Abstract
Purpose
Cold air is a major environmental factor that exacerbates asthma. Transient receptor potential melastatin family member 8 (TRPM8) is a cold-sensing channel expressed in the airway epithelium. However, its role in airway inflammation remains unknown. We investigated the role of TRPM8 in innate immune responses in bronchial epithelial cells and asthmatic subjects.
Methods
The TRPM8 mRNA and protein expression on BEAS2B human bronchial epithelial cells was examined by real-time polymerase chain reaction (PCR), immunofluorescence staining and western blotting. Additionally, interleukin (IL)-4, IL-6, IL-8, IL-13, IL-25 and thymic stromal lymphopoietin (TSLP) levels before and after menthol, dexamethasone and N-(4-tert-butylphenyl)-4-(3-chloropyridin-2-yl) piperazine-1-carboxamide (BCTC) treatments were measured via real-time PCR. TRPM8 protein levels in the supernatants of induced sputum from asthmatic subjects and normal control subjects were measured using enzyme-linked immunosorbent assay, and mRNA levels in sputum cell lysates were measured using real-time PCR.
Results
Treatment with up to 2 mM menthol dose-dependently increased TRPM8 mRNA and protein in BEAS2B cells compared to untreated cells (P < 0.001) and concomitantly increased IL-25 and TSLP mRNA (P < 0.05), but not IL-33 mRNA. BCTC (10 μM) significantly abolished menthol-induced up-regulation of TRPM8 mRNA and protein and IL-25 and TSLP mRNA (P < 0.01). TRPM8 protein levels were higher in the supernatants of induced sputum from asthmatic subjects (n = 107) than in those from healthy controls (n = 19) (P < 0.001), and IL-25, TSLP and IL-33 mRNA levels were concomitantly increased (P < 0.001). Additionally, TRPM8 mRNA levels correlated strongly with those of IL-25 and TSLP (P < 0.001), and TRPM8 protein levels were significantly higher in bronchodilator-responsive asthmatic subjects than in nonresponders.
Conclusions
TRPM8 may be involved in the airway epithelial cell innate immune response and a molecular target for the treatment of asthma.
Keywords: Asthma, transient receptor potential channels, epithelium, interleukin-25, thymic stromal lymphopoietin
INTRODUCTION
Exposure to cold air is a major environmental factor that exacerbates chronic inflammatory airway diseases.1 When cold air is inhaled, heat loss and compensatory thermoregulatory actions occur in the airways.2 Cold air provokes a series of respiratory symptoms, including chest tightness, dyspnea and cough, in patients with chronic airway diseases such as asthma or chronic obstructive pulmonary disease (COPD).3,4,5 Cold temperature-related exacerbation of these diseases is often followed by a subsequent increase in bacterial and viral infections of the airway, infiltration of inflammatory factors, and mucus secretion, suggesting that cold stimuli trigger the exacerbation.6,7 However, the mechanism of cold-induced airway responses has not been well established.
The discovery of thermosensitive ion channels of the transient receptor potential (TRP) family has provided some clues to understanding of the molecular mechanism of temperature detection.8 Transient receptor potential melastatin family member 8 (TRPM8), a nonselective calcium (Ca2+)-permeable cation channel, is expressed on a subset of sensory neurons as well as in a number of nonneuronal areas including respiratory epithelium.9,10 TRPM8 is activated by cold temperatures of less than 25°C and cooling agents, such as menthol or icilin agents. Cooling temperatures below 24°C-28°C start to evoke depolarizing currents of TRPM8; these currents increase with decreasing temperatures and peak near 10°C. Previous studies have identified a cold and menthol-activated TRPM8 variant in lung epithelial cells. Activation of the TRPM8 variant in lung epithelial cells by cold air leads to increased expression of several cytokine and chemokine genes.11 Moreover, TRPM8 expression in bronchial epithelial cells is augmented in patients with COPD, and the TRPM8 channel is responsible for the enhanced expression of mucin 5AC (MUC5AC),12 suggesting that these results show that TRPM8 receptors are potentially involved in the airway inflammatory response induced by cold air in chronic airway diseases.
The airway epithelium has been thought to function mainly as a physical barrier and through mucociliary clearance to impede the access of allergens, air pollutants, irritants, and viruses to lung dendritic cells.13 In the airways of asthmatic patients, the epithelia are fragile, and some areas of the epithelial basement membrane seem to be denuded of ciliated cells, which is a vulnerable condition for the invasion of pathogens or allergens into the airways. Moreover, the epithelium generates a wide range of mediators that can modulate inflammatory responses, either helping maintain homeostasis or enhance inflammation. In particular, the asthmatic airway epithelium is a major source of cytokines and chemokines, and they are actively involved in airway inflammation and repair mechanisms, which are important in both the airway remodeling and mucous metaplastic responses of chronic asthma.14
Cold temperature exposure of the airways can damage respiratory epithelium, and the damaged epithelial cells release inflammatory mediators and recruit inflammatory cells into the airways as observed in cold-air athletes.15,16 In animal models mimicking exercise-induced asthma, the gene expression of various cytokines was increased in bronchoalveolar lavage fluids after exercise in cold air compared with their levels after warm air exposure, and the overall cytokine pattern was predominantly a Th2 profile pattern.17 As the TRP channels function by means of their direct effect on the intracellular levels of cations or through the indirect modulation of a large series of intracellular pathways such as cytokine production, cell differentiation and cytotoxicity,18 we speculated that TRPM8, which is known as a cold receptor, may be involved in the inflammatory process of chronic airway disease. Therefore, we explored the hypothesis that the cold-mediated activation of TRPM8 of airway epithelium is involved in airway inflammation in vitro using bronchial epithelial cells and determine the clinical relevance of TRPM8 expression in patients with asthma ex vivo.
MATERIALS AND METHODS
Chemicals
N-(4-tert-butylphenyl)-4-(3-chloropyridin-2-yl) piperazine-1-carboxamide (BCTC), menthol and dexamethasone were purchased from Sigma Chemical Corp. (St. Louis, MO, USA). Rabbit anti-TRPM8 (C-term) polyclonal antibody was purchased from Abcam (Cambridge, UK). Oligonucleotides were purchased from Bioneer (Daejeon, Korea). SYBR Premix EX Taq was purchased from TaKaRa Biotechnology (Shiga, Japan).
Cell culture
Human bronchial epithelial cells (BEAS2B cells) were used in this study (CRL-9609™; American Type Culture Collection, Rockville, MD, USA). BEAS2B cells were cultured in RPMI-1640 medium (HyClone, Logan, UT, USA) with 10% fetal bovine serum and were maintained at 37°C in a humidified atmosphere of 5% carbon dioxide and 95% air. The cells were maintained at 37°C, between 30% and 90% confluence, in an air-ventilated and humidified incubator maintained with 5% carbon dioxide. For subculturing, the cells were trypsin-dissociated and passaged every 2 to 4 days. The cytotoxicity of each condition was monitored with the Vybrant MTT Cell Proliferation Assay Kit (V-13154; Life Technologies, Carlsbad, CA, USA), showing that no significant toxicity was observed at the concentration of these presented experiments.
Quantitative real-time polymerase chain reaction (PCR)
Total RNA was isolated using TRI REAGENT (Molecular Research Center, Cincinnati, OH, USA). For BEAS2B and human cells from induced sputum, cDNA was prepared from 1 µg RNA using amfiRivert Platinum cDNA Synthesis Master Mix (GenDEPOT, Katy, TX, USA) (42°C, 60 minutes), followed by heating inactivation (70°C, 15 minutes). Real-time PCR for quantitative mRNA expression analyses was performed on a StepOne Plus Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) with a SYBR Premix EX Taq (TaKaRa Biotechnology), and the primers are listed in Supplementary Table S1. Standard PCR conditions were as follows: 95°C for 3 minutes; 40 cycles of 15 seconds at 95°C, 30 seconds at 58°C, and 15 seconds at 72°C; followed by a standard melting curve. Samples were normalized to glyceraldehyde 3-phosphate dehydrogenase. Analyses were performed with the Ct method, which allows for quantitative expression analysis using the formula 2−ΔΔCt. Experiments were reproduced a minimum of 3 times with different passages of cells.
Immunofluorescence
BEAS2B cells were cultured on 22-mm round coverslips (BD Biosciences, Bedford, MA, USA) until 75% confluence and fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 10 minutes. Cells were washed 3 times with PBS, and nonspecific binding was blocked using a solution of 5% bovine serum albumin in PBS. The cells were rinsed 3 times with PBS and incubated at room temperature (RT) for 2 hours with a rabbit polyclonal immunoglobulin G (IgG) antibody fraction specific for human TRPM8 (Abcam, Cambridge, MA, USA), diluted 1:250 in the blocking solution. The cells were washed and treated for 1 hour at RT with an Alexa-Fluor 488 conjugated goat anti-rabbit IgG secondary antibody (Molecular Probes, Eugene, OR, USA) at a dilution of 1:500 in the blocking solution. The nuclei were counterstained blue using 4′,6-diamidino-2-phenylindole (DAPI) at 300 nM dilution in PBS. Controls consisted of untreated cells or cells treated with either primary or secondary antibodies alone. Images were collected using a Zeiss LSM700 laser scanning confocal microscope (Carl Zeiss, Oberkochen, Germany) equipped with filters to visualize green fluorescent protein and DAPI. Immunoreactivity of TRPM8 was detected as green fluorescence.
Western blotting
BEAS2B cells were harvested from 6-well plates, and total protein was extracted with RIPA buffer (T&I, Gangwon, Chuncheon, Korea) containing protease inhibitor cocktail. The Bradford Assay Kit (Bio-Rad, Hercules, CA, USA) was then used to determine the concentrations of each protein sample. For each sample, 20 μg of protein was loaded per well and separated by 8% or 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Separated proteins were then transferred to PVDF membranes (GE Healthcare Life Sciences, Buckinghamshire, UK) at 250 mA for 60 minutes. The membranes were probed with primary antibodies to TRPM8 (1:1,000) (Cat. No. ab109308) and beta actin (1:1,000) (Cat. No. 3700, cell signaling) overnight at 4°C with gentle shaking. After washing, the membrane was incubated with corresponding horseradish peroxidase (HRP)-conjugated secondary antibodies (1:2,000) (#7074; Cell Signaling Technology Inc., Danvers, MA, USA) for 1 hour. Immunoreactivity was visualized with a chemiluminescence substrate (iNtRON Biotechnology) using a western blot imaging system (Bio-Rad).
Participant selection
Asthma was diagnosed by physicians on the basis of the Global Initiative for Asthma guidelines.19 The diagnosis was supported by one or more of the following criteria: an increase in forced expiratory volume in 1 second (FEV1) > 12% and an increase of > 200 mL after inhalation of 400 mcg albuterol, a 20% reduction in FEV1 in response to a provocative concentration of inhaled methacholine (PC20) < 16 mg/mL, or an increase in FEV1 > 20% after 2 weeks of treatment with systemic or inhaled corticosteroids. The patients were recruited from a tertiary hospital. Exacerbation of asthma was defined as an episode of short-term treatment with systemic corticosteroids to manage increased asthmatic symptoms including dyspnea, cough, wheezing, or chest tightness with an FEV1< 80% of the patient's personal best. At the baseline visit, demographic information, including enrollment age, sex, body mass index, tobacco consumption, age of asthma onset, and asthma duration, were collected. All patients underwent a standardized assessment that included sputum analysis, complete blood cell counts with differential counts, serum total IgE levels, chest radiography, spirometry, and allergy skin prick tests with 24 common inhalant allergens (Bencard Co., Brentford, UK). Atopy was defined as a mean wheal diameter > 3 mm over that induced by the saline control in response to common inhalant allergens on skin prick tests. Informed written consent was obtained from each patient. For the mRNA study, sputum samples and clinical data of 37 patients used in this study were provided by the biobank of Soonchunhyang University Bucheon Hospital, a member of the Korean Biobank Network. Normal control subjects aged 20-55 years were recruited by advertisement; these participants answered negatively to a screening questionnaire regarding respiratory symptoms and other allergic diseases, and they had FEV1 values > 80% predicted and normal findings on simple chest radiographs.
Procedure of sputum induction and preparation
Sputum was induced using isotonic saline containing a short-acting bronchodilator. The samples were processed as previously described.20,21 Briefly, all samples with visibly greater solidity were carefully selected and placed in a preweighed Eppendorf tube to which 8 volumes of 0.05% dithiothreitol (Sputolysin; Calbiochem Corp., San Diego, CA, USA) in Dulbecco's PBS were added. Total cell counts were determined using a hemocytometer. Sputum cells were collected by cytocentrifugation, and 500 cells were examined after staining with Diff-Quick (American Scientific Products, Chicago, IL, USA). The remainder of the homogenized sputum sample was centrifuged at 1,000 ×g for 5 minutes; the supernatant was collected and stored at −70°C for subsequent protein analyses.
Quantitative measurement of TRPM-8 using enzyme-linked immunosorbent assay (ELISA)
TRPM8 was measured in duplicate using a sandwich ELISA kit (Cloud-Clone Corp., Katy, TX, USA) according to the manufacturer's instructions. The limit of detection was 0.082 ng/mL. Values below this level were scored as 0 ng/mL for statistical analysis.
Statistical analysis
All analyses were performed using GraphPad Prism 8 software (GraphPad Software Inc., San Diego, CA, USA) and IBM SPSS Statistics (IBM, Armonk, NY, USA). Data are presented as the means ± standard error of the means. A paired t test with a significance level of P < 0.05 was used. To compare the clinical parameters of the study groups, the Kruskal-Wallis, χ2, and Mann-Whitney U tests were used. Spearman's rank correlation coefficient was obtained to evaluate the correlation between TRPM8 mRNA and inflammatory cytokines. A P value of < 0.05 was regarded as significant.
Ethical issues
Informed consent forms were obtained from all study subjects including patients and healthy controls. The study protocol was approved by the Ethics Committee of Hallym University (HALLYM 2018-06-001-001). Clinical data and sputum samples for mRNA assessment were obtained from the biobank of Soonchunhyang University Hospital (SCHBC-BIOBANK-2018-013-01).
RESULTS
Identification of TRPM8 transcript and protein in airway epithelial cells
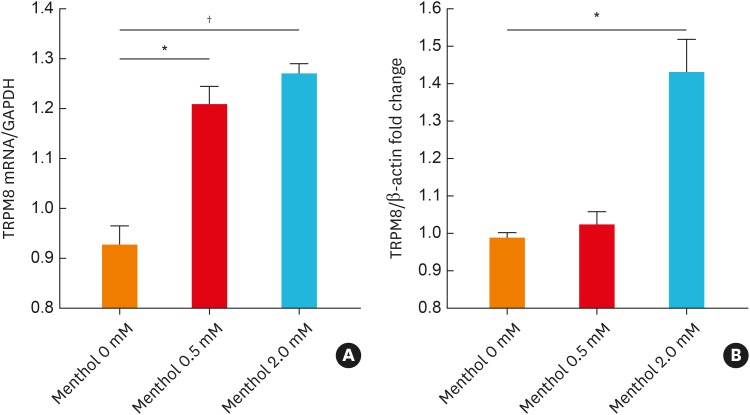
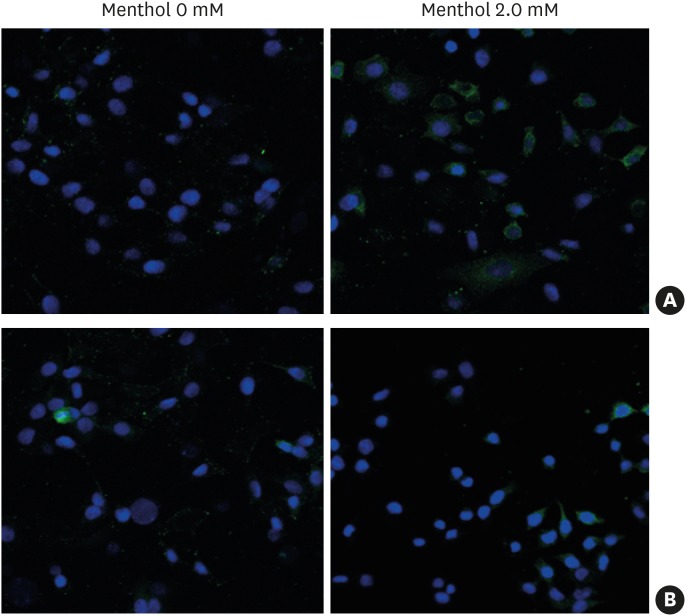
BEAS2B cells were used because they were found to express TRPM8 in our previous study.22 The cells were treated with various concentrations of menthol (0–2.0 mM), and both the mRNA and protein expression of TRPM8 robustly increased in a dose-dependent manner (Fig. 1). The results of ELISA to assess cell supernatants and cell lysates were similar to those of Western blotting (Supplementary Fig. S1). In addition, immunofluorescence staining showed that 2-mM menthol treatment induced an increase in green fluorescence mainly in the plasma membrane and endoplasmic reticulum. Pretreatment with 10 μmol BCTC (a TRPM8 antagonist) for 3 hours attenuated the menthol-induced increase in TRPM8 gene and protein expression (Fig. 2).
Fig. 1. Menthol-induced expression of TRPM8 mRNA and protein in BEAS2B cells. Cells were treated with menthol (0-2.0 mM) for 3 hours, and TRPM8 mRNA and protein levels were then measured using real-time polymerase chain reaction (A) and Western blotting (B), respectively. The data are presented as the means ± standard error of the means of 6 independent experiments.
TRPM8, transient receptor potential melastatin family member 8; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Footnote symbols indicate a statistically significant increases in mRNA and protein expression compared to those in 0-mM methanol-treated cells (paired t test; *P < 0.01; †P < 0.001).
Fig. 2. A representative image of immunofluorescence staining of TRPM8 protein in BEAS2B cells treated with menthol for 3 hours. TRPM8 was detected with Alexa-Fluor 488 (green) and nuclei were counterstained using DAPI (blue). Treatment with menthol (2.0 mM) for 3 hours led to increased green fluorescence in the cytoplasm and plasma membrane (A), which was attenuated by pretreatment with 10 μmol BCTC (B). Control, vehicle-treated cells.
TRPM8, transient receptor potential melastatin family member 8; DAPI, 4′,6-diamidino-2-phenylindole; BCTC, N-(4-tert-butylphenyl)-4-(3-chloropyridin-2-yl) piperazine-1-carboxamide.
TRPM8-mediated alteration in interleukin (IL)-25, IL-33, and thymic stromal lymphopoietin (TSLP) levels in BEAS2B cells
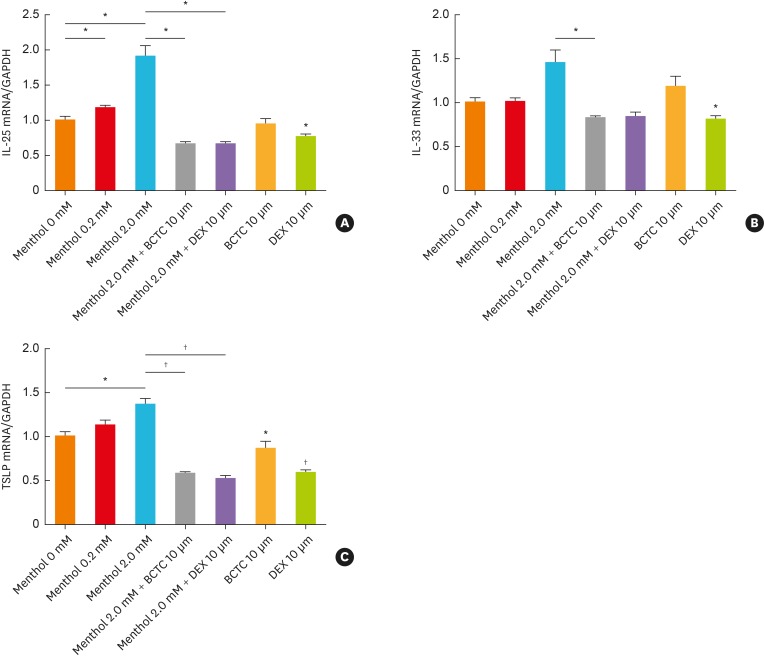
To determine whether TRPM8 is associated with epithelial airway inflammation, we measured IL-25, IL-33, and TSLP levels after menthol treatment to induce TRPM8 activation. As shown in Fig. 3, the mRNA expression of IL-25 and TSLP increased significantly with increasing menthol concentrations (P < 0.05). The mRNA expression of IL-33 tended to increase at the concentration of 2 mM menthol, but the difference was not statistically significant (P = 0.077). When we pretreated the cells with BCTC for 3 hours, the menthol-induced increases in the mRNA expression levels of IL-25, IL-33 and TSLP were reduced (P < 0.05 for IL-25 and IL-33, P < 0.01 for TSLP). The degree of decrement in each cytokine by BCTC was comparable to that with dexamethasone treatment (Fig. 3).
Fig. 3. TRPM8-mediated alterations in IL-25 (A), IL-33 (B), and TSLP (C) mRNA levels in BEAS2B cells. The menthol-induced increase in the expression of each cytokine was attenuated by pretreatment with 10 μmol BCTC and 10 μmol dexamethasone. The data are presented as the means ± standard error of the means of 6 independent experiments.
TRPM8, transient receptor potential melastatin family member 8; IL, interleukin; TSLP, thymic stromal lymphopoietin; BCTC, N-(4-tert-butylphenyl)-4-(3-chloropyridin-2-yl) piperazine-1-carboxamide.
Significant differences between untreated and 2-mM menthol-treated cells were analyzed by an unpaired t test (*P < 0.05; †P < 0.01).
Levels of TRPM8 mRNA and protein in the induced sputum of asthmatic patients
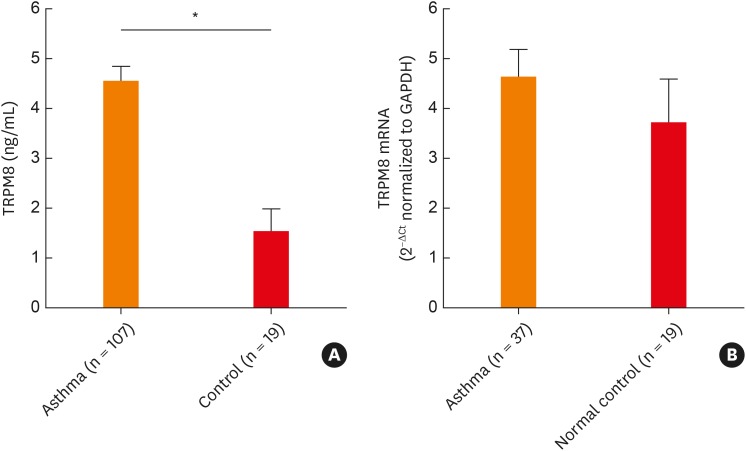
Based on the in vitro data showing the association between TRPM8 and epithelial-driven cytokines, we attempted to determine whether this in vitro finding would be reflected in patients with asthma. Protein levels of TRPM8 were significantly increased in the supernatant of the induced sputum from asthmatic patients compared with normal controls (Fig. 4). TRPM8 mRNA levels were quantitated in 37 asthmatic patients and 19 normal controls among the study subjects. The mRNA levels of TRPM8 tended to increase in the asthmatic patients compared to the controls, although the differences were not statistically significant (P = 0.1145).
Fig. 4. Comparison of TRPM8 protein levels in supernatants and mRNA levels in cell lysates of sputum from asthmatic patients and normal controls. TRPM8 protein (A) and mRNA expression (B) were determined by enzyme-linked immunosorbent assay and quantitative real-time polymerase chain reaction, respectively. The data are presented as the mean ± standard error of the mean of each group (Mann-Whitney U test).
TRPM8, transient receptor potential melastatin family member 8; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
*P < 0.001.
Correlation of TRPM8 levels with cytokines in the induced sputum of asthmatic patients and controls
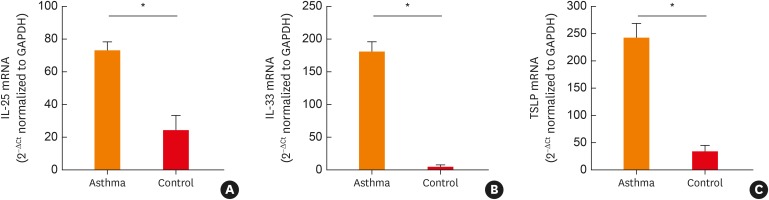
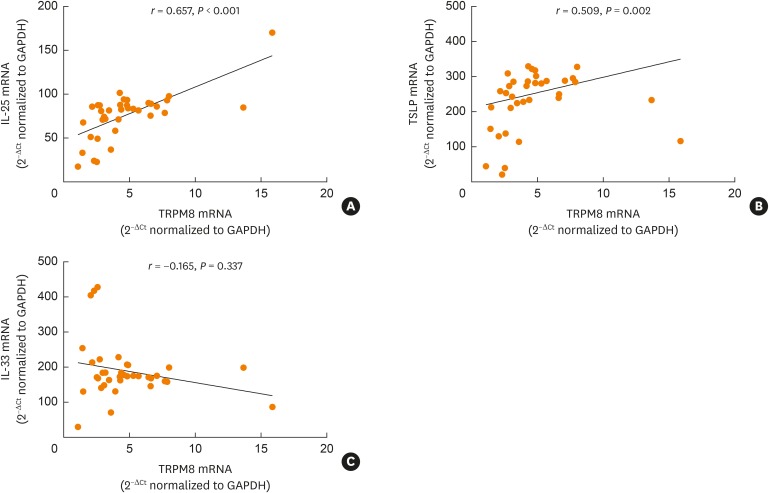
The levels of cytokines, including Th2-related cytokines (IL-4 and IL-13), Th1-related cytokines (IL-1β and tumor necrosis factor [TNF]-α), proinflammatory cytokines (IL-6 and IL-8), and epithelial-driven cytokines (IL-25, IL-33, and TSLP), were measured in the induced sputum of the 37 asthmatic patients and the 19 healthy controls. The levels of IL-4, IL-13, IL-25, IL-33, TSLP, and TNF-α mRNA were significantly increased in the asthmatic patients compared to the controls (Fig. 5 and Supplementary Fig. S2). The levels of IL-6 and IL-8 were comparable between the 2 groups; however, the IL-1β levels were higher in the controls than in the asthmatic patients. Moreover, we observed a significant correlation between TRPM8 and IL-25/TSLP in the induced sputum of patients with asthma (Fig. 6).
Fig. 5. Levels of IL-25 (A), IL-33 (B), and TSLP (C) in the induced sputum of asthmatic patients and normal controls. Levels of mRNA were determined using real-time polymerase chain reaction. The data are presented as the means ± standard error of the means (Mann-Whitney U test).
IL, interleukin; TSLP, thymic stromal lymphopoietin; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
*P < 0.001.
Fig. 6. Correlation of TRPM8 mRNA expression with IL-25 (A), TSLP (B), and IL-33 (C) expression in the sputum of asthmatics. The relationships between TRPM8 and cytokines were assessed by using Spearman rank correlation.
TRPM8, transient receptor potential melastatin family member 8; IL, interleukin; TSLP, thymic stromal lymphopoietin; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
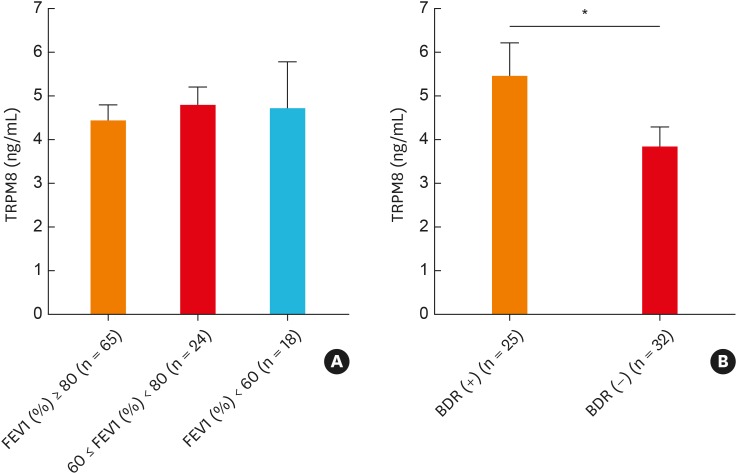
Association between clinical parameters and TRPM8 protein/mRNA expression
To determine the clinical significance of TRPM8 expression, we compared TRPM8 levels according to various clinical parameters. When we compared the TRPM8 level in patients with asthma according to the lung functional parameters FEV1 (%) and bronchodilator response (BDR), the mean value of TRPM8 was higher in patients with a positive BDR than in those with a negative BDR (Fig. 7 and Supplementary Fig. S3). However, the TRPM8 level did not differ according to lung function. The TRPM8 level was not associated with the PC20 value/sputum eosinophilia/atopy status in the study subjects. When we further analyzed the gene expression of TRPM8 and various cytokines according to sputum inflammatory patterns (eosinophilic [sputum eosinophil {EOS} ≥ 3%] vs. noneosinophilic [EOS < 3%]) in patients with asthma, there was no any significant difference in the expression, except for IL-8 gene expression (Supplementary Fig. S4).
Fig. 7. Comparison of TRPM8 protein levels in induced sputum according to the FEV1% predicted value (A) and the BDR (B). A positive BDR is defined as an increase of ≥12% and ≥200 mL in FEV1 15 minutes after inhalation of a short-acting bronchodilator. The data are presented as the mean ± standard error of the mean of each group (Mann-Whitney U test).
TRPM8, transient receptor potential melastatin family member 8; FEV1, forced expiratory volume in 1 second; BDR, bronchodilator response.
*P < 0.05.
DISCUSSION
In the present study, we showed that TRPM8 could significantly enhance the mRNA expression of epithelial-driven cytokines in BEAS2B cells, and that the activation or inhibition of TRPM8 accordingly increased or decreased the levels of these cytokine genes. Furthermore, we found that TRPM8 is expressed in the airways of patients with asthma, and that the degree of TRPM8 gene expression is associated with IL-25 and TSLP. These findings suggest that a cold receptor of TRPM8 is actively involved in the pathogenic role of the innate immune responses in asthmatic airways.
The TRP channels are known as molecular sensors in organisms that participate in the detection or transduction of thermal, chemical, and mechanical stimuli.8 The airway epithelium represents a key frontline defense against environmental exposure and various kinds of pathogenic stimuli, such as temperature, chemicals, and inhaled allergens, through several coordinated immune cells.23 As cold stimuli alone or combined with other factors can cause anatomical and functional alterations in the respiratory tract, we focused on the association between TRPM8 and airway epithelial cells in asthma, and in vitro findings were replicated in human samples. There has been accumulating evidence recently regarding the link between TRPM8 and airway inflammation.24 Sabnis et al.11 have demonstrated that the activation of the TRPM8 variant of bronchial epithelial cells by either cold or menthol regulates the expression of multiple cytokines and chemokines, such as IL-1, IL-4, IL-6, IL-8, IL-13, granulocyte-macrophage colony-stimulating factor, and TNF-α. Studies using a mouse model of asthma have reported that cold stimuli induce inflammatory gene expression, such as IL-6 and IL-8 through TRPM8, and that NF-κB is related to the signaling pathways that induce TRPM8 activation.25 In humans, TRPM8 was found to be elevated in the bronchial epithelium of patients with COPD and to regulate MUC5AC secretion in human bronchial epithelial cells.12 Similarly, upper airways show parallel findings of TRPM8-induced MUC5 gene expression in nasal epithelial cells.26 In our data, we found that TRPM8 strongly correlated with both Th2 cytokines, including IL-4 and IL-13, and epithelial-driven cytokines such as IL-25 and TSLP, in the sputum of patients with asthma, suggesting that cold stimuli activate TRPM8 in epithelial cells, which in turn may induce or perpetuate innate immune responses, contributing to type 2 airway inflammation in patients with asthma.
IL-25, IL-33 and TSLP are well-known epithelial-derived alarmins and can push inflammation toward a type 2 immune response in asthma.14 In this study, the gene expression of these cytokines was significantly increased in the sputum of patients with asthma compared to that of normal controls. Interestingly, an in vitro study showed that IL-25, IL-33 and TSLP were increased following TRPM8 activation, which was attenuated by a TRPM8 antagonist in airway epithelial cells. As TRPM8 is a cold-sensing receptor, we used menthol as an agonist in our study. However, literature reviews show that various stimuli can induce the activation of this receptor and subsequently produce proinflammatory cytokines. Calcium-rich particulate matter and smoking can activate TRPM8,27 and our previous study reported that toluene diisocyanate,22 which is a major cause of occupational asthma, also induces TRPM8 activation in airway epithelial cells. Moreover, human rhinovirus infection can cause up-regulation of TRPM8, which is dependent on viral replication.28 Considering these collective results, we speculated that rather than having a limited role as a cold-sensing receptor, the biological role of TRPM8 in the airways can be activated by various stimuli, including thermal and chemical stimuli as well as environmental viruses with subsequent involvement in the pathogenic mechanisms of type 2 airway inflammation through epithelial cells.
IL-25 is constitutively expressed in epithelial cells, and many immune cells, such as Th2 cells, activated mast cells, basophils, and EOSs, are the source of IL-25.29 When IL-25 is released, it can initiate eosinophilia and up-regulate type 2 cytokine production, mucus overproduction, and airway remodeling in asthma. Viral infection induces IL-25 production in airway epithelial cells in both mice and humans,30 and treatment of IL-25 with rhinovirus-infected airway epithelial cells induces apoptosis and generates reactive oxygen species (ROS), which are attenuated by anti-IL-25 treatment.31 IL-33 is an epithelial cell-derived cytokine, and its receptor (ST2) is expressed on both Th2 cells and ILC2s, inducing both innate and acquired type 2 inflammation in asthma.32 Rhinovirus-infected epithelial cells produce IL-33, which is mediated by oxidative stress in airway epithelial cells during viral infections.33 In addition, numerous studies have demonstrated that several TRP channels are regulated by oxidative stress and that calcium influx through TRP channels may be one mechanism by which oxidative stress mediates cell damage and physiological alterations.34 In particular, oxidative stress can result in up-regulation of TRPM8 in urothelium, and application of a TRPM8 antagonist has been significantly shown to attenuate ROS-induced calcium responses.35 These findings suggest that oxidative stress is one common pathway to link TRPM8- and epithelial-driven cytokines in airway epithelium. TSLP is produced mainly in epithelial cells and acts on immune cells, such as T cells, B cells, dendritic cells, and mast cells, by activating a heteromeric receptor complex composed of the TSLP receptor chain and the IL-7 receptor α chain, leading to the release of inflammatory cytokines. In an atopic dermatitis mouse model, TSLP activates neuronal TRPA1 downstream of the TSLP receptor, suggesting a link between TSLP and TRP channels as well.36 However, further in vitro and in vivo studies are required to determine the association between TSLP, IL-25, IL-33, and TRPM8 in the context of epithelial inflammation in the airways.
In this study, we found that TRPM8 levels increased in patients with asthma compared to controls and correlated with the gene expression of IL-25 and TSLP. As cold stimuli are a common triggering factor of asthma exacerbation and viral infection can induce IL-25 and IL-33 in epithelial cells during asthma exacerbation, we aimed to determine the clinical significance of TRPM8 in our subjects. When we compared TRPM8 protein levels according to asthma status—exacerbated vs. stable states—and lung function severity, we did not find any difference between the 2 groups. Instead, the TRPM8 levels were higher in BDR-positive patients than in BDR-negative patients. Previous studies have demonstrated that BDR-positive patients have more impaired lung function than BDR-negative patients and the loss of asthma control. Furthermore, a persistent BDR despite anti-inflammatory treatment has been associated with greater inhaled corticosteroid doses, lower FEV1, worse asthma control, and higher exacerbation rates.37,38 Therefore, we postulated that TRPM8 is a candidate molecular marker associated with the physiologic response and poor outcomes in asthmatic patients.
Previous studies using an animal model of asthma showed that a TRPM8 agonist inhibited contractions elicited by methacholine in airway smooth muscle39 and proliferation of airway smooth muscle cells,40 which conflicts with our patient data. In addition to airway smooth muscle cells, TRPM8 is expressed in the subpopulation of vagus nerves of the airways. The activation of TRPM8 by cold excites these airway autonomic nerves and subsequently provokes an autonomic nerve reflex to increase airway resistance.41 TRPM8 is expressed in human mast cell lines, and histamine is released by menthol; however, this phenomenon was reversed by a TRPM8 blocker.42 Taken together, these observations indicate that the biological effects of TRPM8 seem to have different dependences on their expressed location. Considering that airway inflammation involves dynamic interplays between different cell systems, such as immune cells, structural cells (e.g., epithelial and smooth muscle cells) and neurons, the exact role of TRPM8 in these interactions and the clinical relevance of TRPM8 require further clarification in both in vitro studies and in vivo studies using a knockout mouse model.
When we measured inflammatory cytokine gene expression in the induced sputum, the expression levels of Th2 cell- and epithelial cell-driven cytokines were higher in asthmatic patients than in healthy controls (Fig. 5 and Supplementary Fig. S2). However, the IL-6 and IL-8 transcript levels did not differ between the 2 groups. As IL-6 and IL-8 are typical proinflammatory cytokines in the airways, their levels should be increased in asthmatic patients. Furthermore, there were no significant relationships between the TRPM8 transcript level and IL-6/IL-8. Previous studies showed that IL-8 mRNA expression was increased in the sputum of asthmatic patients43 and that IL-8 protein expression was correlated with sputum neutrophil counts.44 As IL-1β and TNF-α are also well-known soluble mediators associated with neutrophilic airway inflammation,45 our data are inconsistent with those of previous reports. These discrepancies between data may be explained by the small number of subjects used to examine gene expression and the characteristics of asthma heterogeneity. RNA analysis data were available in only 37 subjects in this study. Furthermore, as shown in Table 1, approximately 70% of the subjects are atopic and have a mean FEV1 of 83%, suggesting that most of them have mild to moderate asthma. However, when we further analyzed differences in cytokine gene expression between subjects with sputum eosinophilia and noneosinophilia, we found a significant increase in IL-8 gene expression in patients with the noneosinophilic phenotype (Supplementary Fig. S4) compared to those with sputum eosinophilia. The gene expression of IL-6, IL-1β, and TNF-α tended to be higher in patients with the noneosinophilic phenotype than in those with the eosinophilic phenotype. Because IL-6, IL-8, IL-1β, and TNF-α are related to neutrophilic inflammation and steroid-refractory severe asthma phenotypes, our study results should be replicated in large cohorts with different phenotypes and inflammatory markers and controls.
Table 1. Baseline characteristics of the study subjects.
| Characteristics | Subjects with asthma | Normal controls | |
|---|---|---|---|
| Number | 107 | 19 | |
| Age (yr) | 53.32 ± 17.13 | 38.16 ± 9.65 | |
| Sex, M:F (% of female) | 46/61 (57.0) | 8:11 (57.9) | |
| NS/ES | 72/35 | 19/0 | |
| Duration of asthma (mon) | 44.96 ± 49.40 | - | |
| FEV1 (% predicted) | 85.19 ± 24.09 | - | |
| FVC (% predicted) | 91.80 ± 15.49 | - | |
| Bronchodilator response (mL) | 235.86 ± 220.27 | - | |
| Bronchodilator response (%) | 10.97 ± 10.40 | - | |
| FEV1/FVC ratio | 71.26 ± 12.04 | - | |
| Methacholine PC20 (mg/mL) | 6.08 ± 6.25 | - | |
| IgE (IU/mL) | 453.25 ± 559.72 | - | |
| Blood eosinophils (%) | 5.67 ± 6.48 | - | |
| Sputum eosinophils (%) | 15.85 ± 23.95 | - | |
| Atopy | 51/73 (69.9) | - | |
| Medication | - | ||
| SABA monotherapy | 6 (5.6) | ||
| LTRA monotherapy | 6 (5.6) | ||
| ICS* | 14 (13.1) | ||
| ICS + LABA* | 75 (70.1) | ||
| ICS + LABA + LAMA* | 6 (5.6) | ||
Values are presented as mean ± standard deviation or number (%).Ex-smokers with fewer than 10 pack-years were included.
FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; ICS, inhaled corticosteroid; IgG, immunoglobulin G; LABA, long-acting beta-agonist; LAMA, long-acting muscarinic antagonist; LTRA, leukotriene receptor antagonist; NS, nonsmoker; ES, ex-smoker; SABA, short-acting beta-2 agonist.
*Patients receiving ICS monotherapy or ICS + LABA ± LAMA combination therapy may have taken both drugs in free combination, or in combination with other controller medications such as methylxanthines or LTRA.
In conclusion, our results demonstrate that the activation of the TRPM8 channel can lead to a significant increase in the epithelial-driven gene expression of IL-25, IL-33, and TSLP in bronchial epithelial cells, and that this increase can be attenuated with a TRPM8 antagonist. The protein and gene expression of TRPM8 were significantly higher in the sputum of patients with asthma compared with that of normal controls. In addition, TRPM8 gene expression strongly correlated with IL-25 and TSLP in patients with asthma. These findings suggest that TRPM8 can be involved in the initiation and perpetuation of airway epithelial immune responses in asthma pathogenesis, and that TRPM8 may be a molecular candidate for treating asthma. However, further experiments will be needed to determine the exact role of the pathogenic mechanism and clinical implication of TRPM8 in chronic airway diseases.
ACKNOWLEDGMENTS
The biospecimens and data used for this study were provided by the biobank of Soonchunhyang University Bucheon Hospital, a member of the Korea Biobank Network. This research was supported by a grant from the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (NFR-2017R1C1B5076565) and a grant from the Hallym University Medical Center Research Fund (01-2012-12).
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
SUPPLEMENTARY MATERIALS
Correlation between TRPM8 gene expression and inflammatory cytokine expression in the sputum of asthmatic patients
Menthol-induced expression of the TRPM8 protein was determined by ELISA using cell supernatants and lysates. Cells were treated with menthol (0-2.0 mM) for 3 hours. The data are presented as the means ± standard error of the means of 3 independent experiments.
Comparison of the inflammatory cytokine gene expression in induced sputum between asthmatic patients (n = 37) and healthy controls (n = 19). Mann-Whitney U test.
Comparison of TRPM8 protein levels in induced sputum according to the methacholine PC20 (A), sputum eosinophil % (B), and atopy status (C) in asthmatic patients. Atopy is defined by a positive skin prick test in response to 1 or more common inhalant allergens. The data are presented as the mean ± standard error of the mean of each group.
Comparison of inflammatory cytokine and TRPM8 gene expression in the sputum of patients with eosinophilic (n = 10) and non-eosinophilic (n = 22) asthma. Sputum eosinophilia was defined as ≥3% eosinophils. Mann-Whitney U test.
References
- 1.D'Amato M, Molino A, Calabrese G, Cecchi L, Annesi-Maesano I, D'Amato G. The impact of cold on the respiratory tract and its consequences to respiratory health. Clin Transl Allergy. 2018;8:20. doi: 10.1186/s13601-018-0208-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giesbrecht GG, Younes M. Exercise- and cold-induced asthma. Can J Appl Physiol. 1995;20:300–314. doi: 10.1139/h95-023. [DOI] [PubMed] [Google Scholar]
- 3.Hyrkäs H, Ikäheimo TM, Jaakkola JJ, Jaakkola MS. Asthma control and cold weather-related respiratory symptoms. Respir Med. 2016;113:1–7. doi: 10.1016/j.rmed.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Donaldson GC, Goldring JJ, Wedzicha JA. Influence of season on exacerbation characteristics in patients with COPD. Chest. 2012;141:94–100. doi: 10.1378/chest.11-0281. [DOI] [PubMed] [Google Scholar]
- 5.Hyrkäs-Palmu H, Ikäheimo TM, Laatikainen T, Jousilahti P, Jaakkola MS, Jaakkola JJ. Cold weather increases respiratory symptoms and functional disability especially among patients with asthma and allergic rhinitis. Sci Rep. 2018;8:10131. doi: 10.1038/s41598-018-28466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim CK, Callaway Z, Gern JE. Viral infections and associated factors that promote acute exacerbations of asthma. Allergy Asthma Immunol Res. 2018;10:12–17. doi: 10.4168/aair.2018.10.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castillo JR, Peters SP, Busse WW. Asthma exacerbations: pathogenesis, prevention, and treatment. J Allergy Clin Immunol Pract. 2017;5:918–927. doi: 10.1016/j.jaip.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moran MM, McAlexander MA, Bíró T, Szallasi A. Transient receptor potential channels as therapeutic targets. Nat Rev Drug Discov. 2011;10:601–620. doi: 10.1038/nrd3456. [DOI] [PubMed] [Google Scholar]
- 9.Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, et al. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- 10.Sabnis AS, Shadid M, Yost GS, Reilly CA. Human lung epithelial cells express a functional cold-sensing TRPM8 variant. Am J Respir Cell Mol Biol. 2008;39:466–474. doi: 10.1165/rcmb.2007-0440OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sabnis AS, Reilly CA, Veranth JM, Yost GS. Increased transcription of cytokine genes in human lung epithelial cells through activation of a TRPM8 variant by cold temperatures. Am J Physiol Lung Cell Mol Physiol. 2008;295:L194–200. doi: 10.1152/ajplung.00072.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li M, Li Q, Yang G, Kolosov VP, Perelman JM, Zhou XD. Cold temperature induces mucin hypersecretion from normal human bronchial epithelial cells in vitro through a transient receptor potential melastatin 8 (TRPM8)-mediated mechanism. J Allergy Clin Immunol. 2011;128:626–634.e1-5. doi: 10.1016/j.jaci.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 13.Whitsett JA, Alenghat T. Respiratory epithelial cells orchestrate pulmonary innate immunity. Nat Immunol. 2015;16:27–35. doi: 10.1038/ni.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitchell PD, O'Byrne PM. Epithelial-derived cytokines in asthma. Chest. 2017;151:1338–1344. doi: 10.1016/j.chest.2016.10.042. [DOI] [PubMed] [Google Scholar]
- 15.Bougault V, Turmel J, St-Laurent J, Bertrand M, Boulet LP. Asthma, airway inflammation and epithelial damage in swimmers and cold-air athletes. Eur Respir J. 2009;33:740–746. doi: 10.1183/09031936.00117708. [DOI] [PubMed] [Google Scholar]
- 16.Karjalainen EM, Laitinen A, Sue-Chu M, Altraja A, Bjermer L, Laitinen LA. Evidence of airway inflammation and remodeling in ski athletes with and without bronchial hyperresponsiveness to methacholine. Am J Respir Crit Care Med. 2000;161:2086–2091. doi: 10.1164/ajrccm.161.6.9907025. [DOI] [PubMed] [Google Scholar]
- 17.Davis MS, Malayer JR, Vandeventer L, Royer CM, McKenzie EC, Williamson KK. Cold weather exercise and airway cytokine expression. J Appl Physiol (1985) 2005;98:2132–2136. doi: 10.1152/japplphysiol.01218.2004. [DOI] [PubMed] [Google Scholar]
- 18.Parenti A, De Logu F, Geppetti P, Benemei S. What is the evidence for the role of TRP channels in inflammatory and immune cells? Br J Pharmacol. 2016;173:953–969. doi: 10.1111/bph.13392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald JM, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31:143–178. doi: 10.1183/09031936.00138707. [DOI] [PubMed] [Google Scholar]
- 20.Park SW, Lee YM, Jang AS, Lee JH, Hwangbo Y, Kim DJ, et al. Development of chronic airway obstruction in patients with eosinophilic bronchitis: a prospective follow-up study. Chest. 2004;125:1998–2004. doi: 10.1378/chest.125.6.1998. [DOI] [PubMed] [Google Scholar]
- 21.Kwon JW, Chang HS, Heo JS, Bae DJ, Lee JU, Jung CA, et al. Characteristics of asthmatics with detectable IL-32γ in induced sputum. Respir Med. 2017;129:85–90. doi: 10.1016/j.rmed.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Kim JH, Jang YS, Jang SH, Jung KS, Kim SH, Ye YM, et al. Toluene diisocyanate exposure induces airway inflammation of bronchial epithelial cells via the activation of transient receptor potential melastatin 8. Exp Mol Med. 2017;49:e299. doi: 10.1038/emm.2016.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gohy ST, Hupin C, Pilette C, Ladjemi MZ. Chronic inflammatory airway diseases: the central role of the epithelium revisited. Clin Exp Allergy. 2016;46:529–542. doi: 10.1111/cea.12712. [DOI] [PubMed] [Google Scholar]
- 24.Grace MS, Baxter M, Dubuis E, Birrell MA, Belvisi MG. Transient receptor potential (TRP) channels in the airway: role in airway disease. Br J Pharmacol. 2014;171:2593–2607. doi: 10.1111/bph.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu H, Hua L, Liu Q, Pan J, Bao Y. Cold stimuli facilitate inflammatory responses through transient receptor potential melastatin 8 (TRPM8) in primary airway epithelial cells of asthmatic mice. Inflammation. 2018;41:1266–1275. doi: 10.1007/s10753-018-0774-y. [DOI] [PubMed] [Google Scholar]
- 26.Liu SC, Lu HH, Fan HC, Wang HW, Chen HK, Lee FP, et al. The identification of the TRPM8 channel on primary culture of human nasal epithelial cells and its response to cooling. Medicine (Baltimore) 2017;96:e7640. doi: 10.1097/MD.0000000000007640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamb JG, Romero EG, Lu Z, Marcus SK, Peterson HC, Veranth JM, et al. Activation of human transient receptor potential melastatin-8 (TRPM8) by calcium-rich particulate materials and effects on human lung cells. Mol Pharmacol. 2017;92:653–664. doi: 10.1124/mol.117.109959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abdullah H, Heaney LG, Cosby SL, McGarvey LP. Rhinovirus upregulates transient receptor potential channels in a human neuronal cell line: implications for respiratory virus-induced cough reflex sensitivity. Thorax. 2014;69:46–54. doi: 10.1136/thoraxjnl-2013-203894. [DOI] [PubMed] [Google Scholar]
- 29.Yao X, Sun Y, Wang W, Sun Y. Interleukin (IL)-25: pleiotropic roles in asthma. Respirology. 2016;21:638–647. doi: 10.1111/resp.12707. [DOI] [PubMed] [Google Scholar]
- 30.Beale J, Jayaraman A, Jackson DJ, Macintyre JD, Edwards MR, Walton RP, et al. Rhinovirus-induced IL-25 in asthma exacerbation drives type 2 immunity and allergic pulmonary inflammation. Sci Transl Med. 2014;6:256ra134. doi: 10.1126/scitranslmed.3009124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuan X, Wang E, Xiao X, Wang J, Yang X, Yang P, et al. The role of IL-25 in the reduction of oxidative stress and the apoptosis of airway epithelial cells with specific immunotherapy in an asthma mouse model. Am J Transl Res. 2017;9:4137–4148. [PMC free article] [PubMed] [Google Scholar]
- 32.Makrinioti H, Toussaint M, Jackson DJ, Walton RP, Johnston SL. Role of interleukin 33 in respiratory allergy and asthma. Lancet Respir Med. 2014;2:226–237. doi: 10.1016/S2213-2600(13)70261-3. [DOI] [PubMed] [Google Scholar]
- 33.Aizawa H, Koarai A, Shishikura Y, Yanagisawa S, Yamaya M, Sugiura H, et al. Oxidative stress enhances the expression of IL-33 in human airway epithelial cells. Respir Res. 2018;19:52. doi: 10.1186/s12931-018-0752-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller BA. The role of TRP channels in oxidative stress-induced cell death. J Membr Biol. 2006;209:31–41. doi: 10.1007/s00232-005-0839-3. [DOI] [PubMed] [Google Scholar]
- 35.Nocchi L, Daly DM, Chapple C, Grundy D. Induction of oxidative stress causes functional alterations in mouse urothelium via a TRPM8-mediated mechanism: implications for aging. Aging Cell. 2014;13:540–550. doi: 10.1111/acel.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson SR, Thé L, Batia LM, Beattie K, Katibah GE, McClain SP, et al. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell. 2013;155:285–295. doi: 10.1016/j.cell.2013.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heffler E, Crimi C, Campisi R, Sichili S, Nicolosi G, Porto M, et al. Bronchodilator response as a marker of poor asthma control. Respir Med. 2016;112:45–50. doi: 10.1016/j.rmed.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 38.Ulrik CS, Frederiksen J. Mortality and markers of risk of asthma death among 1,075 outpatients with asthma. Chest. 1995;108:10–15. doi: 10.1378/chest.108.1.10. [DOI] [PubMed] [Google Scholar]
- 39.Ito S, Kume H, Shiraki A, Kondo M, Makino Y, Kamiya K, et al. Inhibition by the cold receptor agonists menthol and icilin of airway smooth muscle contraction. Pulm Pharmacol Ther. 2008;21:812–817. doi: 10.1016/j.pupt.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 40.Zhang L, An X, Wang Q, He M. Activation of cold-sensitive channels TRPM8 and TRPA1 inhibits the proliferative airway smooth muscle cell phenotype. Lung. 2016;194:595–603. doi: 10.1007/s00408-016-9901-4. [DOI] [PubMed] [Google Scholar]
- 41.Xing H, Ling JX, Chen M, Johnson RD, Tominaga M, Wang CY, et al. TRPM8 mechanism of autonomic nerve response to cold in respiratory airway. Mol Pain. 2008;4:22. doi: 10.1186/1744-8069-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cho Y, Jang Y, Yang YD, Lee CH, Lee Y, Oh U. TRPM8 mediates cold and menthol allergies associated with mast cell activation. Cell Calcium. 2010;48:202–208. doi: 10.1016/j.ceca.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 43.Bullens DM, Truyen E, Coteur L, Dilissen E, Hellings PW, Dupont LJ, et al. IL-17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx? Respir Res. 2006;7:135. doi: 10.1186/1465-9921-7-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gibson PG, Simpson JL, Saltos N. Heterogeneity of airway inflammation in persistent asthma : evidence of neutrophilic inflammation and increased sputum interleukin-8. Chest. 2001;119:1329–1336. doi: 10.1378/chest.119.5.1329. [DOI] [PubMed] [Google Scholar]
- 45.Chang HS, Lee TH, Jun JA, Baek AR, Park JS, Koo SM, et al. Neutrophilic inflammation in asthma: mechanisms and therapeutic considerations. Expert Rev Respir Med. 2017;11:29–40. doi: 10.1080/17476348.2017.1268919. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Correlation between TRPM8 gene expression and inflammatory cytokine expression in the sputum of asthmatic patients
Menthol-induced expression of the TRPM8 protein was determined by ELISA using cell supernatants and lysates. Cells were treated with menthol (0-2.0 mM) for 3 hours. The data are presented as the means ± standard error of the means of 3 independent experiments.
Comparison of the inflammatory cytokine gene expression in induced sputum between asthmatic patients (n = 37) and healthy controls (n = 19). Mann-Whitney U test.
Comparison of TRPM8 protein levels in induced sputum according to the methacholine PC20 (A), sputum eosinophil % (B), and atopy status (C) in asthmatic patients. Atopy is defined by a positive skin prick test in response to 1 or more common inhalant allergens. The data are presented as the mean ± standard error of the mean of each group.
Comparison of inflammatory cytokine and TRPM8 gene expression in the sputum of patients with eosinophilic (n = 10) and non-eosinophilic (n = 22) asthma. Sputum eosinophilia was defined as ≥3% eosinophils. Mann-Whitney U test.