Abstract
Incorporation of hydroxyapatite (HA) into polymer networks is a promising strategy to enhance the mechanical properties and osteoinductivity of the composite scaffolds for bone tissue engineering. In this study, we designed a group of nanocomposite scaffolds based on cross-linkable poly (propylene fumarate) (PPF) and 30 wt% strontium-hydroxyapatite (Sr-HA) nanoparticles. Four different Sr contents (Sr:(Sr+Ca), molar ratio) in the Sr-HA particles were studied: 0% (HA), 5% (Sr5-HA), 10% (Sr10-HA), and 20% (Sr20-HA). Two-dimensional (2D) disks were prepared using a thermal crosslinking method. The structure and surface morphology of different Sr-HA and PPF/Sr-HA composites were characterized using scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDS), transmission electron microscopy (TEM), and atomic force microscopy (AFM). To detect cellular responses in vitro, MC3T3-E1 cells were seeded and cultured on the different PPF/Sr-HA composite disks. Cell morphology after 24 h and 5 d were imaged using Live/Dead live cell staining and SEM, respectively. Cell proliferation was quantified using an MTS assay at 1, 4, and 7 days. Osteogenic differentiation of the cells was examined by alkaline phosphatase (ALP) staining at 10 days and quantified using ALP activity and osteocalcin assays at 7, 14, and 21 days. The sizes of the HA, Sr5-HA, Sr10-HA, and Sr20-HA particles were mainly between 10×20 nm and 10×250 nm, and these nanoparticles were dispersed or clustered in the composite scaffolds. In vitro cell studies showed that the PPF/Sr10-HA scaffold was significantly better than the other three groups (PPF/HA, PPF/Sr5-HA, and PPF/Sr20-HA) in supporting MC3T3-E1 cell adhesion, proliferation, and differentiation. PPF/Sr10-HA may, therefore, serve as a promising scaffold material for bone tissue engineering.
Keywords: nanoparticles, osteogenic differentiation, poly(propylene fumarate), strontium, strontium-substituted hydroxyapatite
INTRODUCTION
Bone tissue engineering focuses on the selection and optimization of material scaffolds, cells, and osteogenic and vascular growth factors for tissue regeneration [1]. The ideal biomaterials for bone regeneration should have characteristics including excellent biocompatibility, osteoconductive activity, osteoinductive activity, and mechanical compatibility [2]. In the past few decades, many scaffold materials have been designed for bone regeneration using various materials, such as poly(propylene fumarate) (PPF), poly(lactic-co-glycolic acid) (PLGA), true bone ceramic, hydroxyapatite (HA), collagen-HA, calcium phosphate, decalcified bone matrix (DBM), fibrin, and titanium [3–11]. However, as scaffolds, these materials do not consistently promote bone repair due to lack of osteoinductive or osteoconductive activity.
PPF is a type of unsaturated linear polyester that can be crosslinked through carbon-carbon double bonds along its backbone to provide effective mechanical properties as a scaffold material [12]. It shows no chemical cytotoxicity and can be degraded into nontoxic poly(acrylic acid-co-fumaric acid), propylene glycol, and fumaric acid by simple hydrolysis of the ester bonds [13]. In addition, it can be printed into a variety of desired three dimensional (3D) shapes. However, its mechanical strength and osteoconductive properties need to be further improved [14]. To compensate for these shortcomings, we previously added different amounts of HA to PPF [14]. We found that a PPF: HA mass ratio of 70:30 not only enhanced the mechanical strength and osteoconductive properties of PPF but also promoted adhesion and proliferation of MC3T3-E1 cells in vitro on the surface of 2D or 3D PPF/HA composites [14,15]. In addition, the incorporation of HA particles in the PPF/HA composites also increased hydrophilicity and protein adsorption on the material surface [14].
PPF/HA composites may be able to serve as scaffold material for bone regeneration with sufficient mechanical strength and good osteoconductivity, but they lack osteoinductive activity. In previous studies, most scaffold materials were combined with recombinant human bone morphogenetic protein 2 (BMP-2), BMP-2-related peptides, collagen, osteoprotegerin, or other small-molecule active peptides to increase their osteoinductive activity [16–20]. By simple physical adsorption, however, the activity-inducing factors will be released very quickly. Recently, strontium, a trace element in the human body, has been found to have the ability to promote bone formation and inhibit osteoclasts [21]. Some studies have reported the effects and mechanisms of strontium-promoted osteogenic differentiation of mesenchymal stem cells, and bone defects were repaired by strontium-substituted HA (Sr-HA) materials [22,23]. These results showed that strontium could induce osteogenesis, and Sr-HA materials could promote osteogenesis.
In this work, we hypothesized that Sr-HA combined with PPF could improve the osteogenic activity of PPF/HA. We had previously fabricated PPF/HA scaffolds with different HA contents and found that scaffolds with 30 wt% HA were optimal in terms of mechanical properties and cell growth [14,24]. We, therefore, maintained the ratio of PPF/Sr-HA at 70:30 and varied the Sr contents (Sr:(Sr+Ca), molar ratio) in the Sr-HA particles: 0% (HA), 5% (Sr5-HA), 10% (Sr10-HA), and 20% (Sr20-HA). We characterized the structure and surface morphology of the different Sr-HA and PPF/Sr-HA composites with scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDS), and transmission electron microscopy (TEM). We also investigated cellular responses in vitro by culturing MC3T3-E1 cells on the different PPF/Sr-HA composite disks and examining cell morphology, cell proliferation, and cell osteogenesis differentiation.
MATERIALS AND METHODS
PPF synthesis
PPF was synthesized through a 2-step procedure, as previously described [25]. First, diethyl fumarate was combined with excess 1,2-propylene glycol, the crosslinking inhibitor hydroquinone, and the catalyst zinc chloride then incubated at 100°C for 1 h and 150°C for 7 h. The formed intermediate was then transesterified under vacuum at 150°C for 7 h to form PPF. The number-average molecular weight (Mn) and weight-average molecular weight (Mw) of PPF was determined by gel permeation chromatography (GPC) on a Waters 717 Plus Autosampler GPC system (Waters; Milford, MA) connected to a high-performance liquid chromatography pump (model 515) and a refractive index detector (model 2410). Monodisperse polystyrene standards (Polysciences; Warrington, PA) with Mn of 474, 6,690, 18,600, and 38,000 g/mol were used to construct the calibration curve. The Mn and Mw of the synthesized PPF were 1,674 and 4,181 g/mol, respectively.
Sr-HA synthesis
HA and Sr-HA were synthesized by a wet chemical method [26] with some modifications (Fig. 1). Briefly, different weights of Ca(NO3)2 and Sr(NO3)2 were separately dissolved in double-distilled water at room temperature. The Sr/(Sr+Ca) molar ratio of the mixed solutions were 0, 0.05, 0.1, and 0.2, respectively. The above precursor solutions of various concentrations were mixed uniformly and heated at 50°C, then (NH4)2·HPO4 solution was added gradually into the different mixed solutions. The reaction system was placed on an ultrasonic shaker to complete dispersion, and the pH was adjusted to 10.0 with NH4OH. The (Ca+Sr)/P ratio of the reaction system was maintained at 5/3, and the mixture was stirred at 400 rpm and kept at 50°C for 5h. Finally, the precipitate was filtered, washed 3 times, sterilized, and dried at 120°C overnight.
Figure 1.
HA (A) and Sr‐HA (B) were synthesized by a wet chemical method.
PPF/Sr-HA preparation
PPF/Sr-HA was prepared as previously described [14, 15]. PPF and HA or Sr-HA were crosslinked separately by free-radical polymerization using the crosslinker 1-vinyl-2-pyrrolidinone (NVP), free radical initiator benzoyl peroxide (BPO), and accelerator N-dimethyl-p-toluidine (DMT), successively. To prepare PPF/HA or PPF/Sr-HA composites, every 1 g of PPF and 0.4 g of NVP were mixed in glass bottles at 37°C for 2 h. HA or Sr-HA powder was added to prepare the PPF/HA or PPF/Sr-HA composite at an HA or Sr-HA composition of 30 wt%. Forty μL of BPO solution (50 mg of BPO in 250 μL of NVP) and 16 μL of DMT solution (20 μL of DMT in 980 μL of methylene chloride) were added and mixed completely. PPF/HA or PPF/Sr-HA composites were then poured into cylindrical molds of 10 mm in diameter and 1 mm in depth and allowed to dry overnight at 60°C to facilitate crosslinking before cooling to room temperature. The crosslinked PPF/HA or PPF/Sr-HA sample disks were removed from the molds for future use.
Sr-HA powder characterization
The surface morphology and elemental composition of HA and Sr-HA powder were examined under SEM and EDS (S-4700; Hitachi Instruments Inc, Tokyo, Japan), respectively. For SEM and element map imaging, the samples were mounted onto an aluminum stub and sputter coated with gold. All samples were detected at 3 kV accelerating voltage.
The morphology of HA and Sr-HA powder was also examined by TEM (JEM 1400, JEOL Inc., Tokyo, Japan). Samples no thicker than 200 nm were placed on an aluminum stub. All samples were detected at 80 kV accelerating voltage.
PPF/Sr-HA disk characterization
The surface morphology of the crosslinked PPF/HA and PPF/Sr-HA disks was examined similarly with SEM. For SEM imaging, the surface of the samples was coated with gold and detected at 3 kV accelerating voltage.
The morphology of HA and Sr-HA particles in the crosslinked PPF/HA and PPF/Sr-HA disks was examined similarly by TEM. The samples were embedded and frozen in liquid nitrogen; then, the blocks were sectioned with a diamond knife into 200-nm–thick sections. All samples were detected at 80 kV accelerating voltage.
Surface morphologies of various types of PPF/HA and PPF/Sr-HA scaffolds were determined using Nanoscope IV PicoFroce Multimode atomic force microscopy (AFM, Bruker). Images of 10 μm × 10 μm (width × length) were collected in contact mode at room temperature [27, 28]. The surface roughness (Rq, Root Mean Square) was calculated and averaged from 5 different areas in the AFM images.
MC3T3-E1 cell culture
For cell attachment and proliferation studies, MC3T3-E1 cells were cultured in vitro using α-Dulbecco’s modified Eagle’s medium (α-DMEM; Gibco), supplemented with 10% fetal bovine serum (Sigma-Aldrich) and 1% penicillin/streptomycin (Gibco). For cell differentiation, the above medium was supplemented with 50 μg/mL ascorbic acid (Sigma-Aldrich) and 10 mM β-glycerophosphate (Sigma-Aldrich). Crosslinked PPF/HA and PPF/Sr-HA disks were immersed in 70% ethanol for 24 h for sterilization, washed with phosphate buffered saline (PBS) (pH 7.4) 3 times, and then prewetted by immersing them in the medium overnight before cell seeding. MC3T3-E1 cells were seeded on the surface of prewetted disks at a density of 2×104 cells/cm2 and incubated at 37°C in a humidified atmosphere of 5% CO2. According to the needs of the experiment, the cells were cultured for different durations.
Cell viability and attachment morphology
Cell attachment and morphology on the surface of PPF/HA and PPF/Sr-HA disks were detected by using the Live/Dead cell double staining kit (Invitrogen) at 24 h post seeding and by SEM at 5 d post seeding. After the culture medium in the plate was discarded, the disks were washed 3 times with PBS to remove residual lipase activity. Each sample was treated with 100 μL prepared assay solution, incubated at 37°C for 15 min, and viewed with confocal laser scanning electron microscopy.
For SEM examination of samples with cells, the cell medium was removed and cells were fixed with Trump solution composed of 1% glutaraldehyde and 4% formaldehyde in PBS, then washed sequentially using PBS and distilled water. The samples were then dehydrated using a graded series of ethanol washes (60%, 70%, 80%, 95%, and 100%) and then critical point dried. Finally, all samples were mounted onto an aluminum stub, sputter-coated with gold, and viewed at 3 kV accelerating voltage.
Cell proliferation
Cell proliferation was quantified using an MTS assay (CellTiter 96 AQueous One Solution Cell Proliferation Assay; Promega, Madison, WI) after 1, 4, and 7 d of cell seeding according to the kit’s instruction. At different time points, the number of living cells on each disk was correlated to the UV absorbance at 490 nm measured by a SpectraMax Plus microplate reader.
Cell differentiation
Alkaline phosphatase (ALP) live staining was examined on the 10th day of osteogenic differentiation, using ALP Live Stain kit (Invitrogen) according to the kit’s instructions. Briefly, after the culture medium was removed, the samples were washed with fresh medium twice and then incubated with prepared ALP Live Stain working solution for 30 min. Afterward, the stain solution was removed and cells were washed twice with fresh medium. All samples were viewed under a fluorescent microscope (ZEISS model AXIO Imager, Germany).
ALP activity was quantified using a Bioassay Systems’ QuantiChrom ALP Assay Kit after 7, 14, and 21 d of cell seeding, according to the kit’s instructions. At different time points, the absorbance of the sample lysate was measured using the SpectraMax Plus microplate reader at a wavelength of 405 nm.
Osteocalcin activity was examined with a Bioassay Systems’ QuantiChrom Calcium Assay Kit after 7, 14, and 21 d of cell seeding according to the kit’s instructions. At different time points, the absorbance of the total cell culture media in each sample was measured using the SpectraMax Plus microplate reader at a wavelength of 612 nm.
Statistical analysis
All measured data were expressed as arithmetic mean ± standard deviation (SD). Analysis of variance (ANOVA) and Tukey’s post-test were performed to assess statistical significance between groups using SPSS 19.0 (SPSS Inc., Chicago, IL, USA). P<0.05 was considered statistically significant.
RESULTS
Sr-HA characterization and morphology
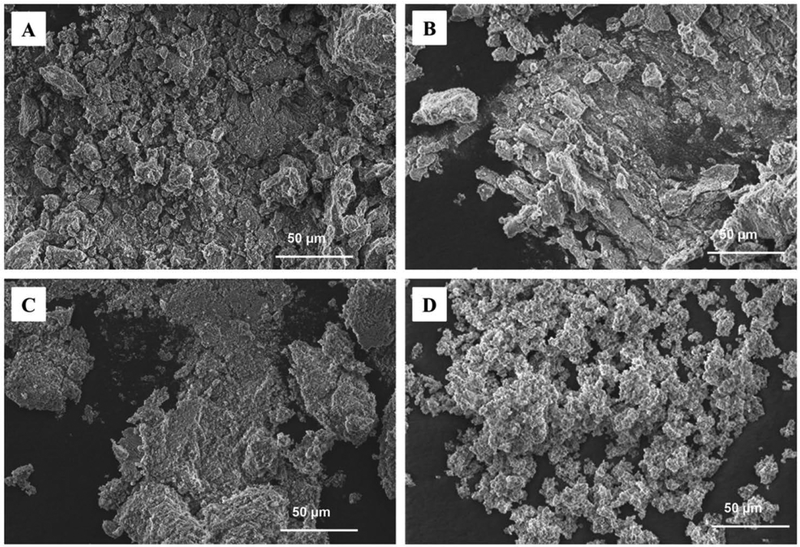
SEM images of HA, Sr5-HA, Sr10-HA, and Sr20-HA showed the formation of apatite-like structures (Fig. 2). An EDS element map confirmed that different amounts of calcium were replaced by strontium in Sr5-HA, Sr10-HA, and Sr20-HA (Fig. 3). Element analysis by EDS indicated that the Sr contents in Sr5-HA, Sr10-HA, and Sr20-HA were 7.93%±0.71%, 13.23%±0.71%, and 27.05%±0.69%, respectively. By TEM, the sizes of HA, Sr5-HA, Sr10-HA, and Sr20-HA particles were mainly between 10×20 nm and 10×250 nm (Fig. 4). HA was observed to be rod-shaped (Fig. 4 A), and Sr5-HA, Sr10-HA, and Sr20-HA were arranged in a flower-like pattern (Fig. 4 B–D), which was most obvious for Sr20-HA (Fig. 4 C).
Figure 2.
SEM images of HA (A), Sr5‐HA (B), Sr10‐HA (C), and Sr20‐HA (D).
Figure 3.
TEM images of HA (A), Sr5‐HA (B), Sr10‐HA (C), and Sr20‐HA (D). Sizes of the particles were primarily between 10 × 20 nm and 10 × 250 nm.
Figure 4.
Fabricated disks of PPF/HA (A), PPF/Sr5‐HA (B), PPF/Sr10‐HA (C), and PPF/Sr20‐HA (D). Surface morphology (E) and internal section morphology (F) of PPF/Sr10‐HA sample.
PPF/Sr-HA characterization and morphology
The macrostructures of PPF/HA, PPF/Sr5-HA, PPF/Sr10-HA, and PPF/Sr20-HA disks are shown in Figure 5 A–D. Because all composite scaffolds have the same ratio of PPF/Sr-HA (70:30) but different amounts of Sr, a representative sample using Sr10-HA was used to view the surface morphology and internal section morphology of PPF/Sr-HA by SEM (Fig. 5E and F). SEM showed that different sizes of white spots (Sr10-HA nanoparticles) were well dispersed on the surface and throughout the samples.
Figure 5.
Fabricated disks of PPF/HA (A), PPF/Sr5‐HA (B), PPF/Sr10‐HA (C), and PPF/Sr20‐HA (D). Surface morphology (E) and internal section morphology (F) of PPF/Sr10‐HA sample.
TEM characterization (Fig. 6) showed that the HA and Sr-HA nanoparticles were dispersed or clustered in the PPF/HA and PPF/Sr-HA samples after simple mixing and crosslinking. The distribution of HA and Sr-HA nanoparticles was similar to previously reported surface grafting with HA particles for PPF/HA composites [14, 29].
Figure 6.
TEM images of PPF/HA (A), PPF/Sr5‐HA (B), PPF/Sr10‐HA(C), and PPF/Sr20‐HA (D).
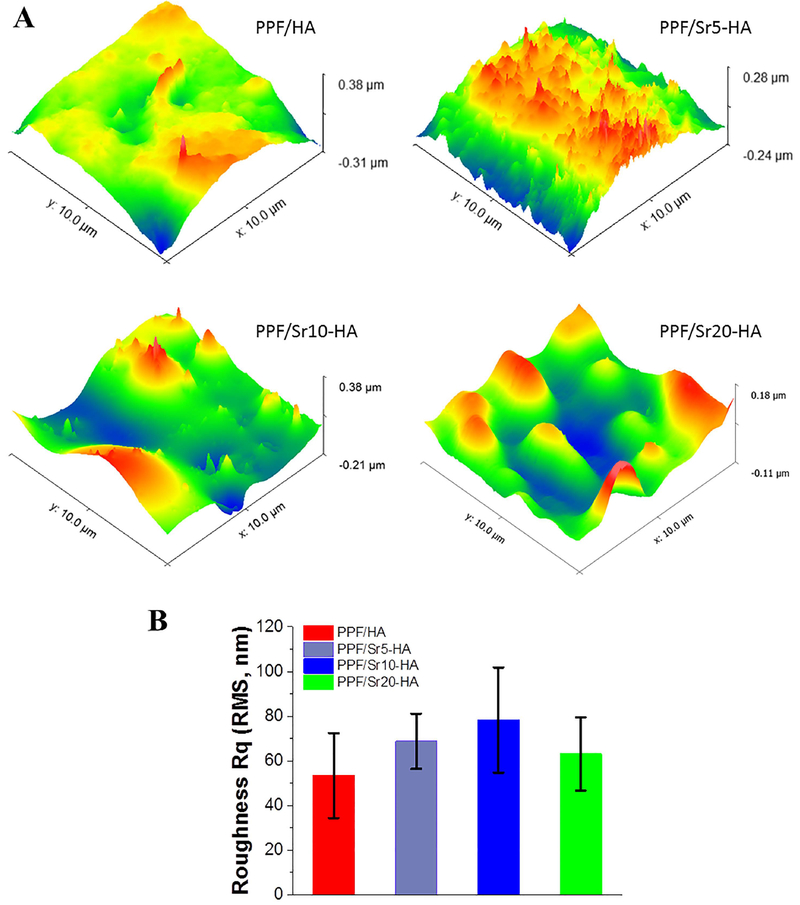
The surface morphological features of the various scaffolds were determined using atomic force microscopy within a 10 μm × 10 μm range. As presented in Fig. 7A, surface patterns varied slightly among the PPF-HA scaffolds with different ratios of strontium substitution. The surface roughness was calculated to be 53.35 ± 19.03 nm, 68.76±12.36 nm, 78.22±23.60 nm, and 63.05±16.43 nm for PPF/HA, PPF/Sr5-HA, PPF/Sr10-HA, and PPF/Sr20-HA scaffolds, respectively (Fig. 7B). Statistical analysis showed no significant differences in surface roughness for these scaffolds (P > 0.05).
Figure 7.
Atomic force micrographs (AFM) of PPF/Sr‐HA disks with different strontium contents. (A) 3D images. (B) Root mean square (RMS) roughness of PPF/ HA, PPF/Sr5‐HA, PPF/Sr10‐HA, and PPF/Sr20‐HA. No significant differences in surface roughness for these four scaffolds (p > 0.05).
Cell viability and attachment morphology
Cell viability on PPF/HA and PPF/Sr-HA nanocomposite scaffolds was detected using the Live/Dead cell viability assay at 24 h. Among the 4 groups of materials (PPF/HA, PPF/Sr5-HA, PPF/Sr10-HA, and PPF/Sr20-HA), cell density was highest on the surface of the PPF/Sr10-HA nanocomposite scaffolds, as seen from the confocal laser scanning electron microscopy images (Fig. 8). The PPF/Sr20-HA group had the lowest viable cell density and the highest dead cell density. This result indicated that cell viability was greatest on the surface of the PPF/Sr10-HA disks.
Figure 8.
Live/dead cell viability assay images of MC3T3‐E1 cells after 24 h seeding on PPF/HA, PPF/Sr5‐HA, PPF/Sr10‐HA, and PPF/Sr20‐HA scaffolds. Cell density on PPF/Sr10‐HA nanocomposite scaffolds was the highest (p < 0.05) among the groups. Green color indicates live cells; red indicates dead cells.
Cell attachment morphology on PPF/HA and PPF/Sr-HA nanocomposite scaffolds was observed by SEM 5 d after cell seeding. The live MC3T3-E1 cells completely adhered to the surfaces of PPF/HA, PPF/Sr5-HA, PPF/Sr10-HA, and PPF/Sr20-HA scaffold materials, and the cells showed long spindle and polygonal shapes. More cells were attached and more protruding cellular processes (for connection between adjacent cells) were found on the PPF/Sr10-HA surface (Fig. 9 A–H). The PPF/Sr10-HA group had considerably more cells than the PPF/HA, PPF/Sr5-HA, and PPF/Sr20-HA groups, while the PPF/Sr20-HA group had the fewest cells on the material surface.
Figure 9.
SEM images of MC3T3‐E1 cells after 5 days of culture on PPF/HA (A, E), PPF/Sr5‐HA (B, F), PPF/Sr10‐HA (C, G), and PPF/Sr20‐HA (D, H) scaffolds. MTS assay analysis of cell proliferation (I) on PPF/HA and PPF/Sr‐HA samples at 1, 4, and 7 days (*p < 0.05).
Cell proliferation
Cell proliferation on PPF/HA and PPF/Sr-HA samples at 1, 4, and 7 d was examined indirectly by the MTS assay. Directly correlating to MTS absorption, the cell density on all groups of scaffolds gradually increased after 1 day of culture, but there was no significant difference between the groups (P>0.05). Cell density in the PPF/Sr10-HA group was significantly higher at 4 and 7 d than in the other 3 groups (P<0.05) (Fig. 9 I). However, in the PPF/Sr20-HA group, the cell density gradually decreased with culture time, possibly due to the higher Sr ion concentrations which inhibited cell growth.
Cell differentiation
ALP live staining showed that the MC3T3-E1 cells accumulated to form multiple layers on all 4 substrates. Green particles were deposited in the cytoplasm of ALP-positive cells after 10 d of culture. From the ALP live stain images, the PPF/Sr10-HA group had substantially more green particles in the cytoplasm than the other 3 groups (Fig. 10A–D). ALP activity (Fig. 10E) was low after 7 d of cell culture, with no significant difference between the groups (P>0.05).
Figure 10.
ALP live staining images showing the number of green particles in the cytoplasm of MC3T3‐E1 cells after 10 days of culture on PPF/Sr10‐HA scaffolds (C) was significantly higher (p < 0.05) than the other groups of (A) PPF/HA, (B) PPF/Sr5‐HA, and (D) PPF/Sr20‐HA. ALP (E) and osteocalcin (F) quantification at 7, 14, and 21 days after cell seeding (*p < 0.05).
At 14 d of cell culture, the ALP activity of all groups increased and reached peak values, with the PPF/Sr10-HA group significantly higher than the other 3 groups (P<0.05). When cells were cultured for 21 d, the ALP activity in each group decreased to varying degrees, but the ALP activity in the PPF/Sr10-HA group was still significantly higher than in the other 3 groups (P<0.05).
With increasing cell culture time, the osteocalcin content of the 4 groups gradually increased (Fig. 10F). At each time point, the osteocalcin content in the PPF/Sr10-HA group was significantly higher than that in the other 3 groups (P<0.05). There was no significant difference in osteocalcin content among the other 3 groups at 7, 14, or 21 d (P>0.05).
DISCUSSION
In this study, PPF and Sr-HA were mixed and prepared into 2D disks with different ratios of Sr-substituted HA (PPF/HA, PPF/Sr5-HA, PPF/Sr10-HA, and PPF/Sr20-HA) by chemical crosslinking. The composite material had a PPF to HA ratio of 70:30 w/w, which as optimized in our previous studies based on enhanced mechanical strength and protein adsorption on the surface of PPF/HA substrates [14, 15]. In the current study, we used strontium to replace part of calcium in HA to obtain Sr contents of 0%, 5%, 10%, and 20% in Sr-HA, in order to further promote osteogenic differentiation. The results of the in vitro experiments confirmed that PPF/Sr10-HA had good cell compatibility and could promote osteogenic differentiation of MC3T3-E1 cells.
Strontium is an essential trace element in the human body; its content in bone is approximately 0.01%. Strontium is also believed to be important for decreasing bone resorption and increasing new bone formation [26]. Strontium-substituted HA-induced osteogenesis is currently a hot topic of research, but the optimal proportion of Sr-HA has not been determined. Yan et al [30] demonstrated that 20% Sr-HA had good osteoconductivity, which could promote better bone growth and improve bone-implant integration. Xu et al [31] reported that Sr-HA could promote the proliferation of MG-63 cells, and 5% Sr-HA composite was optimal for cell activity. Kaygili et al [32] showed that HA with different amounts of strontium ranging from 0.45 atom% to 2.25 atom% could promote bone formation and osteo-integration, and 0.45 atom% HA had the best results. Ni et al. used 10% Sr-HA in the preparation of bone cement [33]. Our previous study confirmed that true bone ceramic (TBC) modification with 10% Sr-HA could better stimulate osteogenesis [34].
Different studies have resulted in different optimal ratios of Sr-HA, which may be attributed to the method of Sr-HA preparation, the material composition other than Sr-HA, and different cell types used for osteogenesis. In the current study, the actual Sr contents of HA, Sr5-HA, Sr10-HA, and Sr20-HA tested by EDS were 0%, 7.93%, 13.23%, and 27.05%, respectively. The results also showed that PPF scaffolds incorporating 10% Sr-HA could promote osteogenesis better than the other 3 groups.
PPF is a promising candidate material for load-bearing applications. However, as a bone scaffold material, its biomechanical and osteoconductive properties need to be further enhanced. In previous studies aiming to improve the biological properties of PPF, we prepared different composite scaffold materials, such as PPF/β-tricalcium phosphate [35], PPF/HA [15], PPF/PLGA [36], and PPF-co-poly(caprolactone) [37]. Among them, PPF/HA nanocomposites increased the mechanical strength and osteoconductive ability of PPF. HA also served as a physical filler for the polymer matrix and changed the chemical properties of the surface as an additive to PPF [15]. In the current study, the use of Sr-HA with osteoinductive activity not only increased the contact area between the surface of PPF and cells but also promoted osteogenesis of cells. The study also confirmed that Sr-HA can promote MC3T3-E1 cell osteogenic differentiation to different degrees, with Sr10-HA having the strongest osteoinductive effect. In addition, the combination of PPF and Sr-HA nanoparticles was helpful for increasing the amount of serum protein adsorbed on the surface of the material and for further promoting cell adhesion, proliferation, and differentiation [38, 39].
Related studies have reported that nanophase ceramics smaller than 100 nm could promote the proliferation of osteoblasts, induce the synthesis of ALP and osteocalcin, and increase the concentration of calcium in the extracellular matrix [40]. In our study, the sizes of HA, Sr5-HA, Sr10-HA, and Sr20-HA particles were mostly between 10×20 nm and 10×250 nm. In vitro cell experiments confirmed that the 4 groups of materials could promote MC3T3-E1 cell proliferation and osteogenic differentiation to varying degrees. A recent study indicated that the osteogenic mechanism of Sr2+ was mainly through the interaction of calcium-sensing receptors with cells to activate inositol-1,4,5-triphosphate production and mitogen-activated protein kinase signaling, along with some nonselective Ca2+ channels, to regulate osteogenic differentiation [41]. Barbara et al [42] reported that when the Sr2+ concentration was 0.1 to 1 mmol/L in long-term culture, strontium could increase the collagen matrix of MC3T3-E1 cells without adverse effects on matrix mineralization. Schumacher et al [43] showed that the optimal concentration of Sr2+ at 1 mmol/L could significantly promote the proliferation and osteogenic differentiation of mesenchymal stem cells, whereas Sr2+ concentration greater than 1 mmol/L significantly inhibited the proliferation of these cells.
In our study, culturing MC3T3-E1 cells on four different substrates, PPF/HA, PPF/Sr5-HA, PPF/Sr10-HA, and PPF/Sr20-HA, showed that the PPF/Sr10-HA group promoted the proliferation activity of MC3T3-E1 cells significantly more than the other 3 groups at 4 and 7 d. In terms of inducing osteogenic differentiation, the ALP activity of the 4 groups reached a peak at 14 d, and the PPF/Sr10-HA group showed significantly more activity than the other 3 groups. At each time point, the osteocalcin content in the PPF/Sr10-HA group was also significantly greater than the other groups. This indicates that the PPF/Sr10-HA material significantly improved the microenvironment for osteogenic differentiation. The adhesion, proliferation, and differentiation of MC3T3-E1 cells were attributed to the improved surface morphology of PPF and the local release of Sr2+.
CONCLUSIONS
In summary, we prepared 4 types of nanocomposites (PPF/HA, PPF/Sr5-HA, PPF/Sr10-HA, and PPF/Sr20-HA) by incorporating HA or Sr-HA nanoparticles into PPF networks. We analyzed the composition and surface morphology of the material types and investigated the adhesion, proliferation, and differentiation of MC3T3-E1 cells on the surface of these materials. The results showed that the PPF/Sr10-HA material was significantly better than the other 3 groups and is a promising scaffold material for bone tissue engineering.
Acknowledgments
This work was supported by NIH R01 AR56212.
Footnotes
CONFLICTS OF INTEREST
There are no conflicts to declare.
REFERENCES
- 1.Garreta E, Gasset D, Semino C, Borros S. Fabrication of a three-dimensional nanostructured biomaterial for tissue engineering of bone. Biomolecular engineering 2007;24(1):75–80. [DOI] [PubMed] [Google Scholar]
- 2.Lluch AV, Ferrer GG, Pradas MM. Surface modification of P (EMA-co-HEA)/SiO2 nanohybrids for faster hydroxyapatite deposition in simulated body fluid? Colloids and Surfaces B: Biointerfaces 2009;70(2):218–225. [DOI] [PubMed] [Google Scholar]
- 3.Kempen DH, Lu L, Zhu X, Kim C, Jabbari E, Dhert WJ, Currier BL, Yaszemski MJ. Development of biodegradable poly (propylene fumarate)/poly (lactic-co-glycolic acid) blend microspheres. I. Preparation and characterization. Journal of Biomedical Materials Research Part A 2004;70(2):283–292. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Yang F, Liu K, Shen H, Zhu Y, Zhang W, Liu W, Wang S, Cao Y, Zhou G. The impact of PLGA scaffold orientation on in vitro cartilage regeneration. Biomaterials 2012;33(10):2926–35. [DOI] [PubMed] [Google Scholar]
- 5.Li J, Lin Z, Zheng Q, Guo X, Lan S, Liu S, Yang S. Repair of rabbit radial bone defects using true bone ceramics combined with BMP-2-related peptide and type I collagen. Materials science and engineering: C 2010;30(8):1272–1279. [Google Scholar]
- 6.Tesavibul P, Chantaweroad S, Laohaprapanon A, Channasanon S, Uppanan P, Tanodekaew S, Chalermkarnnon P, Sitthiseripratip K. Biocompatibility of hydroxyapatite scaffolds processed by lithography-based additive manufacturing. Bio-medical materials and engineering 2015;26(1–2):31–38. [DOI] [PubMed] [Google Scholar]
- 7.He X, Fan X, Feng W, Chen Y, Guo T, Wang F, Liu J, Tang K. Incorporation of microfibrillated cellulose into collagen-hydroxyapatite scaffold for bone tissue engineering. International journal of biological macromolecules 2018;115:385–392. [DOI] [PubMed] [Google Scholar]
- 8.Hamada Y, Fujitani W, Kawaguchi N, Daito K, Niido T, Uchinaka A, Mori S, Kojima Y, Manabe M, Nishida K. The preparation of PLLA/calcium phosphate hybrid composite and its evaluation of biocompatibility. Dental materials journal 2012;31(6):1087–1096. [DOI] [PubMed] [Google Scholar]
- 9.Tan H, Yang B, Duan X, Wang F, Zhang Y, Jin X, Dai G, Yang L. The promotion of the vascularization of decalcified bone matrix in vivo by rabbit bone marrow mononuclear cell-derived endothelial cells. Biomaterials 2009;30(21):3560–6. [DOI] [PubMed] [Google Scholar]
- 10.Noori A, Ashrafi SJ, Vaez-Ghaemi R, Hatamian-Zaremi A, Webster TJ. A review of fibrin and fibrin composites for bone tissue engineering. International journal of nanomedicine 2017;12:4937–4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yin B, Xue B, Wu Z, Ma J, Wang K. A novel hybrid 3D-printed titanium scaffold for osteogenesis in a rabbit calvarial defect model. American journal of translational research 2018;10(2):474–482. [PMC free article] [PubMed] [Google Scholar]
- 12.Wang S, Lu L, Yaszemski MJ. Bone-tissue-engineering material poly (propylene fumarate): correlation between molecular weight, chain dimensions, and physical properties. Biomacromolecules 2006;7(6):1976–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He S, Timmer M, Yaszemski MJ, Yasko A, Engel P, Mikos A. Synthesis of biodegradable poly (propylene fumarate) networks with poly (propylene fumarate)–diacrylate macromers as crosslinking agents and characterization of their degradation products. Polymer 2001;42(3):1251–1260. [Google Scholar]
- 14.Lee K-W, Wang S, Yaszemski MJ, Lu L. Physical properties and cellular responses to crosslinkable poly (propylene fumarate)/hydroxyapatite nanocomposites. Biomaterials 2008;29(19):2839–2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee K-W, Wang S, Dadsetan M, Yaszemski MJ, Lu L. Enhanced cell ingrowth and proliferation through three-dimensional nanocomposite scaffolds with controlled pore structures. Biomacromolecules 2010;11(3):682–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang H, Wang K, Shimer AL, Li X, Balian G, Shen FH. Use of a bioactive scaffold for the repair of bone defects in a novel reproducible vertebral body defect model. Bone 2010;47(2):197–204. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Hong J, Zheng Q, Guo X, Lan S, Cui F, Pan H, Zou Z, Chen C. Repair of rat cranial bone defects with nHAC/PLLA and BMP-2-related peptide or rhBMP-2. Journal of Orthopaedic Research 2011;29(11):1745–1752. [DOI] [PubMed] [Google Scholar]
- 18.Diomede F, D’Aurora M, Gugliandolo A, Merciaro I, Orsini T, Gatta V, Piattelli A, Trubiani O, Mazzon E. Biofunctionalized Scaffold in Bone Tissue Repair. International journal of molecular sciences 2018;19(4):1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jayash SN, Hashim NM, Misran M, Baharuddin N. Local application of osteoprotegerin-chitosan gel in critical-sized defects in a rabbit model. PeerJ 2017;5:e3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang B, Huang J, Xu J, Li X, Li J. Local delivery of a novel PTHrP via mesoporous bioactive glass scaffolds to improve bone regeneration in a rat posterolateral spinal fusion model. RSC Advances 2018;8(22):12484–12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marie PJ. Strontium as therapy for osteoporosis. Current opinion in pharmacology 2005;5(6):633–636. [DOI] [PubMed] [Google Scholar]
- 22.Yang F, Yang D, Tu J, Zheng Q, Cai L, Wang L. Strontium enhances osteogenic differentiation of mesenchymal stem cells and in vivo bone formation by activating Wnt/catenin signaling. Stem cells 2011;29(6):981–991. [DOI] [PubMed] [Google Scholar]
- 23.Rybchyn MS, Slater M, Conigrave AD, Mason RS. An Akt-dependent increase in canonical Wnt signaling and a decrease in sclerostin protein levels are involved in strontium ranelate-induced osteogenic effects in human osteoblasts. Journal of Biological Chemistry 2011;286(27):23771–23779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee K-W, Wang S, Lu L, Jabbari E, Currier BL, Yaszemski MJ. Fabrication and characterization of poly (propylene fumarate) scaffolds with controlled pore structures using 3-dimensional printing and injection molding. Tissue engineering 2006;12(10):2801–2811. [DOI] [PubMed] [Google Scholar]
- 25.Peter SJ, Suggs LJ, Yaszemski MJ, Engel PS, Mikos AG. Synthesis of poly (propylene fumarate) by acylation of propylene glycol in the presence of a proton scavenger. Journal of Biomaterials Science, Polymer Edition 1999;10(3):363–373. [DOI] [PubMed] [Google Scholar]
- 26.Pan H, Li Z, Lam W, Wong J, Darvell B, Luk K, Lu W. Solubility of strontium-substituted apatite by solid titration. Acta Biomaterialia 2009;5(5):1678–1685. [DOI] [PubMed] [Google Scholar]
- 27.Park S, Terzic A Quaternary structure of KATP channel SUR2A nucleotide binding domains resolved by synchrotron radiation X-ray scattering. Journal of Structural Biology 2010; 169: 243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu X, Miller AL 2nd, Park S, Waletzki BE, Zhou Z, Terzic A, Lu L. Functionalized Carbon Nanotube and Graphene Oxide Embedded Electrically Conductive Hydrogel Synergistically Stimulates Nerve Cell Differentiation. ACS Applied Materials and Interfaces 2017; 9(17): 14677–14690. [DOI] [PubMed] [Google Scholar]
- 29.Qiu X, Hong Z, Hu J, Chen L, Chen X, Jing X. Hydroxyapatite surface modified by L-lactic acid and its subsequent grafting polymerization of L-lactide. Biomacromolecules 2005;6(3):1193–1199. [DOI] [PubMed] [Google Scholar]
- 30.Yan J, Sun JF, Chu PK, Han Y, Zhang YM. Bone integration capability of a series of strontium-containing hydroxyapatite coatings formed by micro-arc oxidation. Journal of Biomedical Materials Research Part A 2013;101(9):2465–2480. [DOI] [PubMed] [Google Scholar]
- 31.Xu J, Yang Y, Wan R, Shen Y, Zhang W. Hydrothermal preparation and characterization of ultralong strontium-substituted hydroxyapatite whiskers using acetamide as homogeneous precipitation reagent. The Scientific World Journal 2014;2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaygili O, Keser S, Kom M, Eroksuz Y, Dorozhkin SV, Ates T, Ozercan IH, Tatar C, Yakuphanoglu F. Strontium substituted hydroxyapatites: synthesis and determination of their structural properties, in vitro and in vivo performance. Materials science and engineering: C 2015;55:538–546. [DOI] [PubMed] [Google Scholar]
- 33.Ni G, Lu W, Chiu K, Li Z, Fong D, Luk K. Strontium-containing hydroxyapatite (Sr-HA) bioactive cement for primary hip replacement: An in vivo study. Journal of Biomedical Materials Research Part B: Applied Biomaterials 2006;77(2):409–415. [DOI] [PubMed] [Google Scholar]
- 34.Li J, Yang L, Guo X, Cui W, Yang S, Wang J, Qu Y, Shao Z, Xu S. Osteogenesis effects of strontium-substituted hydroxyapatite coatings on true bone ceramic surfaces in vitro and in vivo. Biomedical Materials 2017;13(1):015018. [DOI] [PubMed] [Google Scholar]
- 35.Peter SJ, Lu L, Kim DJ, Mikos AG. Marrow stromal osteoblast function on a poly (propylene fumarate)/β-tricalcium phosphate biodegradable orthopaedic composite. Biomaterials 2000;21(12):1207–1213. [DOI] [PubMed] [Google Scholar]
- 36.Olthof MG, Kempen DH, Herrick JL, Yaszemski MJ, Dhert WJ, Lu L. Effect of different sustained bone morphogenetic protein-2 release kinetics on bone formation in poly (propylene fumarate) scaffolds. Journal of Biomedical Materials Research Part B: Applied Biomaterials 2018;106(2):477–487. [DOI] [PubMed] [Google Scholar]
- 37.Becker J, Lu L, Runge MB, Zeng H, Yaszemski MJ, Dadsetan M. Nanocomposite bone scaffolds based on biodegradable polymers and hydroxyapatite. Journal of Biomedical Materials Research Part A 2015;103(8):2549–2557. [DOI] [PubMed] [Google Scholar]
- 38.Wei G, Ma PX. Structure and properties of nano-hydroxyapatite/polymer composite scaffolds for bone tissue engineering. Biomaterials 2004;25(19):4749–4757. [DOI] [PubMed] [Google Scholar]
- 39.Vandiver J, Dean D, Patel N, Bonfield W, Ortiz C. Nanoscale variation in surface charge of synthetic hydroxyapatite detected by chemically and spatially specific high-resolution force spectroscopy. Biomaterials 2005;26(3):271–283. [DOI] [PubMed] [Google Scholar]
- 40.Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials 2000;21(17):1803–1810. [DOI] [PubMed] [Google Scholar]
- 41.Caverzasio J Strontium ranelate promotes osteoblastic cell replication through at least two different mechanisms. Bone 2008;42(6):1131–1136. [DOI] [PubMed] [Google Scholar]
- 42.Barbara A, Delannoy P, Denis B, Marie P. Normal matrix mineralization induced by strontium ranelate in MC3T3-E1 osteogenic cells. Metabolism-Clinical and Experimental 2004;53(4):532–537. [DOI] [PubMed] [Google Scholar]
- 43.Schumacher M, Lode A, Helth A, Gelinsky M. A novel strontium (II)-modified calcium phosphate bone cement stimulates human-bone-marrow-derived mesenchymal stem cell proliferation and osteogenic differentiation in vitro. Acta Biomaterialia 2013;9(12):9547–9557. [DOI] [PubMed] [Google Scholar]