Abstract
Purpose
Accumulating evidence has suggested that toll-like receptor 4 (TLR4) is critically involved in the pathogenesis of asthma. The aim of this study was to investigate the role of TLR4 in toluene diisocyanate (TDI)-induced allergic airway inflammation.
Methods
TLR4−/− and wild-type (WT) C57BL/10J mice were sensitized and challenged with TDI to generate a TDI-induced asthma model. B-cell lymphoma 2 (Bcl-2) inhibitors, ABT-199 (4 mg/kg) and ABT-737 (4 mg/kg), were intranasally given to TDI-exposed TLR4−/− mice after each challenge.
Results
TDI exposure led to increased airway hyperresponsiveness (AHR), granulocyte flux, bronchial epithelial shedding and extensive submucosal collagen deposition, which were unexpectedly aggravated by TLR4 deficiency. Following TDI challenge, TLR4−/− mice exhibited down-regulated interleukin-17A and increased colony-stimulating factor 3 in bronchoalveolar lavage fluid (BALF), while WT mice did not. In addition, TLR4 deficiency robustly suppressed the expression of NOD-like receptor family pyrin domain containing 3 and NLR family CARD domain containing 4, decreased caspase-1 activity in TDI-exposed mice, but had no effect on the level of high mobility group box 1 in BALF. Flow cytometry revealed that TDI hampered both neutrophil and eosinophil apoptosis, of which neutrophil apoptosis was further inhibited in TDI-exposed TLR4−/− mice, with marked up-regulation of Bcl-2. Moreover, inhibition of Bcl-2 with either ABT-199 or ABT-737 significantly alleviated neutrophil recruitment by promoting apoptosis.
Conclusions
These data indicated that TLR4 deficiency promoted neutrophil infiltration by impairing its apoptosis via up-regulation of Bcl-2, thereby resulting in deteriorated AHR and airway inflammation, which suggests that TLR4 could be a negative regulator of TDI-induced neutrophilic inflammation.
Keywords: Toll-like receptor 4, asthma, toluene diisocyanate, neutrophlic inflammation, apoptosis
INTRODUCTION
Asthma is a chronic inflammatory airway disorder characterized by airway hyperresponsiveness (AHR), episodes of reversible airflow obstruction, and airway remodeling, currently affecting more than 300 million individuals around the world, with a large socioeconomic burden.1 Although inhaled corticosteroids act as the first-line treatment of asthma, there are still 5%–20% of asthmatics who respond poorly to steroids even at high doses, contributing to the majority of morbidity and mortality associated with the disease.2 Until recently, the etiology and pathogenesis of asthma remains elusive. Thus, understanding the underlying pathophysiology of asthma is of great importance.
Toll-like receptors (TLRs) are a group of pattern recognition receptors abundantly expressed on hematopoietic and non-hematopoietic stromal or structural airway cells that, after ligation with ligands, exert profound immune-modulatory roles in the development of asthma.3 Without diminishing the importance of other members of this superfamily, a growing body of evidence demonstrates that TLR4 is involved in the pathogenesis of asthma.3 Several studies found that blocking TLR4 using antagonists decreased house dust mite (HDM)- or ovalbumin (OVA)-induced airway inflammation.4,5 In spite of similar results obtained in TLR4-deficient mice indicating that TLR4 signaling is required to induce T helper (Th) 2 responses and airway hypersensitivity,6,7,8 other studies showed that experimental asthma models were more easily induced in TLR4-deficient or MyD88-deficient mice,9,10 while chronic exposure to endotoxin (TLR4 agonist) can protect mice from developing HDM-induced asthma by suppressing epithelial cell-derived Th2 response.11 Besides, activation of TLR4 was able to dampen Th2 sensitization and to boost Th17 sensitization in the HDM-induced model, while in the OVA-induced model concomitant activation of TLR4 only hampers Th2 sensitization.12 These opposite results addressed the controversial but vital role of TLR4 in the pathogenesis of asthma. Thus, the aim of this study was to investigate the role of TLR4 in toluene diisocyanate (TDI)-induced asthma and the mechanisms involved.
MATERIALS AND METHODS
Animals
A 6- to 8-week old male TLR4−/− and wild-type (WT) C57BL/10J mice were purchased from the Model Animal Research Center of Nanjing University. All mice were housed under a specific pathogen-free condition and provided with sterile water and irradiated food available ad libitum. All animal care and experimental procedures were in accordance with the guidelines of the Committee of Guangzhou Medical University on the use and care of animals and were authorized by the Animal Subjects Committee of Guangzhou Medical University (Approval No. A2019-048).
Reagents
TDI (≥98.0%), methacholine and acetone were obtained from Sigma-Aldrich (Shanghai, China). The B-cell lymphoma 2 (Bcl-2)-specific inhibitors ABT-199 and ABT-737 were purchased from Selleck (Shanghai, China). Multiplex immunoassays for interleukin (IL)-4, IL-5, IL-13, IL-17A, IL-17F, IL-6, IL-18, C-C motif chemokine 11 (CCL11), chemokine (C-X-C motif) ligand 1 (CXCL1) and colony-stimulating factor 3 (CSF-3) were bought from eBioscience (San Diego, CA, USA). An ELISA detection kit for mouse high mobility group box 1 (HMGB1) was brought from Chondrex Inc. (Redmond, WA, USA), and a Caspase-1/ICE Colorimetric Assay Kit was purchased from R&D system (Minneapolis, MN, USA). Fluorescent dye-conjugated mouse antibodies, Gr-1 (BV421-labeled) and Siglec-F (BB515-labeled), were purchased from BD (San Jose, CA, USA), and cluster of differentiation (CD) 11b (FITC-labeled) was purchased from Biolegend (San Diego, CA, USA). An Annexin V-Alexa Fluor 647/propidium iodide (PI) detection Kit was bought from Bestbio (Shanghai, China). Primary antibodies against HMGB1, TLR4, mucin-5AC (MUC5AC), α-smooth muscle actin (α-SMA), NOD-like receptor family, pyrin domain containing 3 (NLRP3) and caspase-8, were purchased from Abcam (Shanghai, China). Primary antibody against cleaved-caspase-3 was purchased from Cell Signaling Technology (Danvers, MA, USA). Rabbit-anti-NLR family CARD domain containing 4 (NLRC4), Bcl-2, caspase-3 antibodies were purchased from ABclonal (Wuhan, China).
TDI-induced asthma model
Sensitization and antigen challenge with TDI were performed as described elsewhere.13 In the first protocol, all mice were randomized to paralleled 4 groups: 1) vehicle-sensitized, vehicle-challenged WT mice (WT control group); 2) TDI-sensitized, TDI-challenged WT mice (WT TDI group); 3) vehicle-sensitized, vehicle-challenged TLR4−/− mice (TLR4−/− control group); and 4) TDI-sensitized, TDI-challenged, TLR4−/− mice (TLR4−/− TDI group). In the second protocol, mice were also randomly allocated to paralleled 4 groups: 1) TDI-sensitized, TDI-challenged, solvent-treated WT mice (WT TDI group); 2) TDI-sensitized, TDI-challenged, solvent-treated TLR4−/− mice (TLR4−/− TDI group); 3) TDI-sensitized, TDI-challenged, ABT-199-treated TLR4−/− mice (TLR4−/− TDI + ABT-199 group); and 4) TDI-sensitized, TDI-challenged, ABT-737-treated TLR4−/− mice (TLR4−/− TDI + ABT-737 group). Briefly, on days 1 and 8, the mice were dermally immunized with 0.3% TDI on the dorsum of both ears (20 µL per ear). On days 15, 18 and 21, the mice were placed in a horizontal rectangle chamber and challenged for 3 hours each time with 3% TDI by means of compressed air nebulization (NE-C28; Omron, Tokyo, Japan). Control mice were treated by the same procedures with the same amount of vehicle. The vehicle used to dissolve TDI consisted of a mixture of 2 volumes of acetone and 3 volumes of olive oil for sensitization, and 1 volume of acetone and 4 volumes of olive oil for inhalation. The selective Bcl-2 inhibitors, ABT-199 (4 mg/kg) and ABT-737 (4 mg/kg) dissolved in 30% propylene glycol as well as 5% Tween-80 and 65% D5W (5% dextrose in water, pH = 4.2), were intranasally given to the mice 2 hours after each inhalation.14 Sham mice received the same volume of solvent for comparison.
Assessment of AHR
Airway responsiveness to methacholine was analyzed 24 hours after the final challenge with the FinePointe Resistance and Compliance system (DSI-Buxco, St. Paul, MN, USA) as previously described.15 Measurements of lung resistance (RL) were performed in response to increasing concentrations of nebulized methacholine (6.25, 12.5, 25 and 50 mg/mL) every 5 minutes following each nebulization step until a plateau phase was reached. Results are expressed as percentage of baseline RL value for each dose of methacholine.
Bronchoalveolar lavage fluid (BALF) analysis
After blood was taken, the trachea was cannulated, and the lungs were washed twice with 0.8 mL of sterile saline (0.9% NaCl, prewarmed). Total cells in BALF were counted and differential cell counts were determined by cytospin preparation stained with hematoxylin and eosin (H&E). Then the remaining fluids were centrifuged (1,000 g, 10 minutes), and supernatants were stored at −80°C for further analysis. BALF levels of IL-4, IL-5, IL-13, IL-17A, IL-17F, IL-6 and IL-18 as well as eosinophil chemokine CCL11, neutrophil chemokine CXCL1 and CSF-3 were measured using multiplex immunoassay (eBioscience) according to the manufacturer's instructions.
Flow cytometric analysis of BALF and lung single cell suspension
Mice were anesthetized, and the lungs were flushed in situ with 5 mL of phosphate-buffered saline (PBS) via cannulation of the heart to remove the intravascular blood pool. About 80 mg of minced lung tissues were incubated at 37°C for 40 minutes on a rocker with collagenase (200 UI/mL, Sigma-Aldrich) in RPMI 1640 medium. Subsequently, the enzyme-digested lung tissues were passed through 100-mm nylon filters, and ACK lysis buffer was used to remove the red blood cells. Cell-surface marker expression of neutrophils in BALF and lung single cell suspensions were assessed with the fluorescent dye-conjugated mouse antibodies Gr-1 (BV421; #562709, BD), CD11b (FITC; #101206, Biolegend) for 30 minutes at 4°C, and cell-surface marker expression of eosinophils was stained by Gr-1 (BV421; #562709, BD) and Siglec-F (BB515; #564514, BD). Based on surface marker staining, suspensions were cultured with Annexin V (Alexa Fluor 647-labeled) and PI (#BB-41035, Bestbio) to assess apoptosis levels in BAL and lung single cells. Data were acquired with a BD Biosciences Fortessa flow cytometer and analyzed with FlowJo software.
Lung tissue histopathology
After BALF was collected, the left lungs were inflated with 4% neutral buffered formalin for 24 hours and embedded in paraffin using a standard protocol. Lung sections were cut 4-μm thick with a Leica microtome 2030 (Leica Microsystems Nussloch GmbH, Nussloch, Germany), then stained with H&E and Masson's trichrome for blinded histopathological assessment. Peribronchial and perivascular inflammation in H&E-stained slides was assessed using a scoring standard as previously described16: 0 = normal; 1 = infrequent inflammatory cells; 2 = a ring of inflammatory cells 1 cell layer deep; 3 = a ring of inflammatory cells 2-4 cells deep; and 4 = a ring of inflammatory cells more than 4 cells deep. Epithelial denudation was measured by assessing the percentage of the denuded area in the entire circumference of the bronchus.17 Thickness of airway smooth muscle (ASM) was measured by a modification of Cho's method.18 Briefly, the thickness of the peribronchial smooth muscle layer (the transverse diameter) in large airways was measured from the innermost aspect to the outermost aspect of the circumferential smooth muscle layer.19 The presence of collagen fibril deposition with Masson staining was analyzed by ImageJ software. Scoring was performed at a magnification of 200× by examining at least 8-10 image fields of 12 slices from 6 mice per group.
Measurements of HMGB1 and caspase-1 activity
Lung tissues obtained from the experimental mice were snap-frozen in liquid nitrogen and lung homogenates were stored at −80°C. HMGB1 content in BALF, and caspase-1 activity in the lung tissue were measured by an ELISA kit (Chondrex) and a Caspase-1/ICE Colorimetric Assay Kit (R&D system), respectively, according to the manufacturer's instructions.
Immunohistochemistry and western blotting
Lung sections were prepared as previously described, then stained with mouse anti-MUC5AC antibody (#ab3649, Abcam), rabbit anti-α-SMA (#ab32575, Abcam), TLR4 (#ab13556, Abcam), rabbit anti-HMGB1 antibody (#ab79823, Abcam), rabbit anti-NLRP3 antibody (#214185, Abcam), rabbit anti-NLRC4 antibody (#A13117, ABclonal) and rabbit anti-Bcl-2 antibody (#A0208, ABclonal). After washing with PBS, slices were incubated with anti-rabbit secondary antibody for 30 minutes at room temperature. Signals were visualized with a DAB peroxidase substrate kit., and the localization of targeted molecules in the lung was assessed under a light microscope. Lung tissue samples were ground to fine powder under liquid nitrogen and lysates were subjected to SDS-PAGE and western blotting for the detection of the following antigens: TLR4 (#ab13556, Abcam), HMGB1 (#ab79823, Abcam), NLRP3 (#214185, Abcam), NLRC4 (#A13117, ABclonal), Bcl-2 (#A0208, ABclonal), procaspase-3 (#A0214, ABclonal), procaspase-8 (#108333, Abcam), cleaved caspase-3 (#9664s, Cell Signaling Technology) and cleaved caspase-8 (#9429, Cell Signaling Technology). After incubation with an IRDye® 680WC-conjugated secondary antibody (LICOR Biosciences), immunoreactive bands were exposed to an Odyssey® CLx Imager for image capture. Data analysis was performed with ImageJ Software.
Statistical analysis
Prism (version 6; GraphPad, San Diego, CA, USA) and SPSS version 20.0 (SPSS Inc., Chicago, IL, USA) were used for statistical analysis and graphing. Data are expressed as mean ± standard error, and comparisons among groups were made via 1-way analysis of variance accompanied by Dunnett's T3 (equal variances not assumed) post hoc tests for multiple comparisons. P < 0.05 was considered statistically significant. At least 3 independent experiments were repeated.
RESULTS
TLR4 deficiency aggravates TDI-induced airway hyperreactivity and inflammation
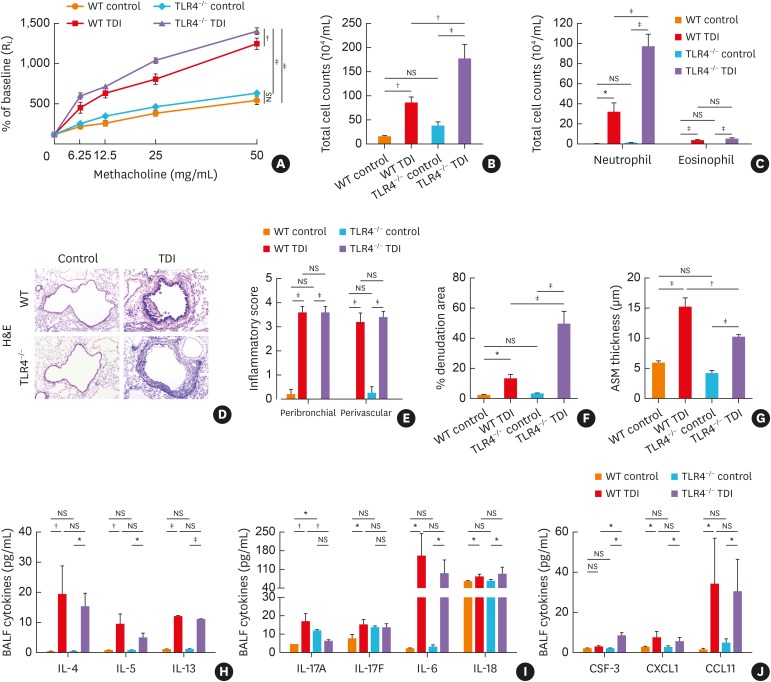
For in vivo studies of the role of TLR4 in TDI-induced airway inflammatory responses, WT and TLR4−/− mice were sensitized with 0.3% TDI (days 1 and 8), followed by 3 challenges using 3% TDI (days 15, 18 and 21). This model shares cardinal features with steroid-insensitive asthma19 including airway neutrophil-dominated inflammation and mixed granulocyte inflammation. As expected, TDI exposure increased airway reactivity to methacholine and inflammatory cell aggregation accompanied with elevated pulmonary expression of TLR4 when compared with control (Fig. 1A-C). TLR4−/− mice displayed significantly exacerbated AHR and a denser accumulation of neutrophils around the airway as compared with WT mice following TDI exposure (Fig. 1A-C). Histological examination of lung sections from the WT TDI group revealed large numbers of infiltrating inflammatory cells around the airways as well as evident epithelial hyperplasia compared with control, and showed no significant differences from that in TLR4-deficient mice. After TDI inhalation, TLR4−/− mice exhibited substantially more neutrophil recruitment as shown by BALF smear (Fig. 1C), and heavier epithelial shedding when compared with WT mice (Fig. 1D and F).
Fig. 1. TLR4 deficiency aggravated TDI-induced airway hyperreactivity and inflammation. (A) Airway hyperresponsiveness was measured by RL. Results are shown as percentage of baseline (n = 4). (B) Numbers of total inflammatory cell in BALF. (C) Numbers of neutrophils and eosinophils in BALF (n = 6). (D) Representative H&E-stained lung sections of different groups. Original magnification was 200×. (E) Semi-quantification of airway inflammation was performed (n = 4–6). (F) Semi-quantification of epithelial denudation was performed (n = 4–6). (G) Analysis of ASM thickness was performed (n = 4–6). (H) Levels of Th2-related cytokines IL-4, IL-5 and IL-13 in BALF (n = 4–6). (I) Levels of Th17-related cytokines IL-17A, IL-17F, IL-6, IL-18 and in BALF (n = 4–6). (J) Levels of neutrophil chemoattractant CSF-3, CXCL1 and eosinophil chemoattractant CCL11 in BALF (n = 4–6).
WT, wild-type; TLR4, toll-like receptor 4; TDI, toluene diisocyanate; RL, lung resistance; H&E, hematoxylin and eosin; BALF, bronchoalveolar lavage fluid; NS, not significant; IL, interleukin; Th, T helper; CCL11, C-C motif chemokine 11; CXCL1, chemokine (C-X-C motif) ligand 1; CSF-3, colony-stimulating factor 3; ASM, airway smooth muscle.
*P < 0.05; †P < 0.01; ‡P < 0.001.
TLR4−/− mice exhibit Th2/Th17 dysregulation after TDI sensitization and challenge
A mixture of Th1, Th2 and Th17 responses have been found to underline TDI-induced asthmatic inflammation.15,19 TDI-exposed WT mice exhibited elevated secretion of Th2-related factors (IL-4, IL-5 and IL-13), Th17-related ILs (IL-17A, IL-17F, IL-6 and IL-18) and eosinophil chemokine CCL11 as well as neutrophil chemokine CSF-3 and CXCL1, in BALF compared with the control group (Fig. 1H-J). TDI-inhaled TLR4−/− mice displayed similar results except down-regulated IL-17A and up-regulated CSF-3 (Fig. 1I and J), of which elevated CSF-3 was proven to inhibit neutrophil apoptosis and to prolong its survival.20
TLR4−/− mice display exacerbated airway remodeling after TDI exposure
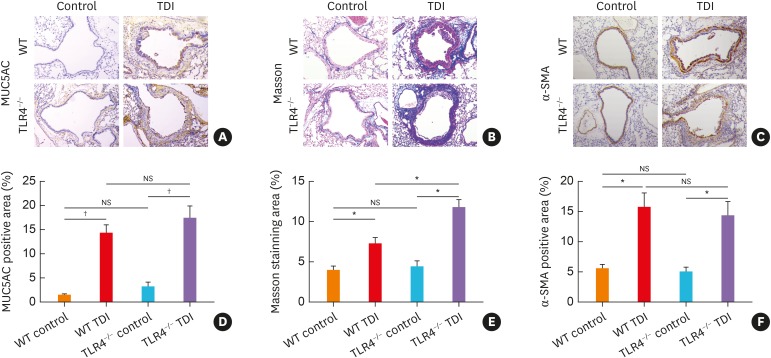
Airway remodeling is an essential pathophysiological feature of asthma.1 To assess whether TLR4 affects the extent of airway remodeling in vivo, lung sections were first stained for MUC5AC, which revealed that the lung expression of MUC5AC in TLR4−/− mice seemed to be slightly higher as compared with WT mice following TDI inhalation though not significant (Fig. 2A and D), indicating that TLR4 deficiency might tend to promote mucus hypersecretion in TDI-induced asthma. The effects of TLR4 deficiency on ASM thickening and collagen deposition induced by TDI were also assessed through α-SMA and trichrome Masson's staining. In accordance with the above results, TLR4 deficiency aggravated collagen deposition in TDI-treated mice (Fig. 2B and E), but reduced ASM thickening (Fig. 1G, Fig. 2C and F).
Fig. 2. TLR4−/− mice exhibited exacerbated airway remodeling after TDI exposure. (A) Representative immunohistochemical staining of MUC5AC in the lung sections of different groups. Original magnification was 200×. (B) Representative Masson trichrome-stained lung sections showing the collagen deposition of different groups. Original magnification was 200×. (C) Representative immunohistochemical staining of α-SMA indicates smooth muscle density. Original magnification was 200×. (D) Semi-quantification of MUC5AC staining was performed by ImageJ software (n = 4–6). (E) Semi-quantification of Masson staining was performed by ImageJ software (n = 4–6). (F) Semi-quantification of α-SMA staining was performed. The percentage of α-SMA positive staining area was measured by ImageJ software (n = 4–6).
WT, wild-type; TLR4, toll-like receptor 4; TDI, toluene diisocyanate; NS, not significant; MUC5AC, mucin-5AC; α-SMA, α-smooth muscle actin.
*P < 0.01; † P < 0.001.
TLR4 deficiency remarkably inhibits the expression of NLRP3 and NLRC4, and suppresses the activity of caspase-1 in TDI-exposed mice
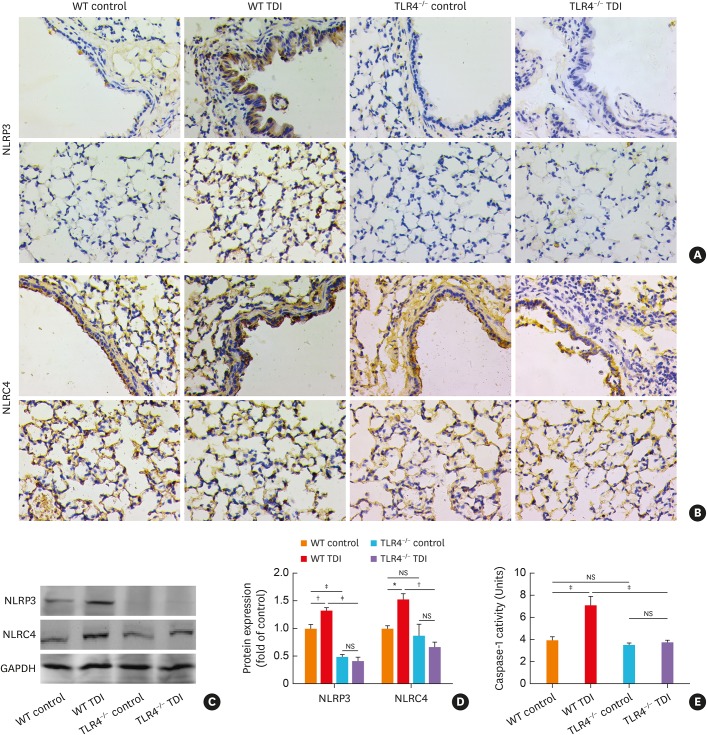
TLR4 signaling is known to mediate the activation of inflammasomes (especially NLRP3 and NLRC4) as the first signal in a broad spectrum of inflammatory processes,21,22,23 so that we detected the expression of NLRP3, NLRC4 and caspase-1 in the TDI immunized and challenged mice. As for NLRP3, immunohistochemistry showed that there was relatively weak staining in the lungs of control mice. TDI exposure led to increased immunoreactivity of NLPR3 especially distributed in the airway epithelium, alveolar epithelium and infiltrating inflammatory cells (Fig. 3A). In line with this, western blotting revealed up-regulated NLRP3 expression in the whole lung after TDI exposure (Fig. 3C and D), while TLR4 deficiency almost eliminated pulmonary NLRP3 expression in TDI-exposed mice (Fig. 3A, C, and D).
Fig. 3. TLR4-deficiency remarkably inhibited the activation of NLRP3, but slightly blunted NLRC4 in TDI-exposed mice. (A) Representative immunohistochemistry of NLRP3 in the bronchial regions (upper panel) and alveolar regions (lower panel). TLR4 deficiency significantly inhibited expression of NLRP3. Original magnification was 400×. (B) Representative immunohistochemistry of NLRC4 in the bronchial regions (upper panel) and alveolar regions (lower panel). TLR4 deficiency in C57/BL10 mice slightly blunted pulmonary expression of NLRC4. Original magnification was 400×. (C, D) Protein expressions of NLRP3, NLRC4 in lung homogenates were detected by Western blotting and densitometric analysis was performed (n = 6). (E) Levels of caspase-1 activity in lung homogenates of different groups (n = 4–6).
WT, wild-type; TLR4, toll-like receptor 4; TDI, toluene diisocyanate; NS, not significant; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; NLRP3, NOD-like receptor family, pyrin domain containing 3; NLRC4, NLR family CARD domain containing 4.
*P < 0.05; †P < 0.01; ‡P < 0.001.
As for NLRC4, there was relatively dense staining in the alveolar epithelia of the lung in control mice. Though it seems likely that TDI sensitization and challenge just slightly elevated NLRC4 expression in the immunohistochemistry of lung sections (Fig. 3B), western blotting showed TDI inhalation robustly raise the expression of NLRC4 in the whole lung (Fig. 3C and D). TLR4 deficiency also slightly repressed pulmonary NLRC4 expression as indicated by immunohistochemistry and western blotting (Fig. 3B-D). Similarly, the activity of caspase-1 was enhanced in TDI-exposed mice, which was completely diminished by the lack of TLR4 (Fig. 3E).
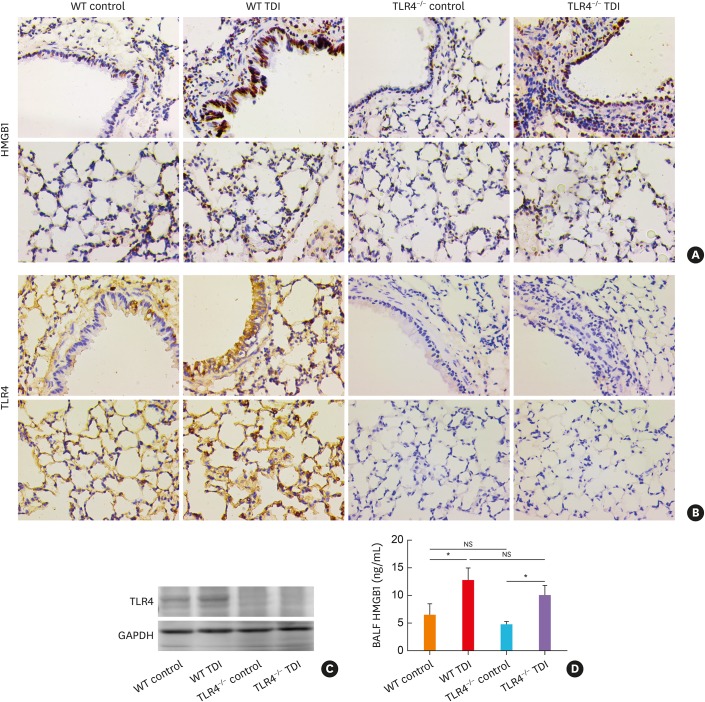
TLR4 deficiency does not affect HMGB1 production in TDI-treated mice
Although the activation of caspase-1 was inhibited by TLR4 deficiency, which has been demonstrated to contribute to TDI-induced airway inflammation in our previous study,15 TLR4 deficiency exacerbated TDI-induced neutrophil infiltration and remodeling. Then, we detected another classic neutrophil chemokine, HMGB1.24 As previously reported, TDI treatment enhanced the expression of HMGB1 in both lung tissue and BALF (Fig. 4A and D). Also, the TDI-exposed TLR4−/− mice exhibited similar pulmonary expression of HMGB1 (Fig. 4A). Consistent with this, ELISA revealed that the secreted HMGB1 level in BALF of TLR4-deficient mice did not differ from that of WT mice after TDI exposure (Fig. 4D).
Fig. 4. TLR4 deficiency did not affect HMGB1 production in TDI-treated mice. (A) Representative immunohistochemistry of HMGB1 in the bronchial regions (upper panel) and alveolar regions (lower panel). Original magnification was 400×. (B) Representative immunohistochemical staining of TLR4 in the bronchial regions (upper panel) and alveolar regions (lower panel). (C) Protein expression of TLR4, HMGB1 in lung homogenates were detected by Western blotting (n = 6). (D) Levels of HMGB1 in BALF of different groups were detected by ELISA (n = 4–6).
WT, wild-type; TLR4, toll-like receptor 4; TDI, toluene diisocyanate; NS, not significant; HMGB1, high mobility group box 1; BALF, bronchoalveolar lavage fluid; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; ELISA, enzyme-linked immunosorbent assay.
*P < 0.05.
TLR4 deficiency impairs neutrophil apoptosis via up-regulation of Bcl-2 in TDI-induced asthma
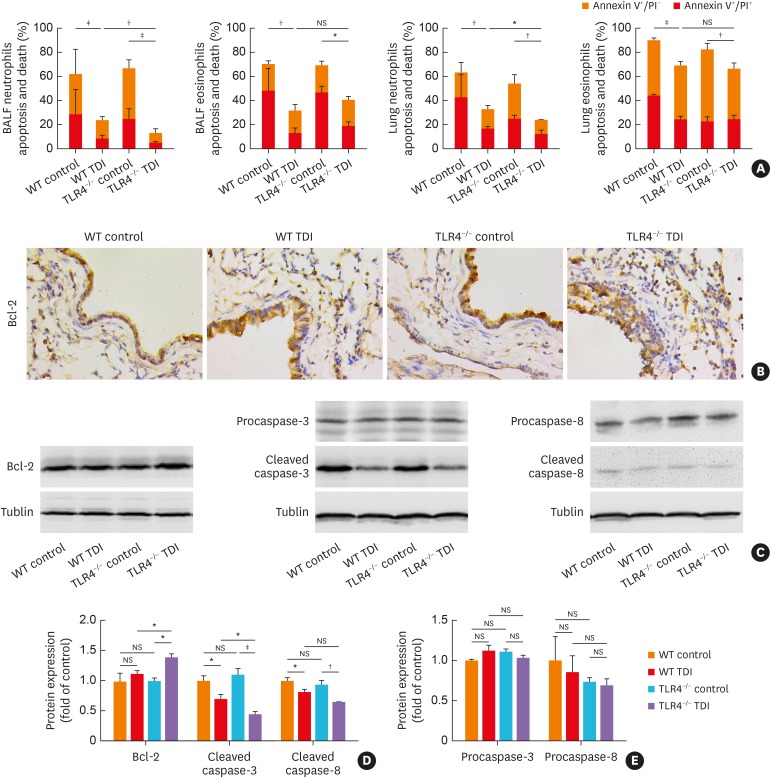
To test the hypothesis that TLR4 deficiency might exacerbate TDI-induced asthma by impairing granulocyte apoptosis, apoptosis of neutrophils and eosinophils was monitored. As expected, TDI exposure dramatically reduced the apoptosis of neutrophils and eosinophils (Fig. 5A). The percentage of neutrophils rather than eosinophils stained positive with annexin V was markedly decreased in TLR4-deficient mice compared with that in WT mice (Fig. 5A), indicating that TLR4 deficiency significantly hampered neutrophil apoptosis, with no obvious effect on eosinophil apoptosis.
Fig. 5. TLR4 deficiency impaired apoptosis of neutrophils, with pulmonary up-regulation of Bcl-2. (A) Numbers of apoptotic ex vivo eosinophils and neutrophils were determined by flow cytometry. (B) Representative immunohistochemistry of Bcl-2 in the bronchial regions. Original magnification was 400×. (C-E) Protein expressions of Bcl-2, procaspase-3, procaspase-8, cleaved caspase-3 and cleaved caspase-8 in lung homogenates were detected by Western blotting and densitometric analysis was performed (n = 6).
WT, wild-type; TLR4, toll-like receptor 4; TDI, toluene diisocyanate; NS, not significant; Bcl-2, B-cell lymphoma 2; BALF, bronchoalveolar lavage fluid; PI, propidium iodide.
*P < 0.05; †P < 0.01; ‡P < 0.001.
Two classic pathways are involved in apoptotic induction: the extrinsic apoptotic pathway (death receptors-initiated caspase-8-dependent) and the intrinsic apoptotic pathway (mitochondrion-initiated caspase-9-dependent pathway), of which, intrinsic apoptosis was strictly controlled by the anti-apoptotic protein Bcl-2, interacting with pro-apoptotic proteins.25 Here, we found that the protein expression of cleaved caspase-3 was markedly decreased in TLR4-deficient mice compared with that in WT mice (Fig. 5C-E) after TDI challenge, indicating that TLR4 deficiency hampered neutrophil apoptosis in a caspase-dependent manner. Besides, TDI inhalation significantly decreased the expression of cleaved caspase-8 in WT mice (Fig. 5A, C and D), which showed no significant differences between TLR4−/− and WT mice (Fig. 5C-E). Moreover, immunohistochemistry and western blotting demonstrated that, after TDI exposure, pulmonary expression of an anti-apoptotic protein Bcl-2 was higher in TLR4−/− mice than in WT mice, though TDI exposure did not increase its expression in WT mice (Fig. 5B-D).
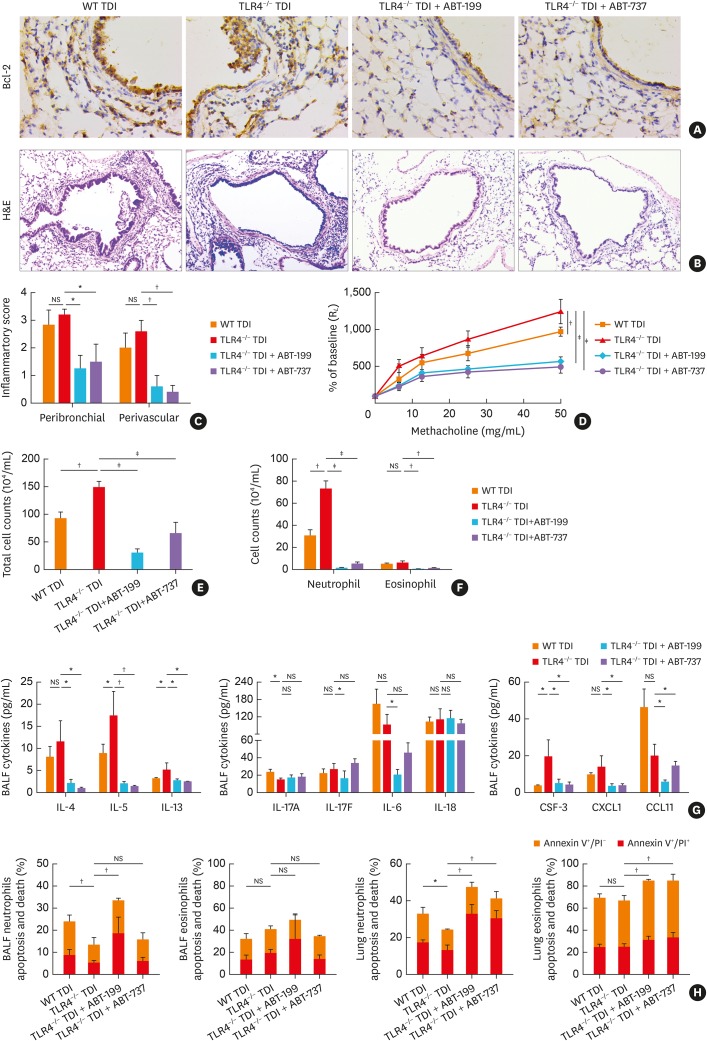
Given that TLR4 deficiency impaired neutrophil apoptosis with the up-regulation of Bcl-2, we used Bcl-2 inhibitors to find out whether this response is modulated by Bcl-2. Immunohistochemistry indicated that either ABT-199 or ABT-737 significantly inhibited the pulmonary expression of Bcl-2 in TLR4-deficient TDI-exposed mice (Fig. 6A). As expected, ABT-199 or ABT-737 treatment in TDI-exposed TLR4-deficient mice markedly attenuated airway inflammation (Fig.6B, C, E, and F), and significantly decreased AHR (Fig. 6D). The levels of IL-4, IL-5, IL-13, IL-17F, IL-6, CSF-3, CXCL1 and CCL11 were also lowered (Fig. 6G). Furthermore, flow cytometry showed that both inhibitors enhanced apoptosis of neutrophils and eosinophils in BALF and/or lung single cell suspension of TLR4-deficient asthmatic mice (Fig. 6H), which implied that TLR4 deficiency might impair neutrophil apoptosis via pulmonary up-regulation of Bcl-2.
Fig. 6. Bcl-2 inhibitors alleviated the aggravated airway inflammation in TDI-exposed TLR4–/– mice. (A) Representative immunohistochemistry of Bcl-2 in the bronchial regions. Original magnification was 400×. (B) Representative H&E-stained lung sections of different groups. Original magnification was 200×. (C) Semi-quantification of peribronchial and perivascular inflammation was performed (n = 5-6). (D) Airway hyperresponsiveness was measured by RL. Results were shown as percentage of baseline (n = 4). (E) Numbers of total inflammatory cell in BALF (n = 5–6). (F) Numbers of neutrophils, and eosinophils in BALF (n = 5–6). (G) Levels of Th2-related cytokines (IL-4, IL-5 and IL-13), Th17-related cytokines (IL-17A, IL-17F, IL-6 and IL-18), eosinophil chemoattractant CCL11, and neutrophil chemoattractant CSF-3, CXCL1 in BALF (n = 4–6). (H) Numbers of apoptotic ex vivo eosinophils and neutrophils were determined.
WT, wild-type; TLR4, toll-like receptor 4; TDI, toluene diisocyanate; NS, not significant; RL, lung resistance; Bcl-2, B-cell lymphoma 2; IL, interleukin; CCL11, C-C motif chemokine 11; CXCL1, chemokine (C-X-C motif) ligand 1; CSF-3, colony-stimulating factor 3; BALF, bronchoalveolar lavage fluid; Th, T helper; H&E, hematoxylin and eosin; PI, propidium iodide.
*P < 0.05; †P < 0.01; ‡P < 0.001.
DISCUSSION
The TDI-challenged asthma model is unique in that it results in aggregation of a large number of neutrophils and a handful of eosinophils into the airways which is insensitive to corticosteroids, and features airway remodeling,19 including ASM cell proliferation, goblet cells hyperplasia and extensive collagen deposition. This study revealed that inhibition of TLR4 using gene knockout mice aggravates TDI-induced AHR and airway inflammation, which is associated with Bcl-2-dependent impaired apoptosis of neutrophils resulted from TLR4 deficiency.
It is well documented that TLR4 engagement, as the first signaling, could trigger the activation of inflammasomes in an array of inflammatory responses.21,22,23 Inhibition of TLR4 would therefore interfere with downstream signals. Previously we have demonstrated that blockade of the NLRP3/caspase-1 axis effectively prevents neutrophil aggregation in TDI-induced asthma.15 Thus, we anticipated that the TLR4-deficient mice would exhibit ameliorated asthmatic inflammation after TDI inhalation. Actually, in the current study, immunohistochemistry and western blotting did indicate that the expressions of NLRP3 and NLRC4 as well as caspase-1 activity were dramatically suppressed by TLR4 deficiency after TDI challenge. However, we observed aggravated airway hypersensitivity, worsened bronchial epithelial shedding and heavier accumulation of neutrophils into the airway in TLR4-deficient mice after TDI inhalation, which are in striking contrast to our expectation. As one of the pattern recognition receptors abundantly expressed in the airway, TLR4 takes an important part in the detection of inspiratory stimuli, and once activated would trigger a list of immune-modulatory responses.3 Increased expressions of TLR4 were found in both serum and induced sputum of patients with asthma.26,27 Data from animal models induced by OVA and HDM revealed a controversial but significant role of TLR4 in the development of asthma.9,10,11,12 In the present study, lack of TLR4 exacerbates TDI-induced asthmatic responses, implying that TLR4 acts to counteract allergic airway inflammation induced by TDI. This is in agreement with what was found in an OVA-induced asthma model sensitized by the subcutaneous route,9 but in disagreement with the data in other HDM- or OVA-induced model immunized by the intranasal route,6,7 where TLR4 was indispensable for generating airway inflammation and Th2 responses. Indeed, TDI-induced asthmatic mice were dermally sensitized with 0.3% TDI on the dorsum of both ears, which was more similar to sensitization by the subcutaneous route but not by the intranasal route. Thus, this might be attributed to different sensitized routes among asthma models, though the exact mechanism is still open to further investigation.
HMGB1 is an identified pro-inflammatory mediator and when localized in the extracellular space, serves as an alarmin through diverse receptors, including TLR2, TLR4, TLR9 and RAGE, to mediate inflammation.28 Clinical studies and animal models have already demonstrated a role of HMGB1 and its receptors in airway inflammation and asthma.29,30,31 In this study, we observed that TDI inhalation gave rise to pulmonary expression of HMGB1, which agrees with previous studies indicating that HMGB1 was significantly up-regulated in OVA- and TDI-induced asthma, contributing to neutrophilia.31,32 However, TLR4−/− mice exhibited similar translocation and secretion of HMGB1 when compared with WT mice after TDI inhalation, accompanied by none-increased release of an array of neutrophil chemokines. These findings could not provide a satisfactory explanation for the aggravated airway inflammation of TLR4−/− mice after TDI exposure and drove us to test the hypothesis that TLR4 deficiency might exacerbate TDI-induced asthma by impairing apoptosis of neutrophils.
Apoptosis plays an essential role in tissue homeostasis of multicellular organisms.25 Infiltrating neutrophils are cleared from inflamed sites by apoptosis, which promotes resolution rather than persistence of tissue injury.33 Thus, attenuation of neutrophil apoptosis may lead to more severe and prolonged inflammatory responses.20 Studies have indicated a principal role of TLR4 in the regulation of neutrophil survival.34,35 In 2010, researchers from USA and China found that the absence of TLR4 worsened neutrophilic inflammation through inhibition of TLR4/IL-1RA signaling.36 Subsequently, the same research team reported that TLR4 promoted neutrophil apoptosis,37 indicating lack of TLR4 would result in airway neutrophil recruitment.36,38 Here, in the present study, TDI-exposed TLR4−/− mice exhibited a decrease in neutrophil apoptosis, contributing to the aggravation of TDI-induced airway inflammation, which suggested that TLR4 acts to promote apoptosis of neutrophils and therefore counteracts airway inflammation in TDI-induced asthma. In contrast, a large number of studies showed that TLR4 agonists delayed neutrophil apoptosis,35,39 while TLR4 antagonists blunted airway inflammation.4,5 Their finding seems opposite to ours given the crucial role of TLR4 in the regulation of neutrophil apoptosis. Yet, it should be noted that ligation of TLR4 to its ligands would result in not only the canonical MyD88-dependent signaling but also pathways independent of MyD88.40 Actually, TLR4 agonists were found to have only a modest direct inhibition of neutrophil apoptosis.35 In addition, TLR4 anti-inflammatory signaling is dependent upon a MyD88-independent pathway.36,41 Thus, it is reasonable that inhibition of TLR4 by gene-knockout mice may result in almost opposite outcomes from intervention of signaling using inhibitors. Further investigations are still needed to define the precise mechanism responsible for our observations.
Accumulating evidence has demonstrated that Bcl-2 plays a crucial role in the regulation of neutrophil apoptosis and could be a promising therapeutic target for steroid-resistant asthma.14,42,43 An anti-apoptotic protein, Bcl-2, could interact with pro-apoptotic proteins, which strictly control intrinsic apoptosis.44 Yet currently, there is limited evidence suggesting a direct link between TLR4 and Bcl-2 in the regulation of neutrophil apoptosis. Liu et al.45 discovered that TLR4 activation down-regulated the anti-apoptotic protein Bcl-2 and inhibited the signaling of the intrinsic apoptotic pathway in THP-1.45 Recently, several studies indicated that inhibition of TLR4 by siRNA or inhibitors increased the protein expression of Bcl-2 in vivo.46,47 In agreement with the results of Liu et al.,45 we detected increased expression of Bcl-2 rather than decreased expression of cleaved caspase-8 in TDI-treated TLR4−/− mice compared with WT mice, indicating that the intrinsic apoptotic pathway was mainly implicated in TLR4-induced neutrophil apoptosis. In addition, we also observed that either ABT-199 or ABT-737 could effectively promote neutrophil apoptosis in TDI-exposed TLR4-deficient mice, thereby markedly alleviating TDI-induced airway inflammation. This suggests that TLR4 may promote neutrophil apoptosis through inhibition of Bcl-2 in TDI-induced asthma.
To our knowledge, this is the first study to investigate the role of TLR4 in TDI-induced asthma and to demonstrate the possible mechanisms involved. However, it is noteworthy that even though Bcl-2 inhibitors could attenuate TDI-induced airway inflammation in TLR4-deficient mice, a direct causality between TLR4 and Bcl-2, especially the exact pathway by which TLR4 regulated Bcl-2, could not be established in the present study. However, our study clearly indicated TLR4 as a negative regulator in TDI-induced asthma and explored its possible mechanism, which provided us some new insights into the pathogenesis of TDI-induced asthma.
In conclusion, these data indicated TLR4 deficiency impaired neutrophil apoptosis via pulmonary up-regulation of Bcl-2, thereby resulting in deteriorated AHR and airway inflammation, which suggests that elevated TLR4 could protect against the development of TDI-induced asthma.
ACKNOWLEDGMENTS
This study was supported by National Key R&D Program of China (2017YFC1310601), National Natural Science Foundation of China (81871266, 81800022), Guangzhou Healthcare Collaborative Innovation Major Project (201604020012), and Scientific and Technological Project of Guangzhou (201604020008, 201804020042).
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
References
- 1.Masoli M, Fabian D, Holt S, Beasley R Global Initiative for Asthma (GINA) Program. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004;59:469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 2.Kim RY, Pinkerton JW, Essilfie AT, Robertson AA, Baines KJ, Brown AC, et al. Role for NLRP3 inflammasome-mediated, IL-1β-dependent responses in severe, steroid-resistant asthma. Am J Respir Crit Care Med. 2017;196:283–297. doi: 10.1164/rccm.201609-1830OC. [DOI] [PubMed] [Google Scholar]
- 3.Zakeri A, Russo M. Dual role of toll-like receptors in human and experimental asthma models. Front Immunol. 2018;9:1027. doi: 10.3389/fimmu.2018.01027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via toll-like receptor 4 triggering of airway structural cells. Nat Med. 2009;15:410–416. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang H, Li T, Han X, Sun J. TLR4 antagonist ameliorates combined allergic rhinitis and asthma syndrome (CARAS) by reducing inflammatory monocytes infiltration in mice model. Int Immunopharmacol. 2019;73:254–260. doi: 10.1016/j.intimp.2019.05.021. [DOI] [PubMed] [Google Scholar]
- 6.Phipps S, Lam CE, Kaiko GE, Foo SY, Collison A, Mattes J, et al. Toll/IL-1 signaling is critical for house dust mite-specific helper T cell type 2 and type 17 [corrected] responses. Am J Respir Crit Care Med. 2009;179:883–893. doi: 10.1164/rccm.200806-974OC. [DOI] [PubMed] [Google Scholar]
- 7.Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. 2002;196:1645–1651. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan AM, Chen HC, Pochard P, Eisenbarth SC, Herrick CA, Bottomly HK. TLR4 signaling in stromal cells is critical for the initiation of allergic Th2 responses to inhaled antigen. J Immunol. 2010;184:3535–3544. doi: 10.4049/jimmunol.0900340. [DOI] [PubMed] [Google Scholar]
- 9.Bortolatto J, Borducchi E, Rodriguez D, Keller AC, Faquim-Mauro E, Bortoluci KR, et al. Toll-like receptor 4 agonists adsorbed to aluminium hydroxide adjuvant attenuate ovalbumin-specific allergic airway disease: role of MyD88 adaptor molecule and interleukin-12/interferon-γ axis. Clin Exp Allergy. 2008;38:1668–1679. doi: 10.1111/j.1365-2222.2008.03036.x. [DOI] [PubMed] [Google Scholar]
- 10.Mirotti L, Alberca Custódio RW, Gomes E, Rammauro F, de Araujo EF, Garcia Calich VL, et al. CpG-ODN shapes alum adjuvant activity signaling via MyD88 and IL-10. Front Immunol. 2017;8:47. doi: 10.3389/fimmu.2017.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Korem T, Zeevi D, Suez J, Weinberger A, Avnit-Sagi T, Pompan-Lotan M, et al. Growth dynamics of gut microbiota in health and disease inferred from single metagenomic samples. Science. 2015;349:1101–1106. doi: 10.1126/science.aac4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barboza R, Câmara NO, Gomes E, Sá-Nunes A, Florsheim E, Mirotti L, et al. Endotoxin exposure during sensitization to Blomia tropicalis allergens shifts TH2 immunity towards a TH17-mediated airway neutrophilic inflammation: role of TLR4 and TLR2. PLoS One. 2013;8:e67115. doi: 10.1371/journal.pone.0067115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yao L, Zhao H, Tang H, Liang J, Liu L, Dong H, et al. The receptor for advanced glycation end products is required for β-catenin stabilization in a chemical-induced asthma model. Br J Pharmacol. 2016;173:2600–2613. doi: 10.1111/bph.13539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tian BP, Xia LX, Bao ZQ, Zhang H, Xu ZW, Mao YY, et al. Bcl-2 inhibitors reduce steroid-insensitive airway inflammation. J Allergy Clin Immunol. 2017;140:418–430. doi: 10.1016/j.jaci.2016.11.027. [DOI] [PubMed] [Google Scholar]
- 15.Chen S, Yao L, Huang P, He Q, Guan H, Luo Y, et al. Blockade of the NLRP3/caspase-1 axis ameliorates airway neutrophilic inflammation in a toluene diisocyanate-induced murine asthma model. Toxicol Sci. 2019;170:462–475. doi: 10.1093/toxsci/kfz099. [DOI] [PubMed] [Google Scholar]
- 16.Yao L, Zhao H, Tang H, Song J, Dong H, Zou F, et al. Chicken IgY facilitates allergic airway inflammation in a chemical-induced murine asthma model by potentiating IL-4 release. Toxicol Lett. 2015;239:22–31. doi: 10.1016/j.toxlet.2015.08.1108. [DOI] [PubMed] [Google Scholar]
- 17.Yao L, Chen S, Tang H, Huang P, Wei S, Liang Z, et al. Transient receptor potential ion channels mediate adherens junctions dysfunction in a toluene diisocyanate-induced murine asthma model. Toxicol Sci. 2019;168:160–170. doi: 10.1093/toxsci/kfy285. [DOI] [PubMed] [Google Scholar]
- 18.Cho JY, Miller M, McElwain K, McElwain S, Broide DH. Combination of corticosteroid therapy and allergen avoidance reverses allergen-induced airway remodeling in mice. J Allergy Clin Immunol. 2005;116:1116–1122. doi: 10.1016/j.jaci.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 19.Chen R, Zhang Q, Chen S, Tang H, Huang P, Wei S, et al. IL-17F, rather than IL-17A, underlies airway inflammation in a steroid-insensitive toluene diisocyanate-induced asthma model. Eur Respir J. 2019;53:1801510. doi: 10.1183/13993003.01510-2018. [DOI] [PubMed] [Google Scholar]
- 20.Matute-Bello G, Liles WC, Radella F, 2nd, Steinberg KP, Ruzinski JT, Jonas M, et al. Neutrophil apoptosis in the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1997;156:1969–1977. doi: 10.1164/ajrccm.156.6.96-12081. [DOI] [PubMed] [Google Scholar]
- 21.Yu R, Jiang S, Tao Y, Li P, Yin J, Zhou Q. Inhibition of HMGB1 improves necrotizing enterocolitis by inhibiting NLRP3 via TLR4 and NF-κB signaling pathways. J Cell Physiol. 2019;234:13431–13438. doi: 10.1002/jcp.28022. [DOI] [PubMed] [Google Scholar]
- 22.Jabir MS, Ritchie ND, Li D, Bayes HK, Tourlomousis P, Puleston D, et al. Caspase-1 cleavage of the TLR adaptor TRIF inhibits autophagy and β-interferon production during Pseudomonas aeruginosa infection. Cell Host Microbe. 2014;15:214–227. doi: 10.1016/j.chom.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 23.Chassaing B, Koren O, Carvalho FA, Ley RE, Gewirtz AT. AIEC pathobiont instigates chronic colitis in susceptible hosts by altering microbiota composition. Gut. 2014;63:1069–1080. doi: 10.1136/gutjnl-2013-304909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Du F, Hawez A, Mörgelin M, Thorlacius H. Neutrophil extracellular trap-microparticle complexes trigger neutrophil recruitment via high-mobility group protein 1 (HMGB1)-toll-like receptors(TLR2)/TLR4 signalling. Br J Pharmacol. 2019;176:3350–3363. doi: 10.1111/bph.14765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hotchkiss RS, Strasser A, McDunn JE, Swanson PE. Cell death. N Engl J Med. 2009;361:1570–1583. doi: 10.1056/NEJMra0901217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wood LG, Garg ML, Gibson PG. A high-fat challenge increases airway inflammation and impairs bronchodilator recovery in asthma. J Allergy Clin Immunol. 2011;127:1133–1140. doi: 10.1016/j.jaci.2011.01.036. [DOI] [PubMed] [Google Scholar]
- 27.Crespo-Lessmann A, Mateus E, Vidal S, Ramos-Barbón D, Torrejón M, Giner J, et al. Expression of toll-like receptors 2 and 4 in subjects with asthma by total serum IgE level. Respir Res. 2016;17:41. doi: 10.1186/s12931-016-0355-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris HE, Andersson U, Pisetsky DS. HMGB1: a multifunctional alarmin driving autoimmune and inflammatory disease. Nat Rev Rheumatol. 2012;8:195–202. doi: 10.1038/nrrheum.2011.222. [DOI] [PubMed] [Google Scholar]
- 29.Watanabe T, Asai K, Fujimoto H, Tanaka H, Kanazawa H, Hirata K. Increased levels of HMGB-1 and endogenous secretory RAGE in induced sputum from asthmatic patients. Respir Med. 2011;105:519–525. doi: 10.1016/j.rmed.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 30.Cuppari C, Manti S, Chirico V, Caruso R, Salpietro V, Giacchi V, et al. Sputum high mobility group box-1 in asthmatic children: a noninvasive sensitive biomarker reflecting disease status. Ann Allergy Asthma Immunol. 2015;115:103–107. doi: 10.1016/j.anai.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 31.Hou C, Kong J, Liang Y, Huang H, Wen H, Zheng X, et al. HMGB1 contributes to allergen-induced airway remodeling in a murine model of chronic asthma by modulating airway inflammation and activating lung fibroblasts. Cell Mol Immunol. 2015;12:409–423. doi: 10.1038/cmi.2014.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liang J, Zhao H, Yao L, Tang H, Dong H, Wu Y, et al. Phosphatidylinositol 3-kinases pathway mediates lung caspase-1 activation and high mobility group box 1 production in a toluene-diisocyanate induced murine asthma model. Toxicol Lett. 2015;236:25–33. doi: 10.1016/j.toxlet.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 33.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 34.Sabroe I, Prince LR, Jones EC, Horsburgh MJ, Foster SJ, Vogel SN, et al. Selective roles for Toll-like receptor (TLR)2 and TLR4 in the regulation of neutrophil activation and life span. J Immunol. 2003;170:5268–5275. doi: 10.4049/jimmunol.170.10.5268. [DOI] [PubMed] [Google Scholar]
- 35.Sabroe I, Dower SK, Whyte MK. The role of Toll-like receptors in the regulation of neutrophil migration, activation, and apoptosis. Clin Infect Dis. 2005;41(Suppl 7):S421–6. doi: 10.1086/431992. [DOI] [PubMed] [Google Scholar]
- 36.Zhao H, Leu SW, Shi L, Dedaj R, Zhao G, Garg HG, et al. TLR4 is a negative regulator in noninfectious lung inflammation. J Immunol. 2010;184:5308–5314. doi: 10.4049/jimmunol.1000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leu SW, Shi L, Xu C, Zhao Y, Liu B, Li Y, et al. TLR4 through IFN-β promotes low molecular mass hyaluronan-induced neutrophil apoptosis. J Immunol. 2011;186:556–562. doi: 10.4049/jimmunol.1001630. [DOI] [PubMed] [Google Scholar]
- 38.Pace E, Giarratano A, Ferraro M, Bruno A, Siena L, Mangione S, et al. TLR4 upregulation underpins airway neutrophilia in smokers with chronic obstructive pulmonary disease and acute respiratory failure. Hum Immunol. 2011;72:54–62. doi: 10.1016/j.humimm.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 39.François S, El Benna J, Dang PM, Pedruzzi E, Gougerot-Pocidalo MA, Elbim C. Inhibition of neutrophil apoptosis by TLR agonists in whole blood: involvement of the phosphoinositide 3-kinase/Akt and NF-kappaB signaling pathways, leading to increased levels of Mcl-1, A1, and phosphorylated Bad. J Immunol. 2005;174:3633–3642. doi: 10.4049/jimmunol.174.6.3633. [DOI] [PubMed] [Google Scholar]
- 40.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 41.Shalaby KH, Al Heialy S, Tsuchiya K, Farahnak S, McGovern TK, Risse PA, et al. The TLR4-TRIF pathway can protect against the development of experimental allergic asthma. Immunology. 2017;152:138–149. doi: 10.1111/imm.12755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geng X, Wang X, Luo M, Xing M, Wu Y, Li W, et al. Induction of neutrophil apoptosis by a Bcl-2 inhibitor reduces particulate matter-induced lung inflammation. Aging (Albany NY) 2018;10:1415–1423. doi: 10.18632/aging.101477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Croker BA, O'Donnell JA, Nowell CJ, Metcalf D, Dewson G, Campbell KJ, et al. Fas-mediated neutrophil apoptosis is accelerated by Bid, Bak, and Bax and inhibited by Bcl-2 and Mcl-1. Proc Natl Acad Sci U S A. 2011;108:13135–13140. doi: 10.1073/pnas.1110358108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Delbridge AR, Strasser A. The BCL-2 protein family, BH3-mimetics and cancer therapy. Cell Death Differ. 2015;22:1071–1080. doi: 10.1038/cdd.2015.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu B, Sun R, Luo H, Liu X, Jiang M, Yuan C, et al. Both intrinsic and extrinsic apoptotic pathways are involved in toll-like receptor 4 (TLR4)-induced cell death in monocytic THP-1 cells. Immunobiology. 2017;222:198–205. doi: 10.1016/j.imbio.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 46.Wang L, Song LF, Chen XY, Ma YL, Suo JF, Shi JH, et al. MiR-181b inhibits P38/JNK signaling pathway to attenuate autophagy and apoptosis in juvenile rats with kainic acid-induced epilepsy via targeting TLR4. CNS Neurosci Ther. 2019;25:112–122. doi: 10.1111/cns.12991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu T, Zhang K, Kan F, Li F, Yu B, Du W, et al. Adeno-associated virus 9-mediated small RNA interference of TLR4 alleviates myocardial ischemia and reperfusion injury by inhibition of the NF-κB and MAPK signaling pathways in rats. Curr Mol Med. 2019;19:127–135. doi: 10.2174/1566524019666190311122521. [DOI] [PubMed] [Google Scholar]