Abstract
Since 2015, endovascular therapy (EVT) has become the standard of care for acute ischemic stroke (AIS) due to large vessel occlusion. It is a safe and highly effective treatment, and its number needed to treat of 2.6 is one of the highest throughout medicine. The ultimate goal when performing EVT is to maximize chances of good outcome through achievement of fast first-pass complete reperfusion, as incomplete and delayed reperfusion increases complication rates and negatively affects outcome. Since EVT has been established as standard of care, new devices have been developed and treatment techniques have been refined. This review provides a brief overview about the rationale for and history of EVT, followed by a detailed step-by-step description of how to perform EVT using the BADDASS (BAlloon guide with large bore Distal access catheter with Dual Aspiration with Stent-retriever as Standard approach), a combined technique, which is in our opinion the safest and most effective way to achieve fast first-pass complete reperfusion. We also discuss treatment strategies for patients with simultaneous high-grade carotid stenosis/pseudoocclusion/occlusion and gaining carotid access in challenging arch anatomy, as these are commonly encountered situations in AIS, and conclude with an outlook on new technologies and future directions of EVT.
Keywords: aspiration, balloon guide catheter, mechanical thrombectomy, endovascular therapy, ischemic stroke, reperfusion, interventional radiology
What Is the Rationale behind Endovascular Stroke Treatment?
Acute ischemic stroke (AIS), particularly if caused by a large vessel occlusion (LVO), is a severely disabling, life-threatening disease. Until recently, intravenous alteplase was the only approved treatment for AIS patients. However, due to complex guidelines with numerous clinical, radiological, and laboratory-related exclusion criteria, only 3 to 5% of AIS patients receive alteplase. 1 Furthermore, the efficacy of alteplase in LVO, which causes the most severe and disabling strokes, is low, with recanalization rates ranging from 7 to 30%. 2 There clearly was the need for another alternative treatment strategy to reduce the morbidity and mortality that results from AIS. It is because of the low alteplase treatment rates and its low efficacy in LVO that endovascular treatment techniques, which rely on mechanical clot retrieval rather than pharmacological recanalization, have been developed. In 2015, five major randomized controlled trials have shown the benefit of endovascular therapy (EVT) compared with intravenous alteplase in AIS patients with LVO, 3 and since then, EVT is considered standard of care. 4
Benefit from an Individual Patient's Perspective
EVT significantly reduces disability in LVO patients (adjusted odds ratio [OR]: 2.49) and the number needed to treat for reduction of disability by at least one point on the modified Rankin scale is 2.6. 3 This translates into a substantial increase in lifetime quality-adjusted life years. 5 The safety profile of EVT is excellent, with no significant differences in mortality and symptomatic intracranial hemorrhage compared with intravenous alteplase treatment alone. 3 Given this powerful treatment option and the low recanalization rates of LVOs with tPA alone, many physicians, including ourselves, now offer EVT routinely beyond guideline recommendations. 6 Furthermore, it has been shown that not only physicians but also patients themselves prefer a more aggressive treatment approach. 7
Benefit from an Economic Perspective
EVT is beneficial not only from an individual's perspective but also from an economic standpoint. Its cost-effectiveness has been proven in industrial nations and developing countries. 8 9 10 11 The initial costs for EVT are higher compared with medical treatment alone because of the expenses for endovascular devices. Besides, continuous endovascular treatment service has to be established in the first place. However, the improvement in outcome that can be achieved through faster and better recanalization outweighs the initial costs. For an average patient in the United States for instance, the net monetary benefit has been estimated to be $17,000 per 1% increase in the final extended thrombolysis in cerebral infarction (eTICI) 2c/3 rate and $10,600 per 10 minutes of time-to-treatment decrease. 5 12
What Is a “Good” EVT Result?
Given the abovementioned considerations, it is clear that fast first-pass complete reperfusion has to be the goal when performing EVT in acute stroke.
Fast Reperfusion: Time Is Brain
In AIS, neurons die at a rapid pace. The average AIS patient loses 1.9 million neurons per minute, 13 and every 30-minute delay in recanalization decreases the chance of a good functional outcome by 8 to 14%. 14 Delayed reperfusion is associated with increased disability and poor functional outcome, 15 16 17 and long onset-to-reperfusion times were one of the main reasons for the neutral outcomes of early EVT trials. Not only outcome but also reperfusion quality deteriorates with increasing time to treatment, probably because of changes in clot composition 18 —another reason to be fast. Nevertheless, late but successful reperfusion is better than no reperfusion. 19
Complete Reperfusion: TICI 2b Is Not Enough
Reperfusion quality (i.e., how well we open a vessel) is a key determinant of patient outcome: higher eTICI grades are strongly associated with good patient outcome. 20 The saying “only what gets measured gets improved,” although originally from the world of finance and business, holds true in medicine as well. Thus, several scores have been developed to assess reperfusion quality after EVT. The TICI score has initially been described in 2003, 21 but the definition of its categories and its application was highly variable and made the assessment of reperfusion quality and comparisons among different studies somewhat complicated. 22 To harmonize reperfusion assessment, a consensus paper was published in 2013 that introduced the modified thrombolysis in cerebral infarction (mTICI) scale. 23 Since then, successful reperfusion has been defined as mTICI 2b/3. However, it is increasingly recognized that with mTICI 2b, reperfusion yields substantially worse outcomes compared with mTICI 3. 24 25 Hence, the eTICI score has introduced a new category of “near-complete” reperfusion (eTICI 2c, Table 1 ). Good outcome rates with eTICI 2c/3 are higher than with mTICI 2b/3, and eTICI 2c/3 has a better predictive value for good outcome compared with mTICI 2b/3. 24 Hence, eTICI 2c/3 is the new benchmark, and eTICI 2c/3 rates are now routinely reported in recent thrombectomy trials. 26 27
Table 1. Extended thrombolysis in cerebral infarction (eTICI) score 24 65 .
| eTICI score | Definition |
|---|---|
| 0 | No perfusion |
| 1 | Antegrade reperfusion past the initial occlusion, but limited distal branch filling with little or slow distal reperfusion |
| 2a | Antegrade reperfusion of less than half of the previously occluded target artery ischemic territory (e.g., in one major division of the MCA and its territory) |
| 2b | Antegrade reperfusion of more than half of the previously occluded target artery ischemic territory (e.g., in two major divisions of the MCA and their territories) |
| 2c | Near complete perfusion except for slow flow/occlusion in a few distal cortical vessels such that at least 90% of the territory is perfused in the parenchymal phase |
| 3 | Complete antegrade reperfusion of the previously occluded target artery ischemic territory, with absence of visualized occlusion in all distal branches |
Abbreviation: MCA, middle cerebral artery.
First-Pass Reperfusion: Getting the Clot Out in One Fell Swoop
The eTICI score reflects the final reperfusion result. However, it is not only the final result that matters but the facility with which we achieve it. Complete recanalization sometimes requires multiple device passes, particularly in carotid terminus occlusions due to the branching nature of terminal internal carotid artery (ICA) clots (carotid T or L occlusions). 28 Multiple device passes not only delay reperfusion, they also yield an increased risk of endothelial injury, thereby compromising procedural safety. First-pass effect (i.e., achieving complete revascularization with a single device pass) is an independent predictor for good outcome and has therefore been proposed as a new measure for the evaluation of thrombectomy devices. Interestingly, the first-pass effect is higher when balloon guide catheters (BGCs) are used (see next section). 29
From Intra-arterial Thrombolysis to Stent-Retrievers: Endovascular Stroke Therapy in the Past and Present
The first endovascular treatment attempts have been made with intra-arterial recombinant prourokinase. In 1999, the PROACT II trial showed that treatment with intra-arterial prourokinase leads to higher recanalization rates and better outcomes, despite higher rates of symptomatic intracranial hemorrhage. 30 However, these positive results did not lead to Food and Drug Administration (FDA) approval of the drug, which soon thereafter was no longer available. The era of mechanical thrombectomy began when the MERCI device, a corkscrew-shaped wire, became available. In 2005, the MERCI trial showed a recanalization rate (defined as TIMI 2/3) of 46% with the device compared with 18% in the historical control arm. 31 This led to FDA approval of the MERCI device. However, early randomized controlled trials with first-generation devices that were conducted between 2004 and 2012 failed to show benefit of EVT. 32 The reasons for this were manifold, such as poor patient selection (vascular imaging was not required in some of the trials 33 34 ) and long onset-to-treatment times. Although the neutral results of these early trials had raised serious concerns about the efficacy of EVT, a second generation of devices, the so-called stent-retrievers were developed. Stent-retrievers are self-expandable stents that are navigated to the site of occlusion via a microcatheter and microwire. They are then deployed within the clot. Once the clot has migrated through the stent struts and is fully integrated into the stent lumen, the stent-retriever is removed, thereby restoring blood flow. Stent-retrievers allowed for better reperfusion and higher good outcome rates compared with first-generation devices, and in 2015, five randomized controlled EVT trials were published, in all of which stent-retrievers were primarily used. HERMES, a meta-analysis summarizing these five trials, showed clear superiority of EVT compared with intravenous alteplase (the only available treatment option at that time). 3 This has led to changes in guidelines and treatment practice, and nowadays EVT using stent-retrievers is the standard of care for AIS patients with LVO. 4
EVT techniques and devices have been constantly refined and several new techniques have recently been developed, including first-line aspiration 26 35 and the so-called combined approaches (i.e., BADDASS, CAPTIVE, 36 and SAVE 37 38 ) in which both stent-retrievers and aspiration are used. This has led to a constant improvement in reperfusion quality over the last few years. While eTICI 2c/3 was achieved only in 31% of patients in the HERMES dataset, 20 more recent trials, in which combined techniques were used, report eTICI 2c/3 rates greater than 50% ( Table 2 ). In our opinion, the BADDASS technique yields the highest potential for fast first-pass complete reperfusion, due to the standardized use of a BGC and double aspiration.
Table 2. Reperfusion quality in different studies.
| Study | eTICI 3 rate | eTICI 2b/3 rate | eTICI 2c/3 rate | Reference |
|---|---|---|---|---|
| HERMES (mainly stent-retriever–based techniques) | – | 71% | 31% | Goyal et al 3 |
| First-line aspiration ASTER | 37.5% | 85.4% | – | Lapergue et al 26 |
| First-line aspiration COMPASS | 38% | 92% | 56% | Turk et al 27 |
| CAPTIVE | 33% | 100% | 79.5% | McTaggart et al 36 |
| SAVE | 28.3% | 93.5% | 54.5% | Brehm et al 66 |
Abbreviation: eTICI, extended thrombolysis in cerebral infarction score.
Note: Gray color indicates combined techniques in which balloon guide catheter, stent-retriever, and dual aspiration are used.
How to optimize fast first-pass complete reperfusion in AIS? In our opinion, when performing EVT, the BAlloon guide with large bore Distal access catheter with Dual Aspiration with Stent-retriever as Standard Approach (BADDASS) technique should be used, as it combines the advantages of primary aspiration, stent-retrievers, and BGC and thus is the most efficient way to reopen an occluded vessel fast and safely. In the following section, we will provide a detailed, step-by-step description of how to perform EVT using such a combined approach. The eight key features of BADDASS are summarized in Table 3 .
Table 3. Key features of BADDASS.
| 1 | Triaxial setup (balloon guide catheter, large bore distal access catheter, microcatheter) |
| 2 | Distal placement of a long stent-retriever |
| 3 | Active push deployment |
| 4 | Removing and reloading the microcatheter |
| 5 | Applying traction to the stent wire |
| 6 | Releasing traction off the stent wire |
| 7 | Balloon inflation and double aspiration |
| 8 | Complete withdrawal of the stent-retriever–clot complex and distal access catheter |
Abbreviation: BADDASS, BAlloon guide with large bore Distal access catheter with Dual Aspiration with Stent-retriever as Standard approach.
Triaxial Setup: Balloon Guide Catheter, Large Bore Distal Access Catheter, and Microcatheter
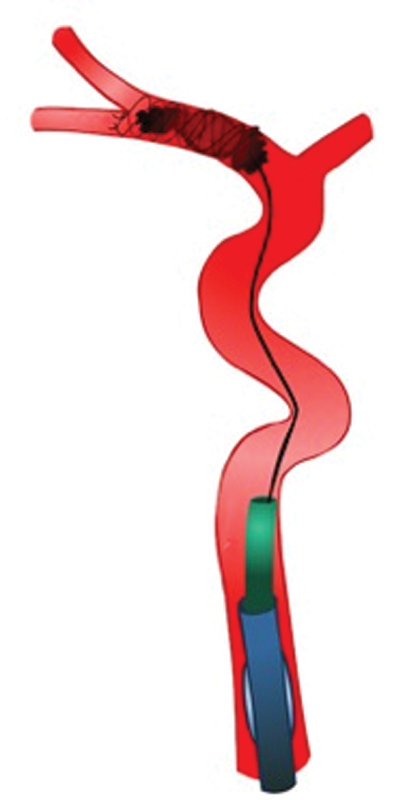
We strongly believe that BGCs should be used by default, as using BGCs is the only way to achieve complete flow arrest and maybe flow reversal. 39 This improves reperfusion times and quality, and, most importantly, patient outcome. 40 41 42 43 After the BGC is placed in the cervical ICA ( Fig. 1 ), a microcatheter is advanced over a microwire through a large bore distal access catheter (DAC). Once the microwire and microcatheter have crossed the occlusion site, the DAC is advanced as distal as possible, but should remain proximal to the ophthalmic artery origin. Ideally, the microwire is J -shaped. This avoids entering small branches and subsequent vessel injury.
Fig. 1.

Placement of a balloon guide catheter in the cervical internal carotid artery.
Distal Placement of a Long Stent-Retriever with the Majority of the Stent beyond the Clot
Generally, the posterior M2 division is the larger, dominant vessel, 44 although variations are common and the neurointerventionalist should study the vascular imaging (ideally multiphase computed tomographic angiography [mCTA]) to be familiar with the individual patient's anatomy. In most cases, it is desirable to advance the microwire and microcatheter to the posterior division, as its course is straighter and its caliber is larger, and thus the risk of vessel injury smaller. Furthermore, first-pass eTICI 2c/3 rates are higher when the posterior division is chosen for stent-retriever placement 44 ( Fig. 2 ). Distal device placement with the majority of the stent deployed beyond the distal clot terminus increases the chance of capturing the clot, particularly when the clot starts slipping distally. 37 The clot terminus can usually be easily identified on the second or third phase of the mCTA. Distal placement also avoids a long stent segment proximal to the clot, which is suboptimal as it prevents advancing the DAC close to the proximal clot interface (see later).
Fig. 2.
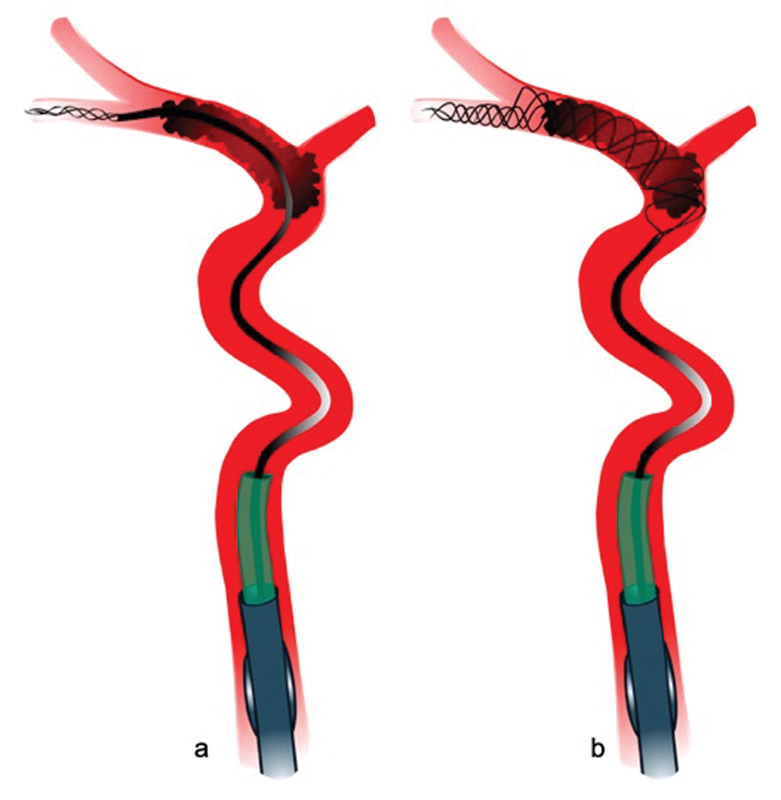
Placement of a long stent-retriever in the inferior M2 trunk, which is usually the dominant vessel ( a ). The stent-retriever is placed distally with approximately two-thirds of the stent beyond the clot ( b ).
Optimizing Clot Integration: Active Push Deployment of the Stent-Retriever
With standard deployment of stent-retrievers (i.e., passive unsheathing), the device's inherent radial force should theoretically ensure the compression of the clot against the vessel wall and subsequent clot migration through the stent struts into its lumen, 45 46 but oftentimes, standard deployment often results in insufficient wall apposition and clot integration ( Fig. 3 ). Thus, when unsheathing the stent-retriever, we recommend to actively push on the stent wire, 47 as this improves wall apposition, clot–device interaction, and clot integration. Of note, too much forward force can lead to telescoping of the stent and vessel wall injury. Thus, we deploy initial part of the stent-retriever passively, and once it is anchored in the vessel, limited forward force should be gently applied to the delivery wire by slightly pushing the stent while unsheathing it ( Fig. 3 ). This so-called active push deployment can improve first-pass reperfusion rates and final reperfusion quality. 47
Fig. 3.
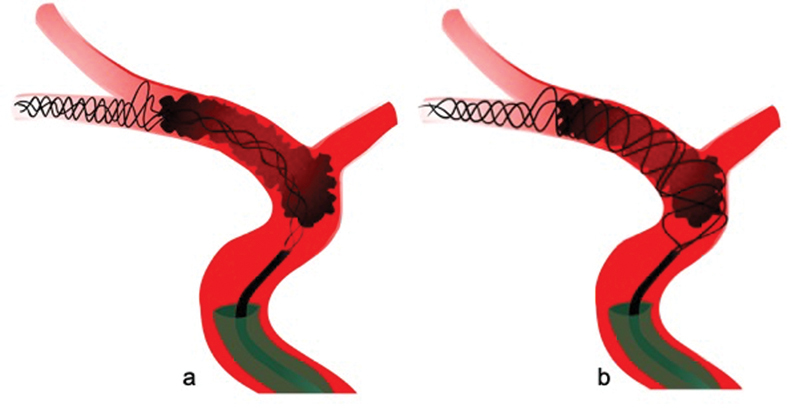
Standard deployment (passive unsheathing) of the stent-retriever results in incomplete opening of the stent, suboptimal interaction with the clot, and impaired clot integration into the stent lumen ( a ). With active push deployment (i.e., slightly pushing the stent wire while unsheathing the stent), the stent is fully opened and integration of the clot into the stent lumen is improved ( b ).
Maximizing Distal Aspiration: Removing the Microcatheter Prior to Clot Retrieval
According to most manufacturers, the microcatheter should be left in situ and overlap with the stent-retriever, both of which are then retrieved into the guide catheter. However, we remove the microcatheter completely out of the patient prior to clot retrieval through slight pulling, because the presence of a microcatheter within the DAC lumen reduces the cross-sectional area, thereby decreasing flow rates and increasing the risk of distal embolization, 48 especially when the microcatheter tip is protruding through the DAC tip 49 ( Fig. 4 ). By removing the microcatheter, flow rates can be increased and efficiency of clot removal can be optimized. 48 Once the microcatheter is removed, it can be immediately flushed and reloaded the microwire, thereby maximizing time efficiency; a second retrieval attempt can be immediately performed in case the first attempt is not successful.
Fig. 4.
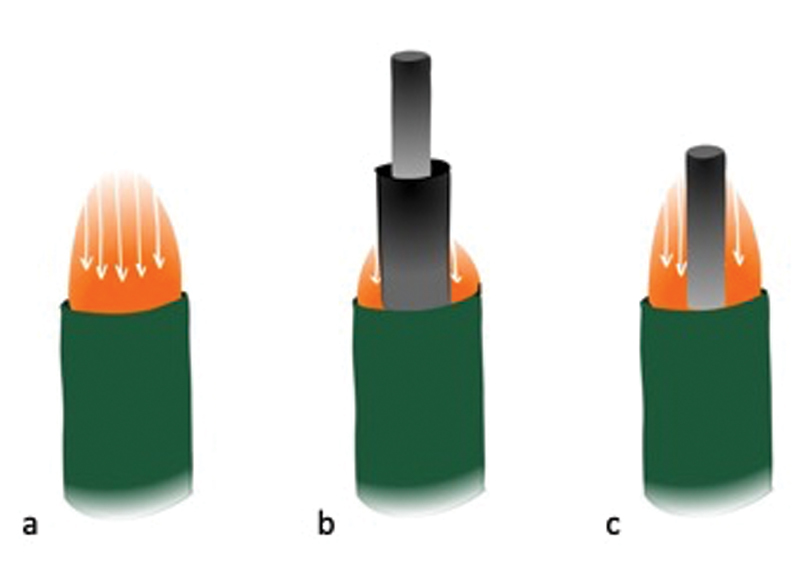
When aspirating through the distal access catheter (DAC), maximal laminar flow is highest in the catheter center of the catheter's cross-sectional area ( a ). A microcatheter in the lumen of the DAC markedly reduces flow rates ( b ), particularly when it protrudes through the DAC tip. When the microcatheter is removed, only the delivery wire remains in the DAC lumen and flow rates are improved ( c ).
Advancing the Large Bore Distal Access Catheter: Applying Traction to the Stent Wire
Once the stent-retriever is deployed, we navigate the DAC as close as possible to the proximal end of the clot, which can be difficult in patients with tortuous vessels. To facilitate forward movement of the DAC, the stent delivery wire, which serves as a guiding structure for the DAC, can be straightened through slight pulling ( Fig. 5 ). Sometimes, the DAC cannot be advanced to the proximal clot interface because of severe tortuosity and/or atherosclerotic disease, although the delivery wire has been straightened. In such cases, reintroducing the microcatheter or selection of a smaller DAC can become necessary.
Fig. 5.
The stent delivery wire, which would naturally follow the curves of the vessel, is straightened through slight pulling. This facilitates advancement of the distal access catheter.
Avoiding Clot Fragmentation: Releasing Traction off the Stent Wire
When the DAC has reached its intended position (i.e., the proximal end of the clot), we release the traction off the delivery wire and ensure that its course is parallel to the DAC and its length equal to the length of the DAC ( Fig. 6 ). Unequal lengths of the DAC and delivery wire could potentially lead to shearing of the clot during clot withdrawal and damage of the DAC tip because the stent-retriever would get pulled into the DAC.
Fig. 6.
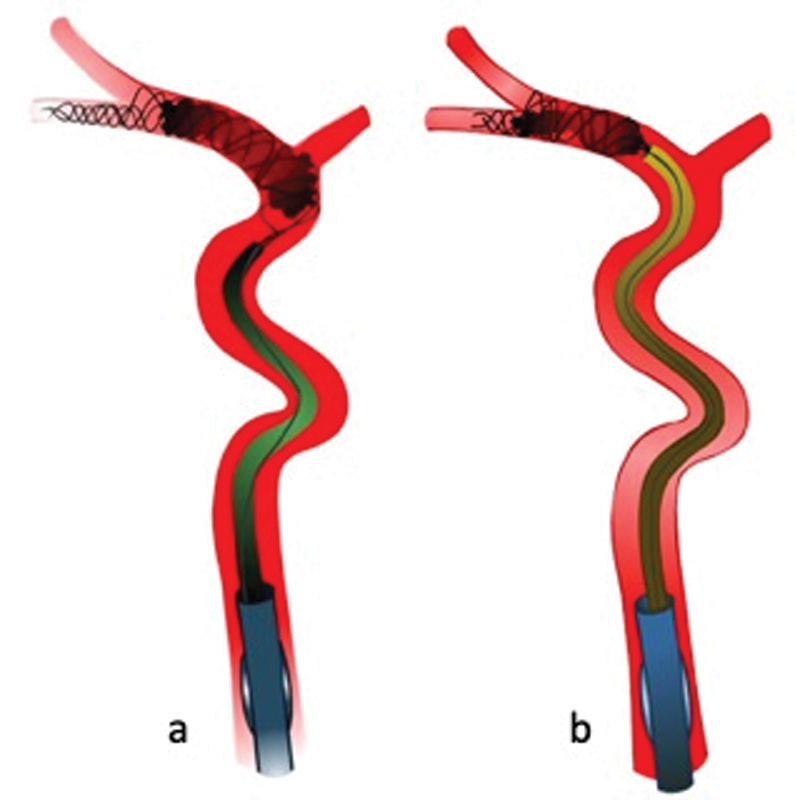
The distal access catheter (DAC) is navigated to the proximal clot interface ( a ). Once the DAC has been advanced as close to the proximal clot terminus as possible, the stent delivery wire is released to ensure that its course is parallel and its length is equal to the DAC ( b ).
Minimizing the Risk of Distal Embolization: Balloon Inflation, Combined Proximal and Distal Aspiration
Once the DAC is placed at the proximal end of the stent-retriever with the engaged clot, the balloon of the guide catheter is inflated to create a state of flow arrest. At the time of initiation of clot retrieval, we apply double aspiration to both the DAC and the BGC (either manually with a syringe or automated aspiration using a double pump, Fig. 7 ), as this decreases procedure times and improves patient outcome. 50 We recommend starting aspiration through the DAC when the catheter is 2 to 3 mm from clot. Applying aspiration earlier might decrease or even reverse flow in collateral vessels, thereby potentially worsening brain ischemia, whereas starting later may increase the risk of distal embolization. As a general rule, we try to keep the total time of aspiration as short as possible.
Fig. 7.

When the distal access catheter (DAC) has entailed the stent-retriever and the proximal clot terminus, and the course of the delivery wire and DAC is parallel, the balloon is inflated and combined proximal and distal aspiration (either manual or automated) is applied at the time of initiation of clot retrieval.
Withdrawal of the Stent-Retriever and Distal Access Catheter
When the DAC has engaged the stent-retriever with the proximal clot terminus, we withdraw both the DAC and stent-retriever with the entrapped clot into the guide catheter as a single unit under continuous double aspiration ( Fig. 8 ). Of note, the stent-retriever/clot complex should not be retrieved into the DAC, as this can damage the DAC tip and lead to clot fragmentation and distal embolization. 36 More distal stent-retriever placement (as described earlier) reduces distal embolization of small clot fragments that might shear off ( Fig. 8 ). Aspiration is maintained until the stent-retriever/clot complex and DAC are fully withdrawn into the BGC and removed out of the patient. We continue the proximal aspiration on the BGC during deflation to remove additional clot fragments from the tip of the BGC.
Fig. 8.
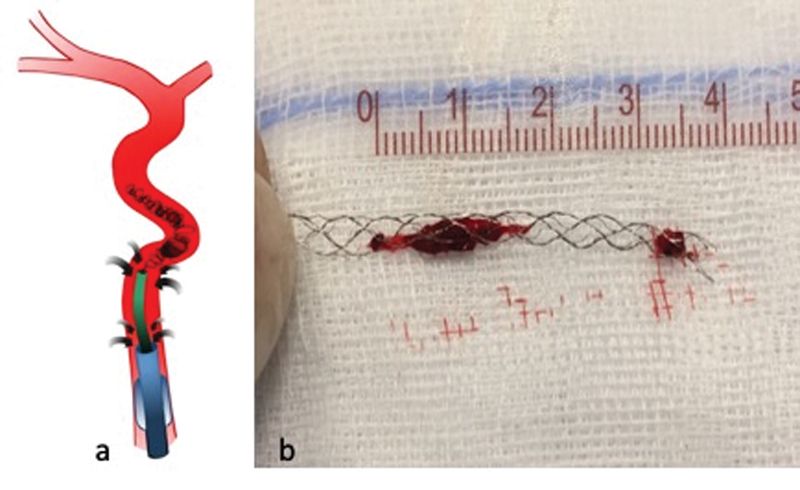
The distal access catheter, stent-retriever, and entrapped clot are withdrawn into the balloon guide catheter under continuous double aspiration ( a ). The distal stent-retriever placement in relation to the clot minimizes the risk of distal embolization: clot fragments that might shear off during the retrieval are likely captured by the distal part of the stent ( b ).
Overcoming High-Grade Carotid Stenosis, Carotid Occlusion, and Pseudoocclusion: Use of Diagnostic Catheters and Wires
Acute stroke patients with tandem lesions (i.e., an occlusion or high-grade stenosis of the extracranial ICA and a combined major intracranial artery occlusion) are challenging to treat. Currently, there is no consensus on the best treatment strategy, and thus, treatment approaches are highly variable. Furthermore, carotid pseudoocclusion (i.e., nonopacification of the vessel due to slow flow caused by a downstream occlusion) commonly mimics extracranial ICA occlusion, as the faint intraluminal opacification caused by slow flow can oftentimes not be appreciated. 51 In case of a tandem lesion, clot retrieval can be approached in two ways: either the intracranial clot is removed first and—if necessary—a carotid stent is put in place after clot retrieval, or the two steps are performed in reverse order. A third option is to perform in parallel both procedures by placing the stent-retriever first and using the wire of the stent-retriever to place a carotid stent. 52 If the intracranial clot is retrieved first, there is, in theory, a small risk of dislocation of thrombotic material, which has accumulated in the poststenotic ICA due to slow flow, into the anterior cerebral artery. However, as per our experience, this risk is almost negligible, while clot retrieval prior to ICA stenting has two major advantages: (1) brain ischemia and infarction is caused by the more distal clot, and fast retrieval of this clot minimizes infarct progression, and (2) when the BGC is placed beyond the ICA stenosis, it can “dotter the lesion,” which, in some cases, is sufficient to dilate the ICA stenosis such that stenting after clot retrieval is not necessary anymore.
High-Grade Stenosis at the ICA Origin with Normal and Patent Distal Vessel Lumen
To navigate fast and safely past a high-grade ICA origin stenosis with normal and patent distal vessel lumen ( Fig. 9a ), a diagnostic wire (e.g., 0.035-in wire, Fig. 9b ) and a diagnostic catheter ( Fig. 9c ) should be used, as they provide the necessary stiffness to overcome the stenosis. Once the diagnostic catheter has passed the stenosis, a BGC is placed in the cervical ICA ( Fig. 9d ) and the procedure is continued as usual.
Fig. 9.
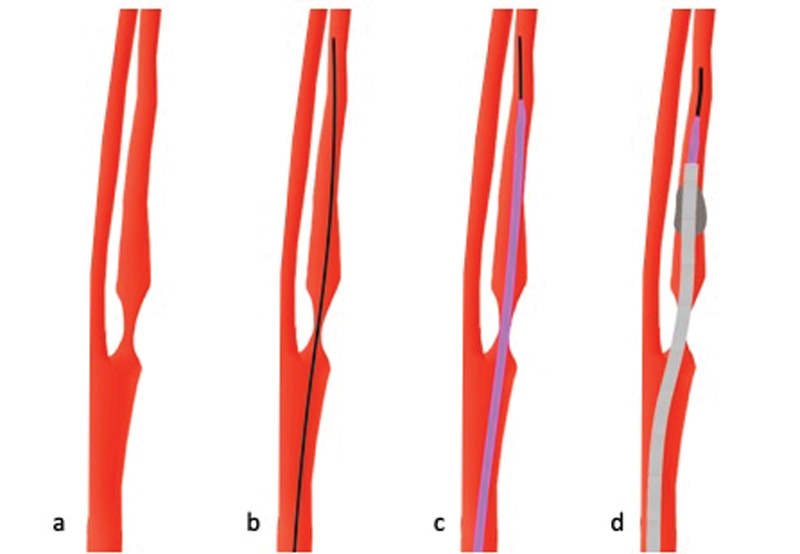
In case of a high-grade stenosis at the internal carotid artery (ICA) origin with a normal poststenotic vessel ( a ), a diagnostic wire (black) is navigated through the stenosis ( b ). A diagnostic catheter (purple) is then advanced past the stenosis ( c ). Once this is done, a balloon-guide catheter (gray) can be placed in the proximal ICA ( d ) and the procedure is continued as usual.
Pseudoocclusion at the ICA Origin
When a flame-shaped contrast extension into the ICA origin is seen ( Fig. 10a and Fig. 11a ), it is not clear whether this represents real extracranial occlusion (usually due to dissection) or pseudoocclusion caused by slow flow due to the intracranial occlusion. mCTA is the advanced imaging modality of choice in AIS 53 54 55 and can help distinguish these two entities: in pseudoocclusion, delayed contrast appearance in the affected ICA can usually be depicted in the delayed phases and the vessel lumen appears slightly hyperdense. 56 If this can be seen ( Fig. 10a ), a pseudoocclusion is more likely and a diagnostic wire ( Fig. 10b ) followed by a diagnostic catheter ( Fig. 10c ) should be advanced past the ICA origin into the proximal ICA. Then, a balloon-guide catheter is navigated past the origin and placed in the proximal ICA ( Fig. 10d ). As soon as this is done, aspiration from the balloon-guide catheter after balloon inflation should be initiated to remove the clotted blood that has accumulated in the ICA due to slow flow 57 ( Fig. 10e ).
Fig. 10.
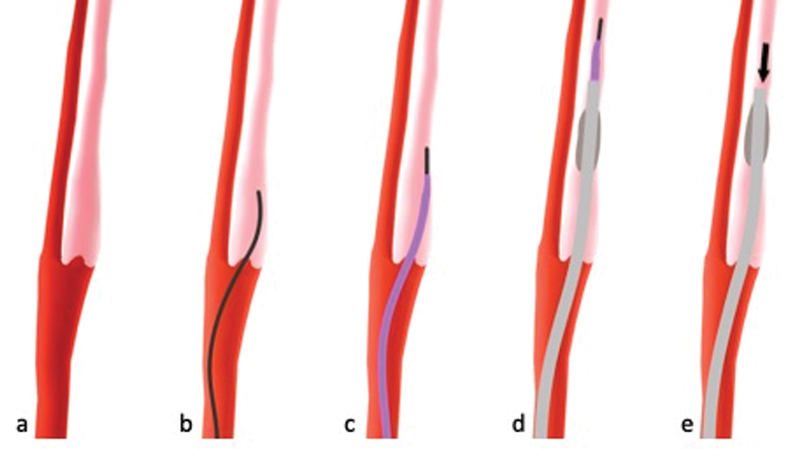
Pseudoocclusion with faint contrast staining (light red) in the internal carotid artery (ICA) in the second and third multiphase computed tomographic angiography phase ( a ). A diagnostic wire ( b ) followed by a diagnostic catheter ( c ) and a balloon-guide catheter ( d ) are advanced past the ICA origin and placed in the proximal ICA. As soon as the balloon-guide catheter is placed properly, aspiration is applied (black arrow) to remove thrombotic material that has accumulated in the ICA due to slow flow ( e ).
Fig. 11.
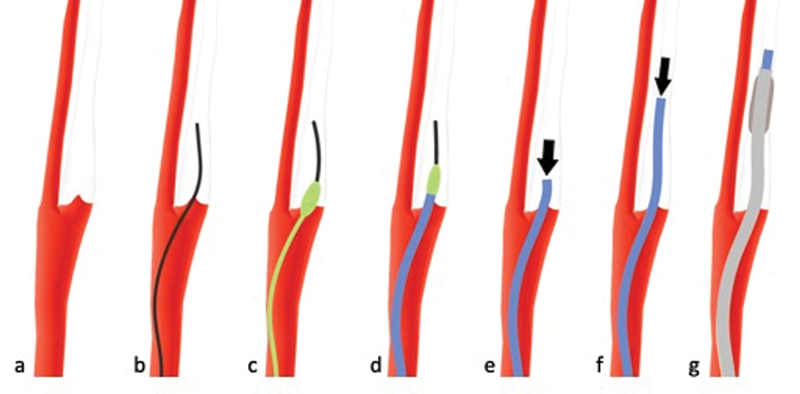
When no contrast staining in the internal carotid artery (ICA) can be appreciated ( a ), it is not clear whether the image constellation represents a real or a pseudoocclusion. In such cases, the risk of clot fragmentation and distal embolization should be minimized by using a microwire ( b ) and a distally fitted microcatheter (green, c ) which allows for facilitated, atraumatic navigation of the distal access catheter (DAC) ( d ) past the ICA origin. Once the DAC (blue) is placed, and while advancing it ( e , f ), suction should be immediately applied to capture clot fragments that could have potentially been sheared off during the previous steps (black arrows). A balloon-guide catheter is then advanced and placed in the cervical ICA ( g ) and the procedure can be continued as usual.
Possible Occlusion or Pseudoocclusion at the ICA Origin
If mCTA is not available or for other reasons it is not possible to discriminate between true and pseudoocclusion ( Fig. 11a ), a less traumatic approach should be chosen to minimize the risk of dislocation and fragmentation of an ICA thrombus with subsequent distal embolization. Therefore, a microwire ( Fig. 11b ) instead of a diagnostic wire, is advanced over the site of the suspected occlusion and a distally fitted microcatheter (a microcatheter with a bulbous part that is designed to match the inner lumen of the DAC; e.g., the Wedge microcatheter, Microvention, Tustin, CA; Fig. 11c ) is used to facilitate navigation of the DAC past the ICA origin ( Fig. 11d ). The DAC is connected to suction as it crosses through the stenosis to capture potentially fragmented thrombotic material that has been sheared off from the ICA origin and can be used to clear out residual clot from the ICA ( Fig. 11e ). The procedure can then be continued as usual. Once the DAC is beyond the stenosis, in our experience, it is relatively easy to advance the BGC over the DAC in the proximal ICA ( Fig. 11f ).
Sometimes, especially in cases with tough, calcified plaque at the ICA origin, the aforementioned, less traumatic approach fails because the microwire and microcatheter are not stiff enough and slip off ( Fig. 12a ). In such cases, a stiffer diagnostic wire and diagnostic catheter, which provide more stability, can be used. Once the catheter is positioned, the diagnostic wire ( Fig. 12b ), followed by the diagnostic catheter ( Fig. 12c ), and a BGC ( Fig. 12d ) are advanced past the occlusion site, and suction immediately applied to the BGC to clear out clotted blood that might have accumulated in the ICA ( Fig. 12e ). This approach carries a small risk of ICA dissection, but it is the only possibility to access the occluded intracranial vessel. Not infrequently, the clot at the ICA origin will encase the guide catheter; in such cases, a balloon is not even necessary to achieve flow arrest, and a regular guide catheter can be used. In cases of chronic carotid occlusions, where the ICA cannot the accessed, a contralateral approach is possible through the anterior communicating artery. 58
Fig. 12.
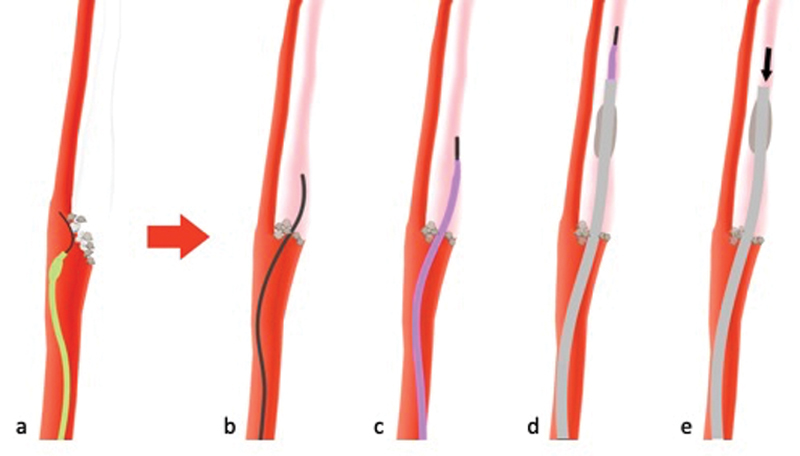
Sometimes, a less traumatic approach is not possible as the microwire and distally fitted microcatheter (green) are not stiff enough and will slip off ( a ). In such cases, a stiffer diagnostic wire and diagnostic catheter (purple) are used, which provide more stability. The wire is navigated past the site of occlusion ( b ), followed by the diagnostic catheter ( c ). Then, a guide catheter (either a balloon guide catheter as shown in d ) or a regular guide catheter) is advanced and aspiration is applied to clear out clot from the internal carotid artery ( e ).
Gaining Access in Patients with Challenging Arch Anatomy—Quadraxial Approach and Appropriate Catheter Choice
Inability to achieve common carotid access is a frequent reason for reperfusion delays and EVT failure. Particularly accessing the left common carotid artery can be difficult when there is an acute angle at the vessel origin. In this case, we recommend using a quadraxial approach with an additional 8-Fr Shuttle sheath (Cook Inc, Bloomington, IN), BGC, a Simmons-2 diagnostic catheter, and a stiff wire (e.g., Glidewire Advantage, Terumo, Japan). This provides more stability to the entire system once carotid access has been established ( Fig. 13 ). Alternatively, a transradial approach can be used, as most left common carotids that are hard to access through the transfemoral route are easily accessed transradially and there are reports of BGC use through the radial artery. 59
Fig. 13.
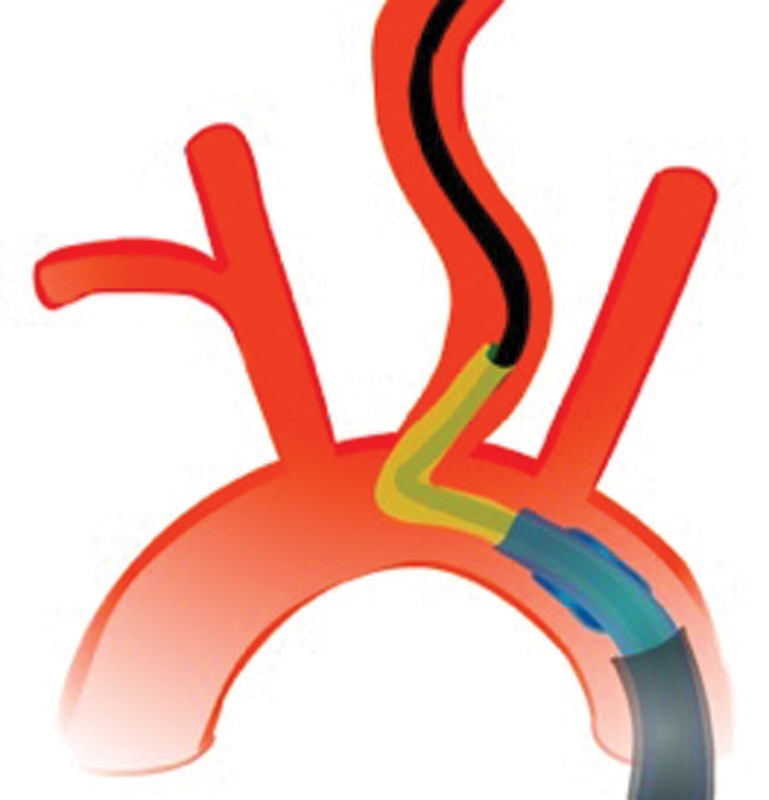
Acute angles at the left common carotid artery origin often require a shuttle in addition to the balloon guide catheter, a Simmons-2 distal access catheter, and stiff diagnostic guidewire (quadraxial approach).
The Simmons-2 catheter's shape is optimized for accessing the CCA in case of an acute angle at the vessel origin and its tip can usually be positioned in the left common carotid artery without difficulty. For a slightly less acute angle (which we see more frequently), we chose the Vitek (VTK) catheter, which can be considered the “work-horse” of left common carotid artery access. Once the catheter tip is in its intended position, it is slightly pulled back to straighten the angle at the common carotid origin. This usually allows us to advance the BGC over the common carotid artery origin ( Fig. 14 ).
Fig. 14.
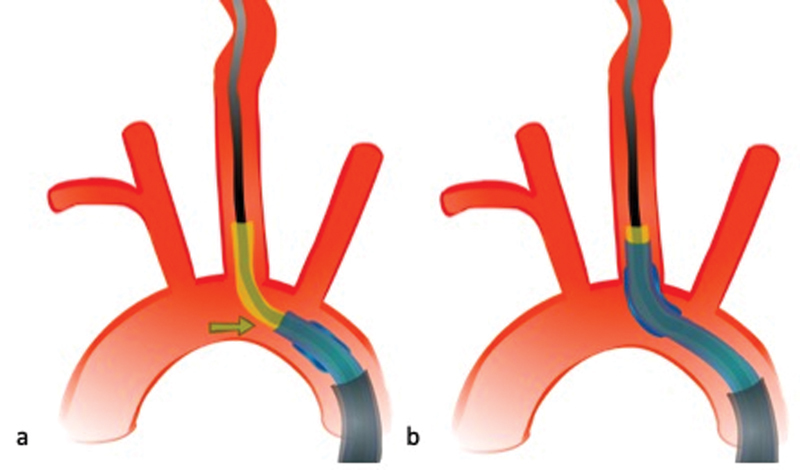
Once the tip of the Simmons-2 catheter is placed in the proximal left common carotid artery, the catheter is slightly pulled back to straighten the angle at the vessel origin ( a ). This allows easy advancement of the balloon guide catheter because the angle it has to overcome is less acute ( b ).
Usually we advance the wire as far distally as possible, sometimes even up to the petrous segment to improve the system's stability. If, despite these measures, support for advancing the BGC is insufficient, the wire can be replaced by an even stiffer wire (e.g., 0.035-in stiff Glidewire or 0.035-in Glidewire Advantage [Terumo, Japan]). Pushing the Simmons-2 or VTK catheter further forward once its tip is positioned in the left common carotid artery has to be avoided, as this will lead to dislocation of the whole system including the guidewire into the aortic arch ( Fig. 15 ). Radial access should be considered the first alternative if the quadraxial approach fails and can be a good first-line approach in patients with known severe atherosclerosis of the femoral arteries and abdominal aorta. Sometimes, the angle at the left common carotid artery origin is such that access is very challenging when coming from the femoral artery but easy from the radial artery. Only in the rare case that both the femoral and radial approach fail, a direct carotid puncture is necessary.
Fig. 15.
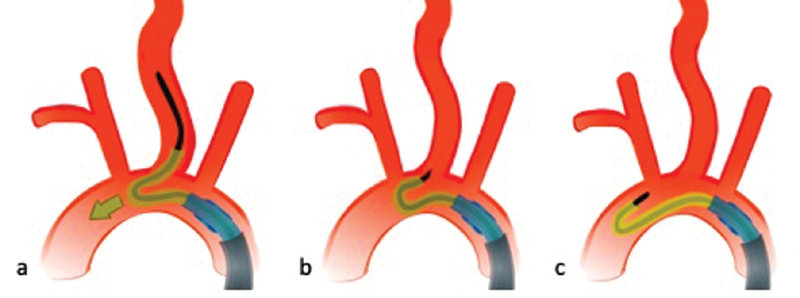
Once its tip is properly positioned in the left common carotid artery, one should not push the Simmons-2 catheter further forward ( a ), as this will lead to dislocation of the whole system including the guidewire into the aortic arch ( b, c ).
The Future of EVT: The Challenge of Getting the Right Patient to the Right Hospital
Current endovascular treatment techniques are already fairly sophisticated, and the scope for further improvement is probably limited. One major problem is that only a small fraction of AIS patients with LVO reach the angiography table. To increase the denominator (i.e., the EVT-eligible treatment population), existing systems of care have to be reorganized and transport paradigms individually tailored to local geography and infrastructure. 60 61 62 Fast first-pass complete reperfusion, which is the focus of this review article, remains a cornerstone for successful stroke treatment. However, nowadays improving prehospital stroke management and intrahospital workflows (e.g., bypassing the CT scanner to get the patient faster to the angiography table: the so-called one-stop management) 63 64 yields an even greater potential for improving patient outcomes.
Conclusion
In conclusion, fast first-pass complete reperfusion should be the ultimate goal when performing EVT in patients with AIS. Ideally, a combined technique (such as BADDASS) should be used, as it yields the greatest chance to achieve this goal and, thus, improve patient outcome. Overcoming high-grade carotid stenosis or (pseudo-)occlusions and tortuous anatomy can be challenging and can be mastered by using stiffer, diagnostic wires/catheters and distally fitted microcatheters. Difficulties in gaining access at the aortic arch level can be overcome by using a quadraxial approach. However, the bottleneck in neurointerventional stroke treatment nowadays is not treatment technique alone, but it is the organization of stroke care: how to get the right patient to the right hospital as fast as possible.
Footnotes
Conflict of Interest None.
References
- 1.Demaerschalk B M, Kleindorfer D O, Adeoye O M et al. Scientific rationale for the inclusion and exclusion criteria for intravenous alteplase in acute ischemic stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2016;47(02):581–641. doi: 10.1161/STR.0000000000000086. [DOI] [PubMed] [Google Scholar]
- 2.Menon B K, Al-Ajlan F S, Najm M et al. Association of clinical, imaging, and thrombus characteristics with recanalization of visible intracranial occlusion in patients with acute ischemic stroke. JAMA. 2018;320(10):1017–1026. doi: 10.1001/jama.2018.12498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goyal M, Menon B K, van Zwam W Het al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials Lancet 2016387(10029):1723–1731. [DOI] [PubMed] [Google Scholar]
- 4.Powers W J, Rabinstein A A, Ackerson T et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49(03):e46–e110. doi: 10.1161/STR.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 5.Kunz W G, Almekhlafi M A, Menon B Ket al. Public health potential of improved reperfusion in thrombectomy for stroke based on HERMES collaboration dataStroke2019. 50(01):Abstract 174)
- 6.Saposnik G, Menon B K, Kashani N et al. Factors associated with the decision-making on endovascular thrombectomy for the management of acute ischemic stroke. Stroke. 2019;50(09):2441–2447. doi: 10.1161/STROKEAHA.119.025631. [DOI] [PubMed] [Google Scholar]
- 7.Wang F, Campbell B CV, Churilov L et al. Insights into variations in preferred selection criteria for acute stroke endovascular therapy. J Neurointerv Surg. 2018;10(06):542–549. doi: 10.1136/neurintsurg-2017-013247. [DOI] [PubMed] [Google Scholar]
- 8.Pan Y, Cai X, Huo X et al. Cost-effectiveness of mechanical thrombectomy within 6 hours of acute ischaemic stroke in China. BMJ Open. 2018;8(02):e018951. doi: 10.1136/bmjopen-2017-018951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arora N, Makino K, Tilden D, Lobotesis K, Mitchell P, Gillespie J. Cost-effectiveness of mechanical thrombectomy for acute ischemic stroke: an Australian payer perspective. J Med Econ. 2018;21(08):799–809. doi: 10.1080/13696998.2018.1474746. [DOI] [PubMed] [Google Scholar]
- 10.Achit H, Soudant M, Hosseini K et al. Cost-effectiveness of thrombectomy in patients with acute ischemic stroke: the THRACE Randomized Controlled Trial. Stroke. 2017;48(10):2843–2847. doi: 10.1161/STROKEAHA.117.017856. [DOI] [PubMed] [Google Scholar]
- 11.Shireman T I, Wang K, Saver J L et al. Cost-effectiveness of solitaire stent retriever thrombectomy for acute ischemic stroke: results from the SWIFT-PRIME Trial (Solitaire With the Intention for Thrombectomy as Primary Endovascular Treatment for Acute Ischemic Stroke) Stroke. 2017;48(02):379–387. doi: 10.1161/STROKEAHA.116.014735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kunz W G, Almekhlafi M A, Menon B K et al. Lifetime quality of life and cost consequences of treatment delays in endovascular thrombectomy for stroke based on HERMES data. J Neurointerv Surg. 2018;10:A1–A2. [Google Scholar]
- 13.Saver J L. Time is brain--quantified. Stroke. 2006;37(01):263–266. doi: 10.1161/01.STR.0000196957.55928.ab. [DOI] [PubMed] [Google Scholar]
- 14.Menon B K, Almekhlafi M A, Pereira V M et al. Optimal workflow and process-based performance measures for endovascular therapy in acute ischemic stroke: analysis of the Solitaire FR thrombectomy for acute revascularization study. Stroke. 2014;45(07):2024–2029. doi: 10.1161/STROKEAHA.114.005050. [DOI] [PubMed] [Google Scholar]
- 15.Saver J L, Goyal M, van der Lugt A et al. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA. 2016;316(12):1279–1288. doi: 10.1001/jama.2016.13647. [DOI] [PubMed] [Google Scholar]
- 16.Menon B K, Sajobi T T, Zhang Y et al. Analysis of workflow and time to treatment on thrombectomy outcome in the Endovascular Treatment for Small Core and Proximal Occlusion Ischemic Stroke (ESCAPE) randomized, controlled trial. Circulation. 2016;133(23):2279–2286. doi: 10.1161/CIRCULATIONAHA.115.019983. [DOI] [PubMed] [Google Scholar]
- 17.Goyal M, Jadhav A P, Bonafe A et al. Analysis of workflow and time to treatment and the effects on outcome in endovascular treatment of acute ischemic stroke: results from the SWIFT PRIME Randomized Controlled Trial. Radiology. 2016;279(03):888–897. doi: 10.1148/radiol.2016160204. [DOI] [PubMed] [Google Scholar]
- 18.Bourcier R, Goyal M, Liebeskind D S et al. Association of time from stroke onset to groin puncture with quality of reperfusion after mechanical thrombectomy: a meta-analysis of individual patient data from 7 randomized clinical trials. JAMA Neurol. 2019;76(04):405–411. doi: 10.1001/jamaneurol.2018.4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Behme D, Gera R G, Tsogkas Iet al. Impact of time on thrombolysis in cerebral infarction score resultsClin Neuroradiol2019 [DOI] [PubMed]
- 20.Liebeskind D S, Bracard S, Guillemin F et al. eTICI reperfusion: defining success in endovascular stroke therapy. J Neurointerv Surg. 2019;11(05):433–438. doi: 10.1136/neurintsurg-2018-014127. [DOI] [PubMed] [Google Scholar]
- 21.Higashida R T, Furlan A J, Roberts H et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. 2003;34(08):e109–e137. doi: 10.1161/01.STR.0000082721.62796.09. [DOI] [PubMed] [Google Scholar]
- 22.Fugate J E, Klunder A M, Kallmes D F. What is meant by “TICI”? AJNR Am J Neuroradiol. 2013;34(09):1792–1797. doi: 10.3174/ajnr.A3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zaidat O O, Yoo A J, Khatri P et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. 2013;44(09):2650–2663. doi: 10.1161/STROKEAHA.113.001972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Almekhlafi M A, Mishra S, Desai J A et al. Not all “successful” angiographic reperfusion patients are an equal validation of a modified TICI scoring system. Interv Neuroradiol. 2014;20(01):21–27. doi: 10.15274/INR-2014-10004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dargazanli C, Fahed R, Blanc R et al. Modified thrombolysis in cerebral infarction 2C/thrombolysis in cerebral infarction 3 reperfusion should be the aim of mechanical thrombectomy: insights from the ASTER Trial (Contact Aspiration Versus Stent Retriever for Successful Revascularization) Stroke. 2018;49(05):1189–1196. doi: 10.1161/STROKEAHA.118.020700. [DOI] [PubMed] [Google Scholar]
- 26.Lapergue B, Blanc R, Gory B et al. Effect of endovascular contact aspiration vs stent retriever on revascularization in patients with acute ischemic stroke and large vessel occlusion: the ASTER Randomized Clinical Trial. JAMA. 2017;318(05):443–452. doi: 10.1001/jama.2017.9644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turk A S, III, Siddiqui A, Fifi J Tet al. Aspiration thrombectomy versus stent retriever thrombectomy as first-line approach for large vessel occlusion (COMPASS): a multicentre, randomised, open label, blinded outcome, non-inferiority trial Lancet 2019393(10175):998–1008. [DOI] [PubMed] [Google Scholar]
- 28.Psychogios M N, Tsogkas I, Brehm A et al. Clot reduction prior to embolectomy: mSAVE as a first-line technique for large clots. PLoS One. 2019;14(05):e0216258. doi: 10.1371/journal.pone.0216258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zaidat O O, Castonguay A C, Linfante I et al. First pass effect: a new measure for stroke thrombectomy devices. Stroke. 2018;49(03):660–666. doi: 10.1161/STROKEAHA.117.020315. [DOI] [PubMed] [Google Scholar]
- 30.Furlan A, Higashida R, Wechsler L et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA. 1999;282(21):2003–2011. doi: 10.1001/jama.282.21.2003. [DOI] [PubMed] [Google Scholar]
- 31.Smith W S, Sung G, Starkman S et al. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke. 2005;36(07):1432–1438. doi: 10.1161/01.STR.0000171066.25248.1d. [DOI] [PubMed] [Google Scholar]
- 32.Khandelwal P, Yavagal D R, Sacco R L. Acute ischemic stroke intervention. J Am Coll Cardiol. 2016;67(22):2631–2644. doi: 10.1016/j.jacc.2016.03.555. [DOI] [PubMed] [Google Scholar]
- 33.Broderick J P, Palesch Y Y, Demchuk A M et al. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med. 2013;368(10):893–903. doi: 10.1056/NEJMoa1214300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ciccone A, Valvassori L; SYNTHESIS Expansion Investigators.Endovascular treatment for acute ischemic stroke N Engl J Med 2013368252433–2434. [DOI] [PubMed] [Google Scholar]
- 35.Lapergue B, Blanc R, Guedin P et al. A direct aspiration, first pass technique (ADAPT) versus stent retrievers for acute stroke therapy: an observational comparative study. AJNR Am J Neuroradiol. 2016;37(10):1860–1865. doi: 10.3174/ajnr.A4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McTaggart R A, Tung E L, Yaghi S et al. Continuous aspiration prior to intracranial vascular embolectomy (CAPTIVE): a technique which improves outcomes. J Neurointerv Surg. 2017;9(12):1154–1159. doi: 10.1136/neurintsurg-2016-012838. [DOI] [PubMed] [Google Scholar]
- 37.Maus V, Behme D, Kabbasch C et al. Maximizing first-pass complete reperfusion with SAVE. Clin Neuroradiol. 2018;28(03):327–338. doi: 10.1007/s00062-017-0566-z. [DOI] [PubMed] [Google Scholar]
- 38.Maus V, Henkel S, Riabikin A et al. The SAVE technique: large-scale experience for treatment of intracranial large vessel occlusions. Clin Neuroradiol. 2019;29(04):669–676. doi: 10.1007/s00062-018-0702-4. [DOI] [PubMed] [Google Scholar]
- 39.Nikoubashman O, Wischer D, Hennemann H M et al. Balloon-guide catheters are needed for effective flow reversal during mechanical thrombectomy. AJNR Am J Neuroradiol. 2018;39(11):2077–2081. doi: 10.3174/ajnr.A5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zaidat O O, Mueller-Kronast N H, Hassan A E et al. Impact of balloon guide catheter use on clinical and angiographic outcomes in the STRATIS Stroke Thrombectomy Registry. Stroke. 2019;50(03):697–704. doi: 10.1161/STROKEAHA.118.021126. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen T N, Malisch T, Castonguay A C et al. Balloon guide catheter improves revascularization and clinical outcomes with the Solitaire device: analysis of the North American Solitaire Acute Stroke Registry. Stroke. 2014;45(01):141–145. doi: 10.1161/STROKEAHA.113.002407. [DOI] [PubMed] [Google Scholar]
- 42.Nguyen T N, Castonguay A C, Nogueira R G et al. Effect of balloon guide catheter on clinical outcomes and reperfusion in Trevo thrombectomy. J Neurointerv Surg. 2019;11(09):861–865. doi: 10.1136/neurintsurg-2018-014452. [DOI] [PubMed] [Google Scholar]
- 43.Brinjikji W, Starke R M, Murad M H et al. Impact of balloon guide catheter on technical and clinical outcomes: a systematic review and meta-analysis. J Neurointerv Surg. 2018;10(04):335–339. doi: 10.1136/neurintsurg-2017-013179. [DOI] [PubMed] [Google Scholar]
- 44.Maus V, Brehm A, Tsogkas I, Henkel S, Psychogios M N. Stent retriever placement in embolectomy: the choice of the post-bifurcational trunk influences the first-pass reperfusion result in M1 occlusions. J Neurointerv Surg. 2019;11(03):237–240. doi: 10.1136/neurintsurg-2018-014114. [DOI] [PubMed] [Google Scholar]
- 45.Nogueira R G, Lutsep H L, Gupta Ret al. Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): a randomised trial Lancet 2012380(9849):1231–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van der Marel K, Chueh J Y, Brooks O W et al. Quantitative assessment of device-clot interaction for stent retriever thrombectomy. J Neurointerv Surg. 2016;8(12):1278–1282. doi: 10.1136/neurintsurg-2015-012209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haussen D C, Rebello L C, Nogueira R G. Optimizing clot retrieval in acute stroke: the push and fluff technique for closed-cell stentrievers. Stroke. 2015;46(10):2838–2842. doi: 10.1161/STROKEAHA.115.010044. [DOI] [PubMed] [Google Scholar]
- 48.Nikoubashman O, Alt J P, Nikoubashman A et al. Optimizing endovascular stroke treatment: removing the microcatheter before clot retrieval with stent-retrievers increases aspiration flow. J Neurointerv Surg. 2017;9(05):459–462. doi: 10.1136/neurintsurg-2016-012319. [DOI] [PubMed] [Google Scholar]
- 49.Froehler M T.Comparison of vacuum pressures and forces generated by different catheters and pumps for aspiration thrombectomy in acute ischemic stroke Intervent Neurol 20176(3-4):199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hopf-Jensen S, Preiß M, Marques L et al. Impact and effectiveness of dual aspiration technique in stent-assisted mechanical thrombectomy: recent improvements in acute stroke management. Cardiovasc Intervent Radiol. 2016;39(11):1620–1628. doi: 10.1007/s00270-016-1404-4. [DOI] [PubMed] [Google Scholar]
- 51.Diouf A, Fahed R, Gaha M et al. Cervical internal carotid occlusion versus pseudo-occlusion at CT angiography in the context of acute stroke: an accuracy, interobserver, and intraobserver agreement study. Radiology. 2018;286(03):1008–1015. doi: 10.1148/radiol.2017170681. [DOI] [PubMed] [Google Scholar]
- 52.Maus V, Behme D, Maurer Cet al. The ReWiSed CARe technique: simultaneous treatment of atherosclerotic tandem occlusions in acute ischemic stroke Clin Neuroradiol 2019 10.1007/s00062-019-00795-z[Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 53.Zerna C, Assis Z, d'Esterre C D, Menon B K, Goyal M. Imaging, intervention, and workflow in acute ischemic stroke: the Calgary approach. AJNR Am J Neuroradiol. 2016;37(06):978–984. doi: 10.3174/ajnr.A4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Menon B K, d'Esterre C D, Qazi E M et al. Multiphase CT angiography: a new tool for the imaging triage of patients with acute ischemic stroke. Radiology. 2015;275(02):510–520. doi: 10.1148/radiol.15142256. [DOI] [PubMed] [Google Scholar]
- 55.García-Tornel A, Carvalho V, Boned Set al. Improving the evaluation of collateral circulation by multiphase computed tomography angiography in acute stroke patients treated with endovascular reperfusion therapies Intervent Neurol 20165(3-4):209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lev M H. Cervical internal carotid artery occlusion versus pseudo-occlusion: Can CT angiography help distinguish these in the acute stroke setting? Radiology. 2018;286(03):1095–1096. doi: 10.1148/radiol.2018172527. [DOI] [PubMed] [Google Scholar]
- 57.Eesa M, Almekhlafi M A, Mitha A P, Wong J H, Goyal M. Manual aspiration thrombectomy through balloon-tipped guide catheter for rapid clot burden reduction in endovascular therapy for ICA L/T occlusion. Neuroradiology. 2012;54(11):1261–1265. doi: 10.1007/s00234-012-1039-3. [DOI] [PubMed] [Google Scholar]
- 58.Maus V, Brehm A, Psychogios M N. Stent retriever embolectomy in acute occlusion of the anterior and middle cerebral artery using a transanterior communicating artery approach. J Vasc Interv Radiol. 2019;30(10):1709–1711. doi: 10.1016/j.jvir.2019.02.021. [DOI] [PubMed] [Google Scholar]
- 59.Maus V, Styczen H, Psychogios M N. Intracranial mechanical thrombectomy using a proximal balloon guide catheter via a transradial access. Interv Neuroradiol. 2019;25(05):508–510. doi: 10.1177/1591019919844850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goyal M, Jadhav A P, Wilson A T, Nogueira R G, Menon B K. Shifting bottlenecks in acute stroke treatment. J Neurointerv Surg. 2016;8(11):1099–1100. doi: 10.1136/neurintsurg-2015-012151. [DOI] [PubMed] [Google Scholar]
- 61.Goyal M, Wilson A T, Mayank D et al. John Nash and the Organization of Stroke Care. AJNR Am J Neuroradiol. 2018;39(02):217–218. doi: 10.3174/ajnr.A5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Holodinsky J K, Williamson T S, Demchuk A M et al. Modeling stroke patient transport for all patients with suspected large-vessel occlusion. JAMA Neurol. 2018;75(12):1477–1486. doi: 10.1001/jamaneurol.2018.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brehm A, Tsogkas I, Maier I L et al. One-stop management with perfusion for transfer patients with stroke due to a large-vessel occlusion: feasibility and effects on in-hospital times. AJNR Am J Neuroradiol. 2019;40(08):1330–1334. doi: 10.3174/ajnr.A6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Psychogios M N, Behme D, Schregel K et al. One-stop management of acute stroke patients: minimizing door-to-reperfusion times. Stroke. 2017;48(11):3152–3155. doi: 10.1161/STROKEAHA.117.018077. [DOI] [PubMed] [Google Scholar]
- 65.Behme D, Tsogkas I, Colla R et al. Validation of the extended thrombolysis in cerebral infarction score in a real world cohort. PLoS One. 2019;14(01):e0210334. doi: 10.1371/journal.pone.0210334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brehm A, Maus V, Tsogkas I et al. Stent-retriever assisted vacuum-locked extraction (SAVE) versus a direct aspiration first pass technique (ADAPT) for acute stroke: data from the real-world. BMC Neurol. 2019;19(01):65. doi: 10.1186/s12883-019-1291-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


