ABSTRACT
Immune checkpoint inhibitors (ICIs) can result in immune-related adverse events which require rapid identification and treatment. Gastrointestinal immune-related adverse events are among the most frequent and severe of these events. ICI colitis can be refractory to current therapies such as corticosteroids and biologic therapy. Fecal microbiota transplantation (FMT) is currently used in cases of recurrent Clostridioides difficile colitis. Many investigations are underway to test the utility of FMT for additional indications, including inflammatory bowel disease (IBD). We present a 71-year-old man with ICI colitis that was nonresponsive to currently defined management options and treated with benefit from FMT.
INTRODUCTION
The indications of immune checkpoint inhibitor (ICI) as a cancer therapy are rapidly expanding. ICI works by blocking inhibitory pathways to improve a patient's immune system response against cancer. Targets of ICI include programmed cell death receptor-1 (PD-1), programmed cell death ligand (PD-L1), and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4). ICIs are associated with several autoimmune adverse events, with diarrhea and colitis being among the most common.1 Help in the identification and management of immune-related adverse events (irAE) can be found in practice guidelines (including the National Comprehensive Cancer Network, the Society for Immunotherapy of Cancer, and the American Society of Clinical Oncology). Treatment is defined by the severity of the toxicity. The histopathology of ICI colitis reveals a pattern distinct from inflammatory bowel disease with dysregulation of GI mucosal immunity. The most frequent findings in ICI colitis include focal neutrophilic cryptitis and neutrophilic infiltration in the lamina propria, followed by excess plasma cells in the lamina propria and lymphocytic cryptitis, whereas biopsies from inflammatory bowel disease are characterized by basal plasmacytosis, lamina propria hypercellularity, mucin depletion, granulomas, crypt abscesses, cryptitis, and ulceration.2,3 Evidence now exists that the gut microbiome is closely associated with the immunomodulatory system and that certain microbiota profiles can influence responses to ICI therapy and potential adverse events.4 Furthermore, microbiome analysis has been shown to identify patients at risk for irAE colitis.5 Not only can the microbiome help to possibly predict the response to treatment or adverse events but also, based on these relationships, it is possible that manipulation of the microbiome could aid in the management of ICI colitis.
CASE REPORT
A 71-year-old man with a medical history of gastric adenocarcinoma with diffusely metastatic disease to the lungs and peritoneum was initiated on pembrolizumab. The patient had no history of autoimmune disease. He developed autoimmune arthritis with cycle 2 of pembrolizumab, which resolved with a course of steroids. He resumed pembrolizumab and shortly after his third dose, he developed symptoms suggestive of ICI colitis with abdominal cramping, pain, and severe diarrhea with greater than 10 bowel movements (BMs) per day. A computed tomography scan showed pancolitis, and a flexible sigmoidoscopy examining only the left colon found severe cobblestoning and edematous folds in a patchy distribution. The inflammation was described as moderate to severe in the rectosigmoid colon, with mild erythema of the left colon and moderate to severe inflammation also at the splenic flexure. A biopsy showed mild active colitis, negative for cytomegalovirus. Intravenous (IV) steroids were given for 7 days. The patient continued to complain of an average of 10 BMs per day and abdominal pain. Owing to a lack of response, infliximab 5 mg/kg was administered. He was discharged on a steroid taper with a subjective mild decrease in BMs frequency.
Six days later, on 50 mg prednisone daily, he returned with abdominal cramping, weight loss, and diarrhea. Stool culture, ova and parasites, and Clostridioides difficile testing were negative. IV steroid treatment was reinitiated with methylprednisolone 125 mg IV daily and the addition of mycophenolate mofetil 500 mg twice daily (these therapies started on day 21 of total steroid therapy). Mesalamine 400 mg 3 times daily was added. After 48 hours, his symptoms worsened with poor oral intake, no change in complaints of abdominal pain, diarrhea, and no improvement on physical examination. Mesalamine was increased to 1,000 mg 4 times daily, methylprednisolone was changed to 2 mg/kg twice daily, and total parenteral nutrition was started. A second dose of infliximab (10 mg/kg) was administered on day 27 of total colitis treatment. The patient continued to experience abdominal cramping, tenesmus, perirectal discomfort, and nonbloody diarrhea hourly. As there was still no improvement after the second infliximab infusion, flexible sigmoidoscopy was repeated. Endoscopically, severe colitis characterized by mucosal edema with significant luminal narrowing, friability, and loss of vascular markings was found in the distal rectosigmoid colon (Figure 1). Given the severity of the inflammation, the endoscope was not advanced beyond the descending colon but the pattern of inflammation was noted to vary from mild to severe in a patchy distribution. Biopsies were obtained showing chronic inflammation with regenerative changes and fibrosis in the lamina propria (Figure 2). Hydrocortisone suppositories 25 mg twice a day were added to the treatment, and the following day, mycophenolate was increased to 1 g twice a day with steroids decreased to 1 mg/kg daily because of side effects. Owing to a lack of improvement, the patient then received a dose of vedolizumab 300 mg on day 35 of the treatment.
Figure 1.
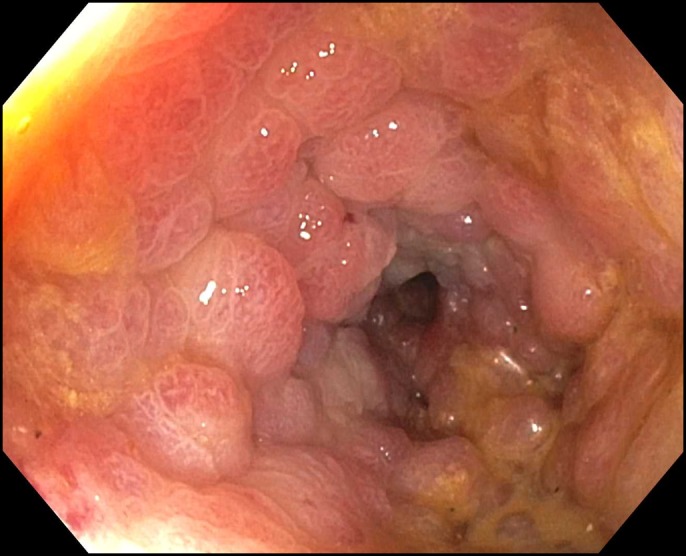
Distal colon with congested mucosa and luminal narrowing.
Figure 2.
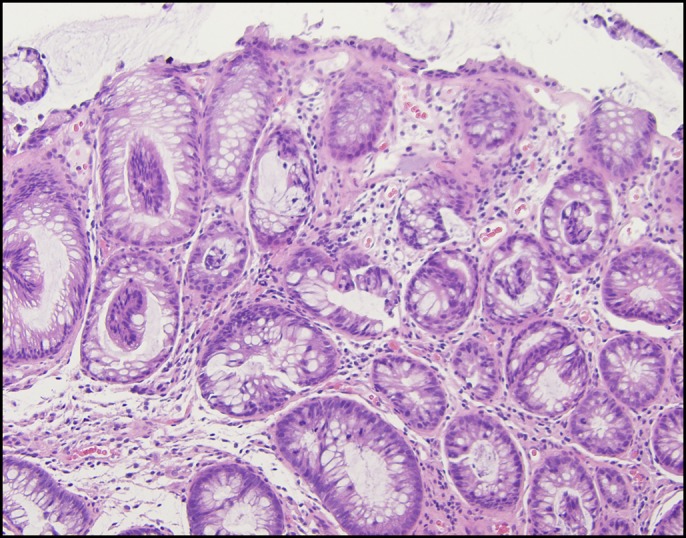
Rectal biopsy showing chronic inflammation with regenerative changes and fibrosis in the lamina propria.
The patient continued to experience symptoms consistent with severe ICI colitis with complaints of more than 10 BMs per day despite current therapy, including diphenoxylate and atropine (Pfizer NY, NY) and loperamide (Johnson & Johnson McNeil Consumer Healthcare Fort, Washington, PA), and abdominal pain requiring IV narcotics. The determination was made to obtain a single-patient investigational new drug approval for compassionate use of fecal microbiota transplantation (FMT) via colonoscopy. Investigational new drug approval was obtained from the FDA and from the institutional IRB. The patient underwent a colonoscopy on day 47, which showed scattered severe inflammation in the entire colon with distortion and narrowing of the lumen with patchy areas of severe edema and some mild to moderate stricturing in both the proximal and distal colon (Figure 3). Donor stool from OpenBiome (Cambridge, MA) was prepared as per OpenBiome's predetermined protocol. Approximately 500 mL of the emulsified donor stool was instilled in the cecum. Two tablets of loperamide were given immediately after the procedure and before bedtime.
Figure 3.
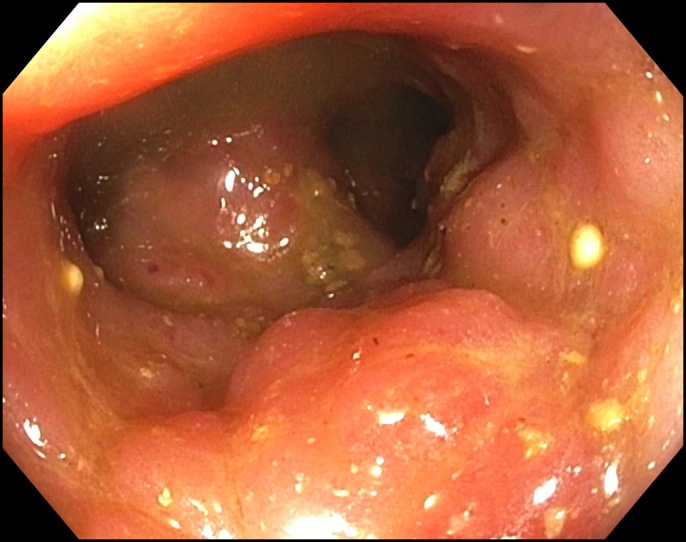
Proximal colon with severe edema and stricturing.
The patient reported improvement in BM frequency on day 2 after FMT. His abdominal pain decreased by day 3, and he was discharged on a steroid taper. Before FMT, the patient was having greater than 10 BMs a day and required IV narcotics for pain control. Ten days after FMT, the patient was having 2 to 3 BMs a day, denied abdominal pain, and indicated that he did not require the oral hydromorphone prescribed at discharge. No adverse effects specific to FMT were identified. He complained of fatigue and exercise intolerance. The patient succumbed due to his oncologic disease 1 month after FMT but did not have any return of colitis symptoms.
DISCUSSION
Risk factors associated with ICI colitis include anti-CTLA therapy, combination ICI therapy, dose of ICI, NSAID use, pre-existing IBD, tumor histology, and aspects of the microbiota (baseline microbiota enriched in Firmicutes and poor in Bacteroides specifically with anti-CLTA-4 therapy).6 Documented therapies for irAE enterocolitis include steroids, infliximab, mesalamine, vedolizumab, tocilizumab, adalimumab, methotrexate, and mycophenolate.6
There is accumulating experimental and clinical evidence that the microbiome is involved in both antitumor and enterocolitis response in patients receiving ICI therapy. There is also evidence that patients who had received antibiotics responded less well to anti-PD-1 therapy.7 The possibility of altering a patient's microbiome via FMT to improve the chances of success of ICI therapy and minimizing adverse events carries much hope and drives much of the current microbiome research. Our patient had severe refractory ICI colitis and experienced a noteworthy improvement after FMT. At present, this is only the second report on FMT in the management of ICI colitis. The previous report described 2 patients with refractory conditions, both of whom experienced substantial benefit after FMT.8 Thus, further investigation of FMT for the management of ICI colitis and possibly other ICI irAE is warranted.
DISCLOSURES
Author contributions: MK Fasanello, KT Robillard, and PM Boland wrote, edited, and approved the final manuscript. AJ Bain edited and approved the final manuscript. K. Kanehira provided the pathology images. KT Robillard is the article guarantor.
Financial disclosure: OpenBiome provided the emulsified donor stool used for FMT free of charge.
Informed consent was obtained for this case report.
REFERENCES
- 1.Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev. 2016;44:51–60. [DOI] [PubMed] [Google Scholar]
- 2.Berman D, Parker SM, Siegel J, et al. Blockade of cytotoxic T-lymphocyte antigen-4 by ipilimumab results in dysregulation of gastrointestinal immunity in patients with advanced melanoma. Cancer Immun. 2010;10:11. [PMC free article] [PubMed] [Google Scholar]
- 3.Feakins RM. Inflammatory bowel disease biopsies: Updated British Society of Gastroenterology reporting guidelines. J Clin Pathol. 2013;66:1005–26. [DOI] [PubMed] [Google Scholar]
- 4.Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti-PD immunotherapy in melanoma patients. Science. 2018;359:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dubin K, Callahan MK, Ren B, et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat Commun. 2016;7:10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soularue E, Lepage P, Colombel JF, et al. Enterocolitis due to immune checkpoint inhibitors: A systematic review. Gut. 2018;67:2056–67. [DOI] [PubMed] [Google Scholar]
- 7.Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91–7. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Wiesnoski DH, Helmink BA, et al. Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat Med. 2018;24:1804–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


