Abstract
Purpose
Recently, there has been a rise in the interest to understand the composition of indoor dust due to its association with lung diseases such as asthma, chronic obstructive pulmonary disease (COPD) and lung cancer. Furthermore, it has been found that bacterial extracellular vesicles (EVs) within indoor dust particles can induce pulmonary inflammation, suggesting that these might play a role in lung disease.
Methods
We performed microbiome analysis of indoor dust EVs isolated from mattresses in apartments and hospitals. We developed diagnostic models based on the bacterial EVs antibodies detected in serum samples via enzyme-linked immunosorbent assay (ELISA) in this analysis.
Results
Proteobacteria was the most abundant bacterial EV taxa observed at the phylum level while Pseudomonas, Enterobacteriaceae (f) and Acinetobacter were the most prominent organisms at the genus level, followed by Staphylococcus. Based on the microbiome analysis, serum anti-bacterial EV immunoglobulin G (IgG), IgG1 and IgG4 were analyzed using ELISA with EV antibodies that targeted Staphylococcus aureus, Acinetobacter baumannii, Enterobacter cloacae and Pseudomonas aeruginosa. The levels of anti-bacterial EV antibodies were found to be significantly higher in patients with asthma, COPD and lung cancer compared to the healthy control group. We then developed a diagnostic model through logistic regression of antibodies that showed significant differences between groups with smoking history as a covariate. Four different variable selection methods were compared to construct an optimal diagnostic model with area under the curves ranging from 0.72 to 0.81.
Conclusions
The results of this study suggest that ELISA-based analysis of anti-bacterial EV antibodies titers can be used as a diagnostic tool for lung disease. The present findings provide insights into the pathogenesis of lung disease as well as a foundation for developing a novel diagnostic methodology that synergizes microbial EV metagenomics and immune assays.
Keywords: Extracellular vesicles; dust; diagnosis; asthma; chronic obstructive pulmonary disease; lung neoplasms, microbiome; bacteria
INTRODUCTION
Due to the importance of the indoor environment and its overall effect on health, interest regarding indoor environments has been steadily rising.1,2 At present, most people spend approximately 87% of their time indoors.3 Indoor dust has particularly significant effects on health as it includes PM10, PM2.5, nanoparticles, bioaerosol and house dust mites. The biological components of indoor dust can induce immune dysfunction and inflammation, leading to inflammatory pulmonary disorders such as asthma and chronic obstructive pulmonary disease (COPD).4,5,6 Asthma, COPD and lung cancer are major lung diseases that cause widespread mortality worldwide.7,8 While cigarette smoking has been suggested to be a common cause of COPD and lung cancer, many patients suffering from these conditions have no history of cigarette smoking. In such cases, it is necessary to view COPD and lung cancer as diseases with complex etiology that may have been caused by factors other than cigarette smoke. These other factors include exposure to gas, dust, indoor biomass fuel combustion or other unknown etiological agents.9
The rise in the number of asthma and COPD patients during the past several decades may be associated with changes in housing styles that have led to increasing amounts of indoor biological contaminants.10,11,12 Although there is limited research on this subject, there is a possibility that the biological particles found in indoor dust can act as etiological agents for lung disease. Indoor dust is known to contain extracellular vesicles (EVs) derived from microorganisms. Bacteria-derived EVs are spherical, lipid-bilayered vesicles with diameters ranging from 20 to 100 nm, produced by both gram-negative and gram-positive bacteria and are common biological ultrafine particles found in the indoor environment.13,14 In vivo testing has shown that airway exposure to bacteria-derived EVs in indoor dust can induce neutrophilic pulmonary inflammation15 and subsequently emphysema in the lungs.16,17 As asthma and COPD are characterized by chronic inflammation of the airways18 and carcinogenesis has been broadly associated with chronic inflammation,19 we hypothesized that exposure to bacterial EVs in indoor dust might be associated with the risk of asthma, COPD and lung cancer.20 Therefore, we performed microbiome analysis of indoor dust microbiota and EVs in order to develop diagnostic models using serum antibodies against microbial EVs dominant in indoor dust.
MATERIALS AND METHODS
Clinical study design
In this study, the subjects were enrolled into 4 groups: asthmatics, COPD patients, lung cancer patients and healthy controls. We enrolled 239 asthmatics, 205 COPD patients and 88 healthy control from Asan Medical Center and 324 lung cancer patients from Dankook University Hospital. The healthy subjects were recruited from the patients who visited the hospital for routine checkups. After the checkup, we selected healthy normal persons with no current pulmonary disease diagnosis and no history of pulmonary disease. We collected the patients' samples regardless of their smoking history. Subjects who lacked sufficient clinical data to meet the inclusion criteria were excluded from the analysis. This study was approved by the Institutional Review Board (IRB) of Asan Medical Center (IRB No. 2014-0360) and of Dankook University Hospital (IRB No. 2012-04-0140). We obtained informed consent from each study participant.
Indoor dust sampling
Dust samples were collected from a mattress from an apartment and 2 hospital mattresses using a vacuum cleaner in Seoul. The sampling was performed in March, June, September and December at the apartment and in July and February at the hospital.
Dust EV isolation
The indoor dust samples mixed in phosphate-buffered saline (PBS) were incubated for 12 hours at 4°C, filtered using gauze and centrifuged at 10,000 g for 15 minutes twice. The pellet comprised of bacterial cells, whereas the supernatant contained the EVs, which was filtered using a 0.45-μm pore-sized vacuum filter and concentrated through ultrafiltration using the QuixStand Benchtop System with a 100-kDa hollow fiber membrane (Amersham Biosciences, Piscataway, NJ, USA). Additional filtration was done using a 0.22-μm vacuum filter to remove any remaining foreign particles and cells. Finally, the EVs were isolated by centrifugation using a 45 Ti rotor (Beckman Instruments, Fullerton, CA, USA) at 150,000 g for 3 hours at 4°C and were diluted in PBS.21,22
DNA extraction and 16S ribosomal DNA (rDNA) amplicon sequencing
DNA samples from the dust microbiota and dust microbial EVs were extracted using a DNeasy PowerSoil Kit (QIAGEN, Hilden, Germany) and then quantified using QIAxpert (QIAGEN). Microbial genomic DNA was amplified using primers specific for the V3-V4 regions of the 16S rDNA. After the libraries were prepared and quantified using QIAxpert (QIAGEN), each amplicon was sequenced with MiSeq (Illumina, SanDiego, CA, USA).
Microbiome analysis of dust microbiota and EVs
Raw reads were filtered using the barcode and primer sequences. High-quality reads that met the following criteria were selected for further analysis: Phred scores higher than 20 and lengths greater than 300 bp. Operational taxonomic units (OTUs) were then clustered using CD-HIT algorithm. Subsequently, based on sequence similarities, taxonomic assignment was performed using UCLUST and QIIME against the Greengenes (ver. 13_8) 16S rDNA sequence database. In the cases where the clusters could not be assigned due to either lack or redundancy in the sequences from the database, the taxa were assigned at the next highest level as denoted by the parentheses next to the taxonomic name.
Enzyme-linked immunosorbent assay (ELISA)
To measure the titers of anti-bacterial EV immunoglobulin G (IgG), IgG1 and IgG4 in serum samples, bacterial EVs were extracted. Staphylococcus aureus, Acinetobacter baumannii, Enterobacter cloacae and Pseudomonas aeruginosa were cultured. When the optical density at 600 nm of the culture reached 1, the bacterial sample was pelleted at 10,000 × g for 20 minutes, and the supernatant was passed through a 0.22 μm bottle top filter to remove any remaining cells. The filtrate was concentrated with a MasterFlex pump system (Cole-Parmer, Vernon Hills, IL, USA) using a 100-kDa Pellicon two Cassette filter membrane (Merck Millipore, Burlington, MA, USA) and subsequently passed through a 0.22-μm bottle top filter. Further, 50 ng of the bacterial EVs isolated from the cultured bacteria were used for coating 96-well plates overnight. IgG1 and IgG4 titers, anti-human IgG, IgG1 and IgG4 antibodies were used for coating instead of bacterial EVs to quantify anti-bacterial EV IgG. The bacterial EV-coated wells were blocked with PBS containing 5% skimmed milk and diluted serum samples were added to these wells. Then, 3,3′,5,5′-Tetramethylbenzidine (TMB) solution was added and after incubation, the reaction was catalyzed by horseradish peroxidase-conjugated anti-IgG, anti-IgG1 and anti-IgG4 antibodies (Abcam, Cambridge, UK). The reaction was stopped using a stop solution and the intensity was measured using a microplate reader at 450 nm. Sensitivity of the bacterial EVs to IgG, IgG1 and IgG4 was defined on an arbitrary basis as a high anti-bacterial EV IgG, IgG1 and IgG4 titer in serum that exceeded the 95th percentile value of the control subjects. In addition, to assess the sensitivity of the analysis method, the limit of detection (LOD) and limit of quantitation (LOQ) were evaluated. The LOD of IgG, IgG1 and IgG4 was 0.011 µg/mL, 0.008 µg/mL and 0.006 µg/mL, respectively. Meanwhile, the LOQ of IgG, IgG1 and IgG4 was 0.027 µg/mL, 0.02 µg/mL and 0.015 µg/mL, respectively.
Statistical analysis
The group values are presented based on the mean and standard deviation calculated for all the subjects within each group. Significant differences between the bacteria and bacterial EVs in indoor dust were tested using the Mann-Whitney U test for continuous variables. In addition, Pearson correlation analysis was performed between samples. Student's t-test was used to compare the serum antibody levels. To develop diagnostic models, logistic regression was applied using biomarkers with significant differences between the clinical groups and included smoking history as a covariate. P values below 0.05 were considered statistically significant. All statistical analyses were performed using R version 3.5.1.
RESULTS
Microbe and microbial EV composition of indoor dust
Metagenomic sequence analysis of 16S rDNA gene amplicons yielded a total of 85,902 valid reads from the bacterial samples (approximately 8,242 and 13,234 reads from the apartment and hospital samples, respectively) and 74,936 valid reads in bacterial EV samples (approximately 8,681 and 10,053 reads from the apartment and hospitals, respectively) (Supplementary Table S1).
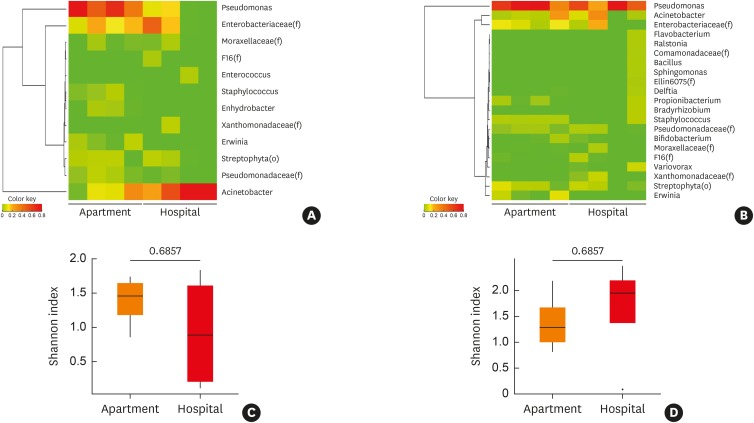
Microbiome analysis of the microbiota and microbial EVs found within indoor dust samples collected from the apartments and hospitals revealed that Proteobacteria was the most abundant taxa at the phylum level with an average abundance of 92.4% (±5.3%) and lowest abundance of 81.2% (Supplementary Fig. S1A). The predominant taxa detected in indoor dust were Gammaproteobacteria at the class level (Supplementary Fig. S1B), Pseudomonadales and Enterobacteriales at the order level (Supplementary Fig. S1C), along with Pseudomonadaceae, Moraxellaceae and Enterobacteriaceae at the family level (Supplementary Fig. S1D). Pseudomonas, Enterobacteriaceae (f) and Acinetobacter were the most abundant taxa at the genus level. Pseudomonas and Acinetobacter were the most abundant in the apartment and hospitals, respectively. In addition, there was a significant difference in the abundance of Pseudomonas, Erwinia, Staphylococcus and Enhydrobacter between the apartment and hospital dust samples (P < 0.05) (over 0.5% in any group) (Fig. 1A, Supplementary Table S1). However, in the case of microbial EVs, Pseudomonas EVs were the most abundant organisms in both the hospital and apartment, while there was no significant difference between bacterial EV taxa (over 0.5% in any group) (Fig. 1B, Supplementary Table S2). Diversity analysis was conducted using the Shannon diversity index to compare the richness and homogeneity between the groups. The results revealed that the alpha diversity was higher in the microbiota isolated from the indoor dust samples from the apartment than from the hospital; however, the results were not significant (Fig. 1C). Meanwhile, microbial EVs showed the opposite trend, although these result were also not significant (Fig. 1D). Seasonal differences were determined between the indoor dust sampling periods. In bacteria, the Acinetobacter population was higher in winter than in other seasons. In addition, Enterobacteriaceae (f) was higher in summer and winter than in spring and fall in the apartment, whereas Pseudomonas and Enterobacteriaceae (f) populations were lower in winter than summer in the hospital. In case of EVs, Acinetobacter was higher in winter than other seasons in the apartment, whereas Acinetobacter and Enterobacteriaceae (f) were higher in summer than in winter in the hospital (Supplementary Fig. S1E).
Fig. 1. Comparison of the microbiome composition and diversity of indoor dust in the apartment and hospital. Taxa that were over 1% in any samples selected for heatmap and Shannon index were analyzed for alpha diversity. Heatmap for microbiome abundance of (A) microbiota in indoor dust at the genus level, (B) heatmap for microbiome abundance of microbial EVs in indoor dust, at the genus level, (C) Shannon index for alpha diversity of microbiota in indoor dust, (D) Shannon index for alpha diversity of microbial EVs in indoor dust.
EV, extracellular vesicle.
*The value outside range mean ± 3standard deviation.
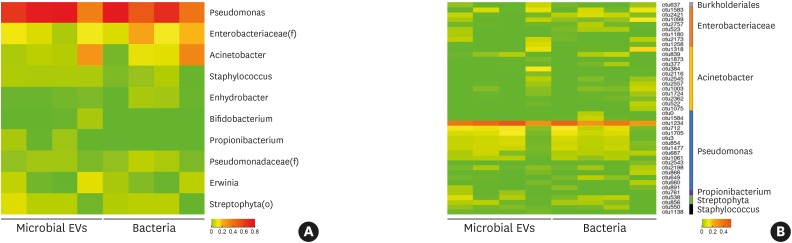
Analysis of indoor dust revealed that the microbiota and microbial EVs from identical samples obtained from the apartment showed a higher correlation with each other (average r = 0.97) than those from the hospital (average r = 0.30). In particular, the bacteria found most abundantly at the phylum level in the apartment, with 94.9% (±2.6%) abundance, was Proteobacteria, which also accounted for 90.1% (±3.3%) of microbial EVs. Further, Pseudomonas, Enterobacteriaceae (f) and Acinetobacter were dominant at the genus level, accounting for 59.4% (±19.7%), 14.9% (±6.4%) and 12.3% (±12.9%) of the microbiota and 64.9% (±22.9%), 7.6% (±4.4%) and 8.6% (±12.3%) of microbial EVs, respectively (Fig. 2A). Followed by Streptophyta (o), Erwinia and Staphylococcus as the most abundant bacterial and microbial EV taxa. OTU analysis of bacteria showed that of all the taxa with over 1% relative abundance, 16 of them belonged to Pseudomonas, 8 belonged to Enterobacteriaceae (f), 13 to Acinetobacter, 2 belonged to Streptophyta (o) and Staphylococcus, and 1 each belonged to Burholderiales (o) and Propinobacterium (Fig. 2B). Acinetobacter baumanii, Acinetobacter schindleri and Acinetobacter lwoffii were estimated to be the most abundant species among the Acinetobacter genus. (Supplementary Fig. S2). P. aeruginosa and E. cloacae were the most abundant species within the Pseudomonas genus (Supplementary Fig. S3) and the Enterobacteriaceae (f) genus, respectively (Supplementary Fig. S4).
Fig. 2. Comparison of the microbiome abundance of indoor dust in the apartment between microbiota and microbial EVs. Taxa that were over 1% in any samples were selected for inclusion in the heatmap. Heatmaps of microbiome abundance based on (A) taxa at the genus level and (B) OTUs.
EV, extracellular vesicle; OTU, operational taxonomic units.
Characteristics of the study subjects
A total of 88 control subjects, 239 asthmatics, 205 COPD patients and 324 lung cancer patients were enrolled (Table). Compared to the control subjects, the mean ages of patients with asthma, COPD and lung cancer were significantly higher (P < 0.001). In addition, COPD and lung cancer patients were statistically more likely to be male (both P < 0.001) and to have smoking history (both P < 0.001) compared to the control subjects, whereas the sex distribution of the asthma patients were similar and they were less likely to have a history of smoking compared to the healthy control subjects. Forced expiratory volume in 1 second was significantly in COPD patients lower than in lung cancer patients.
Table. Demographic findings of the study subjects.
| Variables | Total (male/female) | Age | Smoking (0/1)* | FEV1 (%pred) | |
|---|---|---|---|---|---|
| Healthy controls | 88 (49/39) | 50.8 ± 9.8 | 88 (45/43) | - | |
| Asthma patients | 239 (103/136) | 55.5 ± 14.5 | 239 (174/65) | 83.9 ± 17.9 | |
| COPD patients | 205 (196/9) | 66.4 ± 7 | 205 (0/205) | 47.2 ± 15.3 | |
| Lung cancer patients | 324 (274/50) | 65.7 ± 9 | 324 (79/245) | 74.5 ± 21.9 | |
| P value | |||||
| Healthy vs. asthma | 0.0576 | 0.0008 | 0.0004 | - | |
| Healthy vs. COPD | < 0.0001 | < 0.0001 | < 0.0001 | - | |
| Healthy vs. lung cancer | < 0.0001 | < 0.0001 | < 0.0001 | - | |
| COPD vs. lung cancer | 0.0002 | 0.3044 | < 0.0001 | < 0.0001 | |
Data are shown as mean ± standard deviation or number (%).
COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second.
*Smoking: 0 means the person who does not smoke and 1 means the person who has a history of smoking.
Serum IgG, IgG1 and IgG4 levels against 4 of the dominant bacterial EVs in indoor dust
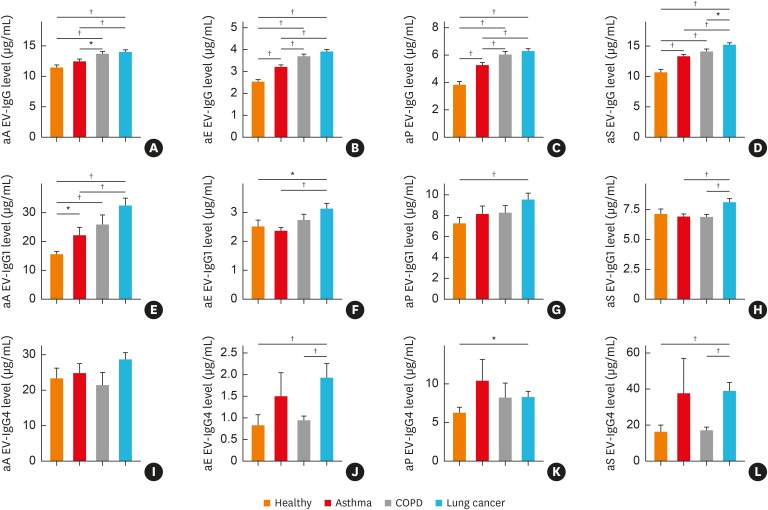
Serum anti-bacterial EV IgG, IgG1 and IgG4 levels were analyzed through ELISA utilizing EV antibodies that targeted S. aureus, A. baumannii, E. cloacae and P. aeruginosa based on their abundance in indoor dust as determined through microbiome analysis. As a result of comparative analysis of control and asthma groups, anti-E. cloacae EV IgG, anti-P. aeruginosa EV IgG, anti-S. aureus EV IgG and anti-A. baumannii EV IgG1 levels were found to be significantly higher (P < 0.05 for each). In addition, comparative analysis of control and COPD subjects showed a significant increase in anti-A. baumannii EV IgG (P < 0.05). Interestingly, there was a significant difference in anti-A. baumannii EV IgG1 levels between asthma and COPD samples with 1.42-fold and 1.66-fold changes, respectively. Comparative analysis of control and lung cancer subjects revealed significantly higher levels of IgG, IgG1 and IgG4 of anti-E. cloacae EV and anti-P. aeruginosa EV (P < 0.05 for each). Notably, anti-A. baumannii EV IgG1, anti-E. cloacae EV IgG4 and anti-S. aureus EV IgG4 showed greater than 2-fold differences. Furthermore, the comparative analysis results of COPD and lung cancer subjects showed significant increases in the levels of IgG, IgG1 and IgG4 of anti-S. aureus EV, and E. cloacae EV IgG4 in lung cancer subjects, especially anti-S. aureus EV IgG4 and anti-E. cloacae EV IgG4 with differences greater than 2-fold (P < 0.05 for each) (Fig. 3, Supplementary Table S3).
Fig. 3. Differences in IgG, IgG1 and IgG4 sensitization to microbial EVs, such as Acinetobacter baumannii, Enterobacter cloacae, Pseudomonas aeruginosa and Staphylococcus aureus among healthy subjects and patients with asthma, COPD or lung cancer. (A) IgG sensitization to A. baumannii EVs, (B) IgG sensitization to E. cloacae EVs, (C) IgG sensitization to P. aeruginosa EVs, (D) IgG sensitization to S. aureus EVs, (E) IgG1 sensitization to A. baumannii EVs, (F) IgG1 sensitization to E. cloacae EVs, (G) IgG1 sensitization to P. aeruginosa EVs, (H) IgG1 sensitization to S. aureus EVs, (I) IgG4 sensitization to A. baumannii EVs, (J) IgG4 sensitization to E. cloacae EVs, (K) IgG4 sensitization to P. aeruginosa EVs, (L) IgG4 sensitization to S. aureus EVs.
IgG, immunoglobulin G; EV, extracellular vesicle; COPD, chronic obstructive pulmonary disease; aE, anti-E. cloacae; aP, anti-P. aeruginosa; aS, anti-S. aureus; aA, anti-A. baumannii.
*P < 0.05, †P < 0.01.
Lung disease diagnostic models based on serum IgG, IgG1 and IgG4 against microbial EVs
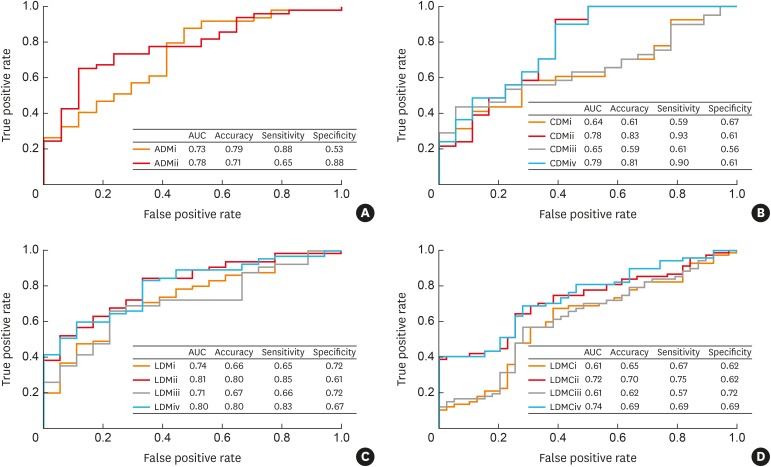
Lung disease diagnostic models were developed using logistic regression, which included whether the subject had smoked before or not as a covariate, while antibodies that showed significant differences in ELISA between clinical groups were selected as biomarkers. The models were created using following four inclusion criteria: i) all significantly different markers as model variables; ii) smoking status added as a covariate with all the markers that showed significant difference; iii) only markers that showed the most significant difference among IgG, IgG1 and IgG4 of each bacterial EV antibody; and iv) smoking status as a covariate while only using markers that showed the most significant difference among IgG, IgG1 and IgG4 of each bacterial EV antibody (Supplementary Table S4). In the asthma diagnostic model (ADM), there was at least 1 significant marker in each applicable EV antibody. The model area under the curve (AUC) increased from 0.73 to 0.78 in the ADMii model compared to the ADMi model (Fig. 4A). In the COPD diagnostic model (CDM), CMDi and CDMiii showed similar results with AUCs of 0.64 and 0.65, respectively. When smoking history was factored in, the AUCs of CDMii and CDMiv increased to 0.78 and 0.79, respectively (Fig. 4B). In the development of a lung cancer diagnostic model (LDM) for healthy controls, LDMi and LDMiii had AUCs of 0.74 and 0.71, respectively. The AUCs of LDMii and LDMiv increased to 0.81, and 0.8, respectively, when smoking history was factored as a covariate (Fig. 4C). Finally, in the LDM for COPD patients (LDMC), LDMCi and LDMCiii both yielded an AUC of 0.61. When smoking status was factored in, the AUCs of the LDMCii and LDMCiv models rose to 0.72 and 0.74, respectively (Fig. 4D).
Fig. 4. Diagnostic models based on enzyme-linked immunosorbent assay and smoking status as covariate for asthma, COPD and lung cancer. (A) ADM vs. healthy controls, (B) CDM vs. healthy controls, (C) LDM vs. healthy controls, (D) LDM vs. COPD patients.
AUC, area under the curve; ADM, asthma diagnostic model; CDM, COPD diagnostic model; LDM, lung cancer diagnostic model; LDMC, LDM for COPD patients.
DISCUSSION
In order to assess the metagenomic characteristics of indoor dust, dust samples were collected from hospital and apartment bed mattresses, and their microbiota as well as the microbial EV compositions were metagenomically analyzed. Pseudomonas, Enterobacter and Acinetobacter bacteria and EVs were the most abundant at the genus level. Furthermore, significant differences among indoor dust samples were determined, depending on the sampling environment. A previous investigation showed that Micrococcus, Staphylococcus, Streptococcus and Corynebacterium were the most abundant genera in indoor dust sampled from kindergartens.23 The factors that most likely contributed to this difference might be the variation in sampling methodology, sampling environment, and lack of bacteria and bacterial EVs separation prior to metagenomic analysis in the previous study. This study demonstrated that although there were differences between the bacteria found within indoor dust in an apartment and hospital, there was considerable taxonomic similarity between the bacteria and bacterial EVs. There were differences between the bacteria sampled from dust on the mattresses in the apartment and hospital due to the variations in the indoor environment conditions, such as temperature, relative humidity, human activity, air quality and sanitation. These indoor environments affect the proliferation and death of bacteria, which is directly linked to the production of bacterial EVs. The results of this study suggest that while there was an increase in the Pseudomonas population in the apartment, it was diminished in the hospital. Therefore, Pseudomonas EVs account for the majority of the microbiota in both the apartment and hospital. On the other hand, since Acinetobacter is neither enriched nor depleted significantly in the apartment and hospital, the proportion of Acinetobacter EVs in samples from the apartment is comparable to that from the hospital. In addition, although sampling was carried out in different seasons, the similarity in the results obtained in this study suggest that the overall composition of indoor dust might not be significantly affected by seasonal variations. One of the limitations of this study is that the dust samples were collected from only 1 area of the mattress and only a few indoor environments: 1 home apartment mattress and 2 hospitals. Future studies should include sampling from different locations and different areas of the mattress such as above the pillow, below the pillow, or the air where components can be inhaled in order to achieve more thorough and accurate indoor dust metagenomic analysis. To the best of our knowledge, however, as this is the first study about metagenomics of the bacterial EVs in indoor dust, the results are valuable as a pilot study in that they indicate the importance of analyzing bacterial EVs in indoor dust.
Recent studies have suggested that non-eosinophilic asthma represents a distinct and stable phenotype with respect to the lower airway pathology and structure.24 Specifically, asthmatic patients have severe, persistent, exacerbated symptoms without any increase in eosinophilic inflammation.25 Persistent asthma may be associated with significant neutrophilic inflammation.26 In addition, exacerbation of COPD symptoms is associated with the neutrophil-predominant inflammatory response.27 It has been seen that neutrophilic inflammation in the airways is induced by bacterial EVs in dust.22 The progression of lower airway inflammation in non-eosinophilic asthma depends on the etiological factor that causes the immune response except inhalant allergens. Based on this, bacterial EVs in dust might be considered to be an important etiological agent for non-eosinophilic asthma,24 bacterial EVs in dust might be important etiological agents for non-eosinophilic asthma.20 COPD and asthma are known risk factors of lung cancer,27,28 and there is evidence supporting the development of cancer due to chronic inflammation.19,29 Therefore, exposure to bacterial EVs in dust might elicit neutrophilic pulmonary inflammation, which might be associated with the development of lung cancer.20
In this study, we targeted S. aureus, A. baumannii, E. cloacae and P. aeruginosa EVs for further analysis as they were determined to be the core metagenomic components of indoor dust. The 6 dominant bacterial EV genera in the indoor dust from apartments were Pseudomonas, Acinetobacter, Enterobacteriaceae (f), Streptophyta (o), Erwinia and Staphylococcus. However, Streptophyta (o) EVs were hard to analyze at lower taxonomic levels. Moreover, although abundant Erwinia EVs were detected in both the apartment samples, they had low abundance in the hospital samples. Therefore, we selected 4 bacterial EVs for further analysis: Pseudomonas, Acinetobacter, Enterobacter and Staphylococcus. These bacterial targets are known for their association with allergic disorders such as atopic dermatitis and asthma.30,31,32 Inhaled bioaerosols function as vehicles for deep-lung as well as environmental transport of airborne infectious agents.33 Furthermore, bioaerosol components have been weakly related to lung disease.34
Recently, there has been a drastic rise in research regarding the association between lung disease and the microbiome. However, there are no published studies that have explored EVs and analyzed the serum IgG levels associated with Staphylococcus, Acinetobacter, Enterobacter and Pseudomonas from patients with COPD, asthma and lung cancer, and healthy subjects. A similar study compared the serum of cystic fibrosis patients and healthy controls, and found an abundance of serum anti-P. aeruginosa IgG1, IgG2, IgG3 and IgG4 in these patients.35 Other studies demonstrated that serum anti-S. aureus enterotoxin IgE was more abundant in patients with COPD and severe asthmatics than in healthy controls.36,37 In general, among the selected markers, P. aeruginosa was most commonly found in bronchiectasis patients.38,39 Meanwhile, S. aureus was one of the most important bacteria associated with acute exacerbations of COPD40 and chronic bacterial colonization seen in the lower respiratory tract of chronic bronchitis patients.41 A. baumannii is a pathogen that has caused public health concern and it is a member of the Acinetobacter genus. It is pervasive in the environment and can survive for extended periods of time in places such as surfaces of hospital floors.42,43 A. baumannii can be clinically present in a wide range of diseases, such as wound infections, urinary tract infections, pneumonia and sepsis.44 Additionally, Enterobacter spp. was discovered commonly in pneumonia patients.45 Collectively, these previous findings support the significance of the core bacterial taxa discovered in indoor dust in relation to lung health and disease.
When diagnostic studies utilizing antibodies such as IgG, IgA and IgM were performed, ELISA assay was found to be an effective diagnostic tool. Troncone et al.46 used IgA and IgG antibodies against tissue transglutaminase for diagnosing celiac disease. Similarly, Kosunen et al.47 attempted to combat H. pylori infection using IgG, IgA and IgM antibodies against Helicobacter pylori. IgG antibodies against dust EVs can be used as a surrogate marker for exposure to dust EVs.20 In previous studies, the immune response of indoor dust-specific antibodies was mainly reported to be IgG1 and IgG4 in allergic patients with diseases such as atopic dermatitis and asthma.22,48 Therefore, we selected the antibodies IgG, IgG1 and IgG4 to determine the immune response to microbial EVs in indoor dust in allergic patients in this study. Another study showed that since IgG2 and IgG3 are also related to pulmonary diseases,49 additional measurement of IgG2 and IgG3 is necessary to build advance diagnostic models. IgG, IgG1 and IgG4 antibodies against A. baumannii, S. aureus, P. aeruginosa and E. cloacae EVs were used as biomarkers in this study, and a diagnostic model was developed and tested using these 4 markers as variables. Although the AUC was relatively low for the CDM, model performance results showed that the efficacy of the 4 markers in diagnosing asthma and lung cancer is promising.
There were significantly larger numbers of patients with smoking history in the COPD and lung cancer groups than the healthy control group. It has been suggested that COPD and lung cancer share a common cause, namely, cigarette smoking. The increase in AUC observed was due to the addition of smoking history as a covariate, providing further evidence that smoking history is an important factor in COPD and lung cancer diagnosis. Due to the significant difference between age and sex of the groups, these factors were omitted as covariates to prevent any misevaluation in the diagnosis of EV antibodies. We observed high potential of diagnostic models using only age or sex for the diagnosis of asthma, COPD and lung cancer, though it is unclear whether this result is unduly affected by the disproportionate age and sex distribution between the clinical groups. The differences observed between age and sex, and whether the subject smokes or not made it evident that it is more likely for male smokers, among healthy subjects, to potentially contract COPD or lung cancer with age than their younger female counterparts. We suggest that fewer markers are advantageous for diagnostic uses since there were no significant differences in the model strength between the i) and ii) models, between iii) and iv) models, or between the ii) model and iv) models. Although the diagnostic tool developed in this study shows strong possibility as a novel diagnosis method, the role played by bacterial EVs, such as A. baumannii, S. aureus, E. cloacae and P. aeruginosa in the development of pulmonary diseases including asthma, COPD and lung cancer still remains unclear. In vivo testing and further cohort study are necessary for further advancement as well as for prediction and prognosis. Future studies should also include clinical information such as age, sex and body mass index for more precise diagnostic capabilities.
We previously showed that anti-indoor dust EV IgG is an independent risk factor for pulmonary diseases.20 Compared to the previous report, this study determined the specific bacterial EVs affecting pulmonary diseases in indoor dust, such as S. aureus, A. baumannii, E. cloacae and P. aeruginosa. We found that anti-dust bacterial EV IgG1 and IgG4 are the primary components of anti-dust bacterial EV IgG as well as important factors of pulmonary diseases. Furthermore, our study demonstrated that diagnosis might be possible using ELISA based on the serum anti-bacterial EV antibodies. In conclusion, in this study, ELISA was used to show that anti-core indoor dust bacterial EV IgG, IgG1 and IgG4 antibody titers in serum were significantly higher in patients with asthma, COPD and lung cancer than in healthy controls, suggesting that this method can be used as a lung disease diagnostic tool based on anti-bacterial EV antibody titers. An additional cohort study is required to determine whether the relationship between exposure to indoor dust EVs and the incidence of asthma, COPD and lung cancer is a casual one or not. This will also help in determining the ability to predict the risk of asthma, COPD and lung cancer and to confirm prognosis after treatment. Taken together, the present findings not only provide an insight into the pathogenesis of asthma, COPD and lung cancer, but offer the basis for developing a novel diagnostic tool.
ACKNOWLEDGMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2016M3A9B6901516 and NRF-2017M3A9F3047497) and by a grant of the KHIDI, funded by the Ministry of Health& Welfare, ROK(HI16C0992).
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
SUPPLEMENTARY MATERIALS
The number of valid reads
Abundance of bacteria and bacterial EVs in the apartment and hospital dust at the genus level
IgG, IgG1 and IgG4 sensitization to bacterial EVs including Acinetobacter baumannii, Enterobacter cloacae, Pseudomonas aeruginosa and Staphylococcus aureus between healthy subjects patients with asthma, COPD or lung cancer
The markers selected for models
Relative abundance of indoor dust bacteria and bacterial EVs in the apartment and hospital at the (A) phylum, (B) class, (C) order, (D) family and (E) genus levels.
Defining species of Acinetobacter.
Defining species of Pseudomonas.
Defining species of Enterobacteriacecae (f).
References
- 1.Lee SC, Chang M. Indoor and outdoor air quality investigation at schools in Hong Kong. Chemosphere. 2000;41:109–113. doi: 10.1016/s0045-6535(99)00396-3. [DOI] [PubMed] [Google Scholar]
- 2.Mendes A, Pereira C, Mendes D, Aguiar L, Neves P, Silva S, et al. Indoor air quality and thermal comfort-results of a pilot study in elderly care centers in Portugal. J Toxicol Environ Health A. 2013;76:333–344. doi: 10.1080/15287394.2013.757213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klepeis NE, Nelson WC, Ott WR, Robinson JP, Tsang AM, Switzer P, et al. The National Human Activity Pattern Survey (NHAPS): a resource for assessing exposure to environmental pollutants. J Expo Anal Environ Epidemiol. 2001;11:231–252. doi: 10.1038/sj.jea.7500165. [DOI] [PubMed] [Google Scholar]
- 4.Kim YK, Oh SY, Jeon SG, Park HW, Lee SY, Chun EY, et al. Airway exposure levels of lipopolysaccharide determine type 1 versus type 2 experimental asthma. J Immunol. 2007;178:5375–5382. doi: 10.4049/jimmunol.178.8.5375. [DOI] [PubMed] [Google Scholar]
- 5.Jeon SG, Oh SY, Park HK, Kim YS, Shim EJ, Lee HS, et al. TH2 and TH1 lung inflammation induced by airway allergen sensitization with low and high doses of double-stranded RNA. J Allergy Clin Immunol. 2007;120:803–812. doi: 10.1016/j.jaci.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 6.Song WJ, Lee JH, Kang Y, Joung WJ, Chung KF. Future risks in patients with severe asthma. Allergy Asthma Immunol Res. 2019;11:763–778. doi: 10.4168/aair.2019.11.6.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 9.Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet. 2009;374:733–743. doi: 10.1016/S0140-6736(09)61303-9. [DOI] [PubMed] [Google Scholar]
- 10.Shendell DG, Mizan SS, Yamamoto N, Peccia J. Associations between quantitative measures of fungi in home floor dust and lung function among older adults with chronic respiratory disease: a pilot study. J Asthma. 2012;49:502–509. doi: 10.3109/02770903.2012.682633. [DOI] [PubMed] [Google Scholar]
- 11.Hansel NN, McCormack MC, Belli AJ, Matsui EC, Peng RD, Aloe C, et al. In-home air pollution is linked to respiratory morbidity in former smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187:1085–1090. doi: 10.1164/rccm.201211-1987OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Husman T. Health effects of indoor-air microorganisms. Scand J Work Environ Health. 1996;22:5–13. doi: 10.5271/sjweh.103. [DOI] [PubMed] [Google Scholar]
- 13.Lee EY, Bang JY, Park GW, Choi DS, Kang JS, Kim HJ, et al. Global proteomic profiling of native outer membrane vesicles derived from Escherichia coli . Proteomics. 2007;7:3143–3153. doi: 10.1002/pmic.200700196. [DOI] [PubMed] [Google Scholar]
- 14.Lee EY, Choi DY, Kim DK, Kim JW, Park JO, Kim S, et al. Gram-positive bacteria produce membrane vesicles: proteomics-based characterization of Staphylococcus aureus-derived membrane vesicles. Proteomics. 2009;9:5425–5436. doi: 10.1002/pmic.200900338. [DOI] [PubMed] [Google Scholar]
- 15.Kim MR, Hong SW, Choi EB, Lee WH, Kim YS, Jeon SG, et al. Staphylococcus aureus-derived extracellular vesicles induce neutrophilic pulmonary inflammation via both Th1 and Th17 cell responses. Allergy. 2012;67:1271–1281. doi: 10.1111/all.12001. [DOI] [PubMed] [Google Scholar]
- 16.Lee J, Lee EY, Kim SH, Kim DK, Park KS, Kim KP, et al. Staphylococcus aureus extracellular vesicles carry biologically active β-lactamase. Antimicrob Agents Chemother. 2013;57:2589–2595. doi: 10.1128/AAC.00522-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim YS, Lee WH, Choi EJ, Choi JP, Heo YJ, Gho YS, et al. Extracellular vesicles derived from gram-negative bacteria, such as Escherichia coli, induce emphysema mainly via IL-17A-mediated neutrophilic inflammation. J Immunol. 2015;194:3361–3368. doi: 10.4049/jimmunol.1402268. [DOI] [PubMed] [Google Scholar]
- 18.Houghton AM. Mechanistic links between COPD and lung cancer. Nat Rev Cancer. 2013;13:233–245. doi: 10.1038/nrc3477. [DOI] [PubMed] [Google Scholar]
- 19.Aggarwal BB, Gehlot P. Inflammation and cancer: how friendly is the relationship for cancer patients? Curr Opin Pharmacol. 2009;9:351–369. doi: 10.1016/j.coph.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim YS, Choi JP, Kim MH, Park HK, Yang S, Kim YS, et al. IgG sensitization to extracellular vesicles in indoor dust is closely associated with the prevalence of non-eosinophilic asthma, COPD, and lung cancer. Allergy Asthma Immunol Res. 2016;8:198–205. doi: 10.4168/aair.2016.8.3.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park KS, Choi KH, Kim YS, Hong BS, Kim OY, Kim JH, et al. Outer membrane vesicles derived from Escherichia coli induce systemic inflammatory response syndrome. PLoS One. 2010;5:e11334. doi: 10.1371/journal.pone.0011334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim YS, Choi EJ, Lee WH, Choi SJ, Roh TY, Park J, et al. Extracellular vesicles, especially derived from gram-negative bacteria, in indoor dust induce neutrophilic pulmonary inflammation associated with both Th1 and Th17 cell responses. Clin Exp Allergy. 2013;43:443–454. doi: 10.1111/cea.12085. [DOI] [PubMed] [Google Scholar]
- 23.Shin SK, Kim J, Ha SM, Oh HS, Chun J, Sohn J, et al. Metagenomic insights into the bioaerosols in the indoor and outdoor environments of childcare facilities. PLoS One. 2015;10:e0126960. doi: 10.1371/journal.pone.0126960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haldar P, Pavord ID. Noneosinophilic asthma: a distinct clinical and pathologic phenotype. J Allergy Clin Immunol. 2007;119:1043–1052. doi: 10.1016/j.jaci.2007.02.042. [DOI] [PubMed] [Google Scholar]
- 25.Turner MO, Hussack P, Sears MR, Dolovich J, Hargreave FE. Exacerbations of asthma without sputum eosinophilia. Thorax. 1995;50:1057–1061. doi: 10.1136/thx.50.10.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gibson PG, Simpson JL, Saltos N. Heterogeneity of airway inflammation in persistent asthma : evidence of neutrophilic inflammation and increased sputum interleukin-8. Chest. 2001;119:1329–1336. doi: 10.1378/chest.119.5.1329. [DOI] [PubMed] [Google Scholar]
- 27.Siva R, Green RH, Brightling CE, Shelley M, Hargadon B, McKenna S, et al. Eosinophilic airway inflammation and exacerbations of COPD: a randomised controlled trial. Eur Respir J. 2007;29:906–913. doi: 10.1183/09031936.00146306. [DOI] [PubMed] [Google Scholar]
- 28.Santillan AA, Camargo CA, Jr, Colditz GA. A meta-analysis of asthma and risk of lung cancer (United States) Cancer Causes Control. 2003;14:327–334. doi: 10.1023/a:1023982402137. [DOI] [PubMed] [Google Scholar]
- 29.Yao H, Rahman I. Current concepts on the role of inflammation in COPD and lung cancer. Curr Opin Pharmacol. 2009;9:375–383. doi: 10.1016/j.coph.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim MH, Choi SJ, Choi HI, Choi JP, Park HK, Kim EK, et al. Lactobacillus plantarum-derived extracellular vesicles protect atopic dermatitis induced by Staphylococcus aureus-derived extracellular vesicles. Allergy Asthma Immunol Res. 2018;10:516–532. doi: 10.4168/aair.2018.10.5.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee SY, Lee E, Park YM, Hong SJ. Microbiome in the gut-skin axis in atopic dermatitis. Allergy Asthma Immunol Res. 2018;10:354–362. doi: 10.4168/aair.2018.10.4.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ege MJ, Mayer M, Schwaiger K, Mattes J, Pershagen G, van Hage M, et al. Environmental bacteria and childhood asthma. Allergy. 2012;67:1565–1571. doi: 10.1111/all.12028. [DOI] [PubMed] [Google Scholar]
- 33.Fiegel J, Clarke R, Edwards DA. Airborne infectious disease and the suppression of pulmonary bioaerosols. Drug Discov Today. 2006;11:51–57. doi: 10.1016/S1359-6446(05)03687-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lacey J, Dutkiewicz J. Bioaerosols and occupational lung disease. J Aerosol Sci. 1994;25:1371–1404. [Google Scholar]
- 35.Pressler T, Pedersen SS, Espersen F, Høiby N, Koch C. IgG subclass antibodies to Pseudomonas aeruginosa in sera from patients with chronic Ps. aeruginosa infection investigated by ELISA. Clin Exp Immunol. 1990;81:428–434. doi: 10.1111/j.1365-2249.1990.tb05351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rohde G, Gevaert P, Holtappels G, Borg I, Wiethege A, Arinir U, et al. Increased IgE-antibodies to Staphylococcus aureus enterotoxins in patients with COPD. Respir Med. 2004;98:858–864. doi: 10.1016/j.rmed.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 37.Bachert C, Gevaert P, Howarth P, Holtappels G, van Cauwenberge P, Johansson SG. IgE to Staphylococcus aureus enterotoxins in serum is related to severity of asthma. J Allergy Clin Immunol. 2003;111:1131–1132. [PubMed] [Google Scholar]
- 38.Davies G, Wells AU, Doffman S, Watanabe S, Wilson R. The effect of Pseudomonas aeruginosa on pulmonary function in patients with bronchiectasis. Eur Respir J. 2006;28:974–979. doi: 10.1183/09031936.06.00074605. [DOI] [PubMed] [Google Scholar]
- 39.King PT, Holdsworth SR, Freezer NJ, Villanueva E, Holmes PW. Microbiologic follow-up study in adult bronchiectasis. Respir Med. 2007;101:1633–1638. doi: 10.1016/j.rmed.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 40.Sethi S, Evans N, Grant BJ, Murphy TF. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 2002;347:465–471. doi: 10.1056/NEJMoa012561. [DOI] [PubMed] [Google Scholar]
- 41.Monsó E, Rosell A, Bonet G, Manterola J, Cardona PJ, Ruiz J, et al. Risk factors for lower airway bacterial colonization in chronic bronchitis. Eur Respir J. 1999;13:338–342. doi: 10.1034/j.1399-3003.1999.13b20.x. [DOI] [PubMed] [Google Scholar]
- 42.Giamarellou H, Antoniadou A, Kanellakopoulou K. Acinetobacter baumannii: a universal threat to public health? Int J Antimicrob Agents. 2008;32:106–119. doi: 10.1016/j.ijantimicag.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 43.Muller-Redetzky H, Suttorp N, Witzenrath M. Experimental models of pneumonia-induced sepsis. Drug Discov Today Dis Models. 2012;9:e23–32. [Google Scholar]
- 44.Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev. 2008;21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.von Baum H, Welte T, Marre R, Suttorp N, Ewig S CAPNETZ study group. Community-acquired pneumonia through Enterobacteriaceae and Pseudomonas aeruginosa: diagnosis, incidence and predictors. Eur Respir J. 2010;35:598–605. doi: 10.1183/09031936.00091809. [DOI] [PubMed] [Google Scholar]
- 46.Troncone R, Maurano F, Rossi M, Micillo M, Greco L, Auricchio R, et al. IgA antibodies to tissue transglutaminase: an effective diagnostic test for celiac disease. J Pediatr. 1999;134:166–171. doi: 10.1016/s0022-3476(99)70410-5. [DOI] [PubMed] [Google Scholar]
- 47.Kosunen TU, Seppälä K, Sarna S, Sipponen P. Diagnostic value of decreasing IgG, IgA, and IgM antibody titres after eradication of Helicobacter pylori . Lancet. 1992;339:893–895. doi: 10.1016/0140-6736(92)90929-w. [DOI] [PubMed] [Google Scholar]
- 48.Boluda L, de la Cuadra B, Berrens L. Binding affinities of allergens from pollen, mites, and house dust for specific IgG subclass antibodies. Allergy. 1996;51:706–711. doi: 10.1111/j.1398-9995.1996.tb02114.x. [DOI] [PubMed] [Google Scholar]
- 49.Klotz M, Blaes F, Funke D, Kalweit G, Schimrigk K, Huwer H. Shift in the IgG subclass distribution in patients with lung cancer. Lung Cancer. 1999;24:25–30. doi: 10.1016/s0169-5002(99)00014-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The number of valid reads
Abundance of bacteria and bacterial EVs in the apartment and hospital dust at the genus level
IgG, IgG1 and IgG4 sensitization to bacterial EVs including Acinetobacter baumannii, Enterobacter cloacae, Pseudomonas aeruginosa and Staphylococcus aureus between healthy subjects patients with asthma, COPD or lung cancer
The markers selected for models
Relative abundance of indoor dust bacteria and bacterial EVs in the apartment and hospital at the (A) phylum, (B) class, (C) order, (D) family and (E) genus levels.
Defining species of Acinetobacter.
Defining species of Pseudomonas.
Defining species of Enterobacteriacecae (f).