Abstract
Background and Purpose
APOE-ε4 genotype is a risk factor for sporadic Alzheimer’s disease and reduced recovery from brain injury. Since data on APOE genotype and dementia associated with TIA/stroke are sparse, we determined the associations in a longitudinal population-based cohort.
Methods
All patients with TIA or stroke (2002–2012) in a defined population of 92 728 (Oxford Vascular Study) had follow-up to 5-years. Pre-event and incident post-event dementia were ascertained through direct patient assessment and follow-up, supplemented by review of hospital/primary care records. Associations between pre- and post-event dementia and APOE genotype (ε4/ε4-homozygous and ε4/ε3-heterozygous versus ε3/ε3) were examined using logistic regression and Cox regression models respectively, adjusted for age, sex, education, cerebrovascular burden (stroke severity, prior stroke, white matter disease), diabetes and dysphasia.
Results
Among 1767 genotyped patients (mean/SD age=73.0/13.0 years, 901 (51%) male, 602 (34%) TIA), 1058 (59.9%) were APOE-ε3/ε3, 403 (22.8%) were ε4/ε3 and 30 (1.7%) were ε4-homozygous. Homozygosity was associated with both pre-event (adjusted odds ratio (OR)=5.81, 95% CI=1.93-17.48, p=0.002) and post-event dementia (adjusted hazard ratio (HR)=3.64, 1.90-7.00, p<0.0001). Association with post-event dementia was maintained after further adjustment for baseline cognitive impairment (HR=2.41, 1.19-4.89, p=0.01). There were no associations overall between ε4/ε3 and pre-event dementia (adjusted OR=1.47, 0.88-2.45, p=0.14) or post-event dementia (HR=1.11, 0.84-1.48, p=0.47).
Conclusions
In patients with TIA and stroke, APOE-ε4 homozygosity was associated with both pre- and post-event dementia. Associations were independent of cerebrovascular burden, and may be mediated through increased neurodegenerative pathology or vulnerability to injury.
Keywords: Apolipoprotein E, dementia, TIA, stroke, Alzheimer’s disease, cohort studies
Introduction
Apolipoprotein E (APOE) is a lipoprotein produced in several organs including the brain with three major isoforms (APOE 2,3,4) encoded by three different alleles located on chromosome 19: APOE-ε4, APOE-ε3 and APOE-ε2.1,2 Allelic frequencies in Caucasians are 15% for APOE-ε4 and 8% for APOE-ε2 with the remainder being APOE-ε3.1,3 The three isoforms differ by single amino acid residues resulting in structural and functional differences.1 The APOE4 isoform has neuropathological effects on neurons, the blood-brain barrier and blood vessels resulting in various clinical manifestations including worse outcomes after brain injury.2,4–7
APOE-ε4 genotype is the major genetic risk factor for sporadic Alzheimer’s disease: ε4/ε4 homozygotes have an odds ratio (OR) of 9-13 compared to ε3/ε3 individuals although risks are substantially lower in ε4 heterozygotes (OR~3).3,8 Dementia free carriers of APOE-ε4 have lower baseline cognitive function and faster rates of cognitive decline9,10 although effects attenuate in the oldest old.11 APOE-ε4 homozygosity has been linked to white matter disease load and progression12,13 but neuropathological studies suggest that effects are mediated by increased Alzheimer pathology.14,15
APOE-ε4 genotype might therefore be expected to impact the risk of dementia associated with stroke. However, there are relatively few data and findings from previous studies are conflicting. Positive association,16–20,21 no effect22 and protective effects have been reported23,24 but numbers with stroke were small (<200) often with limited adjustment for confounders. We therefore undertook a longitudinal population-based study of all TIA and stroke with standardised assessment of confounders and follow-up for dementia to 5-years to determine the association between APOE4-ε4 genotype and dementia before and after TIA and stroke. The current study builds on our previous work validating our methodology to reliably estimate dementia associated with TIA and stroke.25–28
Methods
Data availability
Requests for access to data should be submitted for consideration to the Study Director (peter.rothwell@ndcn.ox.ac.uk).
Patient Cohort and Eligibility
Consecutive patients with TIA or stroke were prospectively recruited from 1st April 2002 to 31st March 2012 as part of the Oxford Vascular Study (OxVASC), a population-based cohort study of all acute vascular events occurring within a defined population of 92 728 covered by around 100 primary care physicians in nine primary care practices in Oxfordshire, UK.29,30 The OXVASC population is 94% Caucasian, 3% Asian, 2% Chinese, and 1% Afro-Caribbean.4 Please see https://www.ahajournals.org/journal/str for further details on the study population and case ascertainment methodology.
Ascertainment of all TIA and ischaemic or haemorrhagic stroke events, including in those with early deaths and TIA/stroke occurring in non-hospitalised/non-referred patients, was achieved by multiple methods and has been shown to be near-complete,31 and to minimise selection biases in determining dementia risk.25 Informed consent (or assent from relatives) was obtained for study interview and follow-up, including ongoing review of all primary care/hospital records and death certificates. The study was approved by the local research ethics committee. Patients eligible for APOE genotype testing were defined as patients surviving to acute assessment, and with consent or assent for blood sampling. Patients who were moribund/unlikely to survive were considered not eligible for genotyping.
Baseline data collection
Patients were assessed by a study clinician as soon as possible after their TIA/stroke. TIA and stroke were defined using the WHO criteria32 (ie patients with relevant infarction on brain imaging but focal neurological symptoms lasting <24 hours were classed as TIA) with review of all cases by the same senior (vascular) neurologist (PMR) throughout the study Please see https://www.ahajournals.org/journal/str for further details on the definitions of cerebrovascular events. Patient data, including education and vascular risk factors, were collected by interview using a standardised form, supplemented by primary care records (see https://www.ahajournals.org/journal/str).29,30 Premorbid functional status was assessed using modified Rankin and Barthel scores, and stroke severity assessed with the NIHSS score.29,30 Baseline brain and vascular imaging and other investigations were performed as reported previously.29,30
Brain imaging and WMD severity grading
For further details on the brain imaging methodology see https://www.ahajournals.org/journal/str. Assessments were made blind to clinical data. A qualitative scale was used (“Oxford scale”) based on the WMD severity score (absent, mild, moderate, or severe) of the Blennow scale for CT scans, and a modified version of the Fazekas scale for MRI scans.33
Follow-up Methodology and Dementia Diagnosis
Multiple methods of follow-up were used to reduce attritional biases in identification of dementia.26 Follow-up interviews were done by trained nurses or study physicians at 1 and 6 months and 1, and 5 years. If clinic follow-up was not possible, patients were assessed at home, or via telephone. Mini-mental-state-examination (MMSE)34 was done at face-to-face interview and telephone-Montreal Cognitive Assessment (T-MoCA) and Telephone Interview for Cognitive Status-modified (TICSm) for those unable to have face-to-face follow-up.35–37
Dementia was defined as pre- or post-event according to whether the diagnosis was made before or after the index event, as described previously (see https://www.ahajournals.org/journal/str).25–28 Briefly, pre-event dementia diagnosis was made using the following information: i) baseline clinical assessment by study physician and discussion with relatives or other informant; ii) any dementia diagnosis, and related consultations and investigations, where available, in the primary care record, with hand-searching of the entire record including individual consultations, clinic letters, and hospitalisation documentation. The diagnosis of pre-event dementia was made by an experienced Consultant physician/geriatrician with subspecialty interest in dementia (STP) using the Diagnostic and Statistical Manual-IV (DSM-IV) criteria after review of the baseline clinical assessment and the medical records.
In patients without pre-event dementia, post-event dementia was diagnosed by STP using the same methodology.25–28 MMSE was done at each follow-up interview, and dementia was diagnosed if MMSE was <24 and remained <24 for all subsequent follow-ups.25–28 In patients with problems interfering with testing, incomplete testing, telephone follow-up or untestability at study interview,28 or without a study follow-up assessment, dementia was diagnosed on the basis of study records where available and hand-searching of primary care, hospital and death records, based on DSM-IV criteria.25–28
APOE genotype testing
Laboratory staff undertaking APOE genotyping were blinded to clinical data. Genomic DNA was extracted from whole blood (collected into vacutainers, K2E, Becton Dickinson) using the QIAamp DNA Blood Midi Kit (Qiagen). The concentration and purity of the DNA was assessed using a Nanodrop 1000 spectrophotometer (Thermo Fisher Scientific UK). The DNA was analysed using the StepOne Real Time PCR analyser (Applied Biosystems) to determine the allelic variants of APOE. The six APOE genotypes (ε2/ε2, ε2/ε3, ε2/ε4, ε3/ε3, ε3/ε4 ε4/ε4) were determined using SNPs rs429358 and rs7412 (Life Technologies). The standard StepOne genotyping method was followed using a 96-well MicroAmp Fast reaction plate (Life Technologies). Each well contained 50ng DNA and one of the two SNPs, in a TaqMan Universal PCR master mix (Applied Biosystems) in a total of 25uL. Results were analysed using the StepOne software. The validity of genotyping was assessed by repeating analyses in 100 samples in an independent lab with investigators blinded to the initial results. Agreement for APOE genotype was found to be 100%.
Statistical Analysis
Baseline characteristics between eligible and tested, eligible but not tested, and not eligible patients were compared using analysis of variance (ANOVA) or χ2 test as appropriate. For tested patients, baseline characteristics between groups with the different APOE genotypes were also compared using analysis of variance (ANOVA) or χ2 test. Cumulative incidence of dementia post-event (i.e. after exclusion of pre-event dementia) was calculated by Kaplan-Meier methods censoring at death as described previously.28 To account for the competing risk of death, we also used Cumulative Incidence Competing Risk methods.
Associations between APOE genotype and dementia were determined by comparing heterozygous (APOE ε4/ε3) or homozygous (APOE ε4/ε4) individuals with ε3/ε3 as the reference group. We excluded those with ε2/ε4 since ε2 is reported as protective against dementia and this group was very small. We also examined APOE-ε2 genotype (ε2/ε3 and ε2/ε2) associations with ε3/ε3 as the reference group.
Associations between APOE-ε4 and APOE-ε2 genotypes and pre-event dementia were determined by binary logistic regression to generate odds ratios (ORs) adjusted for i) age, sex, and education (model 1), ii) age, sex, education and other factors previously reported as associated with pre-event dementia28 including white matter disease, prior stroke, diabetes and also index stroke event severity (NIHSS) and dysphasia occurring after the index stroke (model 2). Stroke severity and dysphasia may be linked to pre-existing dementia because of a shared susceptibility to stroke and dementia.28 Cox regression was used to determine hazard ratios (HRs) for associations between overall 5-year risk of post-event dementia, and separately for early (<1 year) and late (>1 year) post-event dementia (patients with late dementia were excluded from analyses of early dementia and vice versa). Regression analyses were adjusted i) for age, sex, and education (model 1), ii) age, sex, education and other factors reported as associated with dementia after TIA/stroke28 including stroke severity (NIHSS), white matter disease, dysphasia, prior stroke, and diabetes and iii) with further adjustment for baseline cognitive test score (model 3). Similar analyses were done restricted to TIA and minor stroke (defined as NIHSS<3 as per OxVASC protocol) and major stroke (NIHSS>3).28 We also performed competing risks regressions to account for the competing risk of death using cumulative incidence function (CIF) covariate analysis and generated subdistribution hazard ratios (SHRs) for comparison.
We did not examine primary intracerebral haemorrhage (PICH) separately owing to small numbers with this stroke subtype but we undertook sensitivity analyses for pre- and post-event dementia in which PICH was excluded. We preformed further sensitivity analyses excluding recurrent stroke on follow-up in analyses of post-event dementia.
Results
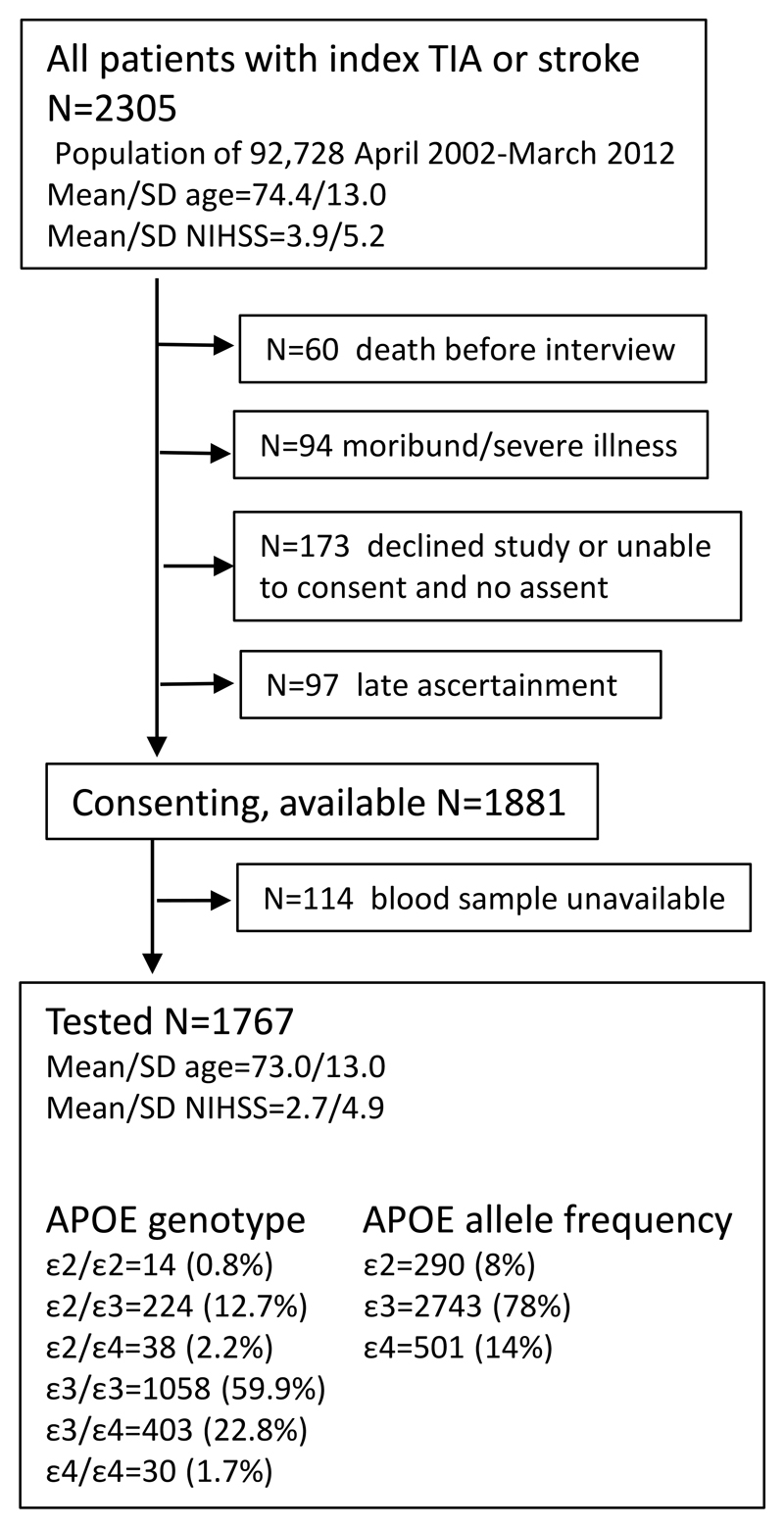
Among 1881 consenting, eligible participants from the source population of 2305 patients recruited from 2002-2012 (mean/sd age 74.4/13.0 years), 1767 (94%) had APOE genotype testing (Figure 1). Non-eligible patients included those with death before interview (n=60), moribund state/severe illness at ascertainment (n=94), declining study bloods/interview or unable to give informed consent and no assent (n=173), and late ascertainment (n=97) (Figure 1). Non-eligible patients and eligible but not tested patients were older with less TIA, greater pre-morbid dependency/disability, moderate/severe white matter disease, more pre- event dementia, higher rates of early death (<31 days), and shorter overall survival time (figure 1, please see https://www.ahajournals.org/journal/str). Non-eligible patients also had higher mean NIHSS and more dysphasia.
Figure.
Flow diagram of all patients with index TIA and stroke events in the source population, number of non-tested patients and reasons for non-test; and number of tested patients and genotype.
APOE-genotype
Among the 1767 tested patients (mean/SD age=73.0/13.0 years, 901 (51%) male, 192 (11%) prior stroke, 602 (34%) TIA, 1100 (62%) ischaemic stroke, and 65 (4%) primary intracerebral haemorrhage), 1058 (59.9%) were APOE-ε3/ε3, 403 (22.8%) were ε4/ε3 and 30 (1.7%) were homozygous (ε4/ε4, figure 1, table 1). The 30 homozygous patients were better educated, but with lower baseline cognition, and more pre-event dementia than heterozygous or APOE-ε3/ε3 patients (table 1). Homozygous patients also tended to be younger (mean/sd age=70.8/11.9 vs 72.3/12.9 and 72.9/13.2 years respectively, p=0.55) with higher pre-morbid dependency but less severe disability, less severe stroke, more post-event dementia and more severe white matter disease than the other groups (table 1).
Table 1.
Distribution of APOE-ε4 and APOE-ε3/ε3 genotypes in tested patients and associated clinical characteristics.
| APOE-ε4 genotype | ||||
|---|---|---|---|---|
| ε3/ε3 N=1058 |
ε4/ε3 N=403 |
ε4/ε4 N=30 |
p | |
| Age, mean/SD years | 72.9/13.2 | 72.3/12.9 | 70.8/11.9 | 0.55 |
| Age ≥ 75 years | 525 (49.6) | 199 (49.4) | 12 (40.0) | 0.58 |
| Male sex | 521 (49.2) | 213 (52.9) | 14 (46.7) | 0.43 |
| Education≤12 years | 721 (68.1) | 255 (63.3) | 16 (53.3) | 0.06 |
| Rankin ≥3* | 163 (15.4) | 55 (13.6) | 7 (23) | 0.31 |
| Barthel <20* | 219 (20.7) | 68 (16.9) | 2 (6.7) | 0.04 |
| Prior stroke | 109 (10.3) | 41 (10.2) | 2 (6.7) | 0.81 |
| Mod./severe WMD | 276/1034 (26.7) | 128/399 (32.1) | 11 (36.7) | 0.07 |
| TIA | 354 (33.5) | 142 (35.2) | 9 (30) | 0.91 |
| Minor stroke | 394 (37.2) | 149 (37.0) | 13 (43) | |
| Major stroke | 310 (29.3)) | 112 (27.8) | 8 (27) | |
| PICH | 38 (3.6) | 14 (3.5) | 2 (6.7) | 0.86 |
| NIHSS mean/SD | 2.8/3.8 | 2.4/3.9 | 2.1/3.8 | 0.45 |
| Dysphasia | 144 (13.6) | 53 (13.2) | 5 (16.7) | 0.86 |
| Low baseline cognitive score | 168/917 (18.3) | 59/366 (16.1) | 10/27 (37.0) | 0.02 |
| Pre-event dementia | 60 (5.7) | 28 (6.9) | 5 (16.7) | 0.04 |
| Post-event dementia | 202/998 (20.2)) | 71/375 (18.9) | 9/25 (30) | 0.12 |
| Post-event dementia (early) | 97/998 (9.7) | 32/375 (8.5) | 3/25 (12) | 0.11 |
| Post-event dementia (late) | 105/998 (10.5) | 39/375 (10.4) | 6/25 (27) | |
| Time to death, mean/SD years | 3.8/2/0 | 3.9/1.9 | 3.8/1.9 | 0.39 |
| Death <31 days | 35 (3.3) | 10 (2.5) | 0 (0) | 0.44 |
Numbers are n (%) unless otherwise specified. Event refers to the index TIA or stroke event. Mod.=moderate, WMD=white matter disease, PICH=primary intracerebral haemorrhage, NIHSS= National Institutes of Health Stroke Scale.
pre-TIA/stroke function.
Pre-event dementia
Pre-event dementia was present in 110/1767 (6%) of genotyped patients of whom 93 were ε4/ε4, ε4/ε3 or ε3/ε3 (table 1). Compared with APOE-ε3/ε3, APOE-ε4 homozygosity was strongly associated with pre-event dementia (OR=4.81 95% CI 1.65-14.06, p=0.004) overall after adjustment for age, sex and education (model 1). After additional adjustment for other factors associated with pre-event dementia (model 2), the OR was broadly similar. Associations were stronger for TIA and minor stroke and did not reach significance for major stroke. There was no association overall between ε4/ε3 status and pre-event dementia after adjustment for all factors in model 2 although there was a significant association for major stroke (OR=2.30, 1.12-4.71, p=0.02, table 2). Exclusion of PICH did not impact the results (table 2).
Table 2.
Odds ratios (OR) for pre-event dementia according to APOE status, unadjusted, and adjusted for age, sex, education (model 1), and for age, sex, education, stroke severity, prior stroke, white matter disease (WMD), diabetes, dysphasia (model 2).
| OR (95% CI) | ||||||
|---|---|---|---|---|---|---|
| Unadjusted | p | Model 1 | p | Model 2 | p | |
| All patients N=1767 | ||||||
| ≥1ε2 | 0.96 (0.52-1.79) | 0.90 | 0.81 (0.52-1.79) | 0.53 | 0.72 (0.37-1.43) | 0.36 |
| ε4/ε3 | 1.24 (0.78-2.00) | 0.37 | 1.37 (0.85-2.22) | 0.19 | 1.47 (0.88-2.45) | 0.14 |
| ε4/ε4 | 3.20 (1.19-8.62) | 0.02 | 4.81 (1.65-14.06) | 0.004 | 5.81 (1.93-17.48) | 0.002 |
| Excluding PICH N=1702 | ||||||
| ≥1ε2 | 0.82 (0.43-1.59) | 0.56 | 0.70 (0.35-1.37) | 0.30 | 0.60 (0.29-1.25) | 0.18 |
| ε4/ε3 | 1.21 (0.76-1.94) | 0.43 | 1.33 (0.82-2.16) | 0.26 | 1.41 (0.84-2.38) | 0.19 |
| ε4/ε4 | 3.39 (1.25-9.20) | 0.02 | 4.98 (1.69-14.70) | 0.004 | 6.06 (2.00-18.48) | 0.002 |
| TIA and minor stroke only N=1251 | ||||||
| ≥1ε2 | 0.59 (0.21-1.69) | 0.33 | 0.49 (0.16-1.45) | 0.20 | 0.34 (0.09-1.22) | 0.10 |
| ε4/ε3 | 0.99 (0.50-2.00) | 0.98 | 1.01 (0.51-2.04) | 0.97 | 0.83 (0.38-1.80) | 0.63 |
| ε4/ε4 | 3.47 (0.98-12.28) | 0.05 | 5.00 (1.26-19.73) | 0.02 | 7.24 (1.66-31.60 | 0.008 |
| Major stroke (NIHSS≥3) only N=516 | ||||||
| ≥1ε2 | 1.26 (0.57-2.79) | 0.56 | 1.22 (0.54-2.76) | 0.64 | 1.23 (0.52-2.91) | 0.64 |
| ε4/ε3 | 1.62 (0.84-3.10) | 0.15 | 1.98 (1.00-3.92) | 0.05 | 2.30 (1.12-4.71) | 0.02 |
| ε4/ε4 | 3.23 (0.62-16.74) | 0.16 | 4.38 (0.76-25.17) | 0.10 | 5.13 (0.80-32.67) | 0.08 |
ε3/ε3 is the reference group for all analyses.
Post-event dementia
In the 1767 APOE genotyped patients, <5% did not complete study interview follow-up until death or end of study (69 (4%) at 6 months, 71 (4%) at 1 year and 73 (4%) at 5 years) in whom all available study data and information from medical records was used to assign dementia diagnoses. Post-event dementia occurred in 345/1657 (21%) genotyped patients without pre-event dementia. APOE ε4/ε4 was strongly associated with all post-event dementia to 5-years follow-up (HR=2.94 95% CI 1.55-5.57, p=0.001) after adjustment for age, sex and education (model 1, table 3, please see https://www.ahajournals.org/journal/str). When other factors associated with post-event dementia were entered (model 2), the association strengthened (3.64, 1.90-7.00, p<0.0001). After further adjustment for baseline cognitive score (model 3), the HR remained broadly similar (table 3). In contrast, no associations were seen in ε4/ε3 individuals after adjustment for age, sex and education or after adjustment for other factors associated with post-event dementia. Results were similar when PICH was excluded and when the 194 patients with recurrent stroke on follow-up, were excluded (table 3). Competing risk analyses and subdistribution hazard ratios showed similar results (please see https://www.ahajournals.org/journal/str).
Table 3.
Hazard ratios (HR) for 5-year incidence of post-event dementia according to APOE status, unadjusted and adjusted for age, sex, education (model 1), and for age, sex, education, stroke severity, prior stroke, white matter disease (WMD), diabetes, dysphasia (model 2), and model 2 adjusted for baseline cognitive score (model 3).
| Hazard ratio (95% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | p | Model 1 | p | Model 2 | p | Model 3 | p | |
| All patients N=1657 | ||||||||
| ≥1ε2 | 1.36 (1.02-1.83) | 0.04 | 1.12 (0.83-1.51) | 0.45 | 1.18 (0.87-1.61) | 0.28 | 1.12 (0.81-1.56) | 0.49 |
| ε4/ε3 | 0.92 (0.70-1.20) | 0.52 | 0.91 (0.69-1.19) | 0.49 | 1.02 (0.77-1.13) | 0.91 | 1.11 (0.8401.48) | 0.47 |
| ε4/ε4 | 2.05 (1.09-3.87) | 0.03 | 2.94 (1.55-5.57) | 0.001 | 3.64 (1.90-7.00) | <0.0001 | 2.41 (1.19-4.89) | 0.01 |
| Excluding PICH N=1596 | ||||||||
| ≥1ε2 | 1.32 (0.98-1.80) | 0.07 | 1.08 (0.80-1.47) | 0.61 | 1.16 (0.85-1.59) | 0.35 | 1.05 (0.74-1.47) | 0.79 |
| ε4/ε3 | 0.92 (0.70-1.22) | 0.57 | 0.91 (0.69-2.00) | 0.49 | 1.02 (0.77-1.35) | 0.90 | 1.08 (0.80-1.44) | 0.62 |
| ε4/ε4 | 1.94 (1.00-3.79) | 0.05 | 2.75 (1.40-5.39) | 0.003 | 3.34 (1.69-6.60) | 0.001 | 2.08 (0.99-4.38) | 0.05 |
| Excluding recurrent stroke on follow-up N=1463 | ||||||||
| ≥1ε2 | 1.34 (0.97-1.87) | 0.08 | 1.10 (0.79-1.54) | 0.57 | 1.09 (0.77-1.54) | 0.63 | 0.95 (0.65-1.38) | 0.78 |
| ε4/ε3 | 0.90 (0.66-1.22) | 0.50 | 0.92 (0.67-1.25) | 0.58 | 0.99 (0.72-.35) | 0.94 | 1.14 (0.82-1.57) | 0.44 |
| ε4/ε4 | 1.60 (0.7103.62) | 0.26 | 3.04 (1.33-6.93) | 0.008 | 4.03 (1.74-9.34) | 0.001 | 3.51 (1.48-8.34) | 0.004 |
| TIA and minor stroke only, N=1199 | ||||||||
| ≥1ε2 | 1.52 (1.00-2.32) | 0.05 | 1.24 (0.89-1.90) | 0.33 | 1.30 (0.84-2.00) | 0.24 | 1.38 (0.87-2.18) | 0.17 |
| ε4/ε3 | 1.12 (0.78-1.62) | 0.52 | 1.07 (0.74-1.55) | 0.71 | 1.05 (0.72-1.52) | 0.80 | 1.10 (0.75-1.61) | 0.62 |
| ε4/ε4 | 2.34 (1.02-5.33) | 0.04 | 3.49 (1.52-8.01) | 0.003 | 3.46 (1.49-8.00) | 0.004 | 1.82 (0.69-4.82) | 0.23 |
| Major stroke (NIHSS≥3) only, N=458 | ||||||||
| ≥1ε2 | 1.14 (0.75-1.71) | 0.55 | 1.01 (0.67-1.53) | 0.96 | 1.07 (0.69-1.66) | 0.77 | 1.02 (0.62-1.66) | 0.95 |
| ε4/ε3 | 0.75 (0.51-1.12) | 0.17 | 0.81 (0.54-1.20) | 0.29 | 0.94 (0.63-1.42) | 0.78 | 1.14 (0.73-1.77) | 0.57 |
| ε4/ε4 | 3.04 (1.11-8.28) | 0.03 | 4.21 (1.51-11.74) | 0.006 | 6.38 (2.15-18.62) | 0.001 | 5.44 (1.78-16.67) | 0.003 |
ε3/ε3 is the reference group for all analyses.
Looking separately at early (<1 year) versus late (>1 year) post-event dementia, homozygous-ε4 status was overall more strongly associated with late vs early post-event dementia (OR=4.42, 2.04-9.59, p<0.0001 vs 2.71, 0.86-8.61, p=0.09) after adjustment for age, sex and education (model 1, table 4). Associations were maintained after adjustment for other factors associated with post-event dementia (model 2, OR=4.88 (2.23-10.70), p<0.0001 for late dementia vs 3.45 (1.06-11.16) p=0.04 for early dementia) although the association with early dementia was no longer significant after adjustment for baseline cognitive score (model 3, HR=2.11, 0.62-7.15, p=0.23, table 4). However, when patients were further stratified by severity of the event, homozygous-ε4 status appeared to impact more on early dementia risk after major stroke and later dementia risk after TIA and minor stroke (please see https://www.ahajournals.org/journal/str).
Table 4.
Hazard ratios (HR) for early (≤1 year) and late (>1 year) post-event dementia according to APOE-ε4 status, unadjusted and adjusted for demographic factors (model 1), and for age, sex, education, stroke severity, prior stroke, white matter disease (WMD), diabetes, dysphasia (model 2), and model 2 adjusted for baseline cognitive score (model 3).
| Hazard ratio (95% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | p | Model 1 | p | Model 2 | p | Model 3 | p | |
| All patients, N=1657 | ||||||||
| ε4/ε3 | ||||||||
| Early | 0.85 (0.57-1.26) | 0.41 | 0.88 (0.59-1.31) | 0.53 | 1.04 (0.69-1.56) | 0.85 | 1.35 (0.87-2.11) | 0.19 |
| Late | 0.98 (0.68-1.41) | 0.92 | 0.96 (0.66-1.38) | 0.81 | 0.97 (0.67-1.40) | 0.87 | 0.96 (0.66-1.40) | 0.84 |
| ε4/ε4 | ||||||||
| Early | 1.52 (0.48-4.78) | 0.48 | 2.71 (0.86-8.61) | 0.09 | 3.45 (1.06-11.16) | 0.04 | 2.11 (0.62-7.15) | 0.23 |
| Late | 2.92 (1.36-6.27) | 0.006 | 4.42 (2.04-9.59) | <0.0001 | 4.88 (2.23-10.70) | <0.0001 | 3.12 (1.29-7.53) | 0.01 |
ε3/ε3 is the reference group for all analyses.
Discussion
Homozygosity for the APOE-ε4 allele was associated with both pre- and post-event dementia in patients with TIA and stroke. Associations were strengthened after adjustment for other factors including stroke severity and white matter disease, suggesting that effects are mediated through mechanisms other than cerebrovascular disease burden. Associations with post-event dementia were also robust to adjustment for baseline cognitive score suggesting that post-event APOE-ε4 effect was not simply mediated through worse cognition at baseline.
The frequencies of the different APOE alleles in our study were similar to those reported in Caucasian populations1,3 The younger age and better education of APOE ε4/ε4 homozygous individuals suggests some survival bias. ε4/ε4 has the highest, and ε2/ε2 the lowest risk of symptomatic vascular disease38 and ε4 is also associated with CAA5,6 and possibly also with hypertension.39,40 Notably, the higher rates of dementia in ε4/ε4 individuals occurred despite the presence of factors (younger age, increased cognitive reserve, less severe stroke) which would be expected to be protective against dementia.
The strength of associations between pre-event dementia and ε4-homozygosity was slightly lower than reported in case control studies of Alzheimer’s disease (OR ~6 vs ~9). ε4/ε3-heterozygous state is associated with OR of ~2-3 for Alzheimer’s disease but we only observed effects on pre-event dementia in major stroke.3,41 Lower rates of genotyping in those with pre-event dementia, severe stroke and early death may have impacted our findings. Associations with post-event dementia were broadly similar to those reported in population-based volunteer cohorts of stroke and dementia although post-stroke cohort studies showed no consistent relationships, and event severity and early/late post-stroke dementia were not examined.16,42,43
ε4/ε4 associations appeared overall stronger for dementia after major stroke than for TIA/minor stroke and for late vs early dementia. Adjustment for baseline cognitive score attenuated the association with dementia more strongly in those with early vs late dementia and with more minor events. Although numbers in these analyses were small, this supports a role for increased susceptibility/reduced brain reserve in in ε4/ε4-associated early post-stroke dementia in keeping with neuropathological evidence that subclinical Alzheimer’s disease may be revealed by the occurrence of stroke.44–46 In addition, our findings indicate that low baseline cognition in those with ε4/ε4 is relatively more important in dementia after TIA/minor stroke whereas faster cognitive decline may be the more dominant factor after major stroke. Stroke may accelerate cognitive decline in ε4/ε4 individuals through facilitating aberrant beta-amyloid processing as suggested by traumatic brain injury studies,2 or through amyloid-associated post-stroke inflammation and increased infarct size.41 Such processes may also contribute to worse recovery after stroke as is seen after other neurological injury.2,5,6
The association between APOE-ε4/ε4 and dementia might not be mediated solely by Alzheimer’s pathology. Recent studies have demonstrated other independent detrimental effects: APOE-ε4 increases transactive response DNA-binding protein of 43 kDa (TDP-43) and thereby hippocampal sclerosis.47 APOE-ε4 is also linked to risk of macroinfarcts and vascular disease in general.15 It should be noted that although our findings were robust to adjustment for stroke severity and leukoaraisois, we were not able to take account of other measures of cerebrovascular burden including silent/microinfarcts, microbleeds, perivascular spaces and superficial siderosis and thus a vascular effect cannot be completely excluded.
The impact of other risk factors for dementia may also be moderated by the presence of APOE-ε4. Female sex increases the risk of Alzheimer’s disease but differential effects of sex by APOE genotype is controversial and reports are inconsistent.3 In our study we did not see different effects of APOE-ε4 in women versus men. Similarly vascular risk factors may act differently on the brain according to APOE status. Diabetes is a risk factor for dementia but the effect of poor glycaemic control on WMD may be exacerbated by APOE-ε4.48 Multiple vascular risk factors may also be more detrimental to WMD integrity in the presence of APOE-ε4.49 However, the high risk of APOE-ε4 alone may mask additional effects of other risk factors on cognitive outcomes.
Our study has some limitations. TIA/stroke and dementia diagnoses were made by PMR and STP respectively rather than by a consensus panel but this ensured consistency of approach over the 15-year time period of this study. We were not able to adjust for other potential associates of stroke-related dementia for example, social and lifestyle factors, depression, treatments, specific stroke subtypes, and lesion characteristics. We were also not able to examine the relationship between APOE genotype and specific sub-types of dementia although mixed pathology is common in older subjects.13,14,44,45 Similarly, we could not examine the associations of APOE genotype with dementia in PICH vs ischaemic stroke owing to small numbers with haemorrhage. The number of patients with the ε4/ε4 genotype was small and therefore the findings, particularly in relation to the associations with subgroups including minor vs major cerebrovascular events and early versus late dementia, should be interpreted with caution. Our study may also have been underpowered to show the reported protective effect of ε2 genotypes Finally, APOE genotype testing was not possible in patients who died acutely, or in whom consent/assent was unavailable and these patients had more severe events and more dementia. This may have resulted in an under-estimation of the association between APOE-ε4 and particularly, pre-event dementia, but this was unavoidable even with our inclusive study design.
In conclusion, APOE-ε4 homozygosity is strongly related to both pre- and post-event dementia probably through neurodegenerative processes including Alzheimer pathology, increased TDP-43, and enhanced stroke injury/reduced recovery. APOE-ε4 status should be included in matching of baseline dementia risk in stroke trials to prevent cognitive decline. Also, the effect of risk factor interventions may differ by APOE status. Further studies are required to determine the impact of APOE genotype on cognitive trajectory after stroke and on dementia sub-type. Pooled analysis of individual patient data from multiple studies will be needed to properly determine associations between stroke-associated dementia and the six different APOE genotypes, and with stroke sub-type and early versus late post-event dementia.
Supplementary Material
Acknowledgements
We acknowledge the use of the facilities of the Acute Vascular Imaging Centre, Oxford. We thank Dr Ramon Luengo-Fernandez for assistance with the cumulative incidence competing risk analyses.
Sources of Funding
The Oxford Vascular Study is funded by the Wellcome Trust, Wolfson Foundation, British Heart Foundation, National Institute of Health Research (NIHR), and the NIHR Oxford Biomedical Research Centre. Sarah Pendlebury is supported by the NIHR Oxford Biomedical Research Centre.
Footnotes
Disclosures: none
References
- 1.Zannis VI, Kardassis D, Zannis EE. Genetic mutations affecting human lipoproteins, their receptors, and their enzymes. Adv Hum Genet. 1993;21:145–319. doi: 10.1007/978-1-4615-3010-7_3. [DOI] [PubMed] [Google Scholar]
- 2.Verghese PB, Castellano JM, Holtzman DM. Apolipoprotein E in Alzheimer's disease and other neurological disorders. Lancet Neurol. 2011;10:241–52. doi: 10.1016/S1474-4422(10)70325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278:1349–56. [PubMed] [Google Scholar]
- 4.Zlokovic Cerebrovascular effects of Apolipoprotein E. JAMA Neurol. 2013;70:440–444. doi: 10.1001/jamaneurol.2013.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rannikmäe K, Kalaria RN, Greenberg SM, Chui HC, Schmitt FA, Samarasekera N, et al. APOE associations with severe CAA-associated vasculopathic changes: collaborative meta-analysis. J Neurol Neurosurg Psychiatry. 2014;85:300–5. doi: 10.1136/jnnp-2013-306485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alberts MJ, Graffagnino C, McClenny C, DeLong D, Strittmatter W, Saunders AM, et al. APOE genotype and survival from intracerebral haemorrhage. Lancet. 1995;346:575. doi: 10.1016/s0140-6736(95)91411-0. [DOI] [PubMed] [Google Scholar]
- 7.Lawrence DW, Comper P, Hutchison MG, Sharma B. The role of apolipoprotein E episilon (ε)-4 allele on outcome following traumatic brain injury: A systematic review. Brain Inj. 2015;29:1018–31. doi: 10.3109/02699052.2015.1005131. [DOI] [PubMed] [Google Scholar]
- 8.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–3. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 9.Caselli RJ, Dueck AC, Osborne D, Sabbagh MN, Connor DJ, Ahern GL, et al. Longitudinal modeling of age-related memory decline and the APOE epsilon4 effect. N Engl J Med. 2009;361:255–63. doi: 10.1056/NEJMoa0809437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Praetorius M, Thorvaldsson V, Hassing LB, Johansson B. Substantial effects of apolipoprotein E ε4 on memory decline in very old age, longitudinal findings from a population-based sample. Neurobiol Aging. 2013;34:2734–9. doi: 10.1016/j.neurobiolaging.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Ganguli M, Lee CW, Snitz BE, Hughes TF, McDade E, Chang CC. Rates and risk factors for progression to incident dementia vary by age in a population cohort. Neurology. 2015;84:72–80. doi: 10.1212/WNL.0000000000001113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Godin O, Tzourio C, Maillard P, Alpérovitch A, Mazoyer B, Dufouil C. Apolipoprotein E genotype is related to progression of white matter lesion load. Stroke. 2009;40:3186–90. doi: 10.1161/STROKEAHA.109.555839. [DOI] [PubMed] [Google Scholar]
- 13.Rojas S, Brugulat-Serrat A, Bargalló N, Minguillón C, Tucholka A, Falcon C, et al. Higher prevalence of cerebral white matter hyperintensities in homozygous APOE-ε4 allele carriers aged 45-75: Results from the ALFA study. J Cereb Blood Flow Metab. 2018;38:250–261. doi: 10.1177/0271678X17707397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mortimer JA, Snowdon DA, Markesbery WR. The effect of APOE-epsilon4 on dementia is mediated by Alzheimer neuropathology. Alzheimer Dis Assoc Disord. 2009;23:152–7. doi: 10.1097/wad.0b013e318190a855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lamar M, Yu L, Rubin LH, James BD, Barnes LL, Farfel JM, et al. APOE genotypes as a risk factor for age-dependent accumulation of cerebrovascular disease in older adults. Alzheimers Dement. 2019;15:258–266. doi: 10.1016/j.jalz.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slooter AJ, Tang MX, van Duijn CM, Stern Y, Ott A, Bell K, et al. Apolipoprotein E epsilon4 and the risk of dementia with stroke. A population-based investigation. JAMA. 1997;277:818–21. doi: 10.1001/jama.277.10.818. [DOI] [PubMed] [Google Scholar]
- 17.Wagle J, Farner L, Flekkøy K, Wyller TB, Sandvik L, Eiklid KL, et al. Cognitive impairment and the role of the APOE epsilon4-allele after stroke--a 13 months follow-up study. Int J Geriatr Psychiatry. 2010;25:833–42. doi: 10.1002/gps.2425. [DOI] [PubMed] [Google Scholar]
- 18.Wagle J, Farner L, Flekkøy K, Wyller TB, Sandvik L, Eiklid KL, et al. Association between APOE epsilon4 and cognitive impairment after stroke. Dement Geriatr Cogn Disord. 2009;27:525–33. doi: 10.1159/000223230. [DOI] [PubMed] [Google Scholar]
- 19.Sachdev PS, Lipnicki DM, Crawford JD, Wen W, Brodaty H. Progression of cognitive impairment in stroke/TIA patients over 3 years. J Neurol Neurosurg Psychiatry. 2014;85:1324–30. doi: 10.1136/jnnp-2013-306776. [DOI] [PubMed] [Google Scholar]
- 20.Zhu L, Fratiglioni L, Guo Z, Basun H, Corder EH, Winblad B, et al. Incidence of dementia in relation to stroke and the apolipoprotein E epsilon4 allele in the very old. Findings from a population-based longitudinal study. Stroke. 2000;31:53–60. doi: 10.1161/01.str.31.1.53. [DOI] [PubMed] [Google Scholar]
- 21.Ballard CG, Morris CM, Rao H, O'Brien JT, Barber R, Stephens S, et al. APOE epsilon4 and cognitive decline in older stroke patients with early cognitive impairment. Neurology. 2004;63:1399–402. doi: 10.1212/01.wnl.0000141851.93193.17. [DOI] [PubMed] [Google Scholar]
- 22.Arpa A, del Ser T, Goda G, Barba R, Bornstein B, Apolipoprotein E. angiotensin-converting enzyme and alpha-1-antichymotrypsin genotypes are not associated with post-stroke dementia. J Neurol Sci. 2003;210:77–82. doi: 10.1016/s0022-510x(03)00026-1. [DOI] [PubMed] [Google Scholar]
- 23.Dik MG, Deeg DJ, Bouter LM, Corder EH, Kok A, Jonker C. Stroke and apolipoprotein E epsilon4 are independent risk factors for cognitive decline: A population-based study. Stroke. 2000;31:2431–6. doi: 10.1161/01.str.31.10.2431. [DOI] [PubMed] [Google Scholar]
- 24.Reitz C, Luchsinger JA, Tang MX, Manly J, Mayeux R. Stroke and memory performance in elderly persons without dementia. Arch Neurol. 2006;63:571–6. doi: 10.1001/archneur.63.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pendlebury ST, Chen PJ, Bull L, Silver L, Mehta Z, Rothwell PM. Oxford Vascular Study. Methodological factors in determining rates of dementia in transient ischemic attack and stroke: (I) impact of baseline selection bias. Stroke. 2015;46:641–6. doi: 10.1161/STROKEAHA.114.008043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pendlebury ST, Chen PJ, Welch SJ, Cuthbertson FC, Wharton RM, Mehta Z, et al. Oxford Vascular Study. Methodological Factors in Determining Risk of Dementia After Transient Ischemic Attack and Stroke: (II) Effect of Attrition on Follow-Up. Stroke. 2015;46:1494–500. doi: 10.1161/STROKEAHA.115.009065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pendlebury ST, Klaus SP, Thomson RJ, Mehta Z, Wharton RM, Rothwell PM. Methodological Factors in Determining Risk of Dementia After Transient Ischemic Attack and Stroke: (III) Applicability of Cognitive Tests. Stroke. 2015;46:3067–73. doi: 10.1161/STROKEAHA.115.010290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pendlebury ST, Rothwell PM, for the Oxford Vascular Study Incidence and prevalence of dementia associated with TIA and stroke: rates and risk factors in a population-based cohort. Lancet Neurology. 2019;18:248–258. doi: 10.1016/S1474-4422(18)30442-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rothwell PM, Coull AJ, Giles MF, Howard SC, Silver LE, Bull LM, et al. Oxford Vascular Study. Change in stroke incidence, mortality, case-fatality, severity, and risk factors in Oxfordshire, UK from 1981 to 2004 (Oxford Vascular Study) Lancet. 2004;363:1925–33. doi: 10.1016/S0140-6736(04)16405-2. [DOI] [PubMed] [Google Scholar]
- 30.Rothwell PM, Coull AJ, Silver LE, Fairhead JF, Giles MF, Lovelock CE, et al. Oxford Vascular Study. Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study) Lancet. 2005;366:1773–83. doi: 10.1016/S0140-6736(05)67702-1. [DOI] [PubMed] [Google Scholar]
- 31.Coull AJ, Silver LE, Bull LM, Giles MF, Rothwell PM. Oxford Vascular (OXVASC) Study. Direct assessment of completeness of ascertainment in a stroke incidence study. Stroke. 2004;35:2041–5. doi: 10.1161/01.STR.0000137605.48864.2f. [DOI] [PubMed] [Google Scholar]
- 32.Advisory Council for the National Institute of Neurological and Communicative Disorders and Stroke, National Institute of Health, Bethesda, Maryland. A classification and outline of cerebrovascular diseases II. Stroke. 1975;6:564–616. doi: 10.1161/01.str.6.5.564. [DOI] [PubMed] [Google Scholar]
- 33.Simoni M, Li L, Paul NL, Gruter BE, Schulz UG, Küker W, et al. Age- and sex-specific rates of leukoaraiosis in TIA and stroke patients: population-based study. Neurology. 2012;79:1215–22. doi: 10.1212/WNL.0b013e31826b951e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 35.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The montreal cognitive assessment, moca: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 36.Pendlebury ST, Mariz J, Bull L, Mehta Z, Rothwell PM. MoCA, ACE-R and MMSE versus the national institute of neurological disorders and stroke-canadian stroke network vascular cognitive impairment harmonization standards neuropsychological battery after TIA and stroke. Stroke. 2012;43:464–469. doi: 10.1161/STROKEAHA.111.633586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pendlebury ST, Welch SJ, Cuthbertson FC, Mariz J, Mehta Z, Rothwell PM. Telephone assessment of cognition after TIA and stroke: TICSm and telephone MoCA vs face-to-face MoCA and neuropsychological battery. Stroke. 2013;44:227–9. doi: 10.1161/STROKEAHA.112.673384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khan TA, Shah T, Prieto D, Zhang W, Price J, Fowkes GR, et al. Apolipoprotein E genotype, cardiovascular biomarkers and risk of stroke: systematic review and meta-analysis of 14,015 stroke cases and pooled analysis of primary biomarker data from up to 60,883 individuals. Int J Epidemiol. 2013;42:475–92. doi: 10.1093/ije/dyt034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niu W, Qi Y, Qian Y, Gao P, Zhu D. The relationship between apolipoprotein E epsilon2/epsilon3/epsilon4 polymorphisms and hypertension: a meta-analysis of six studies comprising 1812 cases and 1762 controls. Hypertens Res. 2009;32:1060–6. doi: 10.1038/hr.2009.164. [DOI] [PubMed] [Google Scholar]
- 40.van Bockxmeer FM, Mamotte CD. Apolipoprotein epsilon 4 homozygosity in young men with coronary heart disease. Lancet. 1992;340:879–80. doi: 10.1016/0140-6736(92)93288-x. [DOI] [PubMed] [Google Scholar]
- 41.Whitehead SN, Cheng G, Hachinski VC, Cechetto DF. Progressive increase in infarct size, neuroinflammation, and cognitive deficits in the presence of high levels of amyloid. Stroke. 2007;38:3245–50. doi: 10.1161/STROKEAHA.107.492660. [DOI] [PubMed] [Google Scholar]
- 42.Ivan CS, Seshadri S, Beiser A, Au R, Kase CS, Kelly-Hayes M, et al. Dementia after stroke: the Framingham Study. Stroke. 2004;35:1264–8. doi: 10.1161/01.STR.0000127810.92616.78. [DOI] [PubMed] [Google Scholar]
- 43.Jin YP, Østbye T, Feightner JW, Di Legge S, Hachinski V. Joint effect of stroke and APOE 4 on dementia risk: the Canadian Study of Health and Aging. Neurology. 2008;70:9–16. doi: 10.1212/01.wnl.0000284609.77385.03. [DOI] [PubMed] [Google Scholar]
- 44.Rajan KB, Aggarwal NT, Schneider JA, Wilson RS, Everson-Rose SA, Evans DA. Role of APOE ε4 Allele and Incident Stroke on Cognitive Decline and Mortality. Alzheimer Dis Assoc Disord. 2016;30:318–323. doi: 10.1097/WAD.0000000000000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study JAMA. 1997;277:813–7. [PubMed] [Google Scholar]
- 46.Esiri MM, Nagy Z, Smith MZ, Barnetson L, Smith AD. Cerebrovascular disease and threshold for dementia in the early stages of Alzheimer's disease. Lancet. 1999;354:919–20. doi: 10.1016/S0140-6736(99)02355-7. [DOI] [PubMed] [Google Scholar]
- 47.Yang HS, Yu L, White CC, Chhatwal JP, Sperling RA, Bennett DA, et al. Evaluation of TDP-43 proteinopathy and hippocampal sclerosis in relation to APOE ε4 haplotype status: a community-based cohort study. Lancet Neurol. 2018;17:773–781. doi: 10.1016/S1474-4422(18)30251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cox SR, Ritchie SJ, Dickie DA, Pattie A, Royle NA, Corley J, et al. Interaction of APOE e4 and poor glycemic control predicts white matter hyperintensity growth from 73 to 76. Neurobiol Aging. 2017;54:54–58. doi: 10.1016/j.neurobiolaging.2017.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang R, Fratiglioni L, Laukka EJ, Lövdén M, Kalpouzos G, Keller L, et al. Effects of vascular risk factors and APOE ε4 on white matter integrity and cognitive decline. Neurology. 2015;84:1128–35. doi: 10.1212/WNL.0000000000001379. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Requests for access to data should be submitted for consideration to the Study Director (peter.rothwell@ndcn.ox.ac.uk).