BACKGROUND
Anterior cruciate ligament (ACL) reconstruction remains the treatment of choice for ACL deficient active individuals involved with sports or activities that involve quick start/stop, cutting, jumping and abrupt change of direction activities. Based on insurance, industry and implant evidence there are approximately 200,000 ACL reconstructions per year in the United States.1 Fortunately, primary reconstructions typically do well, but can fail at a low but significant rate.2–4 While highly successful in the short term there can be problems with primary reconstructions including loss of motion, extensor dysfunction with certain grafts, arthritis and graft failure. This treatise will address graft failure and the multicenter, multi-surgeon group (Multicenter ACL Revision Study – MARS) assembled to study the issues surrounding revision ACL reconstruction.
A variety of studies have evaluated graft failure in the primary ACL reconstruction setting and have found the failure rates to range from ~1–8% in the standard patient and graft setting.2, 4, 5 In the Multicenter Orthopaedic Outcomes Network (MOON) the graft failure rate in primary reconstructions was 3% in the ipsilateral knee and 3% in the contralateral knee at 2-year follow-up.4 In a systematic review evaluating hamstring vs. patellar tendon autografts, Spindler et al. reported a 3.7% overall failure rate.3 Wright et al. in a systematic review of minimum 5-year follow-up of ACL reconstructions found an ipsilateral ACL graft failure rate of 5.8% and a contralateral native ACL failure rate of 11.8%.2
These are reasonably low levels of failure, but the question confronting us was what happens in cases of failure. What expectations of current and future knee health should these patients have? Can they return once again to the activities that resulted in ACL failure? A better understanding of revision results would allow us to better counsel patients as to expected outcomes.
Defining the outcomes patients can expect and the outcomes that truly matter in the ACL revision setting was the impetus for the development of the Multicenter ACL Revision Study (MARS) Group. There was consensus amongst the MARS surgeons that revision ACL reconstruction typically resulted in worse outcomes compared to primary reconstructions. Revision ACL reconstruction was the strongest predictor for worse KOOS scores in a mixed ACL reconstruction cohort early in the MOON experience.6 In a series of ACL reconstructions reported at minimum 5-year follow-up, revision was the strongest predictor for worse outcome across the board, but in the editing process the journal reviewers and Editor requested that the revision ACL reconstructions be removed from the published manuscript due to the small number and percentage of revision reconstructions.6
Unfortunately, little Level 1 or 2 evidence existed at the time to help us confirm this discrepancy in outcomes that we observed between primary and revision ACL reconstructed patients. In a mixed model meta-analysis of 21 studies with minimum 2-year follow-up after revision reconstruction these worse results were demonstrated after revision reconstruction.7 Of the 21 studies, however, only 4 were Level 1 or 2, while 1 was Level 3 and 16 were Level 4 studies. Objective failure (defined as a re-revision, KT-1000 side-to-side difference of >5 mm, or a positive pivot shift) occurred in 13.7±2.7% -- a much higher rate of failure than typically reported in primary ACL reconstruction. Patient-reported outcomes were worse than expected compared with primary ACL results and usually exceeded the known clinically important difference for these outcome scores.
Based upon these findings, the MOON Group reviewed their prospectively collected cohort which began as a mixed primary and revision patient ACL reconstruction cohort.8, 9 Their working hypothesis was that revision ACL reconstruction results in worse outcome compared to primary reconstruction, as measured by validated patient-based outcome measures, including the Marx activity rating scale, Knee injury and Osteoarthritis Outcome Score (KOOS), and International Knee Documentation Committee Subjective form (IKDC). 408/487 (84%) were available at minimum 2-year follow-up with 39/47 (83%) revisions available at 2-year follow-up. At 2 years, median Marx activity level scores had dropped from 12 to 9 points in the primary reconstructions vs. 10 to 6 points in revisions (p=0.009). While the minimally clinically important difference (MCID) is not known for the Marx score, it is assumed on a 16-point scale that 2 points (representing more than a 10% change or difference) would be clinically significant. The IKDC at 2 years was 85.6 for primary ACL reconstructions and 79.6 for revisions, which was statistically different (p=0.005), but not clinically significant (MCID: 11.5 points).10, 11 For the 5 KOOS subscales a difference of 8–10 points is clinically significant. In this study, the KOOS knee-related quality of life (KRQOL) subscale was lower in revisions at 2 years (75 vs. 62.5; p<0.001). The KOOS sports and recreation subscale also was worse for revisions at 2 years (85 vs. 75; p=0.004). KOOS Pain was lower in revisions and potentially clinically significant (91.7 vs. 83.3). KOOS Symptoms (85.7 vs. 78.6) and ADLs (98.7 vs. 97.1) did not demonstrate a clinically significant difference between primary and revision reconstructions.
Thus, the data represented a prospectively collected cohort of primary and revision ACL reconstructions that used identical validated patient-reported outcome measures. While worse scores across the board were observed for revisions, the factors contributing to these worse outcomes were unknown. Unfortunately, revisions represented only 10% of the cohort. We sought to identify predictors for these worse results. To identify predictors, multi-variable analysis requires ~10–15 subjects per variable assessed and with the multiple factors (50–75 or more) potentially contributing to revision ACL reconstruction outcomes, we realized that a comprehensive assessment of important factors in revision ACL outcomes would require a cohort with 750–1000 patients. It became apparent that the MOON Group, with less than 20 members, could not enroll an adequate number of patients quickly enough for this type of study. A simple moon could not get it done, as we needed a planet. With this in mind we set out to establish a larger group of interested sports medicine surgeons.
METHODS
Study Design
We felt the basic approach utilized by the MOON Group would be appropriate for evaluating the revision patient but realized there were different factors involved in revision surgeries that would need to be captured. A small group developed a Standard Operating Procedure (SOP) manual that outlined rules of engagement for surgeons and patients. Very early on, we engaged the American Orthopaedic Society for Sports Medicine (AOSSM), through Bart Mann, the AOSSM research director. He, the AOSSM Research Committee, and the AOSSM society were enthusiastic about the opportunity to engage their members in an important research question. Based on this collaboration, we had access to the AOSSM website and email systems and were able to solicit broad surgeon participation. Interested AOSSM members participated in three training meetings to learn how the study would proceed. Participants were also engaged in designing forms and determining the variables that would be collected and analyzed. Over 100 members originally expressed interest and we currently have 83 surgeons participating at 52 IRB approved sites. The surgeons are a near 50/50 mix of academic and private practice surgeons, adding to the generalizability of our results.12
In determining study design, we debated over a prospective cohort design versus a randomized trial. Ultimately, we believed we did not know a single critical variable to randomize and felt a cohort to determine predictors would best serve a revision series with its rich number of potential factors. We thus chose a prospective longitudinal cohort similar to the classic Framingham study for cardiovascular disease many years ago.13
Surgeon Involvement
Surgeon inclusion was based on AOSSM membership, attendance at one of our training meetings, and a willingness to follow the procedural issues identified in the SOP. This included utilizing a Musculoskeletal Transplant Foundation (MTF) graft if an allograft was chosen for the subsequent revision. Training videos were reviewed for consensus on meniscal and chondral injury classification and treatment and were based upon similar studies performed by members of the group.14, 15 We studied the group’s ability to agree on etiology of failure for ACL reconstructions utilizing intra-articular videos and radiographs and found fair agreement on most items measured.16
Study Logistics
After obtaining informed consent, the patient filled out a 13-page questionnaire prior to their revision ACL surgery that included questions regarding demographics, sports participation, injury mechanism, comorbidities and knee injury history. Within this questionnaire, each participant also completed the Knee injury and Osteoarthritis Outcome Score (KOOS), the International Knee Documentation Committee Subjective form (IKDC) and the Marx activity rating scale. Contained within the KOOS was the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC). Surgeons filled out a 49-page questionnaire at the time of the revision surgery that included the impression of the etiology of the previous failure, physical exam findings, surgical technique utilized, the intra-articular findings and surgical management of meniscal and chondral damage.
Patient follow-up was completed by mail with re-administration of the same questionnaire as the one they completed at baseline. Patients were also contacted by phone or email to determine whether any subsequent surgery had occurred to either knee since their initial revision ACL reconstruction. If so, operative reports were obtained, whenever possible, in order to document pathology and treatment.
Completed data forms were mailed from each participating site to the data coordinating center. Data from both the patient and surgeon questionnaires were scanned with Teleform™ software (OpenText, Waterloo, Ontario, Canada) utilizing optical character recognition, and the scanned data was verified and exported to a master database.
Teleform™ paper forms were utilized so data was available in real time. If starting today we would utilize electronic data capture, but in 2006 this was best practice for us and our coordinating center. Data was housed at Vanderbilt. Our data capture was outstanding; greater than 99% of all patient and surgeon data points were completed when assessed on our first 900 patients (Table 1).
Table 1.
Data completeness within a subset of variables (n=900 cases)
| Outcome Variable | Questionnaire Source Form | # Variables | # Missing / Observations (%) | % Complete |
|---|---|---|---|---|
| Marx Activity Level | Patient | 4 | 23 / 3,600 (0.6%) | 99.4% |
| IKDC | Patient | 19 | 38 / 17,100 (0.2%) | 99.8% |
| KOOS | Patient | 42 | 259 / 37,800 (0.7%) | 99.3% |
| Current graft type | Surgeon | 1 | 1 / 900 (0.1%) | 99.9% |
| Surgical technique | Surgeon | 1 | 2 / 900 (0.2%) | 99.8% |
| Rehabilitation factors | Surgeon | 6 | 35 / 5,400 (0.7%) | 99.3% |
Once 2-year follow-up began we added a full-time central follow-up coordinator whose responsibility was to contact all patients to send out and obtain questionnaires and perform phone/email follow-up. Each site manages their site with their personal health care personnel or research personnel. An electronic newsletter (Figure 1) was developed that arrived monthly to all surgeons and coordinators and acted as an impetus to stimulate patient enrollment listing total and monthly enrollment figures for surgeons. Participating surgeons all had the goal of appearing on the Top 10 list.
Figure 1:
Electronic Newsletter
Keys that helped us early on were frequently based on our experience with MOON and included the use of conference calls and the ability to communicate by email. The group’s experience with IRB helped new sites get approval and the knowledge regarding grants helped immensely in obtaining funding.
We developed a Scientific Advisory Board for advice. This eight-member Board meets at least annually and provides advice and oversight for the study. This has included what studies should be performed via an application form by members. This has been critical for governance issues. The makeup is balanced by geography, gender and practice type.
Unique Aspects of MARS
The MARS Group and study offer many unique aspects for orthopaedics and even for medical research in general. The recruitment of surgeon participants from the broad population of the AOSSM allowed private practice surgeons to participate and make intellectual contributions that might otherwise not be possible in their practice setting. In fact, the final MARS study surgeon breakdown included more private than academic surgeons (55% vs. 45%). Moreover, the inclusion of private practice surgeons improves the generalizability of the results for the entire orthopaedic surgeon population. We were able to accomplish this broad demographic of participants by leveraging the strength of the coordinating center personnel and minimizing local site and surgeon burden. This included assistance with IRB preparation and submission with no central site multicenter IRB available in 2006 when this study was initiated. Additionally, central coordinating site control of phone and questionnaire follow-up provided outstanding follow-up for a population that is sometimes difficult to follow due to changing patient locations. The 52 sites represent the largest number of sites utilized for a single orthopaedic surgery research study and one of the largest number of sites involved in any multicenter medical studies.
The size and prospective nature of our study is unique for this topic. As we designed the study, we were uncertain of the critical factors leading to the worse outcome observed following revision ACL reconstructions. Thus, we felt a randomized controlled trial of one or two variables might be misguided and inappropriate. We believed a prospective longitudinal cohort design would allow us to recruit adequate subjects and allow leeway for the surgeons to appropriately manage what can be challenging technical cases. This has been borne out by examining one key issue – graft choice for the revision reconstruction. This was the most critical issue for many surgeons performing these reconstructions. Our study design allowed accumulation of a large number of subjects that ended up (unintentionally) equally divided between the four main graft options with ~25% for each choice (BPTB autograft, soft tissue autograft, BPTB allograft, soft tissue allograft). More detail of the graft choice study is discussed in the results section.
The size of this study dwarfs all previous revision ACL reconstruction studies. We performed a meta-analysis of the 21 previous studies with minimum 2-year follow-up. In that meta-analysis it was noted that the 21 studies involved follow-up results for 863 patients in total with predominantly Level 4 retrospective case series represented. Thus, in one study (with over 1200 enrolled patients) we have eclipsed the entirety of the previous result reporting for this important orthopaedic problem. The strength of the size of our cohort becomes even more apparent when moving beyond 2-year follow-up. Recently we performed a systematic review of minimum 5-year follow-up of revision ACL reconstructions.17 Wright et al. demonstrated there are only four studies involving 148 patients with 121 followed. We have completed minimum six-year follow-up and have more than 800 patients followed at that time point. We have begun analysis (see Results) of our six-year results and will have information for surgeons that no other cohort can accomplish. Using this approach, we have simultaneously collected the largest cohort ever assembled prospectively of the multiply revised ACL patient: 150 patients (157/1234; 13%) of the cohort had two or more revisions of their ACL reconstructions.
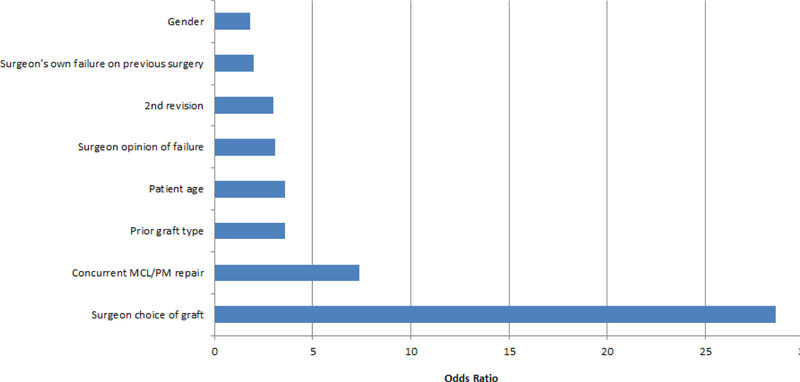
We performed an analysis that represents a unique approach for orthopaedic research. A propensity analysis was performed after our graft choice analysis demonstrated improved results and decreased failure rates if an autograft was utilized.18 Propensity analysis can determine factors that predict decision making for factors when they are not controlled by study design. Propensity analyses flourish when there are more variables so a study of revision ACL reconstructions is a rich milieu to perform this in and would address the concern that graft choice is a fait accompli and that it is predetermined by patient age, sport, previous graft etc. and cannot be impacted by the surgeon choice. Our propensity analysis proved this belief wrong as surgeon was by five times the strongest determinant of the graft utilized for the patient (Figure 2).
Figure 2.
Variables that significantly influence graft choice in a revision ACL reconstruction
Thus, if the surgeon wanted to use an autograft they could typically find a way to accomplish this. We were encouraged by this that our study actually has impact because educating surgeons through manuscripts, reviews and podium presentations is an easier, cheaper approach than any other approach for broadcasting these results i.e. educating patients.
RESULTS
Epidemiology
Follow-up has been very successful despite the challenge of coordinating 52 sites. Our use of central follow-up for this study has been a successful model and provides consistency across the study. The demographics and epidemiology of our cohort was first described in our original MARS publication as well as subsequent publications.12, 19 1234 patients have been enrolled in the study. Median age was 26 with a range from 12–63 years. 518 (42%) were female. For 87% it was their first revision,12 while 13% were undergoing their 2nd or higher revision ACL reconstruction. 73% were injured while playing a sport. The most common sports involved both genders and were soccer and basketball. Associated surgeries included high tibial osteotomies (n=21), medial meniscus transplants (n=34), and lateral meniscus transplants (n=10).
Modern ACL reconstruction with appropriate grafts and well positioned tunnels realistically began in the 1980s. In the 1990s, case series of revision ACL surgery began to appear. In the vast majority of these cases it was felt the etiology of the original ACL graft failure was due to technical issues.20–22 However, in the MARS cohort trauma was more commonly reported as a cause of graft failure (Table 2, Figure 3). This reflects two potential considerations for this finding: 1) improved technical ability with improved training and education; and 2) surgeons self-reporting on their own failures in this cohort: 30% (367/1234) of the patients were the surgeons’ own failures.
Table 2.
Cause of Failure in the MARS Cohort
| # | %* | |
|---|---|---|
| Traumatic | 697 | 56% |
| Technical | 616 | 50% |
| Biologic | 329 | 27% |
| Other | 35 | 3% |
| Blank | 2 | <1% |
the denominator is >100% due to multiple choice option of this question (surgeons were instructed to ‘check all that apply’).
Note: n=426 (35%) of these responses listed a combination of these components as the reason for failure.
Figure 3.
Cause of Failure Venn Diagram
The cause of technical failure in the MARS study was most commonly felt to be due to femoral tunnel malposition (Table 3) with the tibial tunnel the 2nd most common cause. Varus and valgus malalignment were seldom felt to be an issue. (Table 3).
Table 3.
Cause of Technical Failure
| # | |
|---|---|
| Femoral tunnel malposition | 595 |
| Tibial tunnel malposition | 228 |
| Varus / Valgus malalignment | 23 |
| Femoral fixation | 38 |
| Tibial fixation | 18 |
| Autograft source | 17 |
| Allograft source | 66 |
| Posteromedial laxity | 16 |
| Posterolateral laxity | 4 |
Prior graft utilized was autograft 68% of the time, allograft 29% and a combination (autograft + allograft) 3%. The most common previous autograft was patellar tendon, utilized 52% of the time. Patellar tendon was the most common allograft utilized previously for the primary ACL reconstruction (11%). Current graft utilized was 26% patellar tendon autograft, 20% soft tissue autograft, 23% patellar tendon allograft and 25% soft tissue allograft.
Radiographs were obtained for enrolled patients. MARS had required (bilateral standing Anterior-Posterior, full extension lateral) and recommended (Rosenberg, sunrise/merchant, and bilateral long leg standing alignment) radiographs. These radiographs were analyzed for the group and findings collated.23, 24 Sagittal views of the femur demonstrated 42% of femoral tunnels were more than 40% anterior to the posterior cortex. On the tibia sagittal view 49% demonstrated some form of roof impingement on full extension. On longstanding alignment films the average limb alignment was 43% from the medial edge of the tibia.
An interesting use of the cohort involved assessment of osteoarthritis rating scales utilizing the radiographs obtained as part of the study.25 Reliability of different radiographic views as correlated with arthroscopic findings were analyzed. In addition, a multirater osteoarthritis scale study was performed analyzing the Kellgren-Lawrence, IKDC, Albrecht, Fairbank, Brandt and Jäger-Wirth grading scales. 45° flexion posterior-anterior weightbearing radiographs demonstrated the best interobserver reliability as compared to anteroposterior straight standing radiographs. The IKDC grading scale correlated best with the arthroscopic findings of cartilage damage.
Graft Choice
The choice of graft is a major issue for revision ACL reconstructions. Obviously, the patient’s previous graft will alter and potentially bias future choices. Many surgeons prefer allograft due to the complexity of the revision ACL reconstructions. Previous studies in the last few years have demonstrated failure rates for allograft in primary ACL reconstructions to be higher than those noted for autograft reconstructions.26, 27This is especially true in the young active patient. Despite these findings there was no evidence that these higher failure rates would be present in the different clinical situation of a revision ACL reconstruction. Additionally, many surgeons believed that processing was the key to the higher failure rates in allograft reconstructions and that a fresh frozen allograft with no irradiation would perform similarly to an autograft. For that reason, we knew that controlling the source and preparation for our allografts was key. We limited our allograft source to MTF and were able to track the demographics and preparation history for each individual graft utilized in the MARS study. This has given increased validity to our findings outlined later in this review.
We analyzed graft choice as a predictor for outcome as one of the specific aims of our NIH-funded grant.11 The demographics of patients that received autograft and allograft can be seen in Table 4. Allografts were placed in older, less active patients on average.
Table 4.
Graft Choice Demographics
| Autograft Group (n=598) | Allograft Group (n=599) | Auto+Allo Group (n=37) | |
|---|---|---|---|
| Age | 24 (19, 32) | 28 (21, 36) | 21 (18, 28) |
| Gender | |||
| • males (%) | 361 (60%) | 338 (56%) | 17 (47%) |
| • females (%) | 237 (40%) | 261 (44%) | 19 (53%) |
| Marx Activity Level | |||
| • Baseline | 12 (5, 16) | 10 (3, 15) | 12 (8, 16) |
| • 2 year follow-up | 8 (2, 12) | 5 (1, 11) | 10 (3, 13) |
| • 6 year follow-up | 6 (2, 10) | 4 (0, 8) | 8 (3, 10) |
Key: continuous variables are reported as medians (25%, 75% interquartiles). Frequencies are reported as number (percentage).
The form of treatment was known for each allograft and included no radiation (aseptic), light total whole-body irradiation (<1.8 mrad), or rarely terminal radiation (Table 5).
Table 5.
Allograft Treatment
| Sterilization Method | Allograft Cohort (n=636) | Allograft Failure Cohort at 6 years |
|---|---|---|
| Aseptic | 257 (40%) | 15 (42%) |
| Whole Body (1.2–1.8 mRad) | 343 (54%) | 18 (50%) |
| Terminal (0.7–1.0 mRad) | 36 (6%) | 3 (8%) |
The overall re-rupture rate at two years was 3.3% (37/1112): 2.2% (12/545) in autografts, 4.5% (24/534) in allografts, and 3% (1/33) in combination autograft+allografts. Autograft use was 2.78 times less likely to re-rupture (p=0.047; 95% CI=1.01, 7.69). No difference in re-rupture rate was found in soft tissue vs. BTB in either autograft or allograft. IKDC scores improved with autograft reconstruction with an odds ratio (OR) of 1.33 (95% CI: 1.01–1.7; p=0.045). KOOS Sports and Recreation subscale significantly improved with autograft (OR=1.33; CI: 1.02–1.73; p=0.037), as did the KOOS Quality of Life (OR=1.33; CI: 1.03–1.73; p=0.031). The KOOS ADL and Symptoms subscales were not affected by graft choice.
Many people believed that graft choice was a predetermined fate in a revision setting and that the surgeon truly had no choice in determining what graft the patient obtained. To analyze this belief we performed a propensity analysis for graft choice (Figure 2).18 Our analysis demonstrated that the surgeon performing the procedure was far and away the biggest factor on what graft type was chosen for the revision reconstruction, approximately 5 times more impactful than the 2nd most common predictor (which was prior graft). Thus, surgeons truly did have a choice in what graft they utilized. This allows us to modify clinical practice by educating surgeons through publications and podium presentations at major meetings such as the American Academy of Orthopedic Surgeons (AAOS) or AOSSM.
Subsequent 6-year follow-up for the MARS Group has strengthened the graft choice conclusions.28 In an analysis presented at the 2019 AAOS Annual Meeting and currently in manuscript preparation we noted the following. Questionnaire follow-up was obtained on 810 subjects (65%), while phone follow-up was obtained on 949 subjects (76%). Graft choice proved to be a significant predictor of 6-year Marx activity level scores (p=0.024). Specifically, the use of a BTB (bone-patellar tendon-bone) autograft for revision reconstruction predicted improved activity levels compared to a BTB allograft [Odds Ratio (OR)=1.92; 95% confidence intervals (CI)=1.25, 2.94]. Graft choice was not significant in predicting 6-year IKDC, WOMAC, or KOOS outcome scores.
Graft rerupture was reported in 5.8% (55/949) of patients by their 6-year follow-up: 3.4% (16/472) in autografts, 8.3% (37/448) in allografts, and 6.9% (2/29) in combination allograft + autografts. Use of a BTB autograft for revision resulted in patients 4.2 times less likely to sustain a subsequent graft rupture than if a BTB allograft was utilized (p=0.011; 95% CI=1.56, 11.27).Thus, graft choice has increased in significance in predicting re-rupture and sports performance.
Meniscus and Articular Cartilage
Compared to primary reconstructions the revision patient has a much higher chance of having meniscus or articular cartilage damage at the time of revision reconstruction. In our cohort, only 9% of the patients did not have a meniscus tear or grade 2 or worse articular cartilage damage, 90% had at least meniscus or articular cartilage damage and 59% had both at the time of revision (Table 6).
Table 6.
Overall Meniscal and Articular Cartilage Integrity in the MARS Cohort at the time of Enrollment
| Meniscal Pathology | Articular Cartilage Pathology | |||
| Normal | Abnormal | Total | ||
| Normal | 9% | 12% | 21% | |
| Abnormal | 19% | 59% | 78% | |
| Total | 28% | 71% | ||
We analyzed the impact of these meniscus tears and articular cartilage damage on patient outcomes at 2 years.29 Previous lateral meniscectomy prior to the time of revision significantly resulted in worse patient reported outcomes (Table 7); previous medial meniscectomy less so. Interestingly, current meniscal pathology variables documented at the time of the index revision surgery had no significant predictor effect on any 2-year patient outcome measure (Table 7).
Table 7.
Meniscus Impact on 2-Year Patient-Reported Outcomes (significant p values only)
| KOOS | WOMAC | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Structure | Marx | Symptoms | Pain | ADL | Sports / Rec | QOL | IKDC | Stiffness | Pain | ADL |
| Previous Meniscal Pathology | ||||||||||
| • medial | 0.002 | 0.035 | ||||||||
| • lateral | 0.008 | 0.042 | <0.001 | 0.038 | 0.03 | 0.032 | ||||
| Current Meniscal Pathology | ||||||||||
| • medial | ||||||||||
| • lateral | ||||||||||
Key: Wald Chi-Squared Test used to compute overall predictor variable significance. Blank cells indicate non-significant p values of > 0.05. ADL=activities of daily living; Rec=recreation; QOL=quality of life.
Grade 2 or worse articular cartilage damage grade negatively impacted patient-reported outcomes at 2 years. The most significant finding was the consistent negative impact of trochlear groove chondrosis across outcome measures (Table 8).
Table 8.
Articular Cartilage Impact on 2-Year Patient-Reported Outcomes (significant p values only)
| Structure | Marx | KOOS | IKDC | WOMAC | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Symptoms | Pain | ADL | Sports/Rec | QOL | Stiffness | Pain | ADL | |||
| Previous AC Pathology | ||||||||||
| • Yes/no | ||||||||||
| Current AC Pathology | ||||||||||
| • MFC | 0.018 | 0.012 | ||||||||
| • LFC | 0.048 | 0.048 | ||||||||
| • MTP | 0.004 | |||||||||
| • LTP | ||||||||||
| • Patella | ||||||||||
| • Trochlea | 0.034 | <0.001 | 0.011 | 0.01 | <0.001 | |||||
Key: Wald Chi-Squared Test used to compute overall predictor variable significance. Blank cells indicate non-significant p values of > 0.05. ADL=activities of daily living; Rec=recreation; QOL=quality of life; AC=articular cartilage; MFC=medial femoral condyle; LFC=lateral femoral condyle; MTP=medial tibial plateau; LTP=lateral tibial plateau.
MARS analyzed the impact of previous meniscectomy on the presence of chondral damage noted at the time of revision ACL reconstruction. Previous partial meniscectomy was associated with chondral damage (p<0.0001). Meniscus repair appeared to be chondral protective as there was no statistically significant increase in chondral damage noted in the setting of meniscus repair. There was no difference between knees that had not had previous meniscus surgery and those with repair.30
The association of meniscus status, lower extremity alignment and body mass index (BMI) with chondrosis at the time of revision ACL reconstruction was also analyzed.31 The medial compartment had more chondrosis than the lateral compartment (grade 2/3, 42% versus lateral grade 2/3, 26%). The mean weight-bearing line was 0.43 as measured from the medial edge of the tibia. Medial compartment chondrosis was associated with higher BMI (p=0.025), alignment (p=0.002), and “disrupted” medial meniscus status (i.e. a tear that required a repair or a partial meniscectomy; p=0.001). No patient with a weight-bearing line greater than or lateral to 0.625 had grade 4 medial compartment chondrosis. Lateral compartment chondrosis was significantly associated with higher age (p=0.013) and “disrupted” lateral meniscus status (i.e., a tear which required a repair or a partial meniscectomy; p<0.001). Subjects with intact menisci were found to decrease their odds of associated chondrosis by 64% to 84%.31
Predictors of significant chondral surface damage from primary reconstruction to revision reconstruction were analyzed utilizing the MARS and MOON databases.32 One hundred thirty four patients had been enrolled in the MOON cohort and subsequently underwent revision and were enrolled in MARS. Progression of articular cartilage damage was noted in 25.4% (34/134) of patients in the lateral compartment, 23.9% (32/134) in the medial compartment and 23.1% (31/134) in the patellofemoral compartment. For the lateral compartment, patients who had undergone a greater than 33% partial lateral meniscectomy at the time of primary ACL reconstruction had a 16.9 times greater odds of progression of articular cartilage injury than those with an intact lateral meniscus (p=0.001). Patients that underwent a less than 33% partial medial meniscectomy at the time of primary ACL reconstruction had a 4.8 times greater odds of progression of articular cartilage injury then those with an intact medial meniscus at the time of primary reconstruction (p=0.02). For each increase in age by one year there was a 6% increase in the onset of significant chondral damage in the medial compartment and 5% in the lateral compartment (p≤0.02). For the patellofemoral compartment use of an allograft reconstruction at the time of primary ACL reconstruction resulted in a 15 times increase in the risk of progression of articular cartilage injury as compared to a patellar tendon autograft (p<0.001). At the time of revision ACL reconstruction, a 1 unit increase in BMI was associated with a 10% increase in the risk of progression of articular cartilage damage (p=0.046).
Meniscus repair success at two years was analyzed for the cohort.33 In total, 218 patients from 1205 revision ACL reconstructions underwent concurrent meniscal repairs (18% of the cohort). There were 235 repairs performed: 153 medial, 48 lateral and 17 medial + lateral. The vast majority of these repairs (n=178; 76%) were performed with all-inside techniques. Two-year surgical follow-up was obtained on 90% (197/218) of the cohort. Overall, the meniscal repair failure rate was 8.6% (17/197) at 2 years. Of the 17 failures, 15 were medial (13 all-inside, 2 outside-in) and 2 were lateral (both all-inside). Four of the medial failures were treated in conjunction with a subsequent repeat revision reconstruction. Thus, meniscus repair is overall very successful in the revision setting approaching that of repair results in isolated or primary ACL reconstruction settings.
Surgical Factors
Surgical factors were analyzed to determine the impact on patient-reported outcome measures at 2 years.34 A variety of factors were analyzed and in many cases it is difficult to determine intuitively why certain factors impacted outcome. With regards to surgical approach, having undergone a prior arthrotomy decreased IKDC scores (p=0.037, OR=2.43) and decreased all KOOS subscales (p<0.05, OR range =2.38–4.35). A double femoral tunnel resulted in worse KOOS QOL scores (p=0.027, OR=3.13). An ideal tibial position that was not enlarged resulted in worse KOOS, WOMAC, and IKDC scores (p=0.001–0.03, OR range=1.19–2.68). Using a femoral tunnel declared “optimum” vs. drilling an entirely new femoral tunnel resulted in worse KOOS QOL scores (p=0.025, OR=1.79). Undergoing a notchplasty decreased KOOS, IKDC, and WOMAC scores (p=0.013–0.034, OR range=1.40–1.49). Factors that did not impact outcome included blended tunnels and knee position at time of graft fixation.
Graft fixation as a surgical factor impacting outcome was also analyzed. Femoral fixation with a metal screw had better 2-year outcomes in KOOS and WOMAC scores (p=0.01–0.05, OR range=1.41–1.96) when compared to using a bioabsorbable screw, cross-pin or combination. Tibial fixation other than metal screw resulted in worse IKDC (p=0.017, OR=1.67) and WOMAC stiffness (p=0.013, OR=1.72) scores.
Use of biologics and bone grafting was analyzed. Use of biologic enhancement in the revision setting resulted in lower 2-year MARX activity level scores (p=0.025, OR=1.79). Utilizing femoral bone grafts resulted in lower MARX activity scores at 2 years (p=0.048, OR=2.04). Conversely, not bone grafting the tibia resulted in worse KOOS Pain scores (p=0.046, OR=1.95) and WOMAC Pain scores (p=0.004, OR=3.31).
Reoperations following revision ACL reconstruction were analyzed.35 11% of patients underwent subsequent reoperation by 2-year follow-up: 27% were meniscal procedures (69% meniscectomy, 26% repair), 19% were revision ACL reconstructions, 17% were cartilage procedures (61% chondroplasty, 17% microfracture, 13% osteochondral autograft), 11% hardware removal and 9% were performed for arthrofibrosis reasons. Multivariate analysis demonstrated that age less than 20 years old resulted in a doubling of the risk for reoperation as compared to patient’s age range between 20–29 years old. Use of an allograft at the time of revision was a significant predictor for the need for reoperation within 2 years (OR=1.79; p=0.007). Patients with grade 4 chondral damage noted at the time of revision ACL reconstruction were 78% less likely to undergo a reoperation within 2 years. Gender, body mass index, smoking history, Marx activity scale and meniscal treatment (repair, meniscectomy) did not impact the need for reoperation within two years.
Rehabilitation Factors
Rehabilitation factors were analyzed regarding their potential impact on revision ACL reconstruction outcomes. 36 Two rehabilitation factors predicted outcome: 1) use of an ACL derotation brace for return-to-sport had better KOOS sports/rec scores at 2 years (odds ratio=1.50; 95% CI=1.07–2.11; p=0.019), and 2) use of an ACL derotation brace for post-operative rehabilitation period were 2.3 times more likely to have a subsequent surgery by 2 years (OR=2.26; 95% CI=1.11–4.60; p=0.024). Use of an ACL derotation brace at the time of return to sport could not be determined to improve or decrease the graft re-rupture rate. Restricting or allowing of all other factors did not predict outcome including active range of motion, passive range of motion, immediate weight-bearing, and the use of rehabilitative postoperative bracing.
Patient-Reported Outcomes
Predictors both positive and negative for patient reported outcomes measured at 2 years were studied.37 Patient reported outcomes for 2 and 6 years remained stable except steadily decreasing Marx activity level scores (Table 9). Scores for KOOS Quality of Life and Sports/Recreation and IKDC were 15–20 points below those for the same time point in historical primary ACL reconstruction series (Table 9, Figure 4).
Table 9.
Median (IQR) of KOOS, IKDC, and Marx Outcomes over Time of the MARS Cohort
| Score Range | Baseline | 2 Years | 6 Years | |
|---|---|---|---|---|
| IKDC | 0–100 | 52 (38, 63) | 77 (60, 86) | 75 (60, 87) |
| KOOS | ||||
| • symptoms | 0–100 | 68 (54, 82) | 79 (64, 89) | 79 (64, 89) |
| • pain | 0–100 | 75 (58, 86) | 89 (75, 94) | 89 (75, 97) |
| • activities of daily living | 0–100 | 87 (69, 96) | 97 (88, 100) | 97 (87, 100) |
| • sports & recreation | 0–100 | 45 (25, 65) | 75 (55, 90) | 75 (50, 90) |
| • quality of life | 0–100 | 31 (19, 44) | 56 (38, 75) | 62 (44, 75) |
| WOMAC | ||||
| • stiffness | 0–100 | 75 (50, 88) | 75 (62, 100) | 75 (63, 100) |
| • pain | 0–100 | 85 (70, 95) | 95 (80, 100) | 95 (80, 100) |
| • activities of daily living | 0–100 | 87 (69, 96) | 97 (88, 100) | 97 (87, 100) |
| Marx Activity Level | 0–16 | 11 (4, 16) | 7 (2, 12) | 5 (1, 9) |
Figure 4.
Global Patient-Reported Outcomes over Time
The most significant positive predictors of 2-year IKDC scores were having a high baseline IKDC score, high baseline Marx activity level, male gender, and having a longer time between a patient’s last ACL reconstruction, while negative predictors included having a previous lateral meniscectomy, Grades 3/4 trochlear groove or medial tibial plateau (MTP) chondrosis. For KOOS, having a high baseline score and having a longer time between their last ACL reconstruction were significant positive predictors for having better (i.e. higher) 2-year KOOS scores, while having a previous lateral meniscectomy prior to the revision ACL reconstruction was a consistent predictor for having significantly worse (i.e. lower) 2-year KOOS scores. Statistically significant positive predictors for 2-year Marx activity levels included higher baseline Marx activity levels, younger age, male gender, and being a non-smoker. Negative 2-year activity level predictors included having an allograft and a biologic enhancement.
Other Databases
The size and strength of this cohort has encouraged comparison to other registries and cohorts. Our first analysis in this area was to compare it to a prospectively collected primary cohort. During identical enrollment times using a similar collection approach we compared MARS and MOON intra-articular findings.38 Demographics were similar for both populations. Prevalence of medial meniscus tears were fundamentally equivocal (40%) in both populations. New untreated lateral meniscus tears were less common in the revision setting (46% in primaries vs. 34% in revisions; odds ratio (OR)=0.54; p< 0.01). Outerbridge Grade 3 and 4 chondrosis was more common in the patellofemoral joint of revision ACL reconstructions (3–7% in primaries vs. 11–13% in revisions; OR=1.70; p=0.04) and the lateral femoral condyle (5% in primaries vs. 11% in revisions; OR=1.73; p=0.04). No other compartments demonstrated statistically significant differences between revision and primary ACL reconstructions. Both revision and primary ACL reconstructions demonstrated an increased risk of medial and lateral femoral condyle and medial and lateral tibial plateau Grade 3 and 4 chondrosis in the setting of a previous medial or lateral meniscectomy respectively.
The MARS cohort was compared to the Norwegian revision registry and a revision database for the French Arthroscopic Society.39 Patient demographics were very similar. Allograft was more frequently chosen in the United States MARS cohort (29% vs. <1%). Hamstring autograft was most commonly used in Norway (74%) and the most common French graft was a bone patellar tendon bone autograft (70%). Significant articular cartilage injuries were most commonly noted in the MARS cohort. Patient-reported outcomes at baseline were statistically significantly different for KOOS and IKDC for the different patient groups but was only clinically significantly different for the KOOS sports and recreation sub scale where the MARS Group was more than 8 points better at baseline.
CONCLUSIONS
The MARS group was formed over 10 years ago, with the aim of assessing both the short and long-term progression of outcomes following revision ACL reconstruction, and to determine how the initial factors at the time of revision surgery may influence and predict disease progression. This consortium is unique in its size, scope and demographics. The MARS group comprises the most sites involved in a single orthopaedic study with 83 members at 52 sites, covering both academic (44%) and private practice (56%) settings. The study design involves a longitudinal prospective cohort of over 1,200 patients, for whom we have baseline, 2-year and 6-year follow-up on, and which has allowed us to assess modifiable surgical factors in a fashion never before contemplated. While there have been multiple challenges encountered in the MARS study they have for the most part been surmountable. The level of research and the questions that can be asked and answered in a cohort of this size and type is unmatched by any other approach. We believe the study design and scaffolding we have developed for this type of truly multi-surgeon, multicenter research can be a model for future orthopaedic research groups.
Acknowledgements:
This study received funding from the AOSSM, Smith & Nephew, National Football League Charities, and Musculoskeletal Transplant Foundation. This project was partially funded by grant No. 5R01-AR060846 from the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases.
MARS GROUP MEMBERS
Rick W. Wright, MD (Vanderbilt University, Nashville, TN USA); Laura J. Huston, MS (Vanderbilt University, Nashville, TN USA); Amanda K. Haas, MA (Washington University in St. Louis, St. Louis, MO USA); Christina R. Allen, MD (University of California, San Francisco, San Francisco, California USA); Allen F. Anderson, MD† (Tennessee Orthopaedic Alliance, Nashville, TN USA); Daniel E. Cooper, MD (W.B. Carrell Memorial Clinic, Dallas, TX USA); Thomas M. DeBerardino, MD (The San Antonio Orthopaedic Group, San Antonio, TX USA); Warren R. Dunn, MD, MPH (Reedsburg Area Medical Center, Reedsburg, WI USA); Brett (Brick) A. Lantz, MD (Slocum Research and Education Foundation, Eugene, OR USA); Barton Mann, PhD†(AOSSM, Rosemont, IL USA); Kurt P. Spindler, MD (Cleveland Clinic, Cleveland, OH USA); Michael J. Stuart, MD (Mayo Clinic, Rochester, MN USA); Samuel K. Nwosu, MS (Vanderbilt University, Nashville, TN USA); Jacquelyn S. Pennings, PhD (Vanderbilt Unversity, Nashville, TN USA); John P. Albright, MD (University of Iowa Hospitals and Clinics, Iowa City, IA USA), Annunziato (Ned) Amendola, MD (Duke University, Durham, NC USA); Jack T. Andrish, MD (Cleveland Clinic, Cleveland, OH USA); Christopher C. Annunziata, MD (Commonwealth Orthopaedics & Rehabilitation, Arlington, VA USA); Robert A. Arciero, MD (University of Connecticut Health Center, Farmington, CT USA); Bernard R. Bach Jr, MD (Rush University Medical Center, Chicago, IL USA); Champ L. Baker III, MD (The Hughston Clinic, Columbus, GA USA); Arthur R. Bartolozzi, MD (3B Orthopaedics, University of Pennsylvania Health System, Philadelphia, PA USA); Keith M. Baumgarten, MD (Orthopedic Institute, Sioux Falls, SD USA); Jeffery R. Bechler, MD (University Orthopaedic Associates LLC, Princeton, NJ USA); Jeffrey H. Berg, MD (Town Center Orthopaedic Associates, Reston, VA USA); Geoffrey A. Bernas, MD (State University of New York at Buffalo, Buffalo, NY); Stephen F. Brockmeier, MD (University of Virginia, Charlottesville, VA USA); Robert H. Brophy, MD (Washington University in St. Louis, St. Louis, MO USA); Charles A. Bush-Joseph, MD (Rush University Medical Center, Chicago, IL USA); J. Brad Butler V, MD (Orthopedic and Fracture Clinic, Portland, OR USA); John D. Campbell, MD (Bridger Orthopedic and Sports Medicine, Bozeman, MT USA); James L. Carey, MD, MPH (University of Pennsylvania, Philadelphia, PA USA); James E. Carpenter, MD (University of Michigan, Ann Arbor, MI USA); Brian J. Cole, MD (Rush University Medical Center, Chicago, IL USA); Jonathan M. Cooper, DO (HealthPartners Specialty Center, St. Paul, MN USA); Charles L. Cox, MD, MPH (Vanderbilt University, Nashville, TN USA); R. Alexander Creighton, MD (University of North Carolina Medical Center, Chapel Hill, NC USA); Diane L. Dahm, MD (Mayo Clinic, Rochester, MN USA); Tal S. David, MD (Synergy Specialists Medical Group, San Diego, CA USA); David C. Flanigan, MD (The Ohio State University, Columbus, OH USA); Robert W. Frederick, MD (The Rothman Institute/Thomas Jefferson University, Philadelphia, PA USA); Theodore J. Ganley, MD (Children’s Hospital of Philadelphia, Philadelphia, PA USA); Elizabeth A. Garofoli (Washington University in St. Louis, St. Louis, MO USA); Charles J. Gatt Jr, MD (University Orthopaedic Associates LLC, Princeton, NJ USA); Steven R. Gecha, MD (Princeton Orthopaedic Associates, Princeton, NJ USA); James Robert Giffin, MD (Fowler Kennedy Sport Medicine Clinic, University of Western Ontario, London Ontario, Canada); Sharon L. Hame, MD (David Geffen School of Medicine at UCLA, Los Angeles, CA USA); Jo A. Hannafin, MD, PhD (Hospital for Special Surgery, New York, NY USA); Christopher D. Harner, MD (University of Texas Health Center, Houston, TX USA); Norman Lindsay Harris Jr, MD (Grand River Health in Rifle, CO USA); Keith S. Hechtman, MD (UHZ Sports Medicine Institute, Coral Gables, FL USA); Elliott B. Hershman, MD (Lenox Hill Hospital, New York, NY USA); Rudolf G. Hoellrich, MD (Slocum Research and Education Foundation, Eugene, OR USA); Timothy M. Hosea, MD† (University Orthopaedic Associates LLC, Princeton, NJ USA); David C. Johnson, MD, (National Sports Medicine Institute, Leesburg, VA USA); Timothy S. Johnson, MD (National Sports Medicine Institute, Leesburg, VA USA); Morgan H. Jones, MD (Cleveland Clinic, Cleveland, OH USA); Christopher C. Kaeding, MD (The Ohio State University, Columbus, OH USA); Ganesh V. Kamath, MD (University of North Carolina Medical Center, Chapel Hill, NC USA); Thomas E. Klootwyk, MD (Methodist Sports Medicine, Indianapolis, IN USA); Bruce A. Levy, MD (Mayo Clinic Rochester, MN USA); C. Benjamin Ma, MD (University of California, San Francisco, CA USA); G. Peter Maiers II, MD (Methodist Sports Medicine Center, Indianapolis, IN USA); Robert G. Marx, MD (Hospital for Special Surgery, New York, NY USA); Matthew J. Matava, MD (Washington University in St. Louis, St. Louis, MO USA); Gregory M. Mathien, MD (Knoxville Orthopaedic Clinic, Knoxville, TN USA); David R. McAllister, MD (David Geffen School of Medicine at UCLA, Los Angeles, CA USA); Eric C. McCarty, MD (University of Colorado Denver School of Medicine, Denver, CO USA); Robert G. McCormack, MD (University of British Columbia/Fraser Health Authority, British Columbia, Canada); Bruce S. Miller, MD, MS (University of Michigan, Ann Arbor, MI USA); Carl W. Nissen, MD (Connecticut Children’s Medical Center, Hartford, CT USA); Daniel F. O’Neill, MD, EdD (Littleton Regional Healthcare, Littleton, NH USA); Brett D. Owens, MD (Warren Alpert Medical School, Brown University, Providence, RI USA); Richard D. Parker, MD (Cleveland Clinic, Cleveland, OH USA); Mark L. Purnell, MD (Aspen Orthopedic Associates, Aspen, CO USA); Arun J. Ramappa, MD (Beth Israel Deaconess Medical Center, Boston, MA USA); Michael A. Rauh, MD (State University of New York at Buffalo, Buffalo, NY USA); Arthur C. Rettig, MD (Methodist Sports Medicine, Indianapolis, IN USA); Jon K. Sekiya, MD (University of Michigan, Ann Arbor, MI USA); Kevin G. Shea, MD (Intermountain Orthopaedics, Boise, ID USA); Orrin H. Sherman, MD (NYU Hospital for Joint Diseases, New York, NY USA); James R. Slauterbeck, MD (Robert Larner College of Medicine, University of Vermont, Burlington, VT USA); Matthew V. Smith, MD (Washington University in St. Louis, St. Louis, MO USA); Jeffrey T. Spang, MD (University of North Carolina Medical Center, Chapel Hill, NC USA); LTC Steven J. Svoboda, MD (Keller Army Community Hospital, United States Military Academy, West Point, NY USA); Timothy N. Taft, MD (University of North Carolina Medical Center, Chapel Hill, NC USA); Joachim J. Tenuta, MD (Albany Medical Center, Albany, NY USA); Edwin M. Tingstad, MD (Inland Orthopaedic Surgery and Sports Medicine Clinic, Pullman, WA USA); Armando F. Vidal, MD (University of Colorado Denver School of Medicine, Denver, CO USA); Darius G. Viskontas, MD (Royal Columbian Hospital, New Westminster, BC Canada); Richard A. White, MD (Fitzgibbon’s Hospital, Marshall, MO USA); James S. Williams Jr, MD (Cleveland Clinic, Euclid, OH USA); Michelle L. Wolcott, MD (University of Colorado Denver School of Medicine, Denver, CO USA); Brian R. Wolf, MD (University of Iowa Hospitals and Clinics, Iowa City, IA USA); James J. York, MD (Orthopaedic and Sports Medicine Center, LLC, Pasedena, MD)
Footnotes
†We express our appreciation to the late Barton Mann, PHD (AOSSM, Rosemont, IL USA), Timothy M. Hosea, MD (University Orthopaedic Associates LLC, Princeton, NJ USA), and Allen F. Anderson, MD (Tennessee Orthopaedic Alliance, Nashville, TN) whose contribution to this work was of great significance.
BIBLIOGRAPHY
- 1.Mall NA, Chalmers PN, Moric M, et al. 2014. Incidence and trends of anterior cruciate ligament reconstruction in the United States. Am J Sports Med 42: 2363–70. [DOI] [PubMed] [Google Scholar]
- 2.Wright RW, Magnussen RA, Dunn WR and Spindler KP. 2011. Ipsilateral graft and contralateral ACL rupture at five years or more following ACL reconstruction: a systematic review. J Bone Joint Surg Am 93: 1159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spindler KP, Kuhn JE, Freedman KB, et al. 2004. Anterior cruciate ligament reconstruction autograft choice: bone-tendon-bone versus hamstring: does it really matter? A systematic review. Am J Sports Med 32: 1986–95. [DOI] [PubMed] [Google Scholar]
- 4.Wright RW, Dunn WR, Amendola A, et al. 2007. Anterior cruciate ligament revision reconstruction: two-year results from the MOON cohort. J Knee Surg 20: 308–11. [DOI] [PubMed] [Google Scholar]
- 5.Spindler KP and Wright RW. 2008. Clinical practice. Anterior cruciate ligament tear. N Engl J Med 359: 2135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spindler KP, Warren TA, Callison JC Jr., et al. 2005. Clinical outcome at a minimum of five years after reconstruction of the anterior cruciate ligament. J Bone Joint Surg Am 87: 1673–9. [DOI] [PubMed] [Google Scholar]
- 7.Wright RW, Gill CS, Chen L, et al. 2012. Outcome of revision anterior cruciate ligament reconstruction: a systematic review. J Bone Joint Surg Am 94: 531–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wright RW and Fetzer GB. 2007. Bracing after ACL reconstruction: a systematic review. Clin Orthop Relat Res 455: 162–8. [DOI] [PubMed] [Google Scholar]
- 9.Wright R, Spindler K, Huston L, et al. 2011. Revision ACL reconstruction outcomes: MOON cohort. J Knee Surg 24: 289–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wright RW. 2005. Knee sports injury outcome measures. J Knee Surg 18: 69–72. [DOI] [PubMed] [Google Scholar]
- 11.Wright RW. 2009. Knee injury outcomes measures. J Am Acad Orthop Surg 17: 31–9. [DOI] [PubMed] [Google Scholar]
- 12.Mars, Wright RW, Huston LJ, et al. 2010. Descriptive epidemiology of the Multicenter ACL Revision Study (MARS) cohort. Am J Sports Med 38: 1979–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cook NR, Moons KG and Harrell FE Jr. 2010. Assessing predictive performance beyond the Framingham risk score. Jama 303: 1368–9; author reply 1369. [DOI] [PubMed] [Google Scholar]
- 14.Dunn WR, Wolf BR, Amendola A, et al. 2004. Multirater agreement of arthroscopic meniscal lesions. Am J Sports Med 32: 1937–40. [DOI] [PubMed] [Google Scholar]
- 15.Marx RG, Connor J, Lyman S, et al. 2005. Multirater agreement of arthroscopic grading of knee articular cartilage. Am J Sports Med 33: 1654–7. [DOI] [PubMed] [Google Scholar]
- 16.Matava MJ, Arciero RA, Baumgarten KM, et al. 2015. Multirater agreement of the causes of anterior cruciate ligament reconstruction failure: a radiographic and video analysis of the MARS cohort. Am J Sports Med 43: 310–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wright RW, Johnson L, Brophy RH, et al. 2018. Revision Anterior Cruciate Ligament Reconstruction Outcomes at a Minimum of 5-Year Follow-Up: A Systematic Review. J Knee Surg [DOI] [PubMed]
- 18.Mars. 2015. Factors influencing graft choice in revision anterior cruciate ligament reconstruction in the MARS Group. Journal of Knee Surgery epub ahead of print: [DOI] [PMC free article] [PubMed]
- 19.Marsgroup. 2016. The Development and Early to Midterm Findings of the Multicenter Revision Anterior Cruciate Ligament Study. J Knee Surg 29: 528–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson DL, Swenson TM, Irrgang JJ, et al. 1996. Revision anterior cruciate ligament surgery: experience from Pittsburgh. Clin Orthop Relat Res 100–9. [DOI] [PubMed]
- 21.Brown CH Jr. and Carson EW. 1999. Revision anterior cruciate ligament surgery. Clin Sports Med 18: 109–71. [DOI] [PubMed] [Google Scholar]
- 22.Williams RJI, Warren RF, Carson EW and Wickiewicz TL. 1998. Revision Anterior Cruciate Ligament Reconstruction: The Hospital for Special Surgery Experience. Techniques in Orthopaedics 13: 375–383. [Google Scholar]
- 23.Morgan JA, Dahm D, Levy B, et al. 2012. Femoral tunnel malposition in ACL revision reconstruction. J Knee Surg 25: 361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marsgroup. 2013. Radiographic Findings in Revision Anterior Cruciate Ligament Reconstructions from the MARS Cohort. J Knee Surg 26: 239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wright RW and Marsgroup. 2014. Osteoarthritis Classification Scales: Interobserver Reliability and Arthroscopic Correlation. J Bone Joint Surg Am 96: 1145–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeng C, Gao SG, Li H, et al. 2016. Autograft Versus Allograft in Anterior Cruciate Ligament Reconstruction: A Meta-analysis of Randomized Controlled Trials and Systematic Review of Overlapping Systematic Reviews. Arthroscopy 32: 153–63 e18. [DOI] [PubMed] [Google Scholar]
- 27.Kaeding CC, Pedroza AD, Reinke EK, et al. 2015. Risk Factors and Predictors of Subsequent ACL Injury in Either Knee After ACL Reconstruction: Prospective Analysis of 2488 Primary ACL Reconstructions From the MOON Cohort. Am J Sports Med 43: 1583–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mars. 2018. Effect of graft choice on the 6 year outcome of revision anterior cruciate ligament reconstruction in the Multicenter ACL Revision Study (MARS) Cohort. AAOS Abstract
- 29.Marsgroup. 2016. Meniscal and Articular Cartilage Predictors of Clinical Outcome Following Revision Anterior Cruciate Ligament Reconstruction. American Journal of Sports Medicine 44: 1671–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brophy RH, Wright RW, David TS, et al. 2012. Association between previous meniscal surgery and the incidence of chondral lesions at revision anterior cruciate ligament reconstruction. Am J Sports Med 40: 808–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brophy RH, Haas AK, Huston LJ, et al. 2015. Association of Meniscal Status, Lower Extremity Alignment, and Body Mass Index With Chondrosis at Revision Anterior Cruciate Ligament Reconstruction. Am J Sports Med 43: 1616–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mars, Magnussen RA, Borchers JR, et al. 2018. Risk Factors and Predictors of Significant Chondral Surface Change From Primary to Revision Anterior Cruciate Ligament Reconstruction: A MOON and MARS Cohort Study. Am J Sports Med 46: 557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marsgroup. 2018. Meniscus Repair in the Setting of Revision ACL Reconstruction: Results from the MARS Cohort. AJSM
- 34.Mars, Allen CR, Anderson AF, et al. 2017. Surgical Predictors of Clinical Outcomes After Revision Anterior Cruciate Ligament Reconstruction. Am J Sports Med 45: 2586–2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mars, Ding DY, Zhang AL, et al. 2017. Subsequent Surgery After Revision Anterior Cruciate Ligament Reconstruction: Rates and Risk Factors From a Multicenter Cohort. Am J Sports Med 45: 2068–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marsgroup. 2017. Rehabilitation Predictors of Clinical Outcome following Revision ACL Reconstruction. AJSM In Press: [DOI] [PMC free article] [PubMed]
- 37.Marsgroup. 2018. Predictors of Patient Reported Outcomes at Two Years Following Revision ACL Reconstruction. AJSM
- 38.Borchers JR, Kaeding CC, Pedroza AD, et al. 2011. Intra-articular findings in primary and revision anterior cruciate ligament reconstruction surgery: a comparison of the MOON and MARS study groups. Am J Sports Med 39: 1889–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Magnussen RA, Trojani C, Granan LP, et al. 2015. Patient demographics and surgical characteristics in ACL revision: a comparison of French, Norwegian, and North American cohorts. Knee Surg Sports Traumatol Arthrosc 23: 2339–48. [DOI] [PMC free article] [PubMed] [Google Scholar]