Abstract
Purpose
Recent clinical trials have successfully used oral immunotherapy (OIT) to treat food allergies. Probiotics have immunomodulatory effects by balancing Th1/Th2 immunity and enhancing regulatory T-cell activity. In this study, we analyzed the effects of OIT, probiotics alone, and probiotics administered simultaneously with OIT in a mouse model of egg allergy.
Methods
C3H/HeJ mice were sensitized by intragastric administration of ovomucoid (OM) with cholera toxin. For the OIT regime, increasing doses of OM were administered orally to sensitized mice. Lactobacillus casei variety ramnosus (Lcr35) was also administered. The mice were divided into 4 groups: control (no OIT), OIT, Lcr35, and OIT plus Lcr35 (OIT + Lcr35). The effects of OIT and Lcr35 treatment were estimated based on the symptom score, rectal temperature, serum levels of OM-specific immunoglobulin (Ig)E, IgA, IgG1, and IgG2a immediately after and 2 weeks after ceasing treatment and histological staining of the small intestine.
Results
The severity of anaphylaxis decreased in all treatment groups. Simultaneous administration of Lcr35 and OIT decreased the severity of anaphylaxis compared to controls and the OIT group. The protective effects were sustained 2 weeks after ceasing treatment in all treatment groups. A significant decrease in OM-specific IgA, IgG1, and IgG2a levels was observed in both the OIT and OIT plus Lcr35 groups. However, a significant decrease in the OM-specific IgE level was observed only in OIT plus Lcr35 treated mice and was sustained 2 weeks after ceasing treatment. Mucin amounts in the small intestine decreased after OIT, OIT plus Lcr35, and Lcr35 treatment with the lowest in the OIT plus Lcr35 group.
Conclusions
Lcr35 treatment during OIT had some synergic effect for protection against anaphylaxis in a mice model of egg allergy. These findings should be confirmed in future animal studies including more detailed immunological profiles and human studies.
Keywords: Food hypersensitivity; immunotherapy; Lactobacillus rhamnosus; probiotics, IgE
INTRODUCTION
Food allergies are believed to affect between 6% and 8% of young children and 2% of adults,1,2 and are increasing in prevalence.3,4 Allergic reactions to various food allergens can range from mild reactions to anaphylaxis and cause a decrease in body temperature.3,5 Most children develop tolerance during the first 5 years of life. However, some persist until late childhood or adulthood, resulting in significant negative effects on their quality of life. Egg allergy is the most common food allergy along with cow's milk allergy in children,5,6,7 which have a wide spectrum of clinical presentations, including anaphylaxis.
The treatment of food allergies relies on dietary avoidance of the inciting food or food protein until tolerance has developed. In recent years, there has been increasing success in clinical trials of food oral immunotherapy (OIT),8,9,10,11 and OIT is considered a promising treatment for providing protection against allergic reactions caused by accidental food exposure. Clinical trials have mainly focused on egg, milk, and peanut allergies.12 The majority of studies have shown that OIT can induce ‘desensitization,’ the transient ability to tolerate a food that is lost when OIT is stopped.13,14 However, its ability to induce tolerance that lasts after stopping OIT remains unclear.13,14,15,16 Along with safety problems, this restricts the general use of OIT in clinical practice.
The underlying immune mechanisms induced by allergen immunotherapy remain unclear. Possible mechanisms include induction of immunoglobulin (Ig)G4-blocking antibody, loss of effector Th2 cells, and induction of regulatory T cells.17 The mechanisms of clinical tolerance during OIT require further studies.18
Specific modalities have been developed to augment the immune modulatory effects of OIT, such as using adjuvants including anti-IgE, pre/probiotics, and biologics.16,17,19 Among these modalities, probiotics may be the most promising because of their immunomodulatory effects and safety profile.
Probiotics have immunomodulatory effects by balancing Th1/Th2 immunity and enhancing regulatory T-cell activity. Several studies have documented such effects in mouse models of food allergy.20,21,22,23,24 Therefore, some synergistic effects are expected when probiotics are simultaneously administered during OIT. However, few studies have explored the use of probiotics during food OIT in both mice and humans.
We analyzed the effects of OIT, probiotics alone, and the synergistic effects of probiotics during OIT in a mouse model of egg allergy. Moreover, we explored whether probiotics could induce tolerance through a synergistic effect on egg OIT.
MATERIALS AND METHODS
Ethics statement
All mice were treated in accordance with the Guide for the Care and Use of Laboratory Animals as adopted by the Soonchunhyang University College of Medicine. All animal studies were approved by the Institutional Animal Care and Use Committee at Soonchunhyang University Bucheon Hospital (SCHBC_2016_01).
Egg allergy model of mice
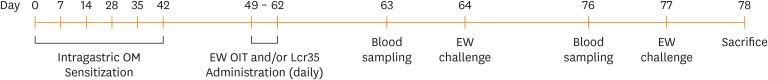
Female, 3-week-old C3H/HeJ mice were sensitized by intragastric administration of 1 mg of ovomucoid (OM; Sigma-Aldrich, St Louis, MO, USA) with 10 µg of cholera toxin (List Biological Laboratories, Campbell, CA, USA) as an adjuvant weekly for 6 weeks. The mice were divided into 4 groups (n = 18 in each group): controls (no OIT), the OIT group, the Lactobacillus casei rhamnosus (Lcr35) group, and the OIT combined with Lcr35 group (OIT + Lcr35). The mouse egg allergy model was generated according to Leonard et al.25 The OIT and Lcr35 (Hanwha Pharma, Seoul, Korea) was administered daily for 2 weeks. On days 64 and 77, allergen challenge was performed. On day 78, mice were sacrificed (Fig. 1). Then we collected the small intestine, and histology analyses were performed.22,23,25,26
Fig. 1. Experimental design of the food allergy model with OIT and probiotic (Lcr35) treatment.
EW, egg white; OM, ovomucoid; OIT, oral immunotherapy.
Administration of OIT
Mice were challenged 1 week after the last sensitization. The OIT was performed daily through the drinking water with egg white protein (EW; Sigma-Aldrich) for 2 weeks. During OIT, the administered dose of EW was increased: 1 mg (days 1 and 2), 5 mg (days 3 and 4), 10 mg (days 5-7), 25 mg (days 8 and 9), and 50 mg (days 10-14). The oral administration was based on a drinking volume of 5 mL/day. Oral administration was performed according to Leonard et al.25
Lcr35 preparation
Lcr35 probiotics used in this experiment were in the form of sterilized lyophilized powder obtained from Hanwha Pharma. Lcr35 probiotics (1 × 109 colony-forming units/250 µL/mouse/day) was reconstituted with 250 µL of sterile phosphate buffered saline. In this method, an oral feeding cannula gavage was attached to the syringe to deliver Lcr35 probiotics. Lcr35 probiotics were orally administered daily to the sensitized mice for 2 weeks (days 49-62) 1 week after the last sensitization.
Allergen challenge
To explore the effects of OIT, Lcr35, and OIT with Lcr35, oral challenges were performed with 25 mg of OM 1 day (63 day) and 14 days (76 day) after the discontinuation of OIT and/or Lcr35. Based on the symptom score of anaphylaxis and rectal temperature measured 30 minutes after the challenge, anaphylaxis severity was graded. Symptom scores were graded as follows: 0 = no symptoms; 1 = scratching around nose and head; 2 = puffiness around the eyes and mouth with reduced activity; 3 = labored respiration, cyanosis around the mouth and tail, or both; 4 = no activity after prodding or tremor and convulsion; and 5 = death.25,26
Measurement of antibodies in serum
OM-specific IgA, IgE, IgG1, and IgG2a levels were quantified using the enzyme-linked immunosorbent assay (ELISA) assay kit (Life diagnostics Inc., West Chester, PA, USA) according to the manufacturer's instructions. Serum extracts were loaded on the ELISA plate. The absorbance was measured at 450 nm on a Microplate Reader.
Histological analyses
The samples of small intestine tissue from mice were flushed with saline, fixed in 4% paraformaldehyde, processed, and infiltrated with paraffin. Paraffin sections were cut with a microtome, deparaffinized, and rehydrated in an ethanol series. Sections were stained with hematoxylin and eosin or with Alcian blue. The number of goblet cells per crypt-villus was measured (5 randomly selected fields/16 mice per group) using ImageJ software (National Institutes of Health, Bethesda, MD, USA). To compare the number of goblet cells per crypt-villus between the 2 groups, Mann-Whitney U test was used.
Statistical analyses
Data were analyzed using SPSS version 18.0 software (SPSS Inc., Chicago, IL, USA) using the 2-sample t test, the Mann-Whitney U test, and Pearson's χ2 test for normally distributed, skewed, and categorical data, respectively. All P values of less than 0.05 were considered to indicate statistical significance. Results are expressed as the mean ± standard error of the mean unless otherwise stated.
RESULTS
Effects of oral administration of OIT and Lcr35 probiotics on the severity of anaphylaxis in a mouse model of egg allergy
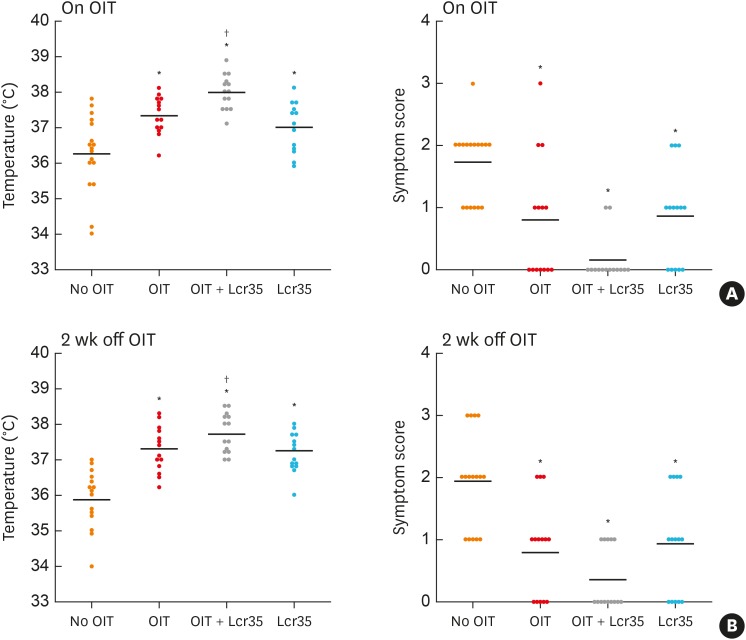
Mice treated with OIT were protected from OM-induced anaphylaxis compared to the control group. Simultaneous administration of Lcr35 during OIT (OIT + Lcr35) significantly reduced the severity of anaphylaxis compared to controls and the OIT group. The administration of Lcr35 alone also decreased the symptom score and had a protective effect on rectal temperature compared to the control group. However, the Lcr35 group did not show significant changes in the symptom score or rectal temperature compared to the OIT group. The clinical protective effects remained 2 weeks after ceasing treatment in all treatment groups. These results support the synergistic immunomodulatory effects of Lcr35 probiotics on OIT in a mouse model of food allergy (Fig. 2).
Fig. 2. Anaphylaxis symptom scores and rectal temperature. Mice were orally challenged with ovomucoid on day 1 and 14 after discontinuation of OIT and/or Lcr35 treatment. (A) Day 1 after discontinuation of OIT (on OIT) and (B) day 14 after discontinuation of OIT.
OIT, oral immunotherapy.
*P < 0.05 compared to the control group (vs. No OIT); †P < 0.05 compared to the OIT group (vs. OIT).
Changes in the serum level of OM-specific IgE and IgA after treatment with OIT and Lcr35 probiotics
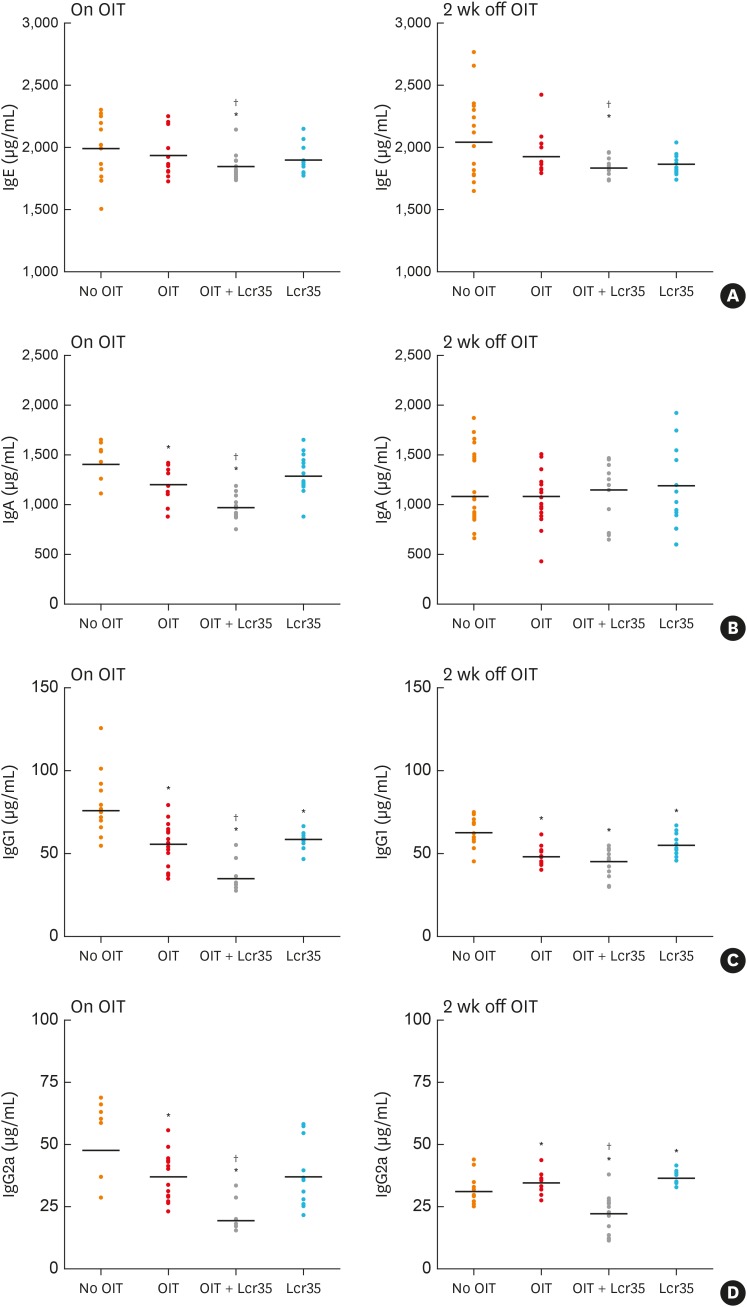
OM-specific Ig levels were measured after treatment. A significant decrease in the OM-specific IgE level was observed only in OIT + Lcr35 treated mice, not in mice treated separately with OIT and Lcr35, and the decrease was sustained 2 weeks ceasing treatment. The level of OM-specific IgA significantly decreased in mice treated with OIT and OIT + Lcr35. Compared to the OIT group, the combined treatment of Lcr35 and OIT significantly reduced the level of OM-specific IgA. However, the difference in OM-specific IgA level between the groups was not significant 2 weeks after ceasing treatment (Fig. 3).
Fig. 3. Serum levels of OM-specific Ig measured via enzyme-linked immunosorbent assay on days 1 (on OIT) and 14 after discontinuation of OIT and/or Lcr35 treatment. (A) OM-specific IgE levels, (B) OM-specific IgA levels, (C) OM-specific IgG1 levels, and (D) OM-specific IgG2a levels.
OM, ovomucoid; OIT, oral immunotherapy; Ig, immunoglobulin.
*P < 0.05 compared to the control group (vs. No OIT); †P < 0.05 compared to the OIT group (vs. OIT).
Changes in the serum level of OM-specific IgG1 and IgG2a levels after treatment with OIT and Lcr35 probiotics
We investigated the effect of OIT and Lcr35 probiotics on the serum level of OM-specific IgG1 (Th2 response) and IgG2 (Th1 response). The levels of OM-specific IgG1 significantly decreased after treatment with OIT, Lcr35, and OIT + Lcr35. The levels of IgG2a, which were expected to increase after treatment, also decreased after each treatment. However, 2 weeks after ceasing treatment, they increased in the OIT and Lcr35 groups compared to control mice. Unlike the OIT and Lcr35 group, mice treated with Lcr35 + OIT had lower levels than controls and the OIT group 2 weeks after ceasing treatment. In all treatment groups, the patterns of OM-specific IgG1 levels 2 weeks after ceasing treatment remained the same as immediately after ceasing treatment (on OIT) (Fig. 3).
Changes in mucus secretion in the small intestine after treatment with OIT and Lcr35 probiotics
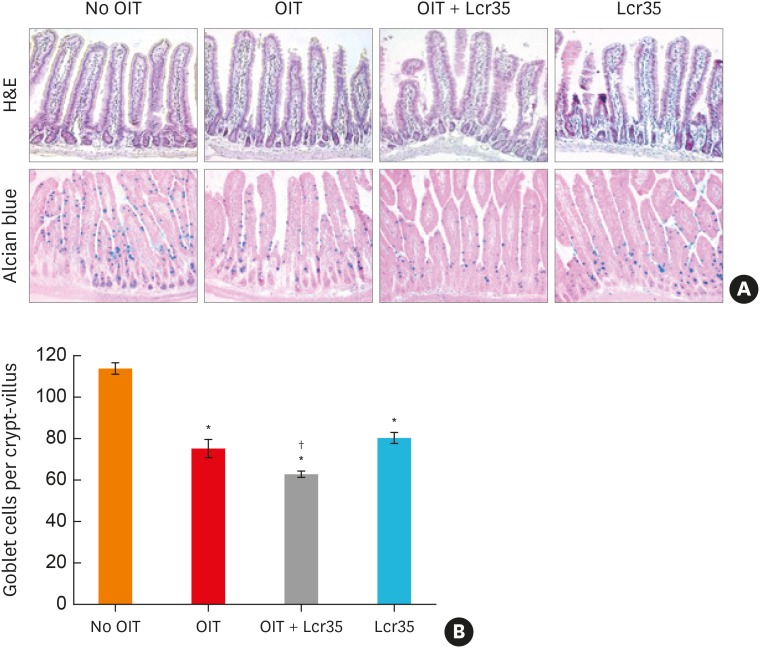
Analyses of mucus expression levels were performed using Alcian blue staining in the small intestine. Acidic mucins are stained blue in the small intestine. In all treatment groups including the Lcr35 group, the amount of mucin significantly decreased compared to the no OIT group. In addition, the decrease in mucin was most significant in the OIT + Lcr35 treatment group (Fig. 4), suggesting that Lcr35 treatment during OIT has a synergistic effect on mucin suppression in a food allergy model.
Fig. 4. Histological findings of the small intestine of mice treated with egg OIT and Lcr35. (A) Hematoxylin and eosin stain (upper, ×200) and Alcian blue stain (lower, ×400). (B) Mucus cell count in the small intestine.
OIT, oral immunotherapy.
*P < 0.05, compared to the control group; †P < 0.05 compared to the OIT group.
DISCUSSION
OIT for the treatment of food allergy induces immune modulation and desensitization,8 but its ability to induce tolerance has not been well-explored. Previous studies have supported the effects of OIT in mouse models of food allergy.25,27,28 Among the modalities under investigation to augment the immunomodulatory effects of OIT, we investigated the effects of oral probiotics during OIT and demonstrated that Lcr35 treatment during OIT has some synergic effect to augment the effects of OIT. Lcr35 treatment alone also had a protective effect against anaphylaxis.
Recent increases in allergic diseases may be associated with a lower incidence of infectious diseases and decreased exposure to microorganisms due to a more hygienic lifestyle. Probiotic microorganisms have immunomodulatory effects on dendritic and T-cell responses, although their immunomodulatory effects depend on the strains. Recently, the use of probiotics has been proposed as a promising treatment modality for the management of allergic diseases. In a murine model of food allergy, oral treatment of a probiotic mixture was effective in redirecting Th2-polarized immune responses towards Th1 and T regulatory responses and had protective effects against allergen-induced anaphylaxis.21,28 Similarly, we showed that the administration of Lcr35 alone had some protective effect against anaphylaxis in a mouse model of egg allergy, but its effect was less significant than OIT treatment.
Previous studies have shown that probiotics do not accelerate tolerance induction in patients with food allergy. Supplementation of L. casei and Bifidobacterium lactis to extensively hydrolyzed formula does not accelerate milk tolerance in infants with allergy to cow's milk.29
Few studies have explored the use of probiotics during food OIT in mice or humans. Two studies (although not for food allergies) showed that probiotics can be useful as an adjuvant for sublingual immunotherapy. One study suggested that Bifidobacterium bifidum is a valid candidate adjuvant for specific immunotherapy of type I allergies.29 According to Moussu et al., 30 and Van Overtvelt et al., 31 lactic acid bacteria can be useful as an adjuvant to enhance sublingual immunotherapy efficacy in a murine asthma model. A recent study in patients with peanut allergy showed that coadministration of a probiotic and peanut OIT may induce sustained unresponsiveness and immune changes,32 and its follow-up study 4 years after treatment cessation provided long-lasting clinical effect and persistent suppression of the allergic immune response to peanut.33 However, that study did not clarify the relative effects of co-administered probiotics during OIT compared to OIT alone. The effect of probiotics plus OIT was only compared with that of placebo, not with that of OIT or probiotics monotherapy. To determine the synergistic effect of probiotics during OIT, it should be compared with the effect of OIT without the administration of probiotics. This is not easy in clinical practice, so we used a mouse egg allergy model to compare the effect of OIT plus probiotics with that of OIT or probiotics alone. We explored whether probiotics have a beneficial effect on the induction of sustained unresponsiveness through synergistic effects on egg OIT.
In the present study, simultaneous administration of Lcr35 during OIT significantly reduced the severity of anaphylaxis and the level of OM-specific IgE and IgA compared to controls and OIT alone. The level of OM-specific IgG1 also significantly decreased compared to controls and the OIT group. The amount of mucin in the small intestine decreased after OIT, OIT + Lcr35, and Lcr35 treatment with the lowest level in the OIT + Lcr35 treatment group. These results support the synergistic immunomodulatory effects of Lcr35 probiotics on OIT in a mouse model of food allergy. However, further studies are required to clarify the synergistic effects of probiotics, including more detailed immunological profiles in mice and humans.
Leonard et al.25 reported that OIT resulted in clinical protection (desensitization) against food-induced anaphylaxis, but not tolerance 2-weeks after discontinuation of OIT in a mouse model. However, in the present study with the same mouse model, the protective effects were sustained 2 weeks after ceasing treatment in all treatment groups, including the OIT group. The explanation for the different results after prolonged off-treatment remains unclear. However, it may be partly due to differences in probiotic species.
Allergen immunotherapy induces the production of allergen-specific IgG, particularly IgG4 functioning as a blocking antibody. In mice, allergen-specific IgG2a inhibits IgE-mediated mast cell activation.34 The effects of immunotherapy may be associated with the ratio of allergen-specific IgE to IgG antibodies.35 However, the importance of IgG-induction in the clinical efficacy of allergen immunotherapy remains controversial. The levels of OVA-specific IgG1 and IgG2a antibodies have been shown to inhibit IgE-triggered mast-cell activation in mice.30 Although previous studies have shown no significant difference in the serum levels of OM-specific IgG1 and IgG2a between OIT-treated mice and controls,25 we observed differences between treated mice and controls. Unexpectedly, the levels of IgG2 significantly decreased after treatment with OIT, Lcr35, and OIT + Lcr35.
It is known that IgA plays a role in protection against allergy and may be important in the development of immune tolerance after OIT.36,37 In a recent study, IgA significantly increased after the rush phase of OIT in patients with egg allergy and then decreased during the maintenance phase.36 Contrary to our expectations, the level of OM-specific IgA decreased after treatment with OIT, Lcr35, and OIT + Lcr35. However, further studies are required to explore the role of allergen-specific IgA during OIT and probiotic treatment.
Overall, we observed that combined treatment of Lcr35 during OIT had a synergistic effect for protection against anaphylaxis and perhaps a partial effect on the development of sustained unresponsiveness in a mouse model of egg allergy. Lcr35 treatment alone also showed some protective effect against anaphylaxis. These findings remain controversial and should be confirmed in human studies. Moreover, further experiments are required, including more detailed immunological profiles such as spleen cell analyses and cytokine profiles.
ACKNOWLEDGMENTS
This study was partly supported by a “Sama Research Grant” in 2016 and the Soonchunhyang University Research Fund.
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
References
- 1.Branum AM, Lukacs SL. Food allergy among children in the United States. Pediatrics. 2009;124:1549–1555. doi: 10.1542/peds.2009-1210. [DOI] [PubMed] [Google Scholar]
- 2.Sampson HA. Food allergy. Part 1: immunopathogenesis and clinical disorders. J Allergy Clin Immunol. 1999;103:717–728. doi: 10.1016/s0091-6749(99)70411-2. [DOI] [PubMed] [Google Scholar]
- 3.Gupta R, Sheikh A, Strachan DP, Anderson HR. Time trends in allergic disorders in the UK. Thorax. 2007;62:91–96. doi: 10.1136/thx.2004.038844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeong K, Kim J, Ahn K, Lee SY, Min TK, Pyun BY, et al. Age-based causes and clinical characteristics of immediate-type food allergy in Korean children. Allergy Asthma Immunol Res. 2017;9:423–430. doi: 10.4168/aair.2017.9.5.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyano-Martínez T, García-Ara C, Díaz-Pena JM, Martín-Esteban M. Prediction of tolerance on the basis of quantification of egg white-specific IgE antibodies in children with egg allergy. J Allergy Clin Immunol. 2002;110:304–309. doi: 10.1067/mai.2002.126081. [DOI] [PubMed] [Google Scholar]
- 6.Park M, Kim D, Ahn K, Kim J, Han Y. Prevalence of immediate-type food allergy in early childhood in Seoul. Allergy Asthma Immunol Res. 2014;6:131–136. doi: 10.4168/aair.2014.6.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perezábad L, Reche M, Valbuena T, López-Fandiño R, Molina E, López-Expósito I. Oral food desensitization in children with IgE-mediated cow's milk allergy: immunological changes underlying desensitization. Allergy Asthma Immunol Res. 2017;9:35–42. doi: 10.4168/aair.2017.9.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burks AW, Jones SM, Wood RA, Fleischer DM, Sicherer SH, Lindblad RW, et al. Oral immunotherapy for treatment of egg allergy in children. N Engl J Med. 2012;367:233–243. doi: 10.1056/NEJMoa1200435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchanan AD, Green TD, Jones SM, Scurlock AM, Christie L, Althage KA, et al. Egg oral immunotherapy in nonanaphylactic children with egg allergy. J Allergy Clin Immunol. 2007;119:199–205. doi: 10.1016/j.jaci.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 10.Kim JD, Kim SY, Kwak EJ, Sol IS, Kim MJ, Kim YH, et al. Reduction rate of specific IgE level as a predictor of persistent egg allergy in children. Allergy Asthma Immunol Res. 2019;11:498–507. doi: 10.4168/aair.2019.11.4.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma G, Im SH. Probiotics as a potential immunomodulating pharmabiotics in allergic diseases: current status and future prospects. Allergy Asthma Immunol Res. 2018;10:575–590. doi: 10.4168/aair.2018.10.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hussey Freeland DM, Fan-Minogue H, Spergel JM, Chatila TA, Nadeau KC. Advances in food allergy oral immunotherapy: toward tolerance. Curr Opin Immunol. 2016;42:119–123. doi: 10.1016/j.coi.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang ML, Martino DJ. Oral immunotherapy and tolerance induction in childhood. Pediatr Allergy Immunol. 2013;24:512–520. doi: 10.1111/pai.12100. [DOI] [PubMed] [Google Scholar]
- 14.Berin MC, Mayer L. Can we produce true tolerance in patients with food allergy? J Allergy Clin Immunol. 2013;131:14–22. doi: 10.1016/j.jaci.2012.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khoriaty E, Umetsu DT. Oral immunotherapy for food allergy: towards a new horizon. Allergy Asthma Immunol Res. 2013;5:3–15. doi: 10.4168/aair.2013.5.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nurmatov U, Venderbosch I, Devereux G, Simons FE, Sheikh A. Allergen-specific oral immunotherapy for peanut allergy. Cochrane Database Syst Rev. 2012;9:CD009014. doi: 10.1002/14651858.CD009014.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berin MC, Shreffler WG. Mechanisms underlying induction of tolerance to foods. Immunol Allergy Clin North Am. 2016;36:87–102. doi: 10.1016/j.iac.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Wawrzyniak M, O'Mahony L, Akdis M. Role of regulatory cells in oral tolerance. Allergy Asthma Immunol Res. 2017;9:107–115. doi: 10.4168/aair.2017.9.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feuille E, Nowak-Wegrzyn A. Allergen-specific immunotherapies for food allergy. Allergy Asthma Immunol Res. 2018;10:189–206. doi: 10.4168/aair.2018.10.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barletta B, Rossi G, Schiavi E, Butteroni C, Corinti S, Boirivant M, et al. Probiotic VSL#3-induced TGF-β ameliorates food allergy inflammation in a mouse model of peanut sensitization through the induction of regulatory T cells in the gut mucosa. Mol Nutr Food Res. 2013;57:2233–2244. doi: 10.1002/mnfr.201300028. [DOI] [PubMed] [Google Scholar]
- 21.Schiavi E, Barletta B, Butteroni C, Corinti S, Boirivant M, Di Felice G. Oral therapeutic administration of a probiotic mixture suppresses established Th2 responses and systemic anaphylaxis in a murine model of food allergy. Allergy. 2011;66:499–508. doi: 10.1111/j.1398-9995.2010.02501.x. [DOI] [PubMed] [Google Scholar]
- 22.Kim H, Kwack K, Kim DY, Ji GE. Oral probiotic bacterial administration suppressed allergic responses in an ovalbumin-induced allergy mouse model. FEMS Immunol Med Microbiol. 2005;45:259–267. doi: 10.1016/j.femsim.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Hougee S, Vriesema AJ, Wijering SC, Knippels LM, Folkerts G, Nijkamp FP, et al. Oral treatment with probiotics reduces allergic symptoms in ovalbumin-sensitized mice: a bacterial strain comparative study. Int Arch Allergy Immunol. 2010;151:107–117. doi: 10.1159/000236000. [DOI] [PubMed] [Google Scholar]
- 24.Perezábad L, Reche M, Valbuena T, López-Fandiño R, Molina E, López-Expósito I. Oral food desensitization in children with IgE-mediated cow's milk allergy: immunological changes underlying desensitization. Allergy Asthma Immunol Res. 2017;9:35–42. doi: 10.4168/aair.2017.9.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leonard SA, Martos G, Wang W, Nowak-Węgrzyn A, Berin MC. Oral immunotherapy induces local protective mechanisms in the gastrointestinal mucosa. J Allergy Clin Immunol. 2012;129:1579–1587.e1. doi: 10.1016/j.jaci.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li XM, Schofield BH, Huang CK, Kleiner GI, Sampson HA. A murine model of IgE-mediated cow's milk hypersensitivity. J Allergy Clin Immunol. 1999;103:206–214. doi: 10.1016/s0091-6749(99)70492-6. [DOI] [PubMed] [Google Scholar]
- 27.Burton OT, Logsdon SL, Zhou JS, Medina-Tamayo J, Abdel-Gadir A, Noval Rivas M, et al. Oral immunotherapy induces IgG antibodies that act through FcγRIIb to suppress IgE-mediated hypersensitivity. J Allergy Clin Immunol. 2014;134:1310–1317.e6. doi: 10.1016/j.jaci.2014.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shin HS, Eom JE, Shin DU, Yeon SH, Lim SI, Lee SY. Preventive effects of a probiotic mixture in an ovalbumin-induced food allergy model. J Microbiol Biotechnol. 2018;28:65–76. doi: 10.4014/jmb.1708.08051. [DOI] [PubMed] [Google Scholar]
- 29.Hol J, van Leer EH, Elink Schuurman BE, de Ruiter LF, Samsom JN, Hop W, et al. The acquisition of tolerance toward cow's milk through probiotic supplementation: a randomized, controlled trial. J Allergy Clin Immunol. 2008;121:1448–1454. doi: 10.1016/j.jaci.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 30.Moussu H, Van Overtvelt L, Horiot S, Tourdot S, Airouche S, Zuercher A, et al. Bifidobacterium bifidum NCC 453 promotes tolerance induction in murine models of sublingual immunotherapy. Int Arch Allergy Immunol. 2012;158:35–42. doi: 10.1159/000330101. [DOI] [PubMed] [Google Scholar]
- 31.Van Overtvelt L, Moussu H, Horiot S, Samson S, Lombardi V, Mascarell L, et al. Lactic acid bacteria as adjuvants for sublingual allergy vaccines. Vaccine. 2010;28:2986–2992. doi: 10.1016/j.vaccine.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 32.Tang ML, Ponsonby AL, Orsini F, Tey D, Robinson M, Su EL, et al. Administration of a probiotic with peanut oral immunotherapy: a randomized trial. J Allergy Clin Immunol. 2015;135:737–744.e8. doi: 10.1016/j.jaci.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 33.Hsiao KC, Ponsonby AL, Axelrad C, Pitkin S, Tang ML, Burks W, et al. Long-term clinical and immunological effects of probiotic and peanut oral immunotherapy after treatment cessation: 4-year follow-up of a randomised, double-blind, placebo-controlled trial. Lancet Child Adolesc Health. 2017;1:97–105. doi: 10.1016/S2352-4642(17)30041-X. [DOI] [PubMed] [Google Scholar]
- 34.Uermösi C, Beerli RR, Bauer M, Manolova V, Dietmeier K, Buser RB, et al. Mechanisms of allergen-specific desensitization. J Allergy Clin Immunol. 2010;126:375–383. doi: 10.1016/j.jaci.2010.05.040. [DOI] [PubMed] [Google Scholar]
- 35.Wachholz PA, Durham SR. Mechanisms of immunotherapy: IgG revisited. Curr Opin Allergy Clin Immunol. 2004;4:313–318. doi: 10.1097/01.all.0000136753.35948.c0. [DOI] [PubMed] [Google Scholar]
- 36.Sugimoto M, Kamemura N, Nagao M, Irahara M, Kagami S, Fujisawa T, et al. Differential response in allergen-specific IgE, IgGs, and IgA levels for predicting outcome of oral immunotherapy. Pediatr Allergy Immunol. 2016;27:276–282. doi: 10.1111/pai.12535. [DOI] [PubMed] [Google Scholar]
- 37.Wright BL, Kulis M, Orgel KA, Burks AW, Dawson P, Henning AK, et al. Component-resolved analysis of IgA, IgE, and IgG4 during egg OIT identifies markers associated with sustained unresponsiveness. Allergy. 2016;71:1552–1560. doi: 10.1111/all.12895. [DOI] [PMC free article] [PubMed] [Google Scholar]