Abstract
Endovascular mechanical thrombectomy has evolved significantly and has become the mainstay and most effective currently available treatment for acute ischemic stroke patients due to large vessel occlusion. Mechanical thrombectomy is presently performed using a stent retriever or stent-like device, an aspiration catheter, or a combination of the two. Much of the literature has focused on the benefits of endovascular mechanical thrombectomy with only limited data about procedural complications and management. Awareness of risk factors and early recognition of these complications can potentially reduce complication rates, improve management, and yield better overall outcomes. In this review, the authors present a description of intraprocedural complications and strategies to prevent and treat these complications.
Keywords: interventional radiology, stroke, complication, hemorrhage, stent
Endovascular mechanical thrombectomy has evolved significantly in the last two decades and has become the mainstay and most effective currently available treatment for acute ischemic stroke patients due to large vessel occlusion. 1 2 Mechanical thrombectomy is presently performed using a stent retriever or stent-like device, an aspiration catheter, or a combination of the two. Much of the literature has focused on the benefits of endovascular mechanical thrombectomy with only limited data about procedural complications and management. The expanded treatment window and growing number of eligible patients may increase the incidence of complications. Indeed, the overall frequency reported in the recent randomized trials approaches 15% based on recent randomized control trials (RCTs). 1 Awareness of risk factors and early recognition of these complications can potentially reduce complication rates, improve management, and yield better overall outcomes.
In this review, we present a description of intraprocedural complications and strategies to prevent and treat these complications. We classified procedural complications into intra- and extracranial complications and discuss anaphylaxis separately. Extracranial complications include access site–related complications, cervical carotid/vertebral dissections, and vasospasm. Intracranial complications include perforations, distal migration of emboli or embolization to a new vascular territory, and device-related complications.
Extracranial Complications
Access Site Complications
Although prolonged time to gain arterial access is not considered in itself a complication, slower times result in delayed clot retrieval and in turn adversely impact the clinical outcomes. 3 Alawieh and colleagues found higher rate of poor functional outcome when procedure time extended beyond 30 minutes. 4 While the transfemoral approach is the most commonly used route, radial, brachial, and direct carotid punctures can also be utilized in patients with arterial tortuosity or severe atherosclerotic disease of the femoral/iliac arteries and aortic arch. 5 6 7 8 Access site hematoma, retroperitoneal hematoma, distal emboli leading to limb ischemia, dissection, pseudoaneurysms, and/or arteriovenous fistula are well-recognized complications that can occur during or after gaining access. 9 The reported rate for puncture site hematoma in RCTs ranges from 2 to 10.7% 10 11 12 13 as opposed to 1 to 2% in non-RCTs. 14 15 16 In a single-center experience, among 473 patients undergoing mechanical thrombectomy including 260 who received tissue plasminogen activator (t-PA), the overall groin complication rate ranged from 0.4 to 0.8%. 3 In the Femoral Arterial Access with Ultrasound Trial (FAUST), ultrasound proved to minimize the number of attempts, increase the first-pass success rate, and reduce venipunctures in 1,004 patients. 17 Hemostasis can be achieved by either manual compression or vascular closure device (VCD). Although some data suggest lower complication rates with VCDs as compared with manual compression, 18 19 20 VCDs are associated with risks and potential complications; so, careful patient selection and experience in their use and adherence to manufacturer recommendations are important. 21
Management
Most groin hematomas are usually managed conservatively by manual compression. It is crucial to focus on the arterial pulse as the landmark to hold pressure, not the skin incision. 22 Depending on the clinical status, some patients require emergent vascular surgery to establish hemostasis and repair flow-limiting dissections or high flow AVFs. Pseudoaneurysms can either be treated by ultrasound-guided compression or direct thrombin injection. 23 Surgical repair for arteriovenous fistulas is the gold standard, though other reported treatment options include covered stent placement ( Fig. 1 ), coil embolization, and glue embolization. 24
Fig. 1.
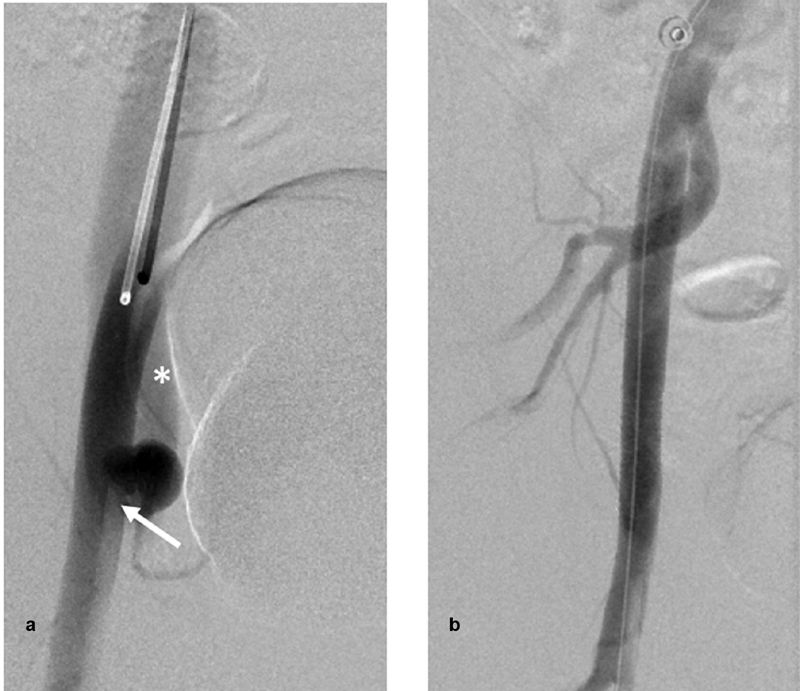
( a ) Digital subtraction angiography (DSA) of the right external iliac artery (EIA) via a left femoral approach anteroposterior view showing a pseudoaneurysm adjacent to the distal tip of the sheath (arrow) with early opacification of the external iliac vein, consistent with arteriovenous fistula (asterisk). ( b ) DSA of the right EIA revealing complete obliteration of aneurysm and the fistula after deploying a Viabahn Gore covered stent across the neck of the aneurysm.
Iatrogenic Cervical Carotid/Vertebral Vessel Dissections
Localized arterial dissections are largely asymptomatic; however, they are associated with increased risk of flow impedance and thromboembolic complications that can lead to poor outcomes. 25 In RCTs, the frequency of arterial dissections ranged between 0.6 and 3.9%. 10 12 13 26 In the case series reported by Goeggel Simonetti et al, vessel dissection occurred in 18 patients (2%) out of 866 who underwent mechanical thrombectomy. 27 Extracranial vessel involvement occurred in 15 patients (mostly cervical internal carotid artery, 14 patients) versus intracranial vascular dissection only in three cases. 27 Interestingly, there was a statistically significant higher incidence in smokers. 27 Localized contrast pocket, double lumen sign, or intimal flap on DSA images aid in identifying such a complication. 25
Management
Depending on the severity of flow limitation and the hemodynamic significance, treatment ranges from conservative management with anticoagulation or dual-antiplatelet therapy (DAP) in asymptomatic non–flow-limiting dissections ( Fig. 2 ) to balloon angioplasty or stenting that may be required to line up the intimal flap with the vessel wall and therefore restore the flow. 25 28 29 Of note, with acute stent placement, the use of antiplatelet therapy should be initiated and hence might be associated with higher risks of bleeding.
Fig. 2.
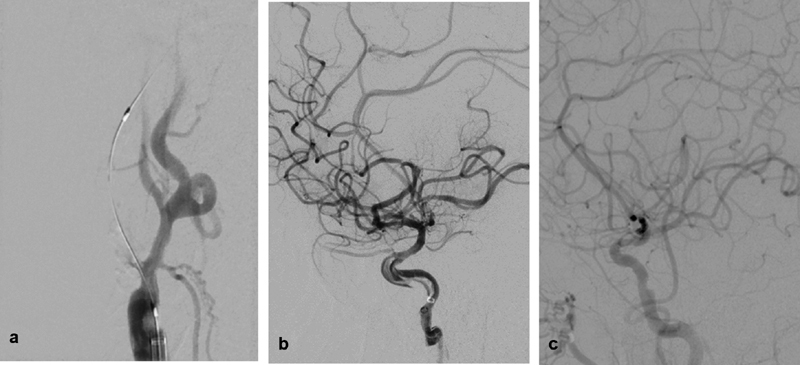
( a ) Digital subtraction angiography (DSA) of the right common carotid artery, right anterior oblique view showing total internal carotid artery (ICA) origin occlusion after microcatheter and a wire were successfully advanced through the occlusion. ( b ) DSA run of the right ICA, lateral view after reperfusion of the intracranial vessels showing a linear/spiral filling defect within the petrocavernous segment of the ICA consistent of dissection. ( c ) Repeat angiography 7 days after the procedure showing complete resolution of the dissection with preserved antegrade flow.
Cervical Carotid/Vertebral Vasospasm
Stable and distal positioning of guiding catheters is necessary during mechanical thrombectomy. However, vasospasm may preclude adequate positioning of guide catheters. Mechanically induced vasospasm results from irritation of the vessel wall during catheter or guidewire manipulation. 30 Spasm can involve any artery, either extracranial, intracranial, or puncture site vessels. 28 31 The rate of vasospasm in RCTs was 3.9 to 23%; however, none were reported to cause clinical deterioration. 9 12 13 32 In a retrospective analysis of 176 acute ischemic stroke patients treated with mechanical thrombectomy, Behme and colleagues reported 5 cases of access vessel vasospasm with no adverse clinical sequelae. 31
Management
In the majority of cases, vasospasm will be self-limited and improve spontaneously within minutes by minimizing irritation of the vessel wall and retracting the catheter. If vasospasm persists and limits antegrade flow, selective injection of a calcium channel blocker such as nimodipine (0.5–1 mg) or 10 mg of either verapamil or nicardipine over several minutes can be considered. 28 33 It is important to monitor for systemic hypotension, as this may be more detrimental than vasospasm, particularly in a patient with acute brain ischemia. 33
Intracranial Complications
Device-Related Complications
Inadvertent stent retriever detachment during thrombectomy is extremely rare (less than 1%) and mostly found in first-generation devices. 34 No standardized way to deal with this complication has been proposed. 34 Capturing the prematurely detached stent has been reported 35 36 37 ; however, associated risks, including vessel dissection, spasm, and/or perforation, have to be considered. Leaving a detached stent can also be an option; however, initiating DAP to prevent stent thrombosis may increase the risk of intracranial hemorrhage (ICH), particularly if the patient received t-PA. 38
Two cases of catheter tip fracture during thrombectomy have been reported with the use of snare/microsnare devices to retrieve the catheter fragments. 39
Vascular Perforation
Two main mechanisms of vessel perforation have been reported: microwire penetration or direct endoluminal trauma and shear forces during the use of stent retriever devices. 28 40 41 While any vessel can be perforated, typically it affects distal intracranial vasculature ( Fig. 3 ). 40 Vessel perforation manifested by contrast extravasation during the procedure is a rare yet drastic complication and, when it occurs, is associated with a high rate of mortality as opposed to angiographically occult postprocedural subarachnoid hemorrhage (SAH) which is characterized by a rather benign course. 40 42 Five recent trials 10 11 12 26 32 reported an incidence of 1.6% (10 perforations) in a total of 634 patients.
Fig. 3.
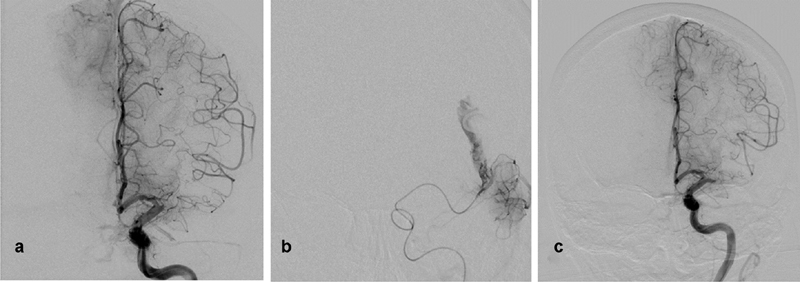
( a ) Digital subtraction angiography (DSA) of the left internal carotid artery (ICA), Towne view showing occluded left middle cerebral artery (MCA) with anterior cerebral artery pial collaterals retrogradely filling the distal MCA territory. ( b ) Microcatheter DSA run of the MCA after first pass using stent retrieval device (Solitaire) revealing contrast extravasation at the distal MCA. ( c ) Guide catheter DSA run of the left ICA showing occluded MCA sealing the microperforation. The presence of collaterals suggests acute on top of chronic occlusion or long-term MCA stenosis.
Fig. 4.
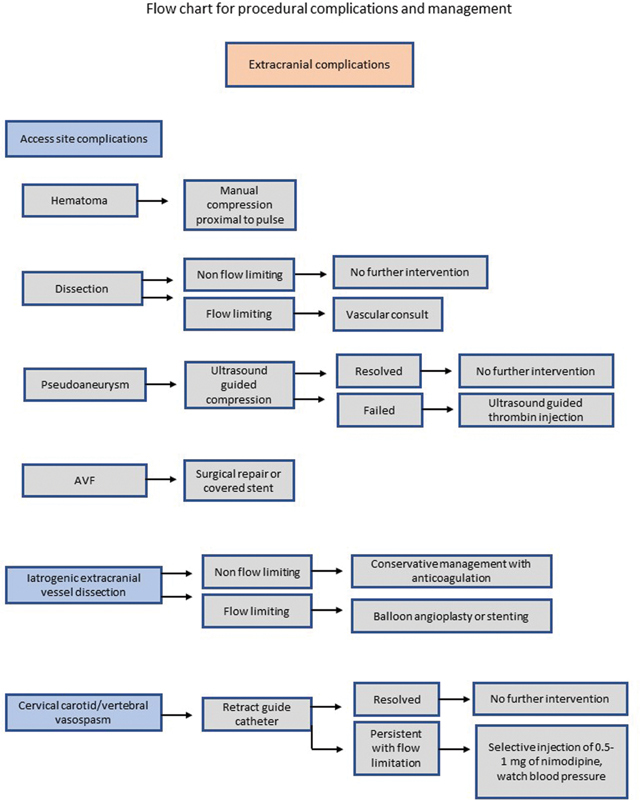
Flow chart for procedural complications and management.
Fig. 5.
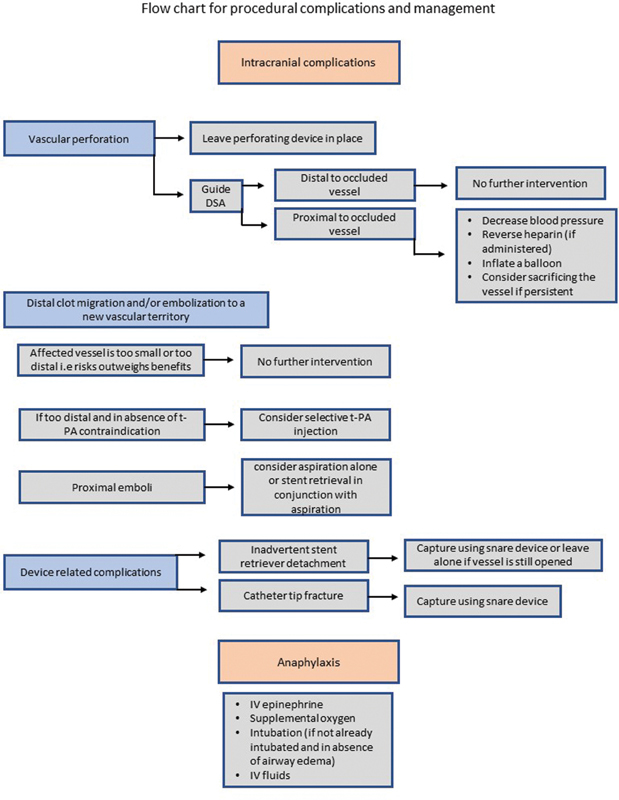
Flow chart for procedural complications and management.
Management
Intracranial vessel perforation manifested by contrast extravasation requires immediate action as it is associated with mortality rates exceeding 50% and an overall poor outcome rate of 75%. 40 Generally, management follows the same rules as those that occur during endovascular treatment of any intracranial lesion: the perforating device should not be pulled back immediately as it may be sealing the site of injury. 43 Sometimes, the clot can achieve hemostasis if the injured vessel is distal to the occlusion. For vessel injuries proximal to the clot, immediate communication with the anesthesia team is important to reduce blood pressure and to watch for Cushing's triad (i.e., increased blood pressure, irregular breathing, and bradycardia) which may indicate imminent brain injury. 44 In patients who received heparin during the procedure, administration of protamine sulfate will rapidly reverse the systemic effect of heparinization. 28 40 41 Reversal of the fibrinolytic effect of t-PA by infusion of platelets and cryoprecipitate is controversial. 40 Inflation of a balloon at the site of injury for several minutes (5–10 minutes) is sometimes needed to achieve hemostasis. 40 45 If bleeding persists despite repeated rounds of balloon inflation, sacrifice of the injured segment with embolic agents or detachable coils can be considered. 9 40 46 Although vessel sacrifice almost certainly results in a larger stroke burden, vessel sacrifice may be the only life-saving option. Follow-up imaging is recommended in these scenarios to exclude pseudoaneurysm formation and evaluate the extent of thromboembolic complications. 47
Distal Clot Migration and/or Embolization to a New Vascular Territory
Migration of clot to a previously unaffected territory may occur during retrieval of a proximal clot. 48 The frequency of migration of emboli to a new vascular territory ranges from 1 to 8.6% in recent clinical trials. 10 11 12 13 26 Bench models have revealed that distal migration of emboli in the same target vessel mostly occurs when catheters cross the occluded segment distal to the clot. 49 The burden of distal embolization is directly proportional to the size of the catheter used to traverse the clot. 49 Several contributing factors are involved such as the type of clot and different types of guide catheters with less frequent embolization to proximal unaffected territory being reported with the use of balloon guide catheters. 48 50
Management
Management depends mainly on the location of emboli and after outweighing risks versus benefits. Based on this notion, nontarget clot migration can be left alone if the affected vessel is too small or distal and it is felt that risks outweigh the potential benefits. If clinically appropriate and in absence of any contraindication, intra-arterial injection of t-PA can be considered in distal migration of the clot. 51 In proximal emboli, the migrated clot can be retracted by aspiration alone or stent retrieval in conjunction with aspiration. 29
Anaphylaxis Emergency
While allergic reactions to contrast media are not uncommon, they are rarely severe and life threatening. 1 Severe anaphylaxis noted by respiratory and/or cardiovascular symptoms should be immediately addressed. 52 Cornerstones of initial management include intravenous (IV) epinephrine, supplemental oxygen, intubation (if not already intubated and in the absence of airway edema), and volume resuscitation with IV fluids. 53 There is little evidence of clear benefit of glucocorticoids in acute settings. 54
Tips and Tricks to Minimize Periprocedural Complications
Endovascular mechanical thrombectomy should be performed by physicians with appropriate stroke training in centers equipped to handle these complex patients. 55 56
Eligible patients for mechanical thrombectomy should be appropriately selected.
Reviewing available cross-sectional images prior to procedure allows better procedural planning in a shorter time period.
Adherence to manufacturer's guidelines and avoiding use of older generations of devices may help reduce device-related complications. 55
Prolonged endovascular procedure time is associated with increased risk of symptomatic ICH. 57 58
Increased number of passes is associated with higher rates of complications and parenchymal hematoma after the procedure. 59 60 61
Minimize traversing the clot with catheter as possible to avoid distal migration of emboli. 49
Postprocedure neurocritical care monitoring with appropriate blood pressure management improves outcomes and helps prevent possible complications. 55 56
Conclusions
Mechanical thrombectomy is the gold standard for acute ischemic stroke patients with large vessel occlusion, and it is incumbent on the interventionalist to anticipate and manage technical complications. Neurointerventionalists, intensivists, and neurologists should be aware of these complications as many are preventable and can be efficiently managed with early recognition.
Footnotes
Conflict of Interest None declared.
References
- 1.Balami J S, White P M, McMeekin P J, Ford G A, Buchan A M. Complications of endovascular treatment for acute ischemic stroke: Prevention and management. Int J Stroke. 2018;13(04):348–361. doi: 10.1177/1747493017743051. [DOI] [PubMed] [Google Scholar]
- 2.Church E W, Gundersen A, Glantz M J, Simon S D. Number needed to treat for stroke thrombectomy based on a systematic review and meta-analysis. Clin Neurol Neurosurg. 2017;156:83–88. doi: 10.1016/j.clineuro.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Shah V A, Martin C O, Hawkins A M, Holloway W E, Junna S, Akhtar N. Groin complications in endovascular mechanical thrombectomy for acute ischemic stroke: a 10-year single center experience. J Neurointerv Surg. 2016;8(06):568–570. doi: 10.1136/neurintsurg-2015-011763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alawieh A, Vargas J, Fargen K M et al. Impact of procedure time on outcomes of thrombectomy for stroke. J Am Coll Cardiol. 2019;73(08):879–890. doi: 10.1016/j.jacc.2018.11.052. [DOI] [PubMed] [Google Scholar]
- 5.Haussen D C, Nogueira R G, DeSousa K G et al. Transradial access in acute ischemic stroke intervention. J Neurointerv Surg. 2016;8(03):247–250. doi: 10.1136/neurintsurg-2014-011519. [DOI] [PubMed] [Google Scholar]
- 6.Mokin M, Snyder K V, Levy E I, Hopkins L N, Siddiqui A H. Direct carotid artery puncture access for endovascular treatment of acute ischemic stroke: technical aspects, advantages, and limitations. J Neurointerv Surg. 2015;7(02):108–113. doi: 10.1136/neurintsurg-2013-011007. [DOI] [PubMed] [Google Scholar]
- 7.Wu C J, Cheng C I, Hung W C et al. Feasibility and safety of transbrachial approach for patients with severe carotid artery stenosis undergoing stenting. Catheter Cardiovasc Interv. 2006;67(06):967–971. doi: 10.1002/ccd.20738. [DOI] [PubMed] [Google Scholar]
- 8.Jadhav A P, Ribo M, Grandhi R et al. Transcervical access in acute ischemic stroke. J Neurointerv Surg. 2014;6(09):652–657. doi: 10.1136/neurintsurg-2013-010971. [DOI] [PubMed] [Google Scholar]
- 9.Akins P T, Amar A P, Pakbaz R S, Fields J D; SWIFT Investigators.Complications of endovascular treatment for acute stroke in the SWIFT trial with solitaire and Merci devices AJNR Am J Neuroradiol 20143503524–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goyal M, Demchuk A M, Menon B K et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019–1030. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 11.Campbell B C, Mitchell P J, Kleinig T J et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372(11):1009–1018. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 12.Jovin T G, Chamorro A, Cobo E et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372(24):2296–2306. doi: 10.1056/NEJMoa1503780. [DOI] [PubMed] [Google Scholar]
- 13.Bracard S, Ducrocq X, Mas J L et al. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol. 2016;15(11):1138–1147. doi: 10.1016/S1474-4422(16)30177-6. [DOI] [PubMed] [Google Scholar]
- 14.Nikoubashman O, Jungbluth M, Schürmann K et al. Neurothrombectomy in acute ischaemic stroke: a prospective single-centre study and comparison with randomized controlled trials. Eur J Neurol. 2016;23(04):807–816. doi: 10.1111/ene.12944. [DOI] [PubMed] [Google Scholar]
- 15.Serles W, Gattringer T, Mutzenbach S et al. Endovascular stroke therapy in Austria: a nationwide 1-year experience. Eur J Neurol. 2016;23(05):906–911. doi: 10.1111/ene.12958. [DOI] [PubMed] [Google Scholar]
- 16.Weber R, Nordmeyer H, Hadisurya J et al. Comparison of outcome and interventional complication rate in patients with acute stroke treated with mechanical thrombectomy with and without bridging thrombolysis. J Neurointerv Surg. 2017;9(03):229–233. doi: 10.1136/neurintsurg-2015-012236. [DOI] [PubMed] [Google Scholar]
- 17.Seto A H, Abu-Fadel M S, Sparling J M et al. Real-time ultrasound guidance facilitates femoral arterial access and reduces vascular complications: FAUST (Femoral Arterial Access With Ultrasound Trial) JACC Cardiovasc Interv. 2010;3(07):751–758. doi: 10.1016/j.jcin.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 18.Sanborn T A, Ebrahimi R, Manoukian S V et al. Impact of femoral vascular closure devices and antithrombotic therapy on access site bleeding in acute coronary syndromes: The Acute Catheterization and Urgent Intervention Triage Strategy (ACUITY) trial. Circ Cardiovasc Interv. 2010;3(01):57–62. doi: 10.1161/CIRCINTERVENTIONS.109.896704. [DOI] [PubMed] [Google Scholar]
- 19.Smilowitz N R, Kirtane A J, Guiry M et al. Practices and complications of vascular closure devices and manual compression in patients undergoing elective transfemoral coronary procedures. Am J Cardiol. 2012;110(02):177–182. doi: 10.1016/j.amjcard.2012.02.065. [DOI] [PubMed] [Google Scholar]
- 20.Arora N, Matheny M E, Sepke C, Resnic F S. A propensity analysis of the risk of vascular complications after cardiac catheterization procedures with the use of vascular closure devices. Am Heart J. 2007;153(04):606–611. doi: 10.1016/j.ahj.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 21.Sheth R A, Walker T G, Saad W E et al. Quality improvement guidelines for vascular access and closure device use. J Vasc Interv Radiol. 2014;25(01):73–84. doi: 10.1016/j.jvir.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 22.Kosmidou I, Karmpaliotis D. Vascular complications after percutaneous coronary intervention.Philadelphia, PA: W.B. Saunders; 2010270–274. [Google Scholar]
- 23.Mishra A, Rao A, Pimpalwar Y. Ultrasound guided percutaneous injection of thrombin: effective technique for treatment of iatrogenic femoral pseudoaneurysms. J Clin Diagn Res. 2017;11(04):TC04–TC06. doi: 10.7860/JCDR/2017/25582.9512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thavarajan D, Bakran A. Iatrogenic arteriovenous fistula in the groin presenting as cardiac failure. NDT Plus. 2009;2(01):46–48. doi: 10.1093/ndtplus/sfn190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis M C, Deveikis J P, Harrigan M R. Clinical presentation, imaging, and management of complications due to neurointerventional procedures. Semin Intervent Radiol. 2015;32(02):98–107. doi: 10.1055/s-0035-1549374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berkhemer O A, Fransen P S, Beumer D et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372(01):11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 27.Goeggel Simonetti B, Hulliger J, Mathier E et al. Iatrogenic vessel dissection in endovascular treatment of acute ischemic stroke. Clin Neuroradiol. 2019;29(01):143–151. doi: 10.1007/s00062-017-0639-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akpinar S H, Yilmaz G.Periprocedural complications in endovascular stroke treatment Br J Radiol 201689(1057):2.0150267E7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Papanagiotou P, White C J. Endovascular reperfusion strategies for acute stroke. JACC Cardiovasc Interv. 2016;9(04):307–317. doi: 10.1016/j.jcin.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 30.Ishihara H, Ishihara S, Niimi J et al. Risk factors and prevention of guiding catheter-induced vasospasm in neuroendovascular treatment. Neurol Med Chir (Tokyo) 2015;55(03):261–265. doi: 10.2176/nmc.oa.2014-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Behme D, Gondecki L, Fiethen S, Kowoll A, Mpotsaris A, Weber W. Complications of mechanical thrombectomy for acute ischemic stroke-a retrospective single-center study of 176 consecutive cases. Neuroradiology. 2014;56(06):467–476. doi: 10.1007/s00234-014-1352-0. [DOI] [PubMed] [Google Scholar]
- 32.Saver J L, Goyal M, Bonafe A et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372(24):2285–2295. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 33.Bauer A M, Rasmussen P A. Treatment of intracranial vasospasm following subarachnoid hemorrhage. Front Neurol. 2014;5:72–72. doi: 10.3389/fneur.2014.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masoud H, Nguyen T N, Martin C Oet al. Inadvertent stent retriever detachment: a multicenter case series and review of device experience FDA reports Intervent Neurol 20164(3-4):75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Youn S W, Kim H K. Refinement of a thrombectomy technique to treat acute ischemic stroke: technical note on microcatheter advance during retrieving self-expandable stent. J Korean Soc Radiol. 2012;67(01):1–6. [Google Scholar]
- 36.Liu K C, Ding D, Starke R M, Geraghty S R, Jensen M E. Intraprocedural retrieval of migrated coils during endovascular aneurysm treatment with the Trevo Stentriever device. J Clin Neurosci. 2014;21(03):503–506. doi: 10.1016/j.jocn.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 37.Kabbani M R, Smith A, Leider M. Endovascular coil retrieval using a TrevoProVue stentriever. BMJ Case Rep. 2014;2014:201. doi: 10.1136/bcr-2014-011181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yub Lee S, Won Youn S, Kyun Kim H, Rok Do Y. Inadvertent detachment of a retrievable intracranial stent: review of manufacturer and user facility device experience. Neuroradiol J. 2015;28(02):172–176. doi: 10.1177/1971400915576650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moshayedi P, Jadhav A P.Direct aspiration catheter fracture and retrieval during neurothrombectomy Intervent Neurol 20187(3-4):148–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mokin M, Fargen K M, Primiani C T et al. Vessel perforation during stent retriever thrombectomy for acute ischemic stroke: technical details and clinical outcomes. J Neurointerv Surg. 2017;9(10):922–928. doi: 10.1136/neurintsurg-2016-012707. [DOI] [PubMed] [Google Scholar]
- 41.Leishangthem L, Satti S R. Vessel perforation during withdrawal of Trevo ProVue stent retriever during mechanical thrombectomy for acute ischemic stroke. J Neurosurg. 2014;121(04):995–998. doi: 10.3171/2014.4.JNS132187. [DOI] [PubMed] [Google Scholar]
- 42.Maus V, Brehm A, Tsogkas I, Henkel S, Psychogios M N. Stent retriever placement in embolectomy: the choice of the post-bifurcational trunk influences the first-pass reperfusion result in M1 occlusions. J Neurointerv Surg. 2019;11(03):237–240. doi: 10.1136/neurintsurg-2018-014114. [DOI] [PubMed] [Google Scholar]
- 43.Doerfler A, Wanke I, Egelhof T et al. Aneurysmal rupture during embolization with Guglielmi detachable coils: causes, management, and outcome. AJNR Am J Neuroradiol. 2001;22(10):1825–1832. [PMC free article] [PubMed] [Google Scholar]
- 44.Nimmo G, Howie A, Grant I. Effects of mechanical ventilation on Cushing's triad. Crit Care. 2009;13 01:P77. [Google Scholar]
- 45.Layton K F, Cloft H J, Kallmes D F. Cerebral aneurysm perforations during treatment with detachable coils. Use of remodelling balloon inflation to achieve hemostasis. Interv Neuroradiol. 2006;12(01):31–35. doi: 10.1177/159101990601200106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Farhat H I, Elhammady M S, Aziz-Sultan M A.N-Butyl-2-cyanoacrylate use in intraoperative ruptured aneurysms as a salvage rescue: case report Neurosurgery 20106701216–217., discussion 217 [DOI] [PubMed] [Google Scholar]
- 47.Nguyen T N, Lanthier S, Roy D. Iatrogenic arterial perforation during acute stroke interventions. AJNR Am J Neuroradiol. 2008;29(05):974–975. doi: 10.3174/ajnr.A0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chueh J Y, Puri A S, Wakhloo A K, Gounis M J. Risk of distal embolization with stent retriever thrombectomy and ADAPT. J Neurointerv Surg. 2016;8(02):197–202. doi: 10.1136/neurintsurg-2014-011491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lapergue B, Blanc R, Gory B et al. Effect of endovascular contact aspiration vs stent retriever on revascularization in patients with acute ischemic stroke and large vessel occlusion: the ASTER randomized clinical trial. JAMA. 2017;318(05):443–452. doi: 10.1001/jama.2017.9644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stampfl S, Pfaff J, Herweh C et al. Combined proximal balloon occlusion and distal aspiration: a new approach to prevent distal embolization during neurothrombectomy. J Neurointerv Surg. 2017;9(04):346–351. doi: 10.1136/neurintsurg-2015-012208. [DOI] [PubMed] [Google Scholar]
- 51.Darkhabani Z, Nguyen T, Lazzaro M A et al. Complications of endovascular therapy for acute ischemic stroke and proposed management approach. Neurology. 2012;79(13) 01:S192–S198. doi: 10.1212/WNL.0b013e31826958e3. [DOI] [PubMed] [Google Scholar]
- 52.Yilmaz R, Yuksekbas O, Erkol Z, Bulut E R, Arslan M N. Postmortem findings after anaphylactic reactions to drugs in Turkey. Am J Forensic Med Pathol. 2009;30(04):346–349. doi: 10.1097/PAF.0b013e3181c0e7bb. [DOI] [PubMed] [Google Scholar]
- 53.Brown S G. Cardiovascular aspects of anaphylaxis: implications for treatment and diagnosis. Curr Opin Allergy Clin Immunol. 2005;5(04):359–364. doi: 10.1097/01.all.0000174158.78626.35. [DOI] [PubMed] [Google Scholar]
- 54.Grunau B E, Wiens M O, Rowe B H et al. Emergency department corticosteroid use for allergy or anaphylaxis is not associated with decreased relapses. Ann Emerg Med. 2015;66(04):381–389. doi: 10.1016/j.annemergmed.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 55.Fiehler J, Cognard C, Gallitelli M et al. European Recommendations on Organisation of Interventional Care in Acute Stroke (EROICAS) Int J Stroke. 2016;11(06):701–716. doi: 10.1177/1747493016647735. [DOI] [PubMed] [Google Scholar]
- 56.White P M, Bhalla A, Dinsmore J et al. Standards for providing safe acute ischaemic stroke thrombectomy services (September 2015) Clin Radiol. 2017;72(02):1750–1.75E11. doi: 10.1016/j.crad.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 57.Soize S, Barbe C, Kadziolka K, Estrade L, Serre I, Pierot L. Predictive factors of outcome and hemorrhage after acute ischemic stroke treated by mechanical thrombectomy with a stent-retriever. Neuroradiology. 2013;55(08):977–987. doi: 10.1007/s00234-013-1191-4. [DOI] [PubMed] [Google Scholar]
- 58.Jiang S, Fei A, Peng Y et al. Predictors of outcome and hemorrhage in patients undergoing endovascular therapy with solitaire stent for acute ischemic stroke. PLoS One. 2015;10(12):e0144452. doi: 10.1371/journal.pone.0144452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baek J H, Kim B M, Heo J H et al. Number of stent retriever passes associated with futile recanalization in acute stroke. Stroke. 2018;49(09):2088–2095. doi: 10.1161/STROKEAHA.118.021320. [DOI] [PubMed] [Google Scholar]
- 60.Kharouba R, Gavriliuc P, Yaghmour N E, Gomori J M, Cohen J E, Leker R R. Number of stentriever passes and outcome after thrombectomy in stroke. J Neuroradiol. 2019;46(05):327–330. doi: 10.1016/j.neurad.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 61.Bourcier R, Saleme S, Labreuche J et al. More than three passes of stent retriever is an independent predictor of parenchymal hematoma in acute ischemic stroke. J Neurointerv Surg. 2019;11(07):625–629. doi: 10.1136/neurintsurg-2018-014380. [DOI] [PubMed] [Google Scholar]


