Abstract
It is generally assumed that allergic asthma originates primarily through sensitization via the respiratory mucosa, but emerging clinical observations and experimental studies indicate that skin exposure to low molecular weight (LMW) agents, i.e. “chemicals,” may lead to systemic sensitization and subsequently develop asthma when the chemical is inhaled. This review aims to evaluate the accumulating experimental evidence that adverse respiratory responses can be elicited upon inhalation of an LMW chemical sensitizer after previous sensitization by dermal exposure. We systematically searched the PubMed and Embase databases up to April 15, 2017, and conducted forward and backward reference tracking. Animal studies involving both skin and airway exposure to LMW agents were included. We extracted 6 indicators of “selective airway hyper-responsiveness” (SAHR)—i.e. respiratory responses that only occurred in previously sensitized animals—and synthesized the evidence level for each indicator into strong, moderate or limited strength. The summarized evidence weight for each chemical agent was graded into high, middle, low or “not possible to assess.” We identified 144 relevant animal studies. These studies involved 29 LMW agents, with 107 (74%) studies investigating the occurrence of SAHR. Indicators of SAHR included physiological, cytological/histological and immunological responses in bronchoalveolar lavage, lung tissue and airway-draining lymph nodes. Evidence for skin exposure-induced SAHR was present for 22 agents; for 7 agents the evidence for SAHR was inconclusive, but could not be excluded. The ability of a chemical to cause sensitization via skin exposure should be regarded as constituting a risk of adverse respiratory reactions.
Keywords: Asthma, occupational asthma; chemicals; causes; skin; airway hypersensitivity; animal model
INTRODUCTION
Asthma is one of the most important lung diseases worldwide. To date, more than 400 causative agents, or asthmagens, have been identified to induce asthma in the workplace.1 Occupational asthma can be induced through either immunological or irritant mechanisms. Some irritants may cause asthma that does not depend on a specific immunological reaction to the offending chemical, but such “irritant-induced asthma” will not be considered in this review.
A critical issue in asthma development relates to where in the body the immune response is initiated, i.e. where sensitization occurs. It is often taken for granted—as reflected by the terms “respiratory sensitizers” and “respiratory sensitization”—that the respiratory tract is the key route for sensitization. However, the assumption that sensitization necessarily occurs via inhalation can be challenged, not only because of the experimental evidence showing that respiratory responses can be elicited by exposing dermally sensitized animals via the airways, but also because of clinical and epidemiological studies indicating that the skin may be the site of initial sensitization.
“Asthmagens” can be classified according to their molecular weight (above or below 5,000 Da). Agents with high molecular weight (HMW) are (glyco)proteins of vegetal, animal or microbiological origin that can cause immunoglobulin E (IgE)-mediated allergy with mainly eosinophilic airway inflammation.2 Agents with low molecular weight (LMW) are synthetic or natural chemicals and some metals that may act as haptens, binding to endogenous proteins to initiate an immunological response.3 The mechanisms of asthma induced by sensitization to LMW agents have not yet been completely elucidated.4 In such chemical-induced asthma, airway inflammation is usually characterized by a mixed neutrophil and eosinophil infiltration, and a specific IgE is not always essential.5,6
The first experimental study investigating the association between skin sensitization and airway hyper-responsiveness was conducted in the guinea pig with toluene diisocyanate (TDI) as the test agent.7 Later, various other animal models of asthma demonstrated that skin exposure to various LMW chemical sensitizers, such as isocyanates and acid anhydrides, could lead to airway hyper-responsiveness upon elicitation via the respiratory route.8,9 In these experimental models, “selectivity” of the airway response is crucial in that it implies that airway responses following respiratory administration of (a small amount of) a chemical can be elicited only in animals that have been previously sensitized via the skin to that chemical and not in control animals that have not been previously sensitized. Meanwhile, animal asthma models have been described in guinea pigs, rats and mice.
The paradigm that skin exposure to an LMW sensitizer can be at the origin of selective airway hyper-responsiveness (SAHR) is recognized to some extent, but not yet accepted widely. Moreover, this paradigm has not been adopted for regulatory purposes. When classifying chemicals, a strict distinction is made between skin (or dermal) sensitizers and respiratory sensitizers, with skin sensitizers defined as agents that may lead to contact dermatitis, and respiratory sensitizers defined as agents that may lead to airway hypersensitivity (mainly asthma) when inhaled.10,11 In other words, for regulatory purposes dermal and respiratory sensitizers are defined according to the organ that is potentially affected by disease, but not to the site of initial sensitization. Several methods have even been developed to differentiate the skin sensitizers defined from respiratory sensitizers,12 although it was accepted that several agents, such as isocyanates and acid anhydrides, can be both dermal and respiratory sensitizers.13,14,15
In this systematic review, we identified animal studies that applied skin exposure to LMW agents followed by airway challenge, and we compared 6 indicators of “SAHR” between groups with and without prior skin exposure. The goal of our review was to reevaluate the validity of the traditional differentiation between respiratory and dermal sensitizers. One of the premises (and conclusions) of our review is that even when immune sensitization initially takes place in a specific organ (e.g. the skin), it has potential consequences for organs at a distant site (e.g. the airways). Consequently, the use of the traditional concepts of “skin sensitizer” and “respiratory sensitizer” may no longer be tenable. We have therefore used these terms only as used in the retrieved documents, and we have introduced the term “SAHR” to indicate an airway response primed by a previous (local or systemic) exposure to a chemical sensitizer, and triggered by airway exposure to the same (or a chemically similar) agent. The definition of terms associated with sensitization in this study is presented in Table 1.
Table 1. The definition of terms associated with sensitization in this study.
| Term | Definition |
|---|---|
| Skin sensitizer | A substance that will lead to an allergic response following skin contact11 |
| Respiratory sensitizer/asthmagen | A substance that will lead to hypersensitivity of the airways following inhalation of the substance11 |
| Sensitizer | A substance that will lead to an allergic response, following the first phase of induction, and the second phase of elicitation, regardless of the site of exposure and reaction |
| Sensitization | An allergic process through the first phase of induction, and the second phase of elicitation, while the exposure sites in 2 phases are not necessary the same organ |
| Selective airway hyper-responsiveness | An airway response that is primed by either local or systemic exposure to a substance, and triggered by airway exposure to the similar substance |
METHODOLOGY
The methodology in this review was based on the Systematic Review Protocol for Animal Intervention Studies.16 This study was not registered on PROSPERO database, since the literature suggests excluding animal studies on PROSPERO because the latter involve different methodologies and objectives.17
Study identification
We conducted a systematic literature search in MEDLINE/PubMed and Embase databases up to April 15, 2017 including non-English studies and all study types. Three groups of keywords were used: 1) the names of 347 LMW skin sensitizers and 192 LMW respiratory sensitizers (asthmagens), 2) respiratory symptoms/signs with related terms AND 3) dermatitis with related terms (Supplementary Table S1). We did not set limits on language or publication year.
The skin sensitizers were accessed from the local lymph node assay (LLNA) database,18 because the LLNA is one of the gold standards to identify skin sensitizers.12,19 The LMW respiratory sensitizers were accessed from an existing list of asthmagenic agents.1 The CAS number of each agent was used to search for corresponding names in the PubChem database,20 and these corresponding names were also included. These searching terms are detailed in Supplementary Tables S2 and S3.
Study screening
The articles retrieved were combined and screened by 2 independent reviewers. The first reviewer (H.C.T.) reviewed all titles and abstracts by using the following inclusion criteria: 1) exposure to LMW agents; 2) either exposure through the skin or symptoms/signs of dermatitis; 3) either exposure through the airway or symptoms/signs of airway hyper-reactivity.
After the first reviewer completed the screening, the articles included and an equal number of randomly chosen non-included articles were forwarded to the second one (S.R.). Cohen's kappa was calculated to assess the inter-rater reliability. In the case of discrepancy, consensus was reached by discussion and consultation of a third reviewer (B.N.).
Additional information source
After the relevant publications had been identified, articles involving animal studies were selected. The references and the citations of the articles were tracked by backward and forward snowball searching via the Scopus database. Duplicates, items that could not be retrieved, or items already included in the previous step were removed. The remaining items were then screened by the first and the second reviewer again, similar to the process described in study screening.
Data extraction and quality assessment
After relevant publications had been selected, the characteristics of each study were extracted, including animal species, animal sex, study designs, studied agents, exposure routes through skin and airway, reported outcome measures, and results.
To assess the quality of the animal studies selected, the Toxicological data Reliability Assessment Tool (ToxRTool) in vivo list was used,21 which provided more detail than SYRCLE's risk of bias tool.22 Based on the score from 21 criteria (Supplementary Table S4), studies were assigned to 1 of the 4 Klimisch score levels23: 1 (reliable without restrictions), 2 (reliable with restrictions), 3 (not reliable) and 4 (not assignable).
Assessment of the evidence level from outcome measures
The outcome measures extracted consist of alterations in skin draining lymph nodes, serum and 6 indicators of SAHR, which include 1) lung function measurements; 2) cytology in bronchoalveolar lavage (BAL); 3) inflammatory biomarkers (proteins, antibody or cytokines) in BAL; 4) pathology change in the airway or lung; 5) cytology in airway draining lymph nodes; and 6) inflammatory biomarkers (cytokines) in airway draining lymph nodes.
To determine whether a chemical leads to skin-induced SAHR, the results from 6 indicators of SAHR in each study were compared between the test group (receiving skin exposure and airway elicitation to the same test agent) and the control group (receiving skin exposure with control vehicle, and airway elicitation with test agent). Skin-induced SAHR was considered present if a significant difference (P < 0.05) between test and control groups was reported in the figure, table or text from the study.
The strength of evidence for each indicator was then synthesized from different studies via the modified Royal College of General Practitioners (RCGP) 3-star system (Table 2).24,25 Strong evidence (★★★) indicated skin-induced SAHR results from at least 2 independent study teams; moderate evidence (★★) indicated skin-induced SAHR results from at least 2 studies, which are not necessarily independent; and limited evidence (★) indicated skin-induced SAHR results from 1 study. An independent study team was defined as one without any overlap of contributing authors.
Table 2. The modified Royal College of General Practitioners 3-star system for evidence assessment.
| Evidence level | Definition |
|---|---|
| ★★★ | Strong evidence: provided by generally consistent findings in multiple, high quality, scientific studies, based on at least 2 independent studies. |
| ★★ | Moderate evidence: provided by generally consistent findings in fewer, smaller or lower quality, scientific studies, based on at least 2 studies. |
| ★ | Limited evidence: provided by one scientific study or inconsistent findings in multiple scientific studies. |
| - | No scientific evidence: no conclusions can be drawn when there are not any studies that meet the criteria. |
Finally, the summarized evidence weight of skin-induced SAHR for each chemical was categorized into 5 levels based on the approach of the Scientific Committee on Health, Environmental and Emerging Risks26: 1) high weight of evidence was given for a strong evidence (★★★) from 1 indicator of SAHR and 1 or more other lines of evidence without conflict; 2) middle weight of evidence was given for a moderate evidence (★★) from at least 1 indicator of SAHR; 3) low weight of evidence was given for limited evidence (★) from the primary indicators of SAHR; 4) weighing of evidence not possible to assess was concluded when no suitable evidence was available; and 5) uncertain weight of evidence indicated conflicting information from different lines of evidence.
RESULTS
The studies included and their characteristics
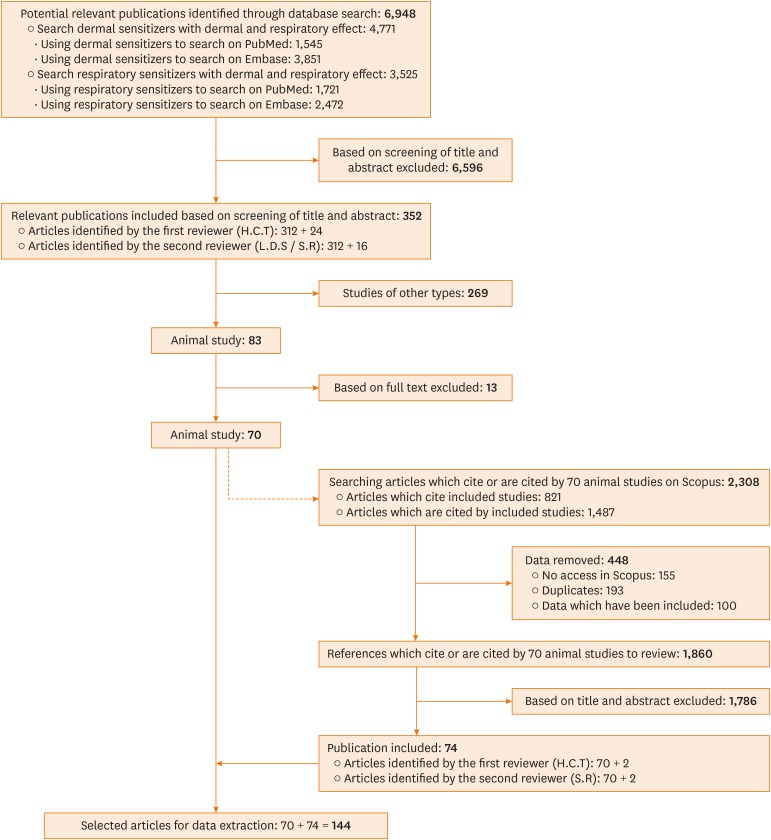
As noted in Figure, the initial literature search yielded 6,948 articles, of which 4,771 related to skin sensitizers and 3,525 related to LMW respiratory sensitizers (asthmagens). The first reviewer screened 6,948 articles based on the title and the abstract, identifying 457 potentially relevant articles. The articles identified by the first reviewer and another 457 randomly selected articles from the non-included articles were checked by the second reviewer without knowing the conclusion of the first reviewer. After the evaluation and comparisons by the second reviewer, 121 articles were removed from the 457 articles identified by the first reviewer, and 16 articles were added, yielding 352 articles, i.e. a Cohen's kappa coefficient of 0.62, with 81% agreement (Supplementary Table S5).
Figure. Flow diagram of systematic literature search.
Among the 352 articles identified, 70 involving animal studies were selected to further track potentially relevant reports. This snowballing process yielded 2,308 articles, of which 448 were first removed, including duplicates, non-retrievable articles and already included articles. Then the first reviewer screened the 1,860 remaining articles and identified 72 potentially relevant articles. The second reviewer screened those 72 articles and an equal number of non-included tracked articles as previously described. A total of 74 additionally tracked articles were finally identified at this tracking step. The Cohen's kappa coefficient at this step was 0.93, with 97% agreement.
Characteristics of the animal studies selected
In total, 144 articles involving animal studies were included (Supplementary Table S6). These articles investigated the association between dermal exposure and respiratory effect, or between respiratory exposure and dermal effect. They were published in 49 different journals, the majority of which were toxicology journals (Supplementary Table S7).
The characteristics of the animal models in the selected studies are shown in Table 3. To evaluate the evolution over time, we employed an early period (before 2005), the recent period (from 2005) and the year 2005 to have approximately similar numbers of articles in each period. The most commonly studied animal type was mouse (n = 78 [54%]), followed by guinea pig and rat. In the recent period, no studies using guinea pigs or mice accounted for the majority (n = 48 [77%]). Male animals were used slightly more frequently than female animals.
Table 3. Characteristics of animals and study designs.
| Characteristics | All studies (n = 144) | 1981–2005 (n = 82) | 2006–2017 (n = 62) | |
|---|---|---|---|---|
| Animal species | ||||
| Mouse | 78 (54) | 30 (37) | 48 (77) | |
| Guinea pig | 40 (28) | 40 (49) | 0 (0) | |
| Rat | 25 (17) | 11 (13) | 14 (23) | |
| Hamster | 1 (1) | 1 (1) | 0 (0) | |
| Sex | ||||
| Male | 76 (53) | 42 (51) | 34 (55) | |
| Female | 61 (42) | 33 (40) | 28 (45) | |
| Both | 4 (3) | 4 (5) | 0 (0) | |
| Unreported | 2 (1) | 2 (2) | 0 (0) | |
| Design | ||||
| Skin exposure followed by airway elicitation | 139 (97) | 77 (94) | 62 (100) | |
| Airway exposure followed by skin elicitation | 6 (4) | 5 (6) | 1 (2) | |
Values are presented as number (%).
Among the 144 articles, only 6 used airway exposure followed by skin elicitation, and the majority used the design of skin exposure followed by some form of airway elicitation (n = 139 [97%]). Detailed representative outcome measures are presented in Supplementary Table S8.
The quality assessment classified most of these studies (n = 98) in Klimisch level 1 (reliable without restrictions). All of the quality criteria, except for housing or feeding conditions, were fulfilled in more than 90% studies (Supplementary Fig. S1). The grading score of ToxRTool criteria for each study is provided in Supplementary Table S9.
Exposure routes
The specific routes and procedures of skin exposure and subsequent airway elicitation are presented in Table 4. The majority of studies in the early period (before 2005) used intradermal injection to induce skin sensitization (n = 31 [40%]), followed by epicutaneous applications simultaneously on the trunk and paws. However, these 2 methods have rarely been used in the recent period (from 2005). Approximately 75% of studies in the recent period used simple epicutaneous application for sensitization, either on the ear (n = 38) or on the trunk (n = 8); another smaller numbers of studies in the recent period exposed animals to chemicals via the trunk on the first day and then via the ear several days later.
Table 4. Exposure routes of the studies identified.
| Method | All studies (n = 139) | 1981–2005 (n = 77) | 2006–2017 (n = 62) | ||
|---|---|---|---|---|---|
| Skin exposure | |||||
| Simple route | |||||
| Epicutaneous application on ear | 41 (29) | 3 (4) | 38 (61) | ||
| Epicutaneous application on trunk | 19 (14) | 11 (14) | 8 (13) | ||
| Epicutaneous application on trunk & paws | 18 (13) | 18 (23) | 0 (0) | ||
| Epicutaneous occlusion on trunk | 3 (2) | 2 (3) | 1 (2) | ||
| Intradermal injection | 33 (24) | 31 (40) | 2 (3) | ||
| Toepad injection | 2 (1) | 2 (3) | 0 (0) | ||
| Combination route | |||||
| Epicutaneous application on trunk, and later on ear | 21 (15) | 8 (10) | 13 (21) | ||
| Cumulative contact enhancement test | 3 (2) | 3 (4) | 0 (0) | ||
| Maximization test | 1 (1) | 1 (1) | 0 (0) | ||
| Intradermal injection followed by epicutaneous application on ear | 1 (1) | 0 (0) | 1 (2) | ||
| Intradermal injection followed by inhalation | 2 (1) | 1 (1) | 1 (2) | ||
| Intradermal injection followed by intratracheal instillation | 1 (1) | 1 (1) | 0 (0) | ||
| Airway elicitation | |||||
| Inhalation | 57 (41) | 34 (44) | 23 (37) | ||
| Intranasal instillation | 43 (31) | 23 (30) | 20 (32) | ||
| Intratracheal instillation | 25 (18) | 15 (19) | 10 (16) | ||
| Intratracheal aerosol application | 5 (4) | 5 (6) | 0 (0) | ||
| Pharyngeal aspiration | 12 (9) | 0 (0) | 12 (19) | ||
Values are presented as number (%).
Data are studies with the design of skin exposure followed and airway elicitation.
In terms of airway elicitation methods, inhalation was most frequently used (n = 57 [41%]), followed by intranasal instillation, with little differences between the early and recent periods. Pharyngeal aspiration has been introduced as a new method in the recent phase (n = 12 [19%]), exceeding intratracheal instillation or intratracheal aerosol application during the same period.
Evidence weight for skin-induced SAHR
A total of 107 articles involving 29 chemicals investigated whether skin-induced SAHR exists. For all 29 chemicals, except 3-carene, EC3 values (concentration required to induce a 3-fold increase in lymph node cell proliferation in the LLNA) were available from the relevant database18 or other references (Supplementary Table S10). The majority of the 29 chemicals proved to be extreme (n = 12) or strong (n = 8) skin sensitizers as defined according to the LLNA classification.27
Of the 6 articles with the design of airway exposure followed by skin elicitation, only 2—TDI28 and dicyclohexylmethane-4,4′-diisocyanate (HMDI)29—showed that airway exposure led to selective skin response upon elicitation. The results of another 4 articles were inconclusive due to the lack of proper control groups.30,31,32,33
Table 5 shows the weight of evidence for 29 retrieved chemicals. Ten of them proved to be known human asthmagens, with definite experimental evidence of skin-induced SAHR for 8 of them, and insufficient evidence for 2 (cobalt chloride [CoCl2] and 3-amino-5-mercapto-1,2,4-triazole [AMT]). Nineteen of the chemicals reviewed are unknown human asthmagens, but 14 of them do present evidence of skin-induced SAHR in experimental animals.
Table 5. Evidence weight of skin-induced selective airway hyper-responsiveness for reviewed chemicals.
| Skin sensitizer | Known asthmagen | Not known asthmagen | ||||
|---|---|---|---|---|---|---|
| Chemicals | No. of studies | Evidence weight | Chemicals | No. of studies | Evidence weight | |
| Extreme | Toluene diisocyanate | 28 | High | 2,4-dinitrofluorobenzene | 10 | High |
| Diphenylmethane diisocyanate | 13 | High | Dinitrochlorobenzene | 10 | High | |
| Hexamethylene diisocynate | 4 | High | Dicyclohexylmethane-4,4′-diisocyanate | 2 | Low | |
| Isophorone diisocyanate | 1 | Low | Oxazolone | 2 | Low | |
| 2,4-dichlorophenoxyacetic acid | 1 | Low | ||||
| Dicarbonyl 4-oxopentanal | 1 | Low | ||||
| Meta-tetramethylene xylene diisocyanate | 1 | Not possible | ||||
| Methylisothiazolinone | 1 | Not possible | ||||
| Strong | Trimellitic anhydride | 35 | High | Picryl (trinitrophenyl) chloride | 6 | Middle |
| Hexahydrophthalic anhydride | 2 | Low | Ammonium hexachloroplatinate | 1 | Low | |
| Cobalt chloride | 1 | Not possible | BRP | 1 | Low | |
| P-tolyl(mono)isocyanate | 1 | Low | ||||
| Phthalic anhydride | 1 | Not possible | ||||
| Moderate | 4,4-methyltetrahydrophthalic anhydride | 1 | Low | Ammonium persulfate | 3 | Middle |
| 3-amino-5-mercapto-1,2,4-triazole | 1 | Not possible | Furathiocarb | 1 | Low | |
| Weak | Furfuryl alcohol | 1 | Low | Piperidinyl chlorotriazine derivative | 1 | Low |
| Methyl salicylate | 1 | Not possible | ||||
| Toluene-2,4-diamine | 1 | Not possible | ||||
| Undetermined | 3-carene | 3 | Middle | |||
*Asthmagens are accessed from the list of Commission des normes, de l'équité, de la santé et de la sécurité du travail (CNESST).1
Of all included chemicals, trimellitic anhydride (TMA) has been studied most often (n = 35), followed by TDI and diphenylmethane diisocyanate (MDI). Six chemicals were identified with high weight of evidence: 5 extreme skin sensitizers (2,4-dinitrofluorobenzene [DNFB], dinitrochlorobenzene [DNCB], hexamethylene diisocyanate, MDI and TDI); and 1 strong skin sensitizer (TMA). Three chemicals were identified with middle weight of evidence: picryl chloride, ammonium persulfate and 3-carene. Thirteen chemicals were identified with low weight of evidence. The evidence weight for each chemical did not change after removal of 9 unreliable studies with Klimisch score 3. It was not possible to weigh the evidence for 7 chemicals, including 2 extreme skin sensitizers (meta-tetramethylene xylene diisocyanate and methylisothiazolinone), 2 strong skin sensitizers (CoCl2 and phthalic anhydride), 1 moderate skin sensitizer (AMT), and 2 weak skin sensitizers (methyl salicylate and toluene-2,4-diamine). Only 1 article was available for each chemical.
Detailed evidence strength for each indicator of skin-induced SAHR
Table 6 shows the evidence strength by RCGP system for 6 representative SAHR indicators. The detailed evidence summaries for individual SAHR indicators are provided in Supplementary Tables S11-S16. The majority of chemicals (n = 17) exhibit SAHR evidence based on alterations in lung function parameters, including rising Penh, resistance, tissue damping, elasticity, and elastance. Specific changes in respiratory pattern were also observed and enhanced responses to methacholine challenge were also frequently observed (Supplementary Table S11).
Table 6. Detailed evidence strength of skin-induced selective airway hyper-responsiveness for different chemicals.
| Chemical | No. of studies | Weight of evidence | Lung function | Cells in BAL | Cytokine, antibody or enzyme in BAL | Pathology of airway or lung | Cells in airway draining LN | Cytokines in airway draining LN | |
|---|---|---|---|---|---|---|---|---|---|
| Extreme skin sensitizers | |||||||||
| Toluene diisocyanate | 28 | High | ★★★ | ★★★ | ★★★ | ★ | ★★★ | ★ | |
| Diphenylmethane diisocyanate | 13 | High | ★★★ | ★★★ | ★★★ | ★★ | ★★ | ★ | |
| 2,4-dinitrofluorobenzene | 10 | High | ★★ | ★★★ | ★★ | ★★ | ND | ND | |
| Dinitrochlorobenzene | 10 | High | ★ | ★★★ | ★ | - | - | - | |
| Hexamethylene diisocynate | 4 | High | - | ★★★ | - | - | ND | ND | |
| Dicyclohexylmethane-4,4′-diisocyanate | 2 | Low | ★ | ND | ND | ND | ND | ND | |
| Oxazolone | 2 | Low | - | ★ | ★ | ★ | ND | ND | |
| 2,4-dichlorophenoxyacetic acid | 1 | Low | ND | ★ | ★ | ND | ★ | ★ | |
| Dicarbonyl 4-oxopentanal | 1 | Low | ★ | ★ | ND | ND | - | ★ | |
| Isophorone diisocyanate | 1 | Low | ★ | ND | ND | ND | ND | ND | |
| Meta-tetramethylene xylene diisocyanate | 1 | Not possible | - | ND | ND | ND | ND | ND | |
| Methylisothiazolinone | 1 | Not possible | - | - | ND | ND | ND | ND | |
| Strong skin sensitizers | |||||||||
| Trimellitic anhydride | 35 | High | ★★★ | ★★★ | ★★★ | ★★★ | ★★★ | ★ | |
| Picryl (trinitrophenyl) chloride | 6 | Middle | ★★ | ND | ★ | ★ | ND | ND | |
| Hexahydrophthalic anhydride | 2 | Low | ★ | ND | ND | ★ | ND | ND | |
| BRP | 1 | Low | ND | - | ★ | ND | - | ★ | |
| Ammonium hexachloroplatinate | 1 | Low | ★ | ★ | - | ND | ★ | ND | |
| P-tolyl(mono) isocyanate | 1 | Low | ★ | ND | ND | ND | ND | ND | |
| Cobalt chloride | 1 | Not possible | ND | - | ND | - | ND | ND | |
| Phthalic anhydride | 1 | Not possible | - | ND | ND | ND | ND | ND | |
| Moderate, weak or undetermined skin sensitizers | |||||||||
| 3-carene | 3 | Middle | ★★ | ND | ND | ND | ND | ND | |
| Ammonium persulfate | 3 | Middle | ★★ | ★★ | ★ | ND | - | - | |
| 4,4-methyltetrahydrophthalic anhydride | 1 | Low | ★ | ND | ND | ND | ND | ND | |
| Furathiocarb | 1 | Low | ND | - | ★ | ND | - | - | |
| Furfuryl alcohol | 1 | Low | ★ | ★ | ND | - | ND | ND | |
| Piperidinyl chlorotriazine derivative | 1 | Low | ★ | ND | ND | ND | ND | ND | |
| 3-amino-5-mercapto-1,2,4-triazole | 1 | Not possible | - | ND | ND | ND | ND | ND | |
| Methyl salicylate | 1 | Not possible | - | ND | ND | ND | ND | ND | |
| Toluene-2,4-diamine | 1 | Not possible | - | - | ND | ND | ND | ND | |
Reference for each category of each chemical is provided in Supplementary Tables S11-S16.
BAL, bronchoalveolar lavage; LN, lymph node; ND, no available data in this indicator; ★★★/★★/★/-, strong/moderate/limited/no scientific evidence under Royal College of General Practitioners 3-star system.
In BAL fluid, evidence for SAHR was provided by rising ratios of neutrophils or eosinophils (Supplementary Table S12), other indicators of inflammation, such as changes in more than ten cytokines or enzymes (Supplementary Table S13), or increased total and specific IgE (for TMA and 2,4-dichlorophenoxyacetic acid).
In lung or airway tissues, pathological changes indicative of skin-induced SAHR varied from pulmonary edema as evidenced by plasma protein exudation, to infiltration by neutrophils or eosinophils, peribronchiolar or perivascular cell accumulation, epithelial damage, and laryngitis (Supplementary Table S14). In airway draining lymph nodes, cellular responses included increased B cells, increased dendritic cells, and influx of eosinophils (Supplementary Table S15) and/or changes in secretion of interleukin (IL)-4, IL-10 and IL-13 (Supplementary Table S16).
DISCUSSION
This systematic review provides a comprehensive and critical assessment of experimental studies investigating if LMW agents are capable of inducing SAHR after having been applied to the skin. To our knowledge, this is the first evidence-based analysis in this field. Our review found 29 LMW agents showing the ability to cause skin-induced SAHR with high evidence for 6 chemicals, middle evidence for 3 chemicals, and low evidence (due to few independent studies) for 13 chemicals. This finding supports the paradigm that the skin may serve as an important route of exposure for asthma development.15
The current review provides evidence that directly links skin exposure to subsequent airway hyper-responsiveness. Almost all of the chemicals reviewed, except 3-carene (also classified as a significant contact allergen),34 had available positive LLNA results. The evidence is mostly seen for extreme or strong skin sensitizers, but not necessarily limited to these categories. Moderate or even weak skin sensitizers were also found to generate skin-induced SAHR.35,36 The evidence weight for 7 chemicals proved not possible to assess because of limited study number. However, we cannot exclude the possibility that some of these agents could still lead to skin-induced SAHR under different doses or frequencies of sensitization or elicitation.
Ten of the agents included are known asthmagens for humans, and 8 of them present evidence of skin-induced SAHR. Of the other 19 agents which are not known as asthmagens, such as DNCB and DNFB, 14 have been demonstrated to be capable of developing skin-induced SAHR in animals (Table 5). The evidence is based mainly on lung function change and inflammatory responses in BAL. This phenomenon indicates that dermal and respiratory sensitizers are not mutually exclusive even though methods have been developed in an attempt to differentiate these 2 groups.37,38
It is noteworthy that the majority of studies included in this review have been published in toxicology journals (Supplementary Table S7), thus suggesting insufficient interest and recognition of this clinically and mechanistically relevant issue by respiratory medicine, dermatology and immunology.
Implications
In humans, many common contact allergens have also been identified as asthmagens,39,40 and occasionally some patients have both allergic contact dermatitis and asthma caused by the same agent.41,42 Moreover, several epidemiological studies showed an association between skin and respiratory symptoms in the workplace.43 For instance, an association between skin exposure to MDI and asthma-like symptoms has been demonstrated.44 Interestingly, in a workforce exposed to di-isocyanates, the prevalence of chemical-induced asthma did not decrease despite reductions in aerial exposure via better collective and personal protective equipment.14,45
In this review, we found that chemicals not generating obvious clinical respiratory symptoms in epidemiological studies, such as DNCB, may still induce subclinical airway responses in in vivo studies. Thus, current methods for defining respiratory sensitizers, which are based mainly on human studies, might neglect potential hazards. According to the evidence that skin exposure may lead to a change in airway susceptibility, monitoring merely the ambient levels of respiratory sensitizers in the environment may underestimate the hazards spread through different exposure routes.
The relationship between skin sensitization and asthma also has applications in diagnostic practices. For example, skin prick testing (SPT) has been used for decades to demonstrate sensitization to an allergen by provoking an immediate IgE-mediated allergic response through introducing the suspected allergen into the skin's surface with a needle.46 In the updated scheme for the diagnosis of asthma in the workplace, SPT is indicated as one of the first-line methods that should be applied.47 However, SPT is used almost exclusively for HMW agents, and its validity is very low (though not entirely absent) for most LMW agents. On the other hand, the use of skin patch testing, which is the gold standard for demonstrating sensitization to LMW chemicals in patients with contact dermatitis, has not been included in the regular etiological diagnosis of chemical-induced asthma.47
Relevance
Throughout this review, we have examined the effects from LMW agents. Compared with LMW agents, animal studies about HMW agents in the skin-airway relationship have been less thoroughly evaluated. The agents studied in recent decades included ovalbumin,48,49,50,51,52,53 house dust mite54,55 and latex.56,57 The methods used for inducing sensitization to HMW agents through skin exposure usually needed either a continuous exposure for more than 72 hours, a skin injury or an intraperitoneal injection. These exposure methods indicate that it is more difficult for HMW agents to enter the skin and initiate subsequent immune reactions. This may also be reflected in the LLNA database for skin sensitizers, which contains only 1 HMW agent (ovalbumin).
This review focused on animal models, which remain important methods to determine whether a chemical is able to elicit airway responses. Even though several techniques have been developed to replace in vivo asthma models,58,59 these techniques are less suitable for investigating the effects of different exposure routes. Compared with in vitro studies, in vivo studies can mimic better the interplay between different chemicals and physiological conditions. Some findings in animals after LMW agent exposure may share similar mechanisms as human respiratory effects.60
Challenges and limitations
During the process of this review, we faced several challenges. One challenge in connecting skin exposure and asthma development is that no widely acknowledged methods exist to identify respiratory sensitizers for animal models to date,11,61 and the identification mainly relies on human data.12 Therefore, we used 6 parameters in this review to identify the evidence of SAHR.
Another challenge is that several variables may alter the effects of chemicals. These variables include animal strain, routes of exposure, and frequencies and dosages of sensitization and elicitation.62 There is no harmonized system to integrate these variables, and thus a formal meta-analysis from the selected studies was not feasible. In addition, it is difficult to simplify the dose-response curve of the allergic effect, because higher exposures in the induction phase may result in lower potency of sensitization.63 The overdoses of airway elicitation may also result in a direct irritant effect, which elicit the process and effect of the sensitization.
In this review, the second reviewer only screened a selected number of studies (n = 914 [13%] in the first screening stage; n = 144 [7.8%] in the second screening stage). This method might increase the false negative rate through excluding some potentially relevant studies. However, we limited the false negative rate through forward and backward reference tracking. Other researchers have validated this method by comparing full systematic reviews with 20% enhanced rapid reviews and concluded that the latter method identified similar numbers of relevant studies.64
Mechanistic considerations
While the pathogenesis of skin and airway sensitization has been extensively studied and some focuses have been put on immunological cells in lung after skin exposure,65 the process from skin exposure to asthma development has not yet been fully delineated.
Based on the existing scientific findings, we hypothesize the following process which links the skin and airway. The skin sensitization begins when haptens penetrate the striatum where the haptens become linked to proteins or macromolecules, sensed by Langerhans cells, and presented to T cells at skin draining lymph nodes.66,67 Once the T cells are activated, they enter the circulatory system61,68 and may then reach the airway tissue or its draining lymph node. Upon the re-exposure to haptens, dendritic cells in the airways can re-stimulate the resident T cell and initiate asthma development.4,69,70 Accordingly, it would be valuable to design experiments that identify and track different cells covering the critical pathway after skin exposure.
CONCLUSION
This review systematically identified studies investigating the association between skin exposure to chemicals and airway hyper-responsiveness in experimental animal models. Although our systematic review of the experimental evidence does not answer the questions as to what makes a chemical an allergen and what are cellular events driving the allergic response and target organs, the available evidence suggests that most of the studied LMW chemicals have the potential to induce SAHR via previous skin exposure. Admittedly, some agents possess only partial evidence mainly because complete outcome measures for each agent have not yet been fully investigated. Studies about TDI, TMA and MDI attracted most attention, while more than half of the LMW agents have been individually tested only once. Despite their small number, studies with the design of airway exposure followed by skin elicitation also support the concept that the immunological effects from the skin and airway interact with each other. Namely, airway exposure leads to selective skin response upon elicitation.
By doing this, we conclude that sensitization is not only an issue of specific organs but also an issue of the immune system in all organs, and the response is dependent on the route of elicitation. Therefore, chemicals that have been identified as skin sensitizers in a series of tests should still be carefully considered able to induce airway adverse response once inhaled because several skin sensitizers also share the characteristics of respiratory sensitizers, although we acknowledge that this concept may not be shared by all experts.71,72 We believe the latter view is to a large extent due to the fact that many skin sensitizers are only rarely or never inhaled. Moreover, we do not dispute that intrinsic chemical properties other than volatility can render some sensitizers more likely to cause dermal disease rather than respiratory disease, and vice versa. However, these chemical determinants are still largely unknown. Accordingly, skin exposure should be taken into account by clinicians dealing with asthma or by surveillance programs in the environment. Furthermore, it cannot be excluded that every chemical sensitizer may elicit the respiratory response upon inhalation, regardless of the route of sensitization.
The exhaustive evidence in this review also brings the concept of “distant sensitization” into asthma development, which means the susceptibility of the airways to a chemical agent may result from prior exposure distant from the airways. This provides an opportunity to reconsider the hazard definition of skin or respiratory sensitizers. The traditional view defined respiratory sensitizers simply as agents that induce airway sensitization via airway exposure. Based on our review, however, this definition is not appropriate for classifying chemicals accurately, since the consequences of sensitization are not necessarily restricted to a specific organ. A chemical may induce respiratory disease after sensitization via dermal contact even when the ambient level is too low to cause sensitization via the respiratory mucosa.
Therefore, we propose to consider the ability of a chemical to cause immune sensitization as a generic property, regardless of the site of sensitization and elicitation of symptoms, by analogy with the ability to cause cancer. Obviously, in terms of “risk”—which depends on both hazard and exposure—and its management, the type of exposure must be taken into account. While in terms of “hazard,” chemicals are considered (and labelled) as carcinogens, regardless of exposure routes or target organs.11 Thus, an agent that causes immunological sensitization should also simply be labelled as “sensitizer.”
In terms of risk assessment, the important issue is whether the offending agent can cause immunological sensitization and whether it can reach the airways. The initiation of the sensitization process and subsequent manifestations depend at least on 1) chemical properties and co-exposures; 2) the mode of exposure; and 3) individual tissue characteristics (e.g. accessibility of antigen-presenting cells across a damaged epithelium). Consequently, if a chemical can lead to allergic contact dermatitis, we should assume that the chemical may also cause asthma or other types of pulmonary hypersensitivity reactions when inhaled by a sensitized person.
To understand the mechanism of airway sensitization, further investigations are warranted with a focus on the pathological change in airway and associated immunological pathways. Lack of established criteria for sensitization in the airways increases the difficulty in analyzing the evidence, and future studies are required to understand the key events of asthma development in order to clarify the criteria. A harmonized system is warranted to construct the model of asthma development.
ACKNOWLEDGMENTS
The first author (Hung-Chang Tsui) received a “Scholarship to Study Abroad” from the Ministry of Education of Taiwan for 2 years (2017/May-2019/May) for his PhD track in KU Leuven. This manuscript is part of his PhD progress.
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
SUPPLEMENTARY MATERIALS
Toxicological data Reliability Assessment Tool quality assessment summary.
Terms used to identify relevant studies with respiratory and skin effect
Skin sensitizers used to identify relevant studies
Respiratory sensitizers used to identify relevant studies
Reliability assessment criteria of toxicological studies (in vivo)
Comparison of the screening between 2 reviewers
List of 144 included studies for data extraction
Top 15 journals with the most articles
Reported outcome measures in studies adopting skin exposure followed by airway elicitation
The grading score of Toxicological data Reliability Assessment Tool criteria for studies investigating skin-induced selective airway hyper-responsiveness
Categorization of reviewed chemicals based on LLNA EC3 value
Strength of evidence for changes of lung function
Strength of evidence for changes of cells in bronchoalveolar lavage
Strength of evidence for changes of protein, antibody or cytokine in bronchoalveolar lavage
Strength of evidence for changes of pathology or histology quantification of airway
Strength of evidence for changes of cells in airway draining lymph node
Strength of evidence for changes of cytokines in airway draining lymph node
REFERENCES
References
- 1.Commission des normes, de l’équité, de la santé et de la sécurité du travail (CNESST) List of agents causing occupational asthma [Internet] Quebec: Commission des normes, de l’équité, de la santé et de la sécurité du travail; 2016. [cited 2016 Dec 10]. Available from: http://www.csst.qc.ca/en/prevention/reptox/occupational-asthma/Pages/occupational-asthma.aspx. [Google Scholar]
- 2.Meca O, Cruz MJ, Sánchez-Ortiz M, González-Barcala FJ, Ojanguren I, Munoz X. Do low molecular weight agents cause more severe asthma than high molecular weight agents? PLoS One. 2016;11:e0156141. doi: 10.1371/journal.pone.0156141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wisnewski AV, Liu Q, Liu J, Redlich CA. Human innate immune responses to hexamethylene diisocyanate (HDI) and HDI-albumin conjugates. Clin Exp Allergy. 2008;38:957–967. doi: 10.1111/j.1365-2222.2008.02982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Vooght V, Hox V, Nemery B, Vanoirbeek JAJ. Mechanisms of occupational asthma caused by low-molecular-weight chemicals. In: Sigsgaard T, Heederik D, editors. Occupational asthma. Basel: Springer; 2010. pp. 141–162. [Google Scholar]
- 5.Park HS, Kim HY, Nahm DH, Son JW, Kim YY. Specific IgG, but not specific IgE, antibodies to toluene diisocyanate-human serum albumin conjugate are associated with toluene diisocyanate bronchoprovocation test results. J Allergy Clin Immunol. 1999;104:847–851. doi: 10.1016/s0091-6749(99)70297-6. [DOI] [PubMed] [Google Scholar]
- 6.Maestrelli P, Boschetto P, Fabbri LM, Mapp CE. Mechanisms of occupational asthma. J Allergy Clin Immunol. 2009;123:531–542. doi: 10.1016/j.jaci.2009.01.057. [DOI] [PubMed] [Google Scholar]
- 7.Karol MH, Hauth BA, Riley EJ, Magreni CM. Dermal contact with toluene diisocyanate (TDI) produces respiratory tract hypersensitivity in guinea pigs. Toxicol Appl Pharmacol. 1981;58:221–230. doi: 10.1016/0041-008x(81)90426-9. [DOI] [PubMed] [Google Scholar]
- 8.Pauluhn J. Respiratory hypersensitivity to trimellitic anhydride in Brown Norway rats: analysis of dose-response following topical induction and time course following repeated inhalation challenge. Toxicology. 2003;194:1–17. doi: 10.1016/s0300-483x(03)00285-3. [DOI] [PubMed] [Google Scholar]
- 9.Tarkowski M, Vanoirbeek JA, Vanhooren HM, De Vooght V, Mercier CM, Ceuppens J, et al. Immunological determinants of ventilatory changes induced in mice by dermal sensitization and respiratory challenge with toluene diisocyanate. Am J Physiol Lung Cell Mol Physiol. 2007;292:L207–14. doi: 10.1152/ajplung.00157.2005. [DOI] [PubMed] [Google Scholar]
- 10.Occupational Safety and Health Administration (OSHA) Health hazard criteria 2012. Appendix A [Internet] Washington, D.C.: Occupational Safety and Health Administration; 2012. [cited 2018 Sep 26]. Available from: https://www.osha.gov/dsg/hazcom/hazcom-appendix-a.html. [Google Scholar]
- 11.United Nations Economic Commission for Europe (UNECE) Globally harmonized system of classification and labelling of chemicals (GHS) [Internet] Geneva: United Nations Economic Commission for Europe; 2015. [cited 2018 Oct 19]. Available from: http://www.unece.org/trans/danger/publi/ghs/ghs_rev06/06files_e.html. [Google Scholar]
- 12.Selgrade MK, Sullivan KS, Boyles RR, Dederick E, Serex TL, Loveless SE. Decision trees for evaluating skin and respiratory sensitizing potential of chemicals in accordance with European regulations. Regul Toxicol Pharmacol. 2012;63:371–380. doi: 10.1016/j.yrtph.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Kimber I. The role of the skin in the development of chemical respiratory hypersensitivity. Toxicol Lett. 1996;86:89–92. doi: 10.1016/0378-4274(96)03678-8. [DOI] [PubMed] [Google Scholar]
- 14.Redlich CA, Herrick CA. Lung/skin connections in occupational lung disease. Curr Opin Allergy Clin Immunol. 2008;8:115–119. doi: 10.1097/ACI.0b013e3282f85a31. [DOI] [PubMed] [Google Scholar]
- 15.Redlich CA. Skin exposure and asthma: is there a connection? Proc Am Thorac Soc. 2010;7:134–137. doi: 10.1513/pats.201002-025RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Vries RBM, Hooijmans CR, Langendam MW, van Luijk J, Leenaars M, Ritskes-Hoitinga M, et al. A protocol format for the preparation, registration and publication of systematic reviews of animal intervention studies. Evid Based Preclin Med. 2015;2:1–9. [Google Scholar]
- 17.Booth A, Clarke M, Dooley G, Ghersi D, Moher D, Petticrew M, et al. The nuts and bolts of PROSPERO: an international prospective register of systematic reviews. Syst Rev. 2012;1:2. doi: 10.1186/2046-4053-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Toxicology Program Interagency Center for the Evaluation of Alternative Toxicological Methods (NICEATM) Murine local lymph node assay (LLNA) database [Internet] Durham: National Toxicology Program Interagency Center for the Evaluation of Alternative Toxicological Methods; 2010. [cited 2016 Nov 20]. Available from: http://ntp.niehs.nih.gov/pubhealth/evalatm/test-method-evaluations/immunotoxicity/nonanimal/index.html. [Google Scholar]
- 19.Anderson SE, Siegel PD, Meade BJ. The LLNA: a brief review of recent advances and limitations. J Allergy (Cairo) 2011;2011:424203. doi: 10.1155/2011/424203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Center for Biotechnology Information (NCBI) PubChem compound [Internet] Bethesda (MD): National Center for Biotechnology Information; 2018. [cited 2018 Jul 9]. Available from: https://www.ncbi.nlm.nih.gov/pccompound?cmd=search. [Google Scholar]
- 21.Schneider K, Schwarz M, Burkholder I, Kopp-Schneider A, Edler L, Kinsner-Ovaskainen A, et al. “ToxRTool”, a new tool to assess the reliability of toxicological data. Toxicol Lett. 2009;189:138–144. doi: 10.1016/j.toxlet.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 22.Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE's risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:43. doi: 10.1186/1471-2288-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klimisch HJ, Andreae M, Tillmann U. A systematic approach for evaluating the quality of experimental toxicological and ecotoxicological data. Regul Toxicol Pharmacol. 1997;25:1–5. doi: 10.1006/rtph.1996.1076. [DOI] [PubMed] [Google Scholar]
- 24.Nicholson PJ, Cullinan P, Taylor AJ, Burge PS, Boyle C. Evidence based guidelines for the prevention, identification, and management of occupational asthma. Occup Environ Med. 2005;62:290–299. doi: 10.1136/oem.2004.016287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baur X, Bakehe P. Allergens causing occupational asthma: an evidence-based evaluation of the literature. Int Arch Occup Environ Health. 2014;87:339–363. doi: 10.1007/s00420-013-0866-9. [DOI] [PubMed] [Google Scholar]
- 26.Scientific Committee on Health, Environmental and Emerging Risks (SCHEER) Memorandum on weight of evidence and uncertainties - revision 2018 [Internet] Luxembourg: Scientific Committee on Health, Environmental and Emerging Risks; 2018. [cited 2019 Jan 1]. Available from: https://ec.europa.eu/health/sites/health/files/scientific_committees/scheer/docs/scheer_o_014.pdf. [Google Scholar]
- 27.European Centre for Ecotoxicology and Toxicology of Chemicals (ECETOC) Contact sensitisation: classification according to potency a commentary [Internet] Brussels: European Centre for Ecotoxicology and Toxicology of Chemicals; 2003. [cited 2018 Oct 19]. Available from: http://www.ecetoc.org/publication/contact-sensitisation-classification-according-to-potency-a-commentary/ [Google Scholar]
- 28.Ebino K, Ueda H, Kawakatsu H, Shutoh Y, Kosaka T, Nagayoshi E, et al. Isolated airway exposure to toluene diisocyanate results in skin sensitization. Toxicol Lett. 2001;121:79–85. doi: 10.1016/s0378-4274(01)00325-3. [DOI] [PubMed] [Google Scholar]
- 29.Stadler J, Karol MH. Experimental delayed hypersensitivity following inhalation of dicyclohexylmethane-4,4′-diisocyanate: a concentration-response relationship. Toxicol Appl Pharmacol. 1984;74:244–249. doi: 10.1016/0041-008x(84)90149-2. [DOI] [PubMed] [Google Scholar]
- 30.Enander I, Ahlstedt S, Nygren H, Björkstén B. Sensitizing ability of derivatives of picryl chloride after exposure of mice on the skin and in the lung. Int Arch Allergy Appl Immunol. 1983;72:59–66. doi: 10.1159/000234841. [DOI] [PubMed] [Google Scholar]
- 31.Karol MH. Concentration-dependent immunologic response to toluene diisocyanate (TDI) following inhalation exposure. Toxicol Appl Pharmacol. 1983;68:229–241. doi: 10.1016/0041-008x(83)90007-8. [DOI] [PubMed] [Google Scholar]
- 32.Sugawara Y, Okamoto Y, Sawahata T, Tanaka K. Skin reactivity in guinea pigs sensitized with 2,4-toluene diisocyanate. Int Arch Allergy Immunol. 1993;100:190–196. doi: 10.1159/000236408. [DOI] [PubMed] [Google Scholar]
- 33.Farraj AK, Harkema JR, Kaminski NE. Topical application versus intranasal instillation: a qualitative comparison of the effect of the route of sensitization on trimellitic anhydride-induced allergic rhinitis in A/J mice. Toxicol Sci. 2006;92:321–328. doi: 10.1093/toxsci/kfj191. [DOI] [PubMed] [Google Scholar]
- 34.Schlede E, Aberer W, Fuchs T, Gerner I, Lessmann H, Maurer T, et al. Chemical substances and contact allergy--244 substances ranked according to allergenic potency. Toxicology. 2003;193:219–259. doi: 10.1016/s0300-483x(03)00266-x. [DOI] [PubMed] [Google Scholar]
- 35.Vanoirbeek JA, Mandervelt C, Cunningham AR, Hoet PH, Xu H, Vanhooren HM, et al. Validity of methods to predict the respiratory sensitizing potential of chemicals: a study with a piperidinyl chlorotriazine derivative that caused an outbreak of occupational asthma. Toxicol Sci. 2003;76:338–346. doi: 10.1093/toxsci/kfg235. [DOI] [PubMed] [Google Scholar]
- 36.Franko J, Jackson LG, Hubbs A, Kashon M, Meade BJ, Anderson SE. Evaluation of furfuryl alcohol sensitization potential following dermal and pulmonary exposure: enhancement of airway responsiveness. Toxicol Sci. 2012;125:105–115. doi: 10.1093/toxsci/kfr271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Jong WH, Arts JH, De Klerk A, Schijf MA, Ezendam J, Kuper CF, et al. Contact and respiratory sensitizers can be identified by cytokine profiles following inhalation exposure. Toxicology. 2009;261:103–111. doi: 10.1016/j.tox.2009.04.057. [DOI] [PubMed] [Google Scholar]
- 38.North CM, Ezendam J, Hotchkiss JA, Maier C, Aoyama K, Enoch S, et al. Developing a framework for assessing chemical respiratory sensitization: a workshop report. Regul Toxicol Pharmacol. 2016;80:295–309. doi: 10.1016/j.yrtph.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 39.Arrandale VH, Liss GM, Tarlo SM, Pratt MD, Sasseville D, Kudla I, et al. Occupational contact allergens: are they also associated with occupational asthma? Am J Ind Med. 2012;55:353–360. doi: 10.1002/ajim.22015. [DOI] [PubMed] [Google Scholar]
- 40.Kimber I, Agius R, Basketter DA, Corsini E, Cullinan P, Dearman RJ, et al. Chemical respiratory allergy: opportunities for hazard identification and characterisation. The report and recommendations of ECVAM workshop 60. Altern Lab Anim. 2007;35:243–265. doi: 10.1177/026119290703500212. [DOI] [PubMed] [Google Scholar]
- 41.De Raeve H, Vandecasteele C, Demedts M, Nemery B. Dermal and respiratory sensitization to chromate in a cement floorer. Am J Ind Med. 1998;34:169–176. doi: 10.1002/(sici)1097-0274(199808)34:2<169::aid-ajim10>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 42.Meuleman L, Goossens A, Linders C, Rochette F, Nemery B. Sensitization to triglycidylisocyanurate (TGIC) with cutaneous and respiratory manifestations. Allergy. 1999;54:752–756. doi: 10.1111/j.1398-9995.1999.00103.x. [DOI] [PubMed] [Google Scholar]
- 43.Chongo-Faruk V. Skin exposure, symptoms and asthma in occupational settings – is there a link? Curr Allergy Clin Immunol. 2017;30:251–257. [Google Scholar]
- 44.Petsonk EL, Wang ML, Lewis DM, Siegel PD, Husberg BJ. Asthma-like symptoms in wood product plant workers exposed to methylene diphenyl diisocyanate. Chest. 2000;118:1183–1193. doi: 10.1378/chest.118.4.1183. [DOI] [PubMed] [Google Scholar]
- 45.Bello D, Herrick CA, Smith TJ, Woskie SR, Streicher RP, Cullen MR, et al. Skin exposure to isocyanates: reasons for concern. Environ Health Perspect. 2007;115:328–335. doi: 10.1289/ehp.9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heinzerling L, Mari A, Bergmann KC, Bresciani M, Burbach G, Darsow U, et al. The skin prick test - European standards. Clin Transl Allergy. 2013;3:3. doi: 10.1186/2045-7022-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vandenplas O, Suojalehto H, Cullinan P. Diagnosing occupational asthma. Clin Exp Allergy. 2017;47:6–18. doi: 10.1111/cea.12858. [DOI] [PubMed] [Google Scholar]
- 48.Spergel JM, Mizoguchi E, Brewer JP, Martin TR, Bhan AK, Geha RS. Epicutaneous sensitization with protein antigen induces localized allergic dermatitis and hyperresponsiveness to methacholine after single exposure to aerosolized antigen in mice. J Clin Invest. 1998;101:1614–1622. doi: 10.1172/JCI1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herrick CA, MacLeod H, Glusac E, Tigelaar RE, Bottomly K. Th2 responses induced by epicutaneous or inhalational protein exposure are differentially dependent on IL-4. J Clin Invest. 2000;105:765–775. doi: 10.1172/JCI8624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kodama M, Asano K, Oguma T, Kagawa S, Tomomatsu K, Wakaki M, et al. Strain-specific phenotypes of airway inflammation and bronchial hyperresponsiveness induced by epicutaneous allergen sensitization in BALB/c and C57BL/6 mice. Int Arch Allergy Immunol. 2010;152(Suppl 1):67–74. doi: 10.1159/000312128. [DOI] [PubMed] [Google Scholar]
- 51.Morita H, Arae K, Ohno T, Kajiwara N, Oboki K, Matsuda A, et al. ST2 requires Th2-, but not Th17-, type airway inflammation in epicutaneously antigen- sensitized mice. Allergol Int. 2012;61:265–273. doi: 10.2332/allergolint.11-OA-0379. [DOI] [PubMed] [Google Scholar]
- 52.Morita H, Arae K, Unno H, Toyama S, Motomura K, Matsuda A, et al. IL-25 and IL-33 contribute to development of eosinophilic airway inflammation in epicutaneously antigen-sensitized mice. PLoS One. 2015;10:e0134226. doi: 10.1371/journal.pone.0134226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Masaki K, Suzuki Y, Kagawa S, Kodama M, Kabata H, Miyata J, et al. Dual role of interleukin-23 in epicutaneously-sensitized asthma in mice. Allergol Int. 2014;63(Suppl 1):13–22. doi: 10.2332/allergolint.13-OA-0632. [DOI] [PubMed] [Google Scholar]
- 54.Aun MV, Saraiva-Romanholo BM, Almeida FM, Brüggemann TR, Kalil J, Martins MA, et al. Sensitization by subcutaneous route is superior to intraperitoneal route in induction of asthma by house dust mite in a murine mode. Einstein (Sao Paulo) 2015;13:560–566. doi: 10.1590/S1679-45082015AO3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deckers J, Sichien D, Plantinga M, Van Moorleghem J, Vanheerswynghels M, Hoste E, et al. Epicutaneous sensitization to house dust mite allergen requires interferon regulatory factor 4-dependent dermal dendritic cells. J Allergy Clin Immunol. 2017;140:1364–1377.e2. doi: 10.1016/j.jaci.2016.12.970. [DOI] [PubMed] [Google Scholar]
- 56.Howell MD, Weissman DN, Jean Meade B. Latex sensitization by dermal exposure can lead to airway hyperreactivity. Int Arch Allergy Immunol. 2002;128:204–211. doi: 10.1159/000064253. [DOI] [PubMed] [Google Scholar]
- 57.Lehto M, Haapakoski R, Wolff H, Majuri ML, Mäkelä MJ, Leino M, et al. Cutaneous, but not airway, latex exposure induces allergic lung inflammation and airway hyperreactivity in mice. J Invest Dermatol. 2005;125:962–968. doi: 10.1111/j.0022-202X.2005.23910.x. [DOI] [PubMed] [Google Scholar]
- 58.Meurs H, Gosens R, Zaagsma J. Airway hyperresponsiveness in asthma: lessons from in vitro model systems and animal models. Eur Respir J. 2008;32:487–502. doi: 10.1183/09031936.00023608. [DOI] [PubMed] [Google Scholar]
- 59.Blume C, Davies DE. In vitro and ex vivo models of human asthma. Eur J Pharm Biopharm. 2013;84:394–400. doi: 10.1016/j.ejpb.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 60.Zosky GR, Sly PD. Animal models of asthma. Clin Exp Allergy. 2007;37:973–988. doi: 10.1111/j.1365-2222.2007.02740.x. [DOI] [PubMed] [Google Scholar]
- 61.Rovida C, Martin SF, Vivier M, Weltzien HU, Roggen E. Advanced tests for skin and respiratory sensitization assessment. ALTEX. 2013;30:231–252. doi: 10.14573/altex.2013.2.231. [DOI] [PubMed] [Google Scholar]
- 62.De Vooght V, Vanoirbeek JA, Luyts K, Haenen S, Nemery B, Hoet PH. Choice of mouse strain influences the outcome in a mouse model of chemical-induced asthma. PLoS One. 2010;5:e12581. doi: 10.1371/journal.pone.0012581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vanoirbeek JA, Tarkowski M, Ceuppens JL, Verbeken EK, Nemery B, Hoet PH. Respiratory response to toluene diisocyanate depends on prior frequency and concentration of dermal sensitization in mice. Toxicol Sci. 2004;80:310–321. doi: 10.1093/toxsci/kfh155. [DOI] [PubMed] [Google Scholar]
- 64.Taylor-Phillips S, Geppert J, Stinton C, Freeman K, Johnson S, Fraser H, et al. Comparison of a full systematic review versus rapid review approaches to assess a newborn screening test for tyrosinemia type 1. Res Synth Methods. 2017;8:475–484. doi: 10.1002/jrsm.1255. [DOI] [PubMed] [Google Scholar]
- 65.Pollaris L, Van Den Broucke S, Decaesteker T, Cremer J, Seys S, Devos FC, et al. Dermal exposure determines the outcome of repeated airway exposure in a long-term chemical-induced asthma-like mouse model. Toxicology. 2019;421:84–92. doi: 10.1016/j.tox.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 66.Karlberg AT, Bergström MA, Börje A, Luthman K, Nilsson JL. Allergic contact dermatitis--formation, structural requirements, and reactivity of skin sensitizers. Chem Res Toxicol. 2008;21:53–69. doi: 10.1021/tx7002239. [DOI] [PubMed] [Google Scholar]
- 67.Kaplan DH, Igyártó BZ, Gaspari AA. Early immune events in the induction of allergic contact dermatitis. Nat Rev Immunol. 2012;12:114–124. doi: 10.1038/nri3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Organisation for Economic Co-operation and Development (OECD) The adverse outcome pathway for skin sensitisation initiated by covalent binding to proteins. Paris: OECD Publishing; 2014. [Google Scholar]
- 69.Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. 2015;16:45–56. doi: 10.1038/ni.3049. [DOI] [PubMed] [Google Scholar]
- 70.Holgate ST, Wenzel S, Postma DS, Weiss ST, Renz H, Sly PD. Asthma. Nat Rev Dis Primers. 2015;1:15025. doi: 10.1038/nrdp.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Burge PS. Occupational asthma and dermatitis: points of contact. Clin Exp Dermatol. 1981;6:235–241. doi: 10.1111/j.1365-2230.1981.tb02299.x. [DOI] [PubMed] [Google Scholar]
- 72.Kimber I, Dearman RJ. What makes a chemical a respiratory sensitizer? Curr Opin Allergy Clin Immunol. 2005;5:119–124. doi: 10.1097/01.all.0000162302.82233.93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Toxicological data Reliability Assessment Tool quality assessment summary.
Terms used to identify relevant studies with respiratory and skin effect
Skin sensitizers used to identify relevant studies
Respiratory sensitizers used to identify relevant studies
Reliability assessment criteria of toxicological studies (in vivo)
Comparison of the screening between 2 reviewers
List of 144 included studies for data extraction
Top 15 journals with the most articles
Reported outcome measures in studies adopting skin exposure followed by airway elicitation
The grading score of Toxicological data Reliability Assessment Tool criteria for studies investigating skin-induced selective airway hyper-responsiveness
Categorization of reviewed chemicals based on LLNA EC3 value
Strength of evidence for changes of lung function
Strength of evidence for changes of cells in bronchoalveolar lavage
Strength of evidence for changes of protein, antibody or cytokine in bronchoalveolar lavage
Strength of evidence for changes of pathology or histology quantification of airway
Strength of evidence for changes of cells in airway draining lymph node
Strength of evidence for changes of cytokines in airway draining lymph node
REFERENCES