathioprine was administered to obtain long-term remission. At present, clinical and endoscopic remission of ileal CD has been achieved using adalimumab in combination with azathioprine (Figure 6).
Figure 4.
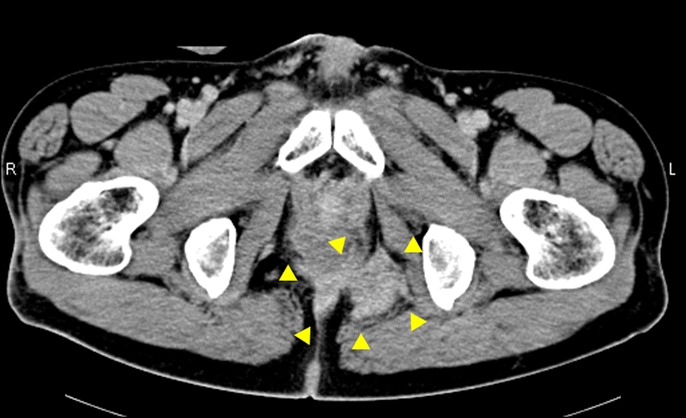
Computed tomography findings of recurrent cancer (arrowheads) in the pelvic cavity.
Figure 5.
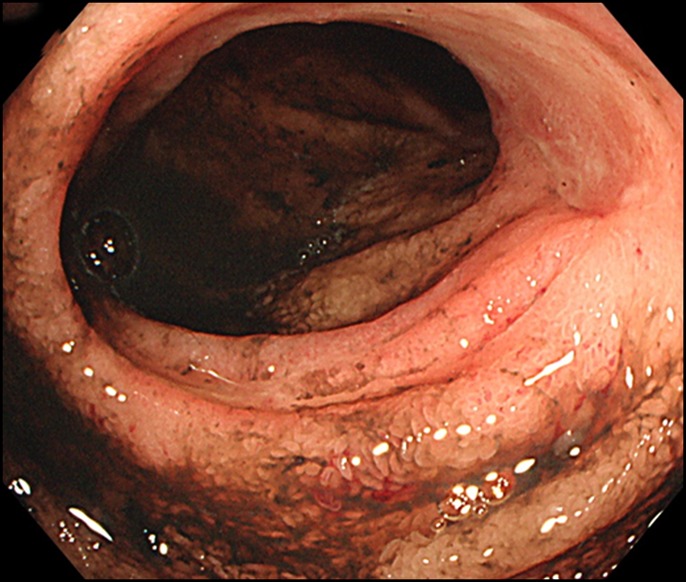
An ileal ulcer in an exacerbation of Crohn's disease.
Figure 6.
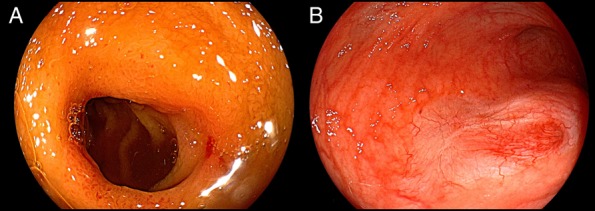
Ileoscopy showing (A) ileal ulcer scars after immunosuppressive therapy and (B) Crohn's disease remission at the site of ileal ureteral anastomosis in the ileal conduit.
DISCUSSION
It is well-known that patients with CD have an increased risk of developing CRC.1–4 Although the prognosis of CRC associated with Crohn's colitis is poorer than that of sporadic colorectal carcinoma, long-term cancer-free survival and clinical remission were obtained with the administration of adjuvant chemotherapy and immunosuppressive therapy in this case.4–6 The administration of both chemotherapy and immunosuppressive therapy is a serious issue for patients with the combination of inflammatory bowel disease (IBD) and cancer.
Regarding chemotherapy for patients with IBD, side effects and therapeutic effects need to be considered. Some studies showed that patients with IBD experienced more CRC treatment alterations than those without IBD, largely because of a higher frequency of treatment delays and gastrointestinal complications during CRC treatment in patients with IBD secondary to an exacerbation of IBD.7–10 Regarding survival of patients who received chemotherapy and of patients with or without IBD, some studies reported that patients with IBD-associated CRC had similar rates of tumor recurrence and survival after postoperative chemotherapy compared with CRC patients without IBD who received postoperative chemotherapy.9,11 On the other hand, among patients with stage IV disease who received chemotherapy, patients with IBD have significantly shorter survival than patients without IBD.9 Although severe diarrhea was seen in this case, postoperative chemotherapy may have contributed to long-term survival. On the other hand, the exacerbation of IBD because of chemotherapy was a problem and the treatment of CD was difficult because no immunosuppressants were used for 5 years before the patient was classified cancer-free.12,13
Regarding immunosuppressive therapy for patients with IBD with a history of cancer, as previous studies have demonstrated an increased risk of neoplasms in patients exposed to immunosuppressants, oncologists and gastroenterologists generally discontinue immunosuppressive therapy for IBD while the patient is receiving chemotherapy and often continue withholding therapy while the patient is in remission from cancer.14–17 A large study of the CESAME prospective observational cohort reported that the independent risk factors for incident cancer in patients with IBD include previous cancer, increased age, and exposure to any immunosuppressant. However, patients with IBD with a history of cancer have an increased risk of developing any (new or recurrent) cancer and immunosuppressive therapy itself has no overall major impact on the cancer risk.18,19 In this case, remission of CD was achieved after total proctocolectomy in the primary surgery; thus, no immunosuppressants were required after the first surgery. When an exacerbation of CD was found during postoperative chemotherapy after recurrent surgery, immunosuppressive therapy was postponed because of the difficulty in determining that the patient was cancer-free. Although the patient's CD was difficult to control for 5 years, the early use of immunosuppressants might have improved the prognosis of CD in the present case.
Based on our case and previous reports, patients with IBD, and a history of recurrent cancer, postoperative chemotherapy was performed for advanced cancer cases, and immunosuppressive therapy after curative resection of cancer might lead to a good prognosis of CD. However, there is no established interval for restarting immunosuppressive therapy for IBD after cancer treatment, and there are limited data on the long-term survival of recurrent cancer cases. Decisions should still be made on an individual basis in a multidisciplinary manner after discussion among the oncologists, gastroenterologists, and other specialists, according to the status of previous cancer and IBD activity.
DISCLOSURES
Author contributions: T. Ueda provided the data and is the article guarantor. T. Tanaka, T. Yokoyama, and T. Sadamitsu wrote the manuscript. H. Fujii, F. Koyama, and A. Yoshimura approved the final manuscript.
Financial disclosure: None to report.
Previous presentation: This case was presented at the seventh Annual meeting of Asian Organization for Crohn's and Colitis; June 14-16, 2019; Taipei, Taiwan.
Informed consent was obtained for this case report.
REFERENCES
- 1.Canavan C, Abrams KR, Mayberry J. Meta-analysis: Colorectal and small bowel cancer risk in patients with Crohn's disease. Aliment Pharmacol Ther. 2006;23(8):1097–104. [DOI] [PubMed] [Google Scholar]
- 2.Ekbom A, Helmick C, Zack M, et al. Increased risk of large-bowel cancer in Crohn's disease with colonic involvement. Lancet. 1990;336(8711):357–9. [DOI] [PubMed] [Google Scholar]
- 3.Gillen CD, Andrews HA, Prior P, et al. Crohn's disease and colorectal cancer. Gut. 1994;355(5):651–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jess T, Gamborg M, Matzen P, et al. Increased risk of intestinal cancer in Crohn's disease: A meta-analysis of population-based cohort studies. Am J Gastroenterol. 2005;100(12):2724–9. [DOI] [PubMed] [Google Scholar]
- 5.Larsen M, Mose H, Gislum M, et al. Survival after colorectal cancer in patients with Crohn's disease: A nationwide population-based Danish follow-up study. Am J Gastroenterol. 2007;102(1):163–7. [DOI] [PubMed] [Google Scholar]
- 6.Ueda T, Inoue T, Nakamoto T, et al. Anorectal cancer in Crohn's disease has a poor prognosis due to its advanced stage and aggressive histological features: A systematic literature review of Japanese patients. J Gastrointest Cancer. 2020;51(1):1–9. [DOI] [PubMed] [Google Scholar]
- 7.Tiersten A, Saltz LB. Influence of inflammatory bowel disease on the ability of patients to tolerate systemic fluorouracil-based chemotherapy. J Clin Oncol. 1996;14(7):2043–6. [DOI] [PubMed] [Google Scholar]
- 8.Naito A, Mizushima T, Takeyama H, et al. Feasibility of chemotherapy in patients with inflammatory bowel disease-related gastrointestinal cancer. Hepatogastroenterology. 2014; 61(132) :942–6. [PubMed] [Google Scholar]
- 9.Axelrad J, Kriplani A, Ozbek U, et al. Chemotherapy tolerance and oncologic outcomes in patients with colorectal cancer with and without inflammatory bowel disease. Clin Colorectal Cancer. 2017;16(3):e205–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nio K, Higashi D, Kumagai H, et al. Efficacy and safety analysis of chemotherapy for advanced colitis-associated colorectal cancer in Japan. Anticancer Drugs. 2016;27(5):457–63. [DOI] [PubMed] [Google Scholar]
- 11.Dugum M, Lin J, Lopez R, et al. Recurrence and survival rates of inflammatory bowel disease-associated colorectal cancer following postoperative chemotherapy: A comparative study. Gastroenterol Rep. 2017(1); 5: 57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dignass A, Assche GV, Lindsay JO, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn's disease: Current management. J Crohns Colitis. 2010;4(1):28–62. [DOI] [PubMed] [Google Scholar]
- 13.Annese V, Beaugerie L, Egan L, et al. European evidence-based consensus: Inflammatory bowel disease and malignancies. J Crohns Colitis. 2015;9(11):945–65. [DOI] [PubMed] [Google Scholar]
- 14.Penn I. The effect of immunosuppression on pre-existing cancers. Transplantation. 1993;55(4):742–7. [DOI] [PubMed] [Google Scholar]
- 15.Beaugerie L, Brousse N, Bouvier AM, et al. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: A prospective observational cohort study. Lancet. 2009;374(9701):1617–25. [DOI] [PubMed] [Google Scholar]
- 16.Kandiel A, Fraser AG, Korelitz BI, et al. Increased risk of lymphoma among inflammatory bowel disease patients treated with azathioprine and 6-mercaptopurine. Gut. 2005;54(8):1121–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poullenot F, Seksik P, Beaugerie L, et al. Risk of incident cancer in inflammatory bowel disease patients starting anti-TNF therapy while having recent malignancy. Inflamm Bowel Dis. 2016;22(6):1362–9. [DOI] [PubMed] [Google Scholar]
- 18.Axelrad J, Bernheim O, Colombel JF, et al. Risk of new or recurrent cancer in patients with inflammatory bowel disease and previous cancer exposed to immunosuppressive and anti-tumor necrosis factor agents. Clin Gastroenterol Hepatol. 2016;14(1):58–64. [DOI] [PubMed] [Google Scholar]
- 19.Beaugerie L, Carrat F, Colombel JF, et al. Risk of new or recurrent cancer under immunosuppressive therapy in patients with IBD and previous cancer. Gut. 2014;63(9):1416–23. [DOI] [PubMed] [Google Scholar]


